Abstract
Background
Chronic obstructive pulmonary disease (COPD) is associated with persistent systemic inflammation. Anti-inflammatory therapies have been shown to decrease acute exacerbations of COPD. The antidiabetic medication metformin decreases oxidative stress and inflammation and may benefit patients with COPD. We aimed at investigating the effect of metformin on health care utilizations in patients with coexisting COPD and diabetes mellitus (DM).
Methods
We studied 5% Medicare beneficiaries with coexisting COPD and DM prescribed metformin or other antidiabetics during the period 2007–2010. The primary outcome was COPD-specific emergency room (ER) visits and hospitalizations; the secondary outcome was all-cause ER visits and hospitalizations over the 2-year follow-up after the index antidiabetic prescription. The effects of metformin were examined by COPD complexity and compared with the effects of other antidiabetic medications.
Results
Among 11,260 patients, 3,193 were metformin users and 8,067 were nonusers. Metformin users were younger, were less sick, were less likely to be on oxygen, and had fewer hospitalizations in the prior year compared with the nonusers. Over a 2-year period, metformin users had lower COPD-specific and all-cause ER visits and hospitalizations (7.11% vs 9.61%, p<0.0001; and 61.63% vs 71.27%, p<0.0001, respectively). In a stratified multivariable analysis, the odds of COPD-specific ER visits and hospitalizations were lower in patients with low-complexity COPD (adjusted odds ratio =0.66, 95% confidence interval =0.52–0.85). However, patients with all COPD complexities get benefits of metformin on all-cause ER visits and hospitalizations.
Conclusion
The use of metformin in patients with coexisting COPD and DM was associated with fewer COPD-specific ER visits and hospitalizations, especially in low-complexity COPD.
Keywords: COPD, diabetes, metformin, ER visits, hospitalization, Medicare
Introduction
The prevalence of chronic obstructive pulmonary disease (COPD) increases with aging, and diabetes mellitus (DM) is a common comorbidity in patients with COPD.1–3 Inflammation is a common feature in all three conditions: COPD, DM, and aging.1,3,4 Various anti-inflammatory therapies such as phosphodiesterase-4 inhibitor, N-acetylcysteine, carbocisteine, and macrolide have been shown to reduce airway inflammation and prevent acute exacerbations of COPD.5–8
Metformin, a biguanide, is a first-line antidiabetic drug with diverse pleotropic properties. Metformin activates both adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways, resulting in reduction in oxidative stress and chronic inflammation.9,10 Animal and clinical studies have suggested other beneficial effects of metformin besides diabetes control. Metformin decreases inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-6, and IL-1, and the inflammatory response of macrophages and induces the production of anti-inflammatory cytokines such as IL-4 and IL-10.11,12 In the animal model, metformin has been shown to decrease eosinophilic inflammation, airway remodeling, and peribronchial and parenchymal fibrosis.13–15 In clinical studies, metformin use in patients with COPD and DM is associated with improvement in respiratory symptoms, St George Respiratory Questionnaire (SGRQ) and transitional dyspnea index scores, respiratory muscle function,16 and overall survival among long-term users.17
It is plausible that metformin use in patients with COPD decreases both local and systemic inflammation and oxidative damage. Our hypothesis was that patients with coexisting COPD and DM on metformin have lower health care utilization as measured by percentage of patients with an emergency room (ER) visits and hospitalizations during a 2-year period. Our primary outcome of interest was COPD-specific ER visits and hospitalizations, and the secondary outcome was all-cause ER visits and hospitalizations.
Methods
Data source
This is a retrospective study using 5% Medicare beneficiaries’ data from the year 2006 to 2012. Over 98% of adults in the USA aged ≥65 years were enrolled in Medicare, which serves >45 million beneficiaries. The Centers for Medicare and Medicaid Services selected a random sample of 5% Medicare beneficiaries based on the eighth and ninth digits (05, 20, 45, 70, and 95) of their health insurance claim number. This population is the standard available for research purposes and has been shown to be representative of the whole cohort. Medicare Beneficiary Summary Files, Medicare Provider Analysis and Review (MedPAR) Files, Outpatient Standard Analytical Files (OutSAFs), Medicare Carrier Files, Durable Medical Equipment (DME) Files, and Prescription Drug Event (PDE) records18 were used for the study. We also used the Chronic Condition Data Warehouse (CCW) to identify patients with coexisting COPD and DM.19 The CCW data were linked by a unique, unidentifiable beneficiary key, which allows researchers to analyze information across the continuum of care. The 27 CCW chronic condition categories include COPD and DM, available from 1999.19 The Research Data Assistance Center provided deidentified data for the research purpose and, due to the nature of the study, patient consent was not required. The study was approved by the University of Texas Medical Branch Institutional Review Board (Project 09-054).
Study cohort
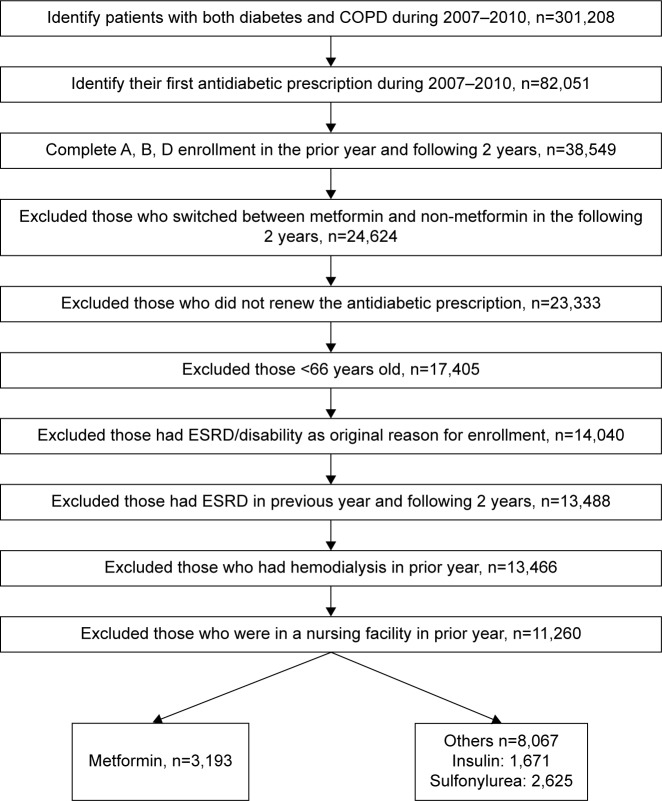
Figure 1 shows the establishment of the study cohort. The study cohort consisted of patients with both COPD and DM, who received antidiabetic treatment between 2007 and 2010. First, we selected beneficiaries who had both COPD and DM between 2007 and 2010 by using CCW chronic condition flags and identified those who had any antidiabetic prescription in the same period. We included those who had their first antidiabetic prescription either in the same year when both COPD and DM were diagnosed or in the year after. The date of the first antidiabetic prescription between 2007 and 2010 was used as the index date.
Figure 1.
Establishment of the cohort.
Abbreviations: COPD, chronic obstructive pulmonary disease; ESRD, end-stage renal disease.
We then selected those who had complete Medicare Parts A, B, and D enrollment in the year prior to and 2 years after the index date. In order to ensure that the effects observed would be associated with a particular antidiabetic, we excluded those who switched between metformin and non-metformin or those who did not renew the antidiabetic prescription. Lastly, we excluded those who were younger than 66 years, who had end-stage renal disease (ESRD)/disability as the original reason for enrollment, who had ESRD in the previous year and in the following 2 years, who had hemodialysis in the prior year, or who were in a nursing facility in the prior year. Hemodialysis was identified by Current Procedural Terminology (CPT) codes 90935, 90937, 90940, and 99512 from OutSAFs and Medicare Carrier Files, and nursing facility services were identified by using CPT codes 99301–99313, 99315, 99316, and 99318 from Medicare Carrier Files. Among these 11,260 patients, 3,193 were metformin users; the rest 8,067 used other antidiabetics, including insulin, sulfonylurea, acarbose, exenatide, glucagon, miglitol, nateglinide, pioglitazone, pramlintide, repaglinide, rosiglitazone, saxagliptin, and sitagliptin.
Measures
The patients were categorized by age, gender, race/ethnicity, and socioeconomic status. We used the Medicare–Medicaid dual eligibility as a proxy of low socioeconomic status. Total hospitalizations in the prior year were identified from MedPAR Files. Oxygen use in the prior year was identified by Healthcare Common Procedure Coding System codes E1390, E1391, and E1392 from DME Files. Spirometry was identified through CPT codes (94010, 94014, 94015, 94016, 94060, 94070, and 94620). Medication for COPD in the follow-up period, including long-acting β-agonists (LABAs), long-acting muscarinic antagonists (LAMAs), inhaled corticosteroids (ICS), ICS with LABAs, short-acting β-agonists, and short-acting muscarinic antagonist, was identified from PDE records.
The following conditions in the prior year were identified by either one inpatient (MedPAR) claim or two outpatient/physician (Carrier) claims at least 30 days apart with the following International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes: gastroesophageal reflux disease (GERD; 530.x–535.x); lung cancer (162.x); anxiety/depression (296.2, 296.3, 296.5, 300.0, 300.4, 309.x, and 311.x); sarcopenia (728.2); osteoporosis (733.0); cardiovascular disease (CVD; including ischemic heart disease; 410.x, 411.x, 412.x, 413.x, and 414.x), cerebrovascular disease (433.x, 434.x, 435.x, 436.x, 437.x, and 438.x), arrhythmia (427.x), heart failure (428.x), and artery disease (440.x, 441.x, 442.x, 443.2x, 443.9x, and 444.x).
To adjust for severity of COPD and DM, COPD severity was categorized as low, moderate, and high complexity as described by Mapel et al,20 and DM was categorized as uncontrolled DM and DM with complications, as published earlier.21
Study outcomes
The primary outcome of this study was COPD-specific ER visits and hospitalizations in the 2 years following the index antidiabetic prescription. The secondary outcome was all-cause ER visits and hospitalizations. ER visit was identified by the provider-assigned revenue codes 0450–0459 and 0981 from claims in OutSAFs. Hospitalizations were identified using MedPAR claims. COPD-specific diagnoses (ER visits and hospitalizations) were identified by claims with ICD-9-CM codes 491.21, 491.22, 491.8, 491.9, 492.8, 493.20, 493.21, 493.22, and 496.x as the primary diagnosis or claims with ICD-9-CM codes 518.81, 518.82, 518.84, and 799.1 as the primary diagnosis and 491.21, 491.22, 493.21, and 493.22 as one of the secondary diagnosis. All others were defined as all-cause ER visits and hospitalizations.
Statistical analyses
We calculated the proportion of patients with COPD-specific and all-cause ER visits and hospitalizations during the follow-up period for metformin user and nonuser groups. The distributions of patient characteristics were calculated, and the difference among groups was examined by χ2 tests or Fisher’s exact test. The difference in COPD-specific and all-cause outcomes among groups was examined by χ2 tests. The effect of group characteristics (metformin users vs nonusers) on the likelihood of having any COPD-specific or all-cause ER visit or hospitalization was examined by logistic regression, adjusted for age, gender, race, dual eligibility, prior-year hospitalization, COPD complexity, diabetes complexity, comorbidities (GERD, anxiety and depression, CVD, sarcopenia, and osteoporosis), prior-year oxygen use, and COPD maintenance medications use. The effect of metformin was stratified by the severity of COPD (low complexity vs moderate/high complexity) on these outcomes. The secondary analysis was performed to study the effect of individual antidiabetic medications (categorized as metformin, sulfonylurea, insulin, and other) on the incidence of COPD-specific and all-cause ER visits and hospitalizations. The “other” group included those who were on one or more antidiabetics other than insulin, metformin, and sulfonylurea. All analyses were performed with SAS Version 9.4 (SAS Inc., Cary, NC, USA). All reported p-values were two-sided, and p<0.05 was considered statistically significant.
Results
Table 1 shows the baseline characteristics of the 11,260 Medicare patients with coexisting COPD and DM who had received antidiabetic medication from 2007 to 2010 and were followed up for 2 years. Of these, 3,193 patients were on metformin and 8,067 were on other antidiabetic medications.
Table 1.
Baseline characteristics of patients with coexisting COPD and DM during 2007–2010 by metformin use
| Patient characteristics | Metformin use
|
p-valuec | |
|---|---|---|---|
| Yesa (n=3,193) | Nob (n=8,067) | ||
| Age (years) | <0.0001 | ||
| 66–74 | 1,501 (47.01) | 2,737 (33.93) | |
| 75–84 | 1,400 (43.85) | 3,958 (49.06) | |
| 85+ | 292 (9.15) | 1,372 (17.01) | |
| Gender | 0.16 | ||
| Male | 1,042 (32.63) | 2,522 (31.26) | |
| Female | 2,151 (67.37) | 5,545 (68.74) | |
| Race/ethnicity | <0.0001 | ||
| White | 2,513 (78.70) | 5,775 (71.59) | |
| Non-white | 680 (21.30) | 2,292 (28.41) | |
| Dual eligibility | 1,268 (39.71) | 4,408 (54.64) | <0.0001 |
| Hospitalizations, prior year | <0.0001 | ||
| 0 | 2,474 (77.48) | 5,509 (68.29) | |
| 1 | 506 (15.85) | 1,592 (19.73) | |
| 2+ | 213 (6.67) | 966 (11.97) | |
| COPD complexity | 0.036 | ||
| Low | 2,354 (73.72) | 5,757 (71.36) | |
| Moderate | 704 (22.05) | 1,958 (24.27) | |
| High | 135 (4.23) | 352 (4.36) | |
| Complications of DM | 472 (14.78) | 2,697 (33.43) | <0.0001 |
| Uncontrolled DM | 672 (21.05) | 2,802 (34.73) | <0.0001 |
| Oxygen use, prior year | 371 (11.62) | 1,180 (14.63) | <0.0001 |
| Spirometry, prior year and following 2 years | 960 (30.07) | 2,319 (28.75) | 0.17 |
| Comorbidities | |||
| GERD, prior year | 382 (11.96) | 987 (12.24) | 0.69 |
| Anxiety/depression, prior year | 261 (8.17) | 639 (7.92) | 0.66 |
| CVD, prior year | 1,335 (41.81) | 4,011 (49.72) | <0.0001 |
| Sarcopenia, prior year | 4 (0.13) | 8 (0.10) | 0.70 |
| Osteoporosis, prior year | 149 (4.67) | 384 (4.76) | 0.83 |
| COPD medication, 2-year follow-up | 0.0001 | ||
| Long-acting medication only | 378 (11.84) | 834 (10.34) | |
| Short-acting medication only | 427 (13.37) | 1,329 (16.47) | |
| Both | 625 (19.57) | 1,598 (19.81) | |
| None | 1,763 (55.21) | 4,306 (53.38) | |
Notes:
Those who had coexisting COPD and DM and received prescription for metformin during 2007–2010.
Those who had coexisting COPD and DM and received antidiabetics prescription other than metformin, such as insulin, sulfonylurea, acarbose, exenatide, glucagon, miglitol, nateglinide, pioglitazone, pramlintide, repaglinide, rosiglitazone, saxagliptin, or sitagliptin during 2007–2010.
χ2 test and Fisher’s exact test for sarcopenia. Data presented as n (%) unless otherwise indicated.
Abbreviations: COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; GERD, gastroesophageal reflux disease.
Metformin users were younger, were less sick with fewer cardiovascular comorbidities, were less likely to be on oxygen, and had better controlled diabetes with fewer complications and fewer hospitalizations in the prior year compared with metformin nonusers. The two groups had no significant difference in sarcopenia, osteoporosis, anxiety and depression, and GERD. In addition, the two groups had no significant difference in receiving spirometry in the prior year and the following 2 years. Metformin nonusers had more complex COPD and were more likely to be on COPD maintenance medications such as LABA/LAMA and/or ICS.
Table 2 shows health care-related outcomes over a 2-year period in metformin users vs nonusers. COPD-specific and all-cause ER visits and hospitalizations were lower in metformin users (7.11% vs 9.61%, p<0.0001; and 61.63% vs 71.27%, p<0.0001, respectively) compared with metformin nonusers. Outcomes varied by COPD complexity. Patients with low-complexity COPD had fewer COPD-specific ER visits and hospitalizations, whereas patients with low- and moderate-complexity COPD had fewer all-cause ER visits and hospitalizations.
Table 2.
ER visits and hospitalizations over a 2-year follow-up period in patients with coexisting COPD and DM
| ER visits and hospitalizations | Metformin users
|
p-valuec | |
|---|---|---|---|
| Yesa n (%) | Nob n (%) | ||
| COPD-specific (overall) | 227 (7.11) | 775 (9.61) | <0.0001 |
| COPD complexity | |||
| Low | 97 (4.12) | 350 (6.08) | 0.0004 |
| Moderate | 108 (15.34) | 370 (18.90) | 0.035 |
| High | 22 (16.30) | 55 (15.63) | 0.86 |
| All-cause (overall) | 1,968 (61.63) | 5,749 (71.27) | <0.0001 |
| COPD complexity | |||
| Low | 1,370 (58.20) | 3,887 (67.52) | <0.0001 |
| Moderate | 496 (70.45) | 1,578 (80.59) | <0.0001 |
| High | 102 (75.56) | 284 (80.68) | 0.21 |
Notes:
Those who had coexisting COPD and DM and received prescription for metformin during 2007–2010.
Those who had coexisting COPD and DM and received antidiabetics prescription other than metformin, such as insulin, sulfonylurea, acarbose, exenatide, glucagon, miglitol, nateglinide, pioglitazone, pramlintide, repaglinide, rosiglitazone, saxagliptin, or sitagliptin during 2007–2010.
χ2 test.
Abbreviations: COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ER, emergency room.
We also examined the interaction effects of severity of COPD and types of antidiabetic users on all-cause and COPD-specific ER visits and hospitalizations. Although the interactions were not clinically significant, it is important to assess the effect of metformin stratified by the severity of COPD (low complexity vs moderate/high complexity) on these outcomes. Table 3 presents the odds of ER visits and hospitalizations based on the complexity of COPD after adjusting for baseline characteristics as shown in Table 1, except spirometry to avoid collinearity with COPD complexity. Only those with low-complexity COPD had significantly lower odds of COPD-specific ER visits and hospitalizations (adjusted odds ratio [OR] =0.66; 95% confidence interval [CI] =0.52–0.85). Metformin users had lower rates of all-cause ER visits and hospitalizations irrespective of COPD complexity.
Table 3.
Odds of ER visits and hospitalizations in patients with coexisting COPD and DM after the initial antidiabetic prescription during 2007–2010
| Antidiabetic use | ER visits or hospitalizations odds ratio (95% confidence interval)a
|
|||
|---|---|---|---|---|
| COPD-specific
|
All-cause
|
|||
| COPD complexity
|
COPD complexity
|
|||
| Low | Moderate/high | Low | Moderate/high | |
| Metformin use | ||||
| Nob | Reference | Reference | Reference | Reference |
| Yesc | 0.66 (0.52–0.85) | 0.82 (0.64–1.04) | 0.73 (0.65–0.81) | 0.72 (0.59–0.88) |
Notes:
Multivariable logistic regression model was used to estimate odds ratio, adjusted by age, gender, race, dual eligibility, prior-year hospitalization, DM complexity, comorbidities (GERD, anxiety and depression, CVD, sarcopenia, and osteoporosis), prior-year oxygen use, and COPD maintenance medications use.
Those who had coexisting COPD and DM and received antidiabetics prescription other than metformin, such as insulin, sulfonylurea, acarbose, exenatide, glucagon, miglitol, nateglinide, pioglitazone, pramlintide, repaglinide, rosiglitazone, saxagliptin, or sitagliptin during 2007–2010.
Those who had coexisting COPD and DM and received prescription for metformin during 2007–2010.
Abbreviations: COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; ER, emergency room; GERD, gastroesophageal reflux disease.
Table 4 shows the effect of each antidiabetic medication on COPD outcomes. Among 8,067 metformin nonusers, 1,671 used only insulin, 2,625 used only sulfonylureas, and 3,771 used other antidiabetics over a 2-year period. Among all the antidiabetics, metformin had the highest reduction in odds for COPD-specific ER visits and hospitalizations, especially in patients with low-complexity COPD.
Table 4.
Odds of ER visits/hospitalizations in patients with coexisting COPD and DM after the index antidiabetic prescription during 2007–2010
| Antidiabetics | ER visits or hospitalizations odds ratio (95% confidence interval)a
|
|||
|---|---|---|---|---|
| COPD-specific
|
All-cause
|
|||
| COPD complexity
|
COPD complexity
|
|||
| Low | Moderate/high | Low | Moderate/high | |
| Insulinb | Reference | Reference | Reference | Reference |
| Metforminc | 0.48 (0.35–0.67) | 0.73 (0.53–1.01) | 0.59 (0.50–0.69) | 0.57 (0.42–0.78) |
| Sulfonyluread | 0.60 (0.44–0.83) | 0.81 (0.59–1.11) | 0.69 (0.58–0.81) | 0.68 (0.50–0.93) |
| Othere | 0.70 (0.53–0.93) | 0.94 (0.71–1.25) | 0.86 (0.73–0.99) | 0.85 (0.63–1.13) |
Notes:
Multivariable logistic regression model was used to estimate odds ratio, adjusted by age, gender, race, dual eligibility, prior-year hospitalization, DM complexity, comorbidities (GERD, anxiety and depression, CVD, sarcopenia, and osteoporosis), prior-year oxygen use, and COPD maintenance medications use.
Those who had coexisting COPD and DM and received prescription for insulin during 2007–2010.
Those who had coexisting COPD and DM and received prescription for metformin during 2007–2010.
Those who had coexisting COPD and DM and received prescription for sulfonylurea during 2007–2010.
Those who had coexisting COPD and DM and received antidiabetics prescription other than metformin, insulin, and sulfonylurea, such as acarbose, exenatide, glucagon, miglitol, nateglinide, pioglitazone, pramlintide, repaglinide, rosiglitazone, saxagliptin, or sitagliptin during 2007–2010.
Abbreviations: COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; ER, emergency room; GERD, gastroesophageal reflux disease.
Discussion
Our study results can be summarized as follows: patients with coexisting COPD and DM who used metformin had lower health care utilization as compared to nonusers. The most benefit is found in patients with low COPD complexity.
The findings of our study build upon prior studies of metformin use in patients with COPD. Sexton et al conducted an open-label observational study in 17 patients with COPD and diet-controlled diabetes or impaired glucose tolerance. Metformin was given daily to all participants for 6 months. In comparison with baseline, follow-up at 26 weeks showed improvement in transitional dyspnea score, SGRQ score, and improved respiratory muscle strength PImax.16 In a single-center retrospective study, Hitchings et al followed 130 patients with COPD and DM, finding that, overall, metformin users had better survival (5.2 years, 95% CI =4.5–5.8, vs 1.9 years, 95% CI =1.1–2.6).17 Li et al showed that metformin users had fewer asthma-related hospitalizations (OR =0.21, 95% CI =0.07–0.63) and asthma exacerbation (OR =0.39, 95% CI =0.19–0.79) as compared to nonusers.22
The biological plausibility of the beneficial effects of metformin in patients with COPD is likely related to its pleotropic properties. Metformin is shown to decrease airway inflammation, airway remodeling, peribronchial and parenchymal fibrosis, and pro-inflammatory cytokines and to induce the production of anti-inflammatory cytokines.11–15 Skeletal muscle dysfunction is common in elderly adults23 and in those with COPD3,24 and DM.25 Sarcopenia is associated with poor outcomes in COPD.24,26 Metformin enhances skeletal muscle function by improving mitochondrial biogenesis in muscle and activating AMPK.27,28
With worsening airflow limitation, the degree of inflammation worsens in patients with COPD, predisposing them to systemic comorbidities.29,30 Indeed, cardiovascular and other comorbidities are higher in COPD patients with moderate and severe diseases as compared to those with mild disease,31–33 perhaps reflecting that the common inflammatory pathway is more amenable to modification in the earlier stages of the disease. In advanced COPD, metformin may not be able to reduce excessive spillover of pulmonary inflammation, or merely reducing inflammation may not be enough to abate pulmonary symptoms. This inflammatory effect likely explains the benefit of metformin in patients with low COPD complexity.
Congestive heart failure (CHF) and chronic kidney disease (CKD) are common in patients with COPD and diabetes.33 All-cause ER visits and hospitalizations were decreased in both low-complexity and moderate-/high-complexity patients and consistent with the recent findings of a systematic review by Crowley et al that CHF and CKD patients on metformin had fewer CHF readmissions and lower all-cause mortality.34 The role of metformin in the exacerbation of COPD without diabetes was studied by Hitchings et al. After metformin was given to patients during an acute exacerbation of COPD for 4 weeks, there was no significant difference in inflammatory markers as C-reactive protein (CRP) or in clinical parameters measured by the COPD Assessment Test score and the EXAcerbations of Chronic Pulmonary Disease Tool score.35 The null effects were likely due to the short duration of therapy. Sexton et al found that 6 months of metformin improved respiratory symptoms and respiratory muscle strength and resulted in a trend toward a decrease in CRP.16 Cameron et al found that inflammatory markers were below the baseline after 8–12 months of metformin use.11 Apparently, a longer duration of therapy is needed to show the beneficial effect of metformin.
Limitations
Our study findings should be interpreted within the context of the following limitations. First, our study is limited to fee-for-service Medicare beneficiaries and may not be generalizable to the non-Medicare population. Second, we used ICD-9-CM codes to establish a diagnosis of COPD and DM; these codes do not reflect disease severity. Administrative database can only provide the percentage of patients with COPD who had a spirometry during the study period, but not the actual values to determine whether or not a patient has airway obstruction. However, we used an administrative algorithm to capture the COPD and DM severity based on prior publications.20,21 Third, systemic chronic diseases interact with each other. Whether the beneficial effects of metformin are due to better control of diabetes, in a less sick patient with fewer exacerbations, or the direct effects of metformin altering the natural course of COPD is uncertain and beyond the scope of our study. Fourth, the surrogate of medication use is prescription refill, and the use of this variable might not capture the actual use of and adherence to antidiabetics and COPD maintenance medication. Fifth, other cointerventions to prevent exacerbations such as smoking cessation, pulmonary rehabilitation, vaccination status, or the use of macrolides, roflumilast and mucolytic, were not examined. Sixth, the results of any observational study are subject to possible selection bias and unmeasured confounding. This is a hypothesis-generating study, and a randomized control trial should be performed to examine the effect of metformin in patients with COPD and DM.
Conclusion
Metformin use in Medicare patients with coexisting COPD and DM was associated with lower odds of COPD-specific ER visits and hospitalizations in patients with low-complexity COPD.
Acknowledgments
The authors thank Sarah Toombs-Smith, PhD, ELS, for help with the preparation of the manuscript. This work was supported by a grant (R01-HS020642) from the Agency for Healthcare Research. Funders had no role in the preparation, approval, or submission of the results. The abstract was presented in Annual CHEST Meeting, October 24, 2016, Los Angeles, CA (http://journal.chestnet.org/article/S0012-3692(16)57195-5/pdf).
Footnotes
Disclosure
Gulshan Sharma serves on the Advisory Board of Theravance Biopharma, Mylan, and Sunovion pharmaceutical companies. The other authors have no conflicts of interest in this work.
References
- 1.MacNee W. Is chronic obstructive pulmonary disease an accelerated aging disease? Ann Am Thorac Soc. 2016;13(Supplement_5):S429–S437. doi: 10.1513/AnnalsATS.201602-124AW. [DOI] [PubMed] [Google Scholar]
- 2.Department of Noncommunicable Disease Prevention and Health Promotion, WHO . Active Ageing – A Policy Framework. Geneva: WHO; 2002. p. 59. [Google Scholar]
- 3.Rogliani P, Lucà G, Lauro D. Chronic obstructive pulmonary disease and diabetes. COPD Res Prac. 2015;1:3. [Google Scholar]
- 4.Faner R, Sobradillo P, Noguera A, et al. The inflammasome pathway in stable COPD and acute exacerbations. ERJ Open Res. 2016;2(3):00002–2016. doi: 10.1183/23120541.00002-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agustí A, Calverley PM, Decramer M, Stockley RA, Wedzicha JA. Prevention of exacerbations in chronic obstructive pulmonary disease: knowns and unknowns. Chronic Obstr Pulm Dis. 2014;1(2):166–184. doi: 10.15326/jcopdf.1.2.2014.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:81–90. doi: 10.2147/COPD.S89849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman I. Pharmacological antioxidant strategies as therapeutic interventions for COPD. Biochim Biophys Acta. 2012;1822(5):714–728. doi: 10.1016/j.bbadis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(7):CD001287. doi: 10.1002/14651858.CD001287.pub5. [DOI] [PubMed] [Google Scholar]
- 9.Pryor R, Cabreiro F. Repurposing metformin: an old drug with new tricks in its binding pockets. Biochem J. 2015;471(3):307–322. doi: 10.1042/BJ20150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K, Colley T, Mercado N. Geroprotectors as a novel therapeutic strategy for COPD, an accelerating aging disease. Int J Chron Obstruct Pulmon Dis. 2012;7:641–652. doi: 10.2147/COPD.S28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron AR, Morrison VL, Levin D, et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ Res. 2016;119(5):652–665. doi: 10.1161/CIRCRESAHA.116.308445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyun B, Shin S, Lee A, et al. Metformin down-regulates TNF-α secretion via suppression of scavenger receptors in macrophages. Immune Netw. 2013;13(4):123–132. doi: 10.4110/in.2013.13.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calixto MC, Lintomen L, André DM, et al. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PLoS One. 2013;8(10):e76786. doi: 10.1371/journal.pone.0076786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park CS, Bang BR, Kwon HS, et al. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem Pharmacol. 2012;84(12):1660–1670. doi: 10.1016/j.bcp.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Takasaka N, Yoshida M, et al. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res. 2016;17(1):107. doi: 10.1186/s12931-016-0420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sexton P, Metcalf P, Kolbe J. Respiratory effects of insulin sensitisation with metformin: a prospective observational study. COPD. 2014;11(2):133–142. doi: 10.3109/15412555.2013.808614. [DOI] [PubMed] [Google Scholar]
- 17.Hitchings AW, Archer JR, Srivastava SA, Baker EH. Safety of metformin in patients with chronic obstructive pulmonary disease and type 2 diabetes mellitus. COPD. 2015;12(2):126–131. doi: 10.3109/15412555.2015.898052. [DOI] [PubMed] [Google Scholar]
- 18.Research Data Assistance Center [homepage on the Internet] CMS data file. Centers for medicare and medicaid services; [Accessed August 26, 2017]. Available from: https://www.resdac.org/cms-data. [Google Scholar]
- 19.Chronic Conditions Data Warehouse [homepage on the Internet] Condition categories. [Accessed August 26, 2017]. Available from: https://www.ccwdata.org/web/guest/condition-categories.
- 20.Mapel DW, Dutro MP, Marton JP, Woodruff K, Make B. Identifying and characterizing COPD patients in US managed care. A retrospective, cross-sectional analysis of administrative claims data. BMC Health Serv Res. 2011;11:43. doi: 10.1186/1472-6963-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo YF, Goodwin JS, Chen NW, Lwin KK, Baillargeon J, Raji MA. Diabetes mellitus care provided by nurse practitioners vs primary care physicians. J Am Geriatr Soc. 2015;63(10):1980–1988. doi: 10.1111/jgs.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CY, Erickson SR, Wu CH. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology. 2016;21(7):1210–1218. doi: 10.1111/resp.12818. [DOI] [PubMed] [Google Scholar]
- 23.Papa EV, Dong X, Hassan M. Skeletal muscle function deficits in the elderly: current perspectives on resistance training. J Nat Sci. 2017;3(1) pii: e272. [PMC free article] [PubMed] [Google Scholar]
- 24.Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213–218. doi: 10.1136/thoraxjnl-2014-206440. [DOI] [PubMed] [Google Scholar]
- 25.Sreekumar R, Nair KS. Skeletal muscle mitochondrial dysfunction & diabetes. Indian J Med Res. 2007;125(3):399–410. [PubMed] [Google Scholar]
- 26.Costa TM, Costa FM, Moreira CA, Rabelo LM, Boguszewski CL, Borba VZ. Sarcopenia in COPD: relationship with COPD severity and prognosis. J Bras Pneumol. 2015;41(5):415–421. doi: 10.1590/S1806-37132015000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 29.Barceló B, Pons J, Fuster A, et al. Intracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary disease. Clin Exp Immunol. 2006;145(3):474–479. doi: 10.1111/j.1365-2249.2006.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perret J, McDonald C, Apostolopoulos V. Elevated serum interleukin-5 levels in severe chronic obstructive pulmonary disease. Acta Biochim Biophys Sin (Shanghai) 2017;49(6):560–563. doi: 10.1093/abbs/gmx030. [DOI] [PubMed] [Google Scholar]
- 31.Dal Negro RW, Bonadiman L, Turco P. Prevalence of different comorbidities in COPD patients by gender and GOLD stage. Multidiscip Respir Med. 2015;10(1):24. doi: 10.1186/s40248-015-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blasi F, Neri L, Centanni S, Falcone F, Di Maria G. Clinical characterization and treatment patterns for the frequent exacerbator phenotype in chronic obstructive pulmonary disease with severe or very severe airflow limitation. COPD. 2017;14(1):15–22. doi: 10.1080/15412555.2016.1232380. [DOI] [PubMed] [Google Scholar]
- 33.Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–475. doi: 10.1183/09059180.00008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowley MJ, Diamantidis CJ, McDuffie JR, et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166(3):191–200. doi: 10.7326/M16-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hitchings AW, Lai D, Jones PW, Baker EH, Metformin in COPD Trial Team Metformin in severe exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2016;71(7):587–593. doi: 10.1136/thoraxjnl-2015-208035. [DOI] [PMC free article] [PubMed] [Google Scholar]