Abstract
Cytosine DNA methylation is highly conserved epigenetic modification involved in a wide range of biological processes in eukaryotes. It was established and maintained by cytosine-5 DNA methyltransferases (C5-MTases) in plants. Through genome-wide identification, eight putative SmC5-MTase genes were identified from the genome of Salvia miltiorrhiza, a well-known traditional Chinese medicine material and an emerging model medicinal plant. Based on conserved domains and phylogenetic analysis, eight SmC5-MTase genes were divided into four subfamilies, including MET, CMT, DRM and DNMT2. Genome-wide comparative analysis of the C5-MTase gene family in S. miltiorrhiza and Arabidopsis thaliana, including gene structure, sequence features, sequence alignment and conserved motifs, was carried out. The results showed conservation and divergence of the members of each subfamily in plants. The length of SmC5-MTase open reading frames ranges widely from 1,152 (SmDNMT2) to 5,034 bp (SmMET1). The intron number of SmC5-MTases varies between 7 (SmDRM1) and 20 (SmCMT1 and SmCMT2b). These features were similar to their counterparts from Arabidopsis. Sequence alignment and conserved motif analysis showed the existence of highly conserved and subfamily-specific motifs in the C5-MTases analyzed. Differential transcript abundance was detected for SmC5-MTases, implying genome-wide variance of DNA methylation in different organs and tissues. Transcriptome-wide analysis showed that the transcript levels of all SmC5-MTase genes was slightly changed under yeast extract and methyl jasmonate treatments. Six SmC5-MTases, including SmMET1, SmCMT1, SmCMT2a, SmCMT2b, SmCMT3 and SmDRM1, were salicylic acid-responsive, suggesting the involvement of SmC5-MTases in salicylic acid-dependent immunity. These results provide useful information for demonstrating the role of DNA methylation in bioactive compound biosynthesis and Dao-di herb formation in medicinal plants.
Keywords: Yeast extract, Salvia miltiorrhiza, Methyl jasmonate, C5-MTase, Cytosine DNA methylation, Salicylic acid
Introduction
Cytosine DNA methylation, a dominating epigenetic modification mechanism, plays vital roles in gene expression regulation, transposon silencing, gene imprinting, and X chromosome inactivation (Chan, Henderson & Jacobsen, 2005; Law & Jacobsen, 2010). It is widespread in eukaryotes and displays distinct characteristics in specific species (Law & Jacobsen, 2010). In animals, cytosine methylation mostly occurs in CG sites with the exception of embryonic stem cells and neurons (Lister et al., 2009; Lister et al., 2013). DNA methylation in plants usually occurs at CG sites. It also occurs at CHG and CHH sites, where H represents any nucleotide except G (Cokus et al., 2008).
DNA methylation of each sequence context in plants can be maintained or established de novo through distinct mechanisms (Niederhuth & Schmitz, 2014). There are two main types of DNA methyltranferases (C5-MTases) involved in DNA methylation maintenance. DNA methyltransferase 1 (MET1), homologous to mammalian Dnmt1, maintains the CG context methylation status through recognizing the hemimethylated CG sites and methylating the newly synthesized DNA strand during DNA replication (Finnegan & Dennis, 1993; Kankel et al., 2003; Law & Jacobsen, 2010). Chromomethylase 3 (CMT3), a plant-specific DNA methyltransferase, maintains CHG context methylation through a self-reinforcing loop interacted with dimethylation of histone 3 on Lysine 9 (H3K9me2) (Du et al., 2012). De novo methylation is mainly established by RNA-directed DNA methylation (RdDM), in which domains-rearranged methyltransferases (DRMs) are guided to target locus to direct methylation of all three sequence contexts via 24-nucleotide short interfering RNAs (Cao & Jacobsen, 2002a; Cao & Jacobsen, 2002b; Law & Jacobsen, 2010). Although DNA methylation of all sequence contexts can be established de novo by RdDM, CHH context is the primary target of RdDM. In addition to RdDM, asymmetrical CHH methylation may also be established and maintained in H3K9me2 regions by CHROMOMETHYLASE 2 (CMT2), another CMT subfamily member (Stroud et al., 2014; Zemach et al., 2013). CMT2- and RdDM-mediated DNA methylation targets CHH sites at different position of the genome (Gent et al., 2013; Stroud et al., 2014; Zemach et al., 2013). The status of cytosine DNA methylation is dynamic. The balance of methylation and non-methylation is also regulated by passive or active DNA demethylation. Passive DNA demethylation occurs in newly synthesized DNA strand by dysfunction of DNA methyltransferase, whereas active demethylation is an outcome of replacement of methylated cytosine with nonmethylated cytosine under the catalysis of DEMETER-like DNA glyscosylases (Law & Jacobsen, 2010; Zhang & Zhu, 2012; Zhu, 2009).
Increasing evidence demonstrates that DNA methylation is involved in the regulation of many important biological processes, including leaf growth (Candaele et al., 2014), seed development (Xing et al., 2015), hybrid vigor (Kawanabe et al., 2016), fruit ripening (Liu et al., 2015; Zhong et al., 2013), and secondary metabolism (Conde et al., 2017). Due to the importance of DNA methylation, C5-MTase genes have been widely identified from various plant species, such as Arabidopsis (Ashapkin, Kutueva & Vanyushin, 2016), rice (Ahmad et al., 2014), tomato (Cao et al., 2014), soybean (Garg et al., 2014), maize (Qian et al., 2014), peanut (Wang et al., 2016), and globe artichoke (Gianoglio et al., 2017). Based on conserved domain and phylogenetic analysis, C5-MTase genes can be divided into the four DNA methyltransferase subfamilies, including MET, CMT, DRM, and DNMT2. The function of a few C5-MTases has been analyzed. AtMET1 was found to regulate plant morphology and flowering time through the maintenance of CG methylation in Arabidopsis (Finnegan, Peacock & Dennis, 1996). The Arabidopsis drm1drm2cmt3 triple mutant exhibited pleiotropic developmental defects, including developmental retardation, plant size reduction, and partial sterility (Cao & Jacobsen, 2002a). Null function of OsMET1-2 caused abnormal seed development and swift seedling lethality in rice (Hu et al., 2014). Functions of the other C5-MTases are largely unknown.
Salvia miltiorrhiza, a member of the Labiatae family, has been widely and successfully used to treat dysmenorrheal, coronary heart diseases and amenorrhoea (Cheng, 2006a; Cheng, 2006b). It is also an emerging model system for traditional Chinese medicine (TCM) studies (Li et al., 2009; Ma et al., 2012; Wang, Morris-Natschke & Lee, 2007; Xu et al., 2015). The genome of S. miltiorrhiza has been decoded recently and abundant transcriptome data are available (Xu et al., 2016; Zhang et al., 2015). Many bioactive compound biosynthesis-related genes have been identified (Li & Lu, 2014; Ma et al., 2012; Xu et al., 2015; Zhang et al., 2014). However, little is known about DNA methylation-related genes in S. miltiorrhiza. With long-term goal to explore biological role of DNA methylation in medicinal plants and to test whether DNA methylation is involved in the regulation of bioactive compound biosynthesis and Dao-di herb formation, we carried out genome-wide identification and characterization of the SmC5-MTase gene family in S. miltiorrhiza.
Materials & Methods
SmC5-MTase gene identification
Sequences of 11 Arabidopsis AtC5-MTase proteins were downloaded from the Arabidopsis Information Resource (TAIR, http://www.arabidopsis.org). Arabidopsis AtC5-MTase proteins were blast-analyzed against the S. miltiorrhiza 99-3 whole genome sequence using tBLASTn algorithm (Altschul et al., 1997; Xu et al., 2016). S. miltiorrhiza C5-MTase gene models were predicted from retrieved genomic DNA sequences through alignment with C5-MTase genes from other plants and S. miltiorrhiza transcriptome data (http://www.ncbi.nlm.nih.gov/sra) using the BLASTx and BLASTn program, respectively (http://www.ncbi.nlm.nih.gov/blast/). Obtained raw genomic sequences, open reading frame (ORF) sequences and deduced protein sequences were listed in Data S1.
Gene structure determination, protein sequence analysis and phylogenetic tree construction
Gene structures were determined on the Gene Structure Display Server (GSDS 2.0, http://gsds.cbi.pku.edu.cn/index.php) using the coding sequences and the corresponding genomic sequences as inputs. Protein amino acid number, molecular weight (Mw) and theoretical isoelectric point (pI) were analyzed using the EXPASY PROTOPARAM tool (http://www.expasy.org/tools/protparam.html). Multiple sequence alignment was carried out using ClustalW (Larkin et al., 2007). Conserved motifs were determined using the MEME suite (Bailey et al., 2009). The neighbor-joining (NJ) tree was constructed using MEGA version 7.0 (Kumar, Stecher & Tamura, 2016). Node robustness was detected using the bootstrap method (1,000 replicates).
RNA extraction and quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Roots, stems, leaves and flowers of 2-year-old S. miltiorrhiza (line 99-3) plants grown in a field nursery at the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, were collected in August and stored in liquid nitrogen until use. Total RNA was extracted from three biological replicates for each tissue using the Quick RNA isolation kit (Huayueyang Biotechnology, Beijing, China). Each biological replicate represents independent single plant. RNA integrity and quantity were analyzed using agarose gel and NanoDrop 2000C Spectrophotometer (Thermo Scientific, Waltham, MA, USA), respectively. Total RNA was reverse-transcribed using the PrimeScript™ RT reagent kit (TaKaRa, Japan). qRT-PCR primers listed in Table S1 were designed using Primer3 (http://frodo.wi.mit.edu/primer3/). qRT-PCR analysis was conducted in triplicate on the CFX96™ real-time PCR detection system (Bio-Rad, Hercules, CA, USA) for each biological replicate using SYBR premix Ex Taq™ kit (TaKaRa, Shiga, Japan) as described (Ma et al., 2012). The 2−ΔΔCt method was used to evaluate relative transcript levels (Livak & Schmittgen, 2001). Differential transcript abundance among tissues was determined by the one-way ANOVA (analysis of variance) method using IBM SPSS 20 software (IBM Corporation, Armonk, NY, USA).
RNA-seq data and bioinformatic analysis
Transcriptome data of periderm, phloem and xylem from S. miltiorrhiza roots was downloaded from SRA database of NCBI (SRA accession number SRR1640458) (Xu et al., 2015). RNA-seq reads from S. miltiorrhiza hairy roots treated with yeast extract (YE) and methyl jasmonate (MeJA) were downloaded from SRA database under the accession number SRP111399 (Zhou et al., 2017). RNA-seq data for S. miltiorrhiza cell cultures treated with salicylic acid (SA) were downloaded from SRA database under the accession number SRX1423774 (Zhang et al., 2016). Differential transcript abundance was analyzed using TopHat2.0.12 and Cufflinks2.2.1 (Trapnell et al., 2012). Heat maps were created using R statistical software (Le Meur & Gentleman, 2012).
Results
Genome-wide identification and sequence feature analysis of SmC5-MTase genes
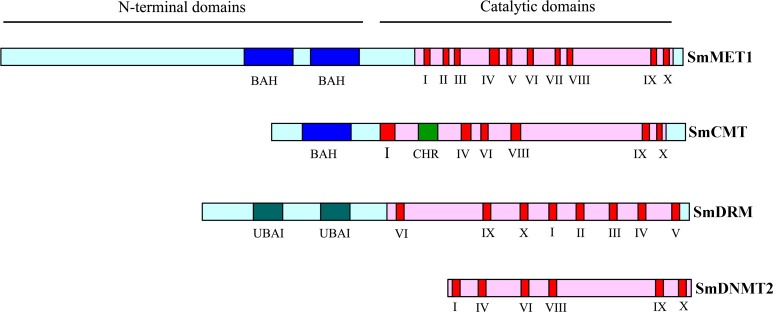
Blast analysis of eleven Arabidopsis cytosine DNA methyltransferase proteins against the whole S. miltiorrhiza 99-3 genome sequence resulted in the identification of eight SmC5-MTase genes. The number of SmC5-MTase genes in S. miltiorrhiza is comparable with that in other plants, such as Arabidopsis (Ashapkin, Kutueva & Vanyushin, 2016), rice (Ahmad et al., 2014) and maize (Qian et al., 2014), which contain eleven, ten and eight C5-MTases, respectively. The presence and distribution of conserved domains and motifs was confirmed by PFAM analysis of protein sequences (Fig. 1). Although the conserved catalytic C-terminal domains with conserved motifs are ubiquitous in all identified SmC5-MTase proteins, the N-terminals have diverse combinations of conserved domains among subfamilies (Fig. 1). It allows us to easily name newly identified genes. C5-MTases with two bromo adjacent homology (BAH) domains in the N-terminal belong to the MET subfamily, whereas C5-MTases having only one BAH domain belong to the CMT subfamily. C5-MTases containing ubiquitin associated (UBA) domains in the N-terminal were classified as the DRM subfamily. In addition, C5-MTases without N-terminal conserved domain belong to the DNMT2 subfamily. Accordingly, the eight SmC5-MTase genes were named SmMET1, SmCMT1, SmCMT2a, SmCMT2b, SmCMT3, SmDRM1, SmDRM2, and SmDNMT2, respectively (Table 1).
Figure 1. Schematic structures of SmC5-MTase proteins.
Table 1. Sequence features and intron numbers of C5-MTases in S. miltiorrhiza and Arabidopsis.
| Gene name | Gene model | Gene length | No. of intron | ORF (bp) | Protein (aa) | MW (kDa) | pI |
|---|---|---|---|---|---|---|---|
| SmMET1 | MG602207 | 9,207 | 15 | 5,034 | 1,677 | 189.4 | 7.54 |
| SmCMT1 | MG602211 | 4,685 | 20 | 2,448 | 815 | 91.8 | 6.30 |
| SmCMT2a | MG602209 | 4,913 | 12 | 2,874 | 957 | 107.7 | 8.03 |
| SmCMT2b | MG602210 | 8,816 | 20 | 2,808 | 935 | 105.3 | 6.97 |
| SmCMT3 | MG602208 | 6,666 | 19 | 2,460 | 819 | 91.6 | 4.89 |
| SmDRM1 | MG602212 | 4,185 | 7 | 1,830 | 609 | 68.6 | 4.97 |
| SmDRM2 | MG602213 | 5,757 | 8 | 2,070 | 689 | 76.5 | 5.10 |
| SmDNMT2 | MG602214 | 3,284 | 9 | 1,152 | 383 | 43.7 | 5.61 |
| AtMET1 | AT5G49160.1 | 5,686 | 10 | 4,605 | 1,534 | 172.4 | 5.97 |
| AtMET2a | AT4G14140.2 | 5,787 | 9 | 4,638 | 1,545 | 174.8 | 6.09 |
| AtMET2b | AT4G08990.1 | 5,716 | 10 | 4,539 | 1,512 | 171.2 | 5.79 |
| AtMET3 | AT4G13610.1 | 6,210 | 12 | 4,215 | 1,404 | 160.2 | 7.24 |
| AtCMT1 | AT1G80740.1 | 4,438 | 19 | 2,376 | 791 | 89.2 | 5.99 |
| AtCMT2 | AT4G19020.1 | 6,411 | 22 | 3,888 | 1,295 | 145.0 | 8.64 |
| AtCMT3 | AT1G69770.1 | 4,999 | 20 | 2,520 | 839 | 94.9 | 6.57 |
| AtDRM1 | AT5G15380.1 | 3,480 | 9 | 1,875 | 624 | 70.9 | 5.16 |
| AtDRM2 | AT5G14620.1 | 3,150 | 9 | 1,881 | 626 | 70.4 | 5.12 |
| AtDRM3 | AT3G17310.2 | 3,462 | 7 | 2,133 | 710 | 79.7 | 4.87 |
| AtDnmt2 | AT5G25480.1 | 2,131 | 8 | 1,152 | 383 | 43.7 | 5.97 |
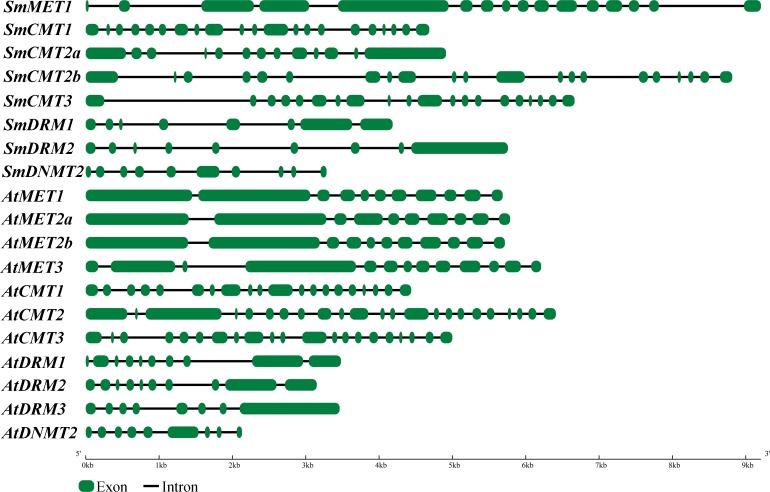
The length of ORFs of SmC5-MTase genes varies from 1,152 bp to 5,034 bp (Table 1). The deduced proteins vary between 383 amino acids and 1,677 amino acids, respectively. The theoretical isoelectric point (pI) varies from 4.89 (SmCMT3) to 8.03 (SmCMT2a) and the molecular weight (Mw) varies from 43.7 kDa (SmDNMT2) to 189.4 kDa (SmMET1) (Table 1). These sequence features were similar to their counterparts in Arabidopsis. On the other hand, the genomic DNA length of SmC5-MTase genes was obviously greater than their Arabidopsis counterparts (Table 1). Gene structure analysis showed that introns were responsible for the enlargement of SmC5-MTase genes (Fig. 2). The underlying mechanism of intron size expansion is unknown.
Figure 2. Gene structures of C5-MTases. in S.miltiorrhiza and Arabidopsis.
Sequence alignment and conserved motif analysis in C5-MTases
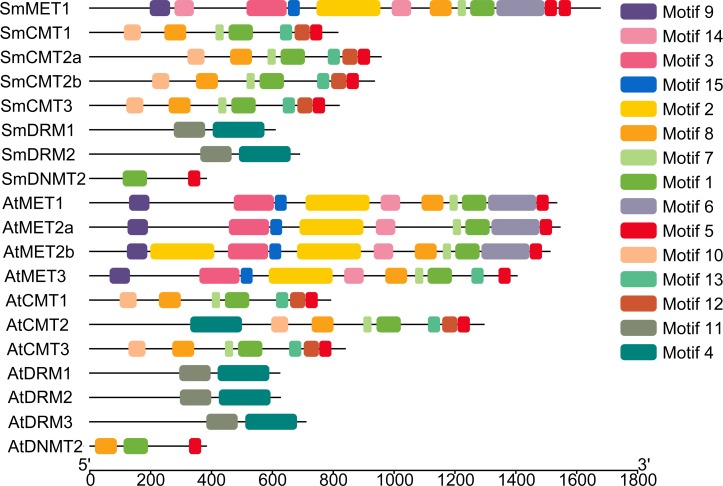
C5-MTases contain N- and C-terminal domains, which are necessary for establishment and maintenance of cytosine DNA methylation at distinct sequence contexts. In order to understand the conservation and divergence of these conserved domains in SmC5-MTases, multiple sequence alignment was performed for C5-MTases from S. miltiorrhiza and Arabidopsis. Four highly conserved motifs, including I, IV, VI and X (Malone, Blumenthal & Cheng, 1995), were found in the C-terminal methyltransferase domain of all C5-MTases analyzed (Figs. S1–S5). Among them, motifs I and X are involved in S-adenosyl-L-methionine (AdoMet) binding, motif IV has the proline-cysteine doublet acting as the functional catalytic site, and motif VI has a glutamic acid involved in target cytosine binding (Malone, Blumenthal & Cheng, 1995). In addition to the highly conserved motifs, various less conserved motifs were found in the C-terminal domain (Figs. S1–S5). These motifs could be associated with gene-specific functions. Compared with the C-terminal domain, the N-terminal domain is more diverse. The N-terminal of SmMET1 contains two replication foci domains (RFD) and two BAH domains (Fig. S1). Except for SmCMT2a, which lacks the Chr domain, the N-terminal of SmCMTs contains a BAH domain and a chromo (Chr) domain (Figs. S2–S3). The N-terminal of SmDRMs has two conserved UBA domains (Fig. S4). The divergence of the N-terminal domains could be important for distinct roles of different SmC5-MTases.
To further explore sequence conservation and divergence of SmC5-MTases, MEME motif search tool was used to identify conserved motifs in SmC5-MTases and AtC5-MTases. A total of 15 conserved motifs were identified. The length of motifs ranges from 28 to 100 amino acids (Table 2). The number of motifs in each C5-MTase varies between 2 and 10. Motifs 1, 4, 5, 6, 7, 8, 12 and 13 are located in the C-terminal DNA methyltransferase domain (Fig. 3). Among them, motifs 1, 5, 7 and 8 are highly conserved in 12–14 C5-MTases, whereas motifs 4, 6, 12 and 13 are conserved in less than ten C5-MTases. The other seven, including motifs 2, 3, 9, 10, 11, 14 and 15, are located in the N-terminal regions of C5-MTases. Among them, motifs 2, 3, 9, 14 and 15 only exist in the MET subfamily. Motifs 2 and 14 correspond to two BAH domains, whereas motifs 3 and 9 are located in the two RFD domains. Motifs 10 and 11 only exist in the CMT and the DRM subfamily, respectively. Motif 10 is specific to the BAH domain of the CMT subfamily, however motif 11 is not the UBA domain of the DRM subfamily (Fig. 3). Motifs commonly existing in C5-MTases are probably associated with the conserved functions of C5-MTases, whereas those specific to a few C5-MTases seem to be related to gene-specific functions.
Table 2. Consensus sequences of fifteen motifs identified in S. miltiorrhiza and Arabidopsis C5-MTases.
| Motif | Width | Best possible match |
|---|---|---|
| 1 | 81 | FLDIVDYLKPKYVLMENVVDFVRFNKGQLFRYALASLLEMGYQVRFGIMAAGAYGLPQFRKRVFIWAAAPEEVLPZWPLPT |
| 2 | 100 | EIKWDGEILGKTSAGEPLYGRALVGGEKVVVGGAVILEVDDPDETPLIYFVEYMFESSDHSKMLHGKLLQRGSETVLGTAANERELFLTNECLTVQLKDI |
| 3 | 100 | MIFVSJRTDMAWYRLGKPSKQYAPWFEPVLKTVRVGISILTMLKRESRVAKLSYADVIKRLCGLEENDKAYISSKLLDVERYVVVHGQIILQLFEEYPDD |
| 4 | 100 | VQRYVLEQCKKWNLVWVGKNKLAPLEPDELEKILGFPKNHTRGGGISLTERYKSLGNSFQVDTVAYHLSVLKPJFPNGINVLSLFSGIGGAEVALHRLGI |
| 5 | 41 | LHPEQDRVLSVRECARLQGFPDSYKFSGTIKEKYRQIGNAV |
| 6 | 100 | PGLKISLPRGLHYDAVRNTKFGAPFRPITVRDTIGDLPPVENGESKTNKEYKTTPVSWFQKKIRGNMSVLTDHICKGLNELNLIRCKKIPKRPGADWRDL |
| 7 | 28 | ILPLPGQVDFICGGPPCQGISGYNRFRB |
| 8 | 73 | LLDLYSGCGAMSTGLCJGAKJSGVKLVTKWAVDJNSYACESLKLNHPETQVRNEAAEDFLDLLKEWEKLCKKY |
| 9 | 68 | GVRFQSFGRVENWNISGYEDGSPVIWISTALADYDCRKPAKKYKKJYDYFFEKACACVEVYKSLSKNP |
| 10 | 57 | FIGKIVEFFETTDGESYFRVQWFYRAEDTVIKNQASLIDKKRVFYSEIMBDNPLDCJ |
| 11 | 100 | HRSLPDKARGPPYFYYENVALTPKGVWAKISRFLYDIQPEFVDSKYFSAAARKRGYIHNLPIENRFQIQPPPPLTIQEAFPLSKRWWPSWDKRKKLNCIL |
| 12 | 51 | ERVKLPSGKPLVPDYALSFEDGKSKKPFGRLWWDETVPTVVTRPEPHNQAI |
| 13 | 41 | LYDHRPLKLNEDDYERVCQIPKRKGANFRDLPGVIVGPDNV |
| 14 | 65 | LKESRKASKASFQVKVRRFYRPEDISAEKAYASDIQELYYSQDTYILPPEAIZGKCEVRKKSDMP |
| 15 | 40 | NLNPRAGIAPVVSKMKAMQATTTRLVNRIWGEFYSIYSPE |
Figure 3. Conserved motifs of S. miltiorrhiza and Arabidopsis C5-MTase proteins identified using the MEME search tool.
Phylogenetic analysis of C5-MTases in S. miltiorrhiza and other plant species
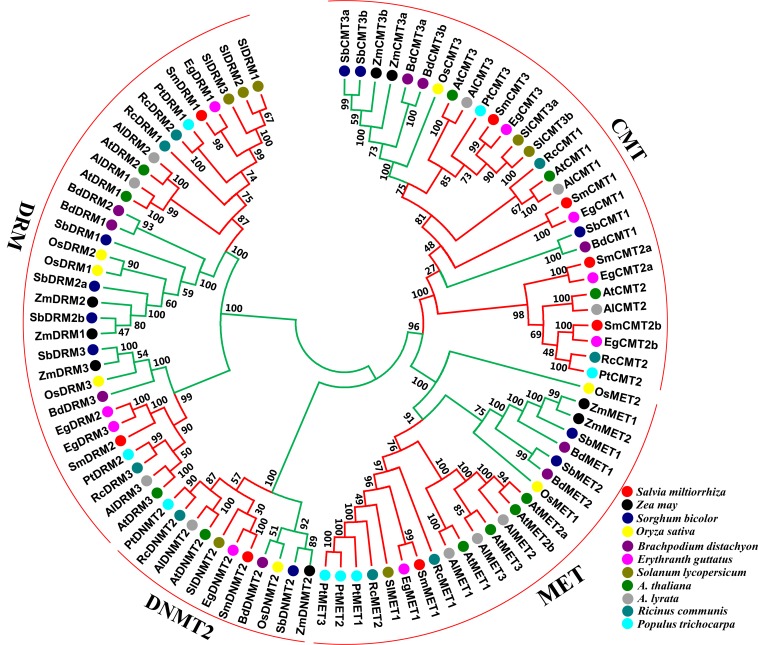
To understand the phylogenetic relationships and evolutionary history of plant C5-MTases, an unrooted neighbor-joining phylogenetic tree was constructed for full-length protein sequences of C5-MTases from S. miltiorrhiza and other 10 plant species (Table S2). Four subfamilies, including MET, DNMT2, CMT and DRM, are exhibited in the tree (Fig. 4). It is consistent with previous studies on other plants (Cao et al., 2014; Qian et al., 2014), confirming the domain based classification and nomenclature. The MET subfamily contains SmMET1 and can be further divided into dicot and monocot groups. The CMT subfamily can be grouped into three clades, including CMT1, CMT2 and CMT3. Among them, CMT1 and CMT3 can be further divided into dicot and monocot groups, whereas CMT2 exists in dicots only. The DRM subfamily can be divided into two dicot and two monocot groups. Similarly, The DNMT2 subfamily can be divided into dicot and monocot groups. The results imply that each subfamily is phylogenetically monophyletic and includes dicot and monocot evolutionary lineages. Taken together, each of the MET and the DNMT2 subfamilies contains a SmC5-MTase, whereas the CMT and the DRM subfamilies include four and two SmC5-MTases, respectively. SmC5-MTases have close phylogenetic relationships with the counterparts from Erythranthe guttatus (Fig. 4). It is consistent with the fact that both S. miltiorrhiza and E. guttatus are members of the Lamiales order.
Figure 4. Phylogenetic analysis of C5-MTase proteins from eleven plant species.
Dicots and monocots are shown with red and green colors, respectively.
Transcript abundance analysis of SmC5-MTase genes in S. miltiorrhiza
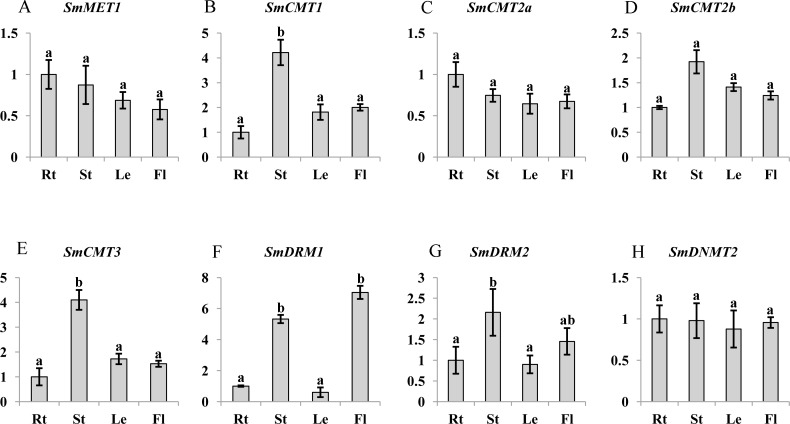
C5-MTases are important for plant growth and development (Cao & Jacobsen, 2002a; Finnegan, Peacock & Dennis, 1996; Hu et al., 2014). To preliminarily understand the function of SmC5-MTases, transcript abundance of genes in roots, stems, leaves and flowers of 2-year-old, field nursery-grown S. miltiorrhiza plants were analyzed. Transcripts of all eight SmC5-MTase genes could be detected in organs analyzed, although differential transcript levels were observed. SmMET1, which may be responsible for CG methylation maintenance, shows relatively uniform transcript levels in roots, stems, leaves and flowers of S. miltiorrhiza (Fig. 5). It is consistent with the transcript abundance patterns of AtMET1 in vegetative organs (Ashapkin, Kutueva & Vanyushin, 2016). Among the four CMT subfamily members, SmCMT1 and SmCMT3 exhibited similar transcript abundance patterns, showing more stem-specific pattern, whereas no significant difference was observed for the transcript levels of SmCMT2a and SmCMT2b in the organs analyzed (Fig. 5). The transcript abundance patterns of SmCMTs are consistent with their phylogenetic relationships (Fig. 4). The transcript level of SmDRM1 was significantly higher in flower and stem than root and leaf (Fig. 5). SmDRM2 was higher in stem, followed by flower, and less in root and leaf (Fig. 5). The transcript abundance patterns of SmDRMs are consistent with the role of DRM subfamily members in de novo methylation (Cao & Jacobsen, 2002a; Cao & Jacobsen, 2002b; Law & Jacobsen, 2010). SmDNMT2 exhibited similar transcript levels in all tissues analyzed, suggesting its importance in plant development.
Figure 5. Transcript abundance of SmMET1 (A), SmCMT1 (B), SmCMT2a (C), SmCMT2b (D), SmCMT3 (E), SmDRM1 (F), SmDRM2 (G), and SmDNMT2 (H) in roots (Rt), Stems (St), leaves (Le) and flowers (Fl) of S. miltiorrhiza.
Transcript level was analyzed using quantitative real time RT-PCR. SmUBQ10 was used as a reference. qRT-PCR was performed in triplicates for each independent biological replicate. Transcript levels in roots were arbitrarily set to 1 and the levels in other tissues were given relative to this. One-way ANOVA was performed using IBM SPSS 20 software. P < 0.05 was considered statistically significant and represented by different letters. Error bars indicate the standard deviations for three biological replicates.
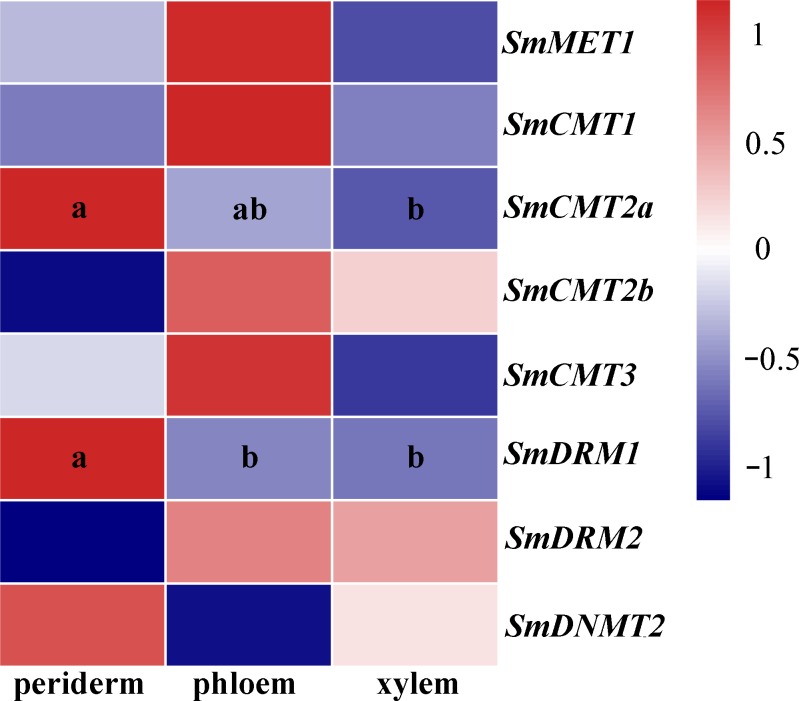
S. miltiorrhiza roots are major medicinal materials of various TCMs. To further investigate possible roles of SmC5-MTases in S. miltiorrhiza, RNA-seq data from periderm, phloem and xylem of roots were analyzed. Differential transcript abundance was observed for SmCMT2a and SmDRM1 (Fig. 6, Table S3), indicating the role of SmCMT2a and SmDRM1 in the biosynthesis of bioactive compounds.
Figure 6. Transcript levels of SmC5-MTases in periderm, phloem and xylem of S.miltiorrhiza roots.
Differential transcript abundance was analyzed using cufflinks software. P < 0.05 was considered statistically significant and was indicated by different letters. Absence of letters indicates that no significant different was observed among three tissues.
Responses of SmC5-MTases to yeast extract and methyl jasmonate treatments
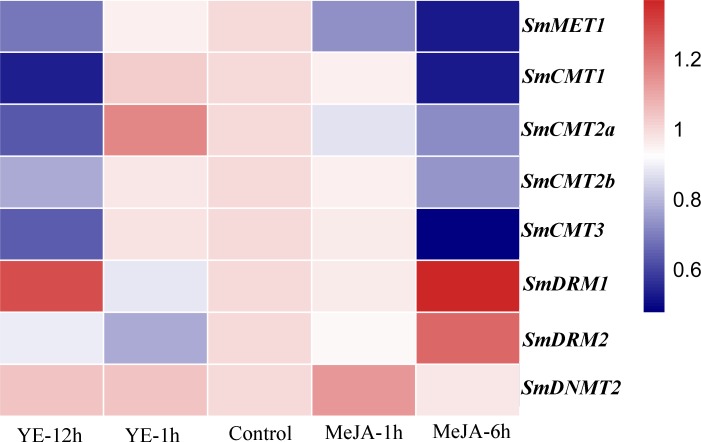
Yeast extract (YE) and methyl jasmonate (MeJA) are effective elicitors for tanshinone and phenolic acid production in S. miltiorrhiza (Chen et al., 2010; Kai et al., 2012; Zhou et al., 2017). The responses of SmC5-MTases to YE and MeJA treatments were analyzed using RNA-seq data from S. miltiorrhiza hairy roots (Zhou et al., 2017). The transcripts of all SmC5-MTase genes were detected in hairy roots (Fig. 7, Table S3). Compared with non-treated control, five SmC5-MTases, including SmMET1, SmCMT1, SmCMT2a, SmCMT2b and SmCMT3, were slightly down-regulated at the time-point of 12 h of YE treatment and at the time-point of 6 h of MeJA treatment. SmDRM1 and SmDRM2 were slightly down-regulated at the time-point of 1 h after YE and MeJA treatments, whereas they were up-regulated with treatment time extension. SmDNMT2 was slightly up-regulated at the time-point of 1 h after two treatments, whereas it was down-regulated after 6 h MeJA treatment. Although all SmC5-MTases exhibited differential transcript abundance compared with control, the variance was not significant. It could be due to the short treatment time. Further analysis of SmC5-MTase genes in response to treatment with extended treatment time will help to elucidate the role of SmC5-MTases in secondary metabolite biosynthesis.
Figure 7. Responses of SmC5-MTase genes to YE and MeJA treatments in S. miltiorrhiza hair roots.
Transcript levels were analyzed at 0, 1 and 12 h of YE treatment and at 0, 1 and 6 h of MeJA treatment. Differential analysis was performed using cufflinks software. P < 0.05 was considered statistically significant.
Salicylic acid-responsive SmC5-MTases
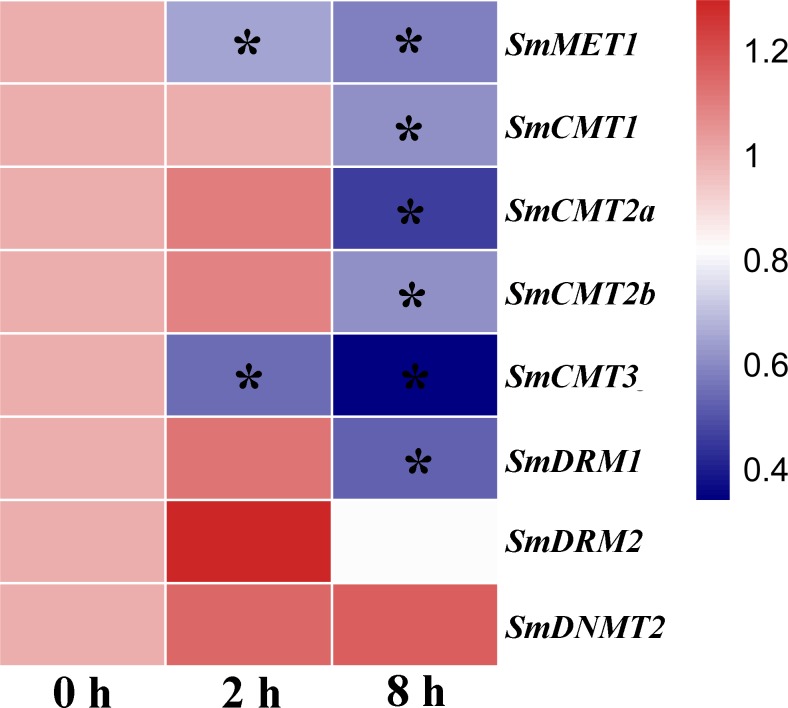
DNA methylation plays a vital role in plant defense by regulating the expression of a subset of stress responsive genes and is involved in priming of salicylic acid (SA)-dependent immunity (López Sánchez et al., 2016; Yu et al., 2013). In order to investigate the potential role of SmC5-MTases in plant defense, RNA-seq data from SA-treated S. miltiorrhiza cell cultures was analyzed (Zhang et al., 2016). The transcripts of all SmC5-MTase genes were determined in S. miltiorrhiza cell cultures and the majority of them were significantly suppressed at 8 h after SA treatment (Fig. 8, Table S3), suggesting the significance of SmC5-MTases in SA-dependent defenses.
Figure 8. Responses of SmC5-MTase genes to SA treatment in S. miltiorrhiza cell cultures.
Transcript levels were detected at 0, 2 and 8 h of SA treatment. Differential analysis was performed using cufflinks software. P < 0.05 was considered statistically significant. ∗presents significant differential transcript abundance compared with 0 h.
Discussion
Lipophilic tanshinones and hydrophilic phenolic acids are two main bioactive compounds from the roots/rhizome of S. miltiorrhiza. Understanding the regulatory mechanism of their biosynthesis and metabolism is important for S. miltiorrhiza quality improvement. Most of the studies about S. miltiorrhiza gene function concentrate on key enzyme genes and transcription factors associated with secondary metabolism (Li et al., 2015; Ma et al., 2012; Xu et al., 2015). The significance of DNA methylation has not been revealed in S. miltiorrhiza. C5-MTases are core elements of DNA methylation and play important roles in various biological processes (Candaele et al., 2014; Xing et al., 2015). As the first step to elucidate DNA methylation regulation in S. miltiorrhiza, we carried out genome-wide identification of SmC5-MTase genes. A total of eight such genes were identified and characterized. Integrative analysis of gene structures, sequence features, conserved domains, conserved motifs and phylogenetic relationships showed that SmC5-MTase genes could be divided into four subfamilies, including MET, CMT, DRM and DNMT2 (Cao et al., 2014; Qian et al., 2014). Arabidopsis has four MET genes, whereas S. miltiorrhiza contains only one (Fig. 4), suggesting the loss of MET genes during S. miltiorrhiza evolution. Although all types of CMTs, including CMT1, CMT2 and CMT3, exit in S. miltiorrhiza genome, CMT2 possesses two paralogs, SmCMT2a and SmCMT2b. It indicates the occurrence of CMT2 gene duplication. Gene loss and duplication imply functional redundancy and divergence of SmC5-MTase genes.
Although the number of each S. miltiorrhiza SmC5-MTase gene subfamily is different from that of Arabidopsis, the conserved domains are similar (Figs. S1–S5). Gene structures and sequence features also show the conservation of S. miltiorrhiza and Arabidopsis C5-MTase genes belonging to a subfamily, although intron size of SmC5-MTase genes exhibits apparent enlargement compared with their Arabidopsis counterparts (Table 1, Fig. 2). The results are confirmed by phylogenetic analysis of C5-MTase full-length protein sequences from sixteen plants (Fig. 4). Based on the phylogenetic tree, each C5-MTase subfamilies can be further divided into dicot and monocot groups. It indicates the origin of C5-MTase genes before dicot and monocot divergence.
DNA methyltransferases are responsible for establishment and maintenance of DNA methylation (Niederhuth & Schmitz, 2014). The transcript abundance of SmC5-MTases exhibited specific spatiotemporal patterns in S. miltiorrhiza (Fig. 5). It may result in variance of genome-wide cytosine DNA methylation in different S. miltiorrhiza tissues. During the development of Arabidopsis flowers, a number of cytosine sites were methylated de novo (Yang et al., 2015). De novo methylation could be caused by DRMs through the RdDM process (Cao & Jacobsen, 2002a; Cao & Jacobsen, 2002b). Significantly high transcript levels of SmDRMs in flowers and stems may result in de novo methylation of cytosine sites during S. miltiorrhiza flower and stem development. Sequence alignment (Fig. S4) and phylogenetic analysis (Fig. 4) showed that SmDRM1 and SmDRM2 were counterparts of AtDRM2 and AtDRM3, respectively. AtDRM2 has been showed to require catalytically mutated AtDRM3 for normal RdDM process in Arabidopsis (Henderson et al., 2010). It shows that SmDRM1 probably also requires assistance of SmDRM2 to accomplish RdDM process in S. miltiorrhiza. CMTs are plant-specific DNA methylation enzymes with the CHR domain. All of the CMTs identified and functionally characterized possess the CHR domain (Bewick et al., 2017). However, SmCMT2a lacks the CHR domain compared with other SmCMTs (Figs. S2–S3) in S. miltiorrhiza. Since other conserved domains exist (Fig. S3), SmCMT2a could play a role differing from the canonical CMTs. It is also possible that SmCMT2a has lost its biochemical activity. The function of SmCMT2a remains to be elucidated. Although DNMT2 contains highly conserved C-terminal methyltransferase domain and is able to interact with type-2 histone deacetylases (AtHD2s) in Arabidopsis (Song et al., 2010), its function remains largely unclear (Goll et al., 2006; Ponger & Li, 2005; Vieira et al., 2017). In this study, we found that SmDNMT2 showed no differential transcript abundance in all tissues analyzed and various treatments (Figs. 5– 8). It is different from other SmC5-MTase genes, implying functional specificity of SmDNMT2.
Recent studies suggest that DNA methylation is involved in regulation of secondary metabolism (Bharti et al., 2015; Conde et al., 2017). Consistently, SmCMT2a and SmDRM1 exhibited significant differential transcript abundance in periderm, phloem and xylem (Fig. 6, Table S3). Although the transcript abundance difference is not statistically significant, difference was observed for all SmC5-MTase genes in response to YE and MeJA treatment (Fig. 7, Table S3). Dynamic changes of SmC5-MTases could regulate the expression of functional genes, including many secondary metabolism-related genes. In addition, the majority of SmC5-MTase genes were significantly down-regulated by SA (Fig. 8). Analysis of DNA methylation mutant showed that DNA methylation could enhance resistance to the biotrophic pathogen Hyaloperonospora arabidopsidis (Hpa) through regulating SA-dependent defense genes in trans or cis (López Sánchez et al., 2016). Similar mechanism could also exist in S. miltiorrhiza.
Conclusions
Eight SmC5-MTase genes were identified using whole genome sequence and RNA-seq data from S. miltiorrhiza. Based on phylogenetic tree and conserved domain distribution, SmC5-MTase genes were divided into four subfamilies, including SmMET, SmCMT, SmDRM and SmDNMT2. Comparative analysis of SmC5-MTases and AtC5-MTases revealed the conservation and divergence of plant C5-MTases. The transcript abundance analysis of SmC5-MTase genes suggests functional importance of SmC5-MTases in secondary metabolism and stress response in S. miltiorrhiza. The results provide useful information for understanding the role of DNA methylation in medicinal plant development and bioactive compound biosynthesis.
Supplemental Information
Raw data.
Funding Statement
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-3-016), the Natural Science Foundation of China (31370327, 31570667 and 81773836), and the Beijing Natural Science Foundation (5152021). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jiang Li performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Caili Li performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Shanfa Lu conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
References
- Ahmad et al. (2014).Ahmad F, Huang X, Lan HX, Huma T, Bao YM, Huang J, Zhang HS. Comprehensive gene expression analysis of the DNA (cytosine-5) methyltransferase family in rice (Oryza sativa L.) Genetics & Molecular Research Gmr. 2014;13:5159–5172. doi: 10.4238/2014.July.7.9. [DOI] [PubMed] [Google Scholar]
- Altschul et al. (1997).Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashapkin, Kutueva & Vanyushin (2016).Ashapkin VV, Kutueva LI, Vanyushin BF. Plant DNA methyltransferase genes: multiplicity, expression, methylation patterns. Biochemistry. 2016;81:141–151. doi: 10.1134/S0006297916020085. [DOI] [PubMed] [Google Scholar]
- Bailey et al. (2009).Bailey TL, Bodén M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research. 2009;37:202–208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick et al. (2017).Bewick AJ, Niederhuth CE, Ji L, Rohr NA, Griffin PT, Leebens-Mack J, Robert J, Schmitz RJ. The evolution of chromomethylases and gene body DNA methylation in plants. Genome Biology. 2017;18:1–13. doi: 10.1186/s13059-017-1195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti et al. (2015).Bharti P, Mahajan M, Vishwakarma AK, Bhardwaj J, Yadav SK. AtROS1 overexpression provides evidence for epigenetic regulation of genes encoding enzymes of flavonoid biosynthesis and antioxidant pathways during salt stress in transgenic tobacco. Journal of Experimental Botany. 2015;66:5959–5969. doi: 10.1093/jxb/erv304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candaele et al. (2014).Candaele J, Demuynck K, Mosoti D, Beemster GT, Inze D, Nelissen H. Differential methylation during maize leaf growth targets developmentally regulated genes. Plant Physiology. 2014;164:1350–1364. doi: 10.1104/pp.113.233312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao et al. (2014).Cao D, Ju Z, Gao C, Mei X, Fu D, Zhu H, Luo Y, Zhu B. Genome-wide identification of cytosine-5 DNA methyltransferases and demethylases in Solanum lycopersicum. Gene. 2014;550:230–237. doi: 10.1016/j.gene.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Cao & Jacobsen (2002a).Cao X, Jacobsen SE. Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proceedings of the National Academy of Sciences of the United States of America. 2002a;99(Suppl 4):16491–16498. doi: 10.1073/pnas.162371599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao & Jacobsen (2002b).Cao X, Jacobsen SE. Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Current Biology. 2002b;12:1138–1144. doi: 10.1016/S0960-9822(02)00925-9. [DOI] [PubMed] [Google Scholar]
- Chan, Henderson & Jacobsen (2005).Chan SW, Henderson IR, Jacobsen SE. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nature Reviews Genetics. 2005;6(5):351–360. doi: 10.1038/nrg1601. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2010).Chen ZW, Zhang JJ, Zhao SJ, Wang ZT, Zhi-Bi HU. Effects of methyl jasmonate on the accumulation of phenolic acids in Salvia miltiorrhiza hairy root. Journal of Chinese Pharmaceutical Sciences. 2010;45:970–974. [Google Scholar]
- Cheng (2006a).Cheng TO. Cardiovascular effects of Danshen. International Journal of Cardiology. 2006a;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Cheng (2006b).Cheng TO. Danshen: a popular Chinese cardiac herbal drug. Journal of the American College of Cardiology. 2006b;47:1499–1500. doi: 10.1016/j.jacc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Cokus et al. (2008).Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature. 2008;452:215–219. doi: 10.1038/nature06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde et al. (2017).Conde D, Moreno-Cortes A, Dervinis C, Ramos-Sanchez JM, Kirst M, Perales M, Gonzalez-Melendi P, Allona I. Overexpression of DEMETER, a DNA demethylase, promotes early apical bud maturation in poplar. Plant Cell Environment. 2017;40:2806–2819. doi: 10.1111/pce.13056. [DOI] [PubMed] [Google Scholar]
- Du et al. (2012).Du J, Zhong X, Bernatavichute YV, Stroud H, Feng S, Caro E, Vashisht AA, Terragni J, Chin HG, Tu A, Hetzel J, Wohlschlegel JA, Pradhan S, Patel DJ, Jacobsen SE. Dual binding of chromomethylase domains to h3k9me2-containing nucleosomes directs DNA methylation in plants. Cell. 2012;151:167–180. doi: 10.1016/j.cell.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan & Dennis (1993).Finnegan EJ, Dennis ES. Isolation and identification by sequence homology of a putative cytosine methyltransferase from Arabidopsis thaliana. Nucleic Acids Research. 1993;21:2383–2388. doi: 10.1093/nar/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan, Peacock & Dennis (1996).Finnegan EJ, Peacock WJ, Dennis ES. Reduced DNA methylation in Arabidopsis thaliana results in abnormal plant development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8449–8454. doi: 10.1073/pnas.93.16.8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg et al. (2014).Garg R, Kumari R, Tiwari S, Goyal S. Genomic survey, gene expression analysis and structural modeling suggest diverse roles of DNA methyltransferases in legumes. PLOS ONE. 2014;9:e88947. doi: 10.1371/journal.pone.0088947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent et al. (2013).Gent JI, Ellis NA, Guo L, Harkess AE, Yao Y, Zhang X, Dawe RK. CHH islands: de novo DNA methylation in near-gene chromatin regulation in maize. Genome Research. 2013;23:628–637. doi: 10.1101/gr.146985.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoglio et al. (2017).Gianoglio S, Moglia A, Acquadro A, Comino C, Portis E. The genome-wide identification and transcriptional levels of DNA methyltransferases and demethylases in globe artichoke. PLOS ONE. 2017;12:e0181669. doi: 10.1371/journal.pone.0181669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll et al. (2006).Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- Henderson et al. (2010).Henderson IR, Deleris A, Wong W, Zhong X, Chin HG, Horwitz GA, Kelly KA, Pradhan S, Jacobsen SE. The de novo cytosine methyltransferase DRM2 requires intact UBA domains and a catalytically mutated paralog DRM3 during RNA–directed DNA methylation in Arabidopsis thaliana. PLOS Genetics. 2010;6:e1001182. doi: 10.1371/journal.pgen.1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2014).Hu L, Li N, Xu C, Zhong S, Lin X, Yang J, Zhou T, Yuliang A, Wu Y, Chen YR, Cao X, Zemach A, Rustgi S, Von Wettstein D, Liu B. Mutation of a major CG methylase in rice causes genome-wide hypomethylation, dysregulated genome expression, and seedling lethality. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10642–10647. doi: 10.1073/pnas.1410761111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai et al. (2012).Kai G, Xu H, Wang J, Zhou C, Zhou W, Qi Y, Xiao J, Wang Y, Zhang L. Molecular mechanism of elicitor-induced tanshinone accumulation in Salvia miltiorrhiza hairy root cultures. Acta Physiologiae Plantarum. 2012;34:1421–1433. doi: 10.1007/s11738-012-0940-z. [DOI] [Google Scholar]
- Kankel et al. (2003).Kankel MW, Ramsey DE, Stokes TL, Flowers SK, Haag JR, Jeddeloh JA, Riddle NC, Verbsky ML, Richards EJ. Arabidopsis MET1 cytosine methyltransferase mutants. Genetics. 2003;163:1109–1122. doi: 10.1093/genetics/163.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanabe et al. (2016).Kawanabe T, Ishikura S, Miyaji N, Sasaki T, Wu LM, Itabashi E, Takada S, Shimizu M, Takasaki-Yasuda T, Osabe K, Peacock WJ, Dennis ES, Fujimoto R. Role of DNA methylation in hybrid vigor in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E6704–e6711. doi: 10.1073/pnas.1613372113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, Stecher & Tamura (2016).Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology & Evolution. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin et al. (2007).Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Law & Jacobsen (2010).Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nature Reviews Genetics. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur & Gentleman (2012).Le Meur N, Gentleman R. Analyzing biological data using R: methods for graphs and networks. Methods in Molecular Biology. 2012;804:343–373. doi: 10.1007/978-1-61779-361-5_19. [DOI] [PubMed] [Google Scholar]
- Li et al. (2015).Li C, Li D, Shao F, Lu S. Molecular cloning and expression analysis of WRKY transcription factor genes in Salvia miltiorrhiza. BMC Genomics. 2015;16:1–21. doi: 10.1186/1471-2164-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li & Lu (2014).Li C, Lu S. Genome-wide characterization and comparative analysis of R2R3-MYB transcription factors shows the complexity of MYB-associated regulatory networks in Salvia miltiorrhiza. BMC Genomics. 2014;15:1–12. doi: 10.1186/1471-2164-15-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2009).Li YG, Song L, Liu M, Hu ZB, Wang ZT. Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma (Danshen) Journal of Chromatography A. 2009;1216:1941–1953. doi: 10.1016/j.chroma.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Lister et al. (2013).Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, Lucero J, Huang Y, Dwork AJ, Schultz MD, Yu M, Tonti-Filippini J, Heyn H, Hu S, Wu JC, Rao A, Esteller M, He C, Haghighi FG, Sejnowski TJ, Behrens MM, Ecker JR. Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146):629–640. doi: 10.1126/science.1237905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister et al. (2009).Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu R, How-Kit A, Stammitti L, Teyssier E, Rolin D, Mortain-Bertrand A, Halle S, Liu M, Kong J, Wu C. A DEMETER-like DNA demethylase governs tomato fruit ripening. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:10804–10809. doi: 10.1073/pnas.1503362112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- López Sánchez et al. (2016).López Sánchez A, Stassen HM, Furci L, Smith LM, Ton J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. The Plant Journal. 2016;88:361–374. doi: 10.1111/tpj.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al. (2012).Ma Y, Yuan L, Wu B, Li X, Chen S, Lu S. Genome-wide identification and characterization of novel genes involved in terpenoid biosynthesis in Salvia miltiorrhiza. Journal of Experimental Botany. 2012;63:2809–2823. doi: 10.1093/jxb/err466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone, Blumenthal & Cheng (1995).Malone T, Blumenthal RM, Cheng X. Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. Journal of Molecular Biology. 1995;253:618–632. doi: 10.1006/jmbi.1995.0577. [DOI] [PubMed] [Google Scholar]
- Niederhuth & Schmitz (2014).Niederhuth CE, Schmitz RJ. Covering your bases: inheritance of DNA methylation in plant genomes. Molecular Plant. 2014;7:472–480. doi: 10.1093/mp/sst165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponger & Li (2005).Ponger L, Li WH. Evolutionary diversification of DNA methyltransferases in eukaryotic genomes. Molecular Biology and Evolution. 2005;22:1119–1128. doi: 10.1093/molbev/msi098. [DOI] [PubMed] [Google Scholar]
- Qian et al. (2014).Qian Y, Xi Y, Cheng B, Zhu S. Genome-wide identification and expression profiling of DNA methyltransferase gene family in maize. Plant Cell Reports. 2014;33:1661–1672. doi: 10.1007/s00299-014-1645-0. [DOI] [PubMed] [Google Scholar]
- Song et al. (2010).Song Y, Wu K, Dhaubhadel S, An L, Tian L. Arabidopsis DNA methyltransferase AtDNMT2 associates with histone deacetylase AtHD2s activity. Biochemical & Biophysical Research Communication. 2010;396:187–192. doi: 10.1016/j.bbrc.2010.03.119. [DOI] [PubMed] [Google Scholar]
- Stroud et al. (2014).Stroud H, Do T, Du J, Zhong X, Feng S, Johnson L, Patel DJ, Jacobsen SE. Non-CG methylation patterns shape the epigenetic landscape in Arabidopsis. Nature Structural & Molecular Biology. 2014;21:64–72. doi: 10.1038/nsmb.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell et al. (2012).Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira et al. (2017).Vieira GC, Vieira GF, Sinigaglia M, Vld SV. Linking epigenetic function to electrostatics: the DNMT2 structural model example. PLOS ONE. 2017;12:e0178643. doi: 10.1371/journal.pone.0178643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang P, Gao C, Bian X, Zhao S, Zhao C, Xia H, Song H, Hou L, Wan S, Wang X. Genome-wide identification and comparative analysis of cytosine-5 DNA methyltransferase and demethylase families in wild and cultivated Peanut. Frontiers in Plant Science. 2016;7:1–14. doi: 10.3389/fpls.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Morris-Natschke & Lee (2007).Wang XH, Morris-Natschke SL, Lee KH. Developments in the chemistry and biology of the bioactive constituents of Tanshen. Medicinal Research Reviews. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- Xing et al. (2015).Xing MQ, Zhang YJ, Zhou SR, Hu WY, Wu XT, Ye YJ, Wu XX, Xiao YP, Li X, Xue HW. Global analysis reveals the crucial roles of DNA methylation during rice seed development. Plant Physiology. 2015;168:1417–1432. doi: 10.1104/pp.15.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2016).Xu H, Song J, Luo H, Zhang Y, Li Q, Zhu Y, Xu J, Li Y, Song C, Wang B, Sun W, Shen G, Zhang X, Qian J, Ji A, Xu Z, Luo X, He L, Li C, Sun C, Yan H, Cui G, Li X, Li X, Wei J, Liu J, Wang Y, Hayward A, Nelson D, Ning Z, Peters RJ, Qi X, Chen S. Analysis of the genome sequence of the medicinal plant Salvia miltiorrhiza. Molecular Plant. 2016;9:949–952. doi: 10.1016/j.molp.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2015).Xu Z, Peters RJ, Weirather J, Luo H, Liao B, Zhang X, Zhu Y, Ji A, Zhang B, Hu S, Au KF, Song J, Chen S. Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. The Plant Journal. 2015;82:951–961. doi: 10.1111/tpj.12865. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang H, Chang F, You C, Cui J, Zhu G, Wang L, Zheng Y, Qi J, Ma H. Whole-genome DNA methylation patterns and complex associations with gene structure and expression during flower development in Arabidopsis. The Plant Journal. 2015;81:268–281. doi: 10.1111/tpj.12726. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2013).Yu A, Lepere G, Jay F, Wang J, Bapaume L, Wang Y, Abraham AL, Penterman J, Fischer RL, Voinnet O, Navarro L. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2389–2394. doi: 10.1073/pnas.1211757110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach et al. (2013).Zemach A, Kim MY, Hsieh PH, Coleman-Derr D, Eshed-Williams L, Thao K, Harmer SL, Zilberman D. The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell. 2013;153:193–205. doi: 10.1016/j.cell.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2015).Zhang G, Tian Y, Zhang J, Shu L, Yang S, Wang W, Sheng J, Dong Y, Chen W. Hybrid de novo genome assembly of the Chinese herbal plant danshen (Salvia miltiorrhiza Bunge) Gigascience. 2015;4:62–65. doi: 10.1186/s13742-015-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang & Zhu (2012).Zhang H, Zhu JK. Active DNA demethylation in plants and animals. Cold Spring Harbor Symposia on Quantitative Biology. 2012;77:161–173. doi: 10.1101/sqb.2012.77.014936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2014).Zhang L, Wu B, Zhao D, Li C, Shao F, Lu S. Genome-wide analysis and molecular dissection of the SPL gene family in Salvia miltiorrhiza. Journal of Integrative Plant Biology. 2014;56:38–50. doi: 10.1111/jipb.12111. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang X, Dong J, Liu H, Wang J, Qi Y, Liang Z. Transcriptome sequencing in response to salicylic acid in Salvia miltiorrhiza. PLOS ONE. 2016;11:e0147849. doi: 10.1371/journal.pone.0147849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong et al. (2013).Zhong S, Fei Z, Chen YR, Zheng Y, Huang M, Vrebalov J, McQuinn R, Gapper N, Liu B, Xiang J, Shao Y, Giovannoni JJ. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nature Biotechnology. 2013;31:154–159. doi: 10.1038/nbt.2462. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2017).Zhou W, Huang Q, Wu X, Zhou Z, Ding M, Shi M, Huang F, Li S, Wang Y, Kai G. Comprehensive transcriptome profiling of Salvia miltiorrhiza for discovery of genes associated with the biosynthesis of tanshinones and phenolic acids. Scientific Reports. 2017;7:10554. doi: 10.1038/s41598-017-10215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu (2009).Zhu JK. Active DNA demethylation mediated by DNA glycosylases. Annual Review of Genetics. 2009;43:143–166. doi: 10.1146/annurev-genet-102108-134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data.
Data Availability Statement
The following information was supplied regarding data availability: