Abstract
Introduction
Distinct motor subtypes of Parkinson's disease (PD) have been described through both clinical observation and through data-driven approaches. However, the extent to which motor subtypes change during disease progression remains unknown. Our objective was to determine motor subtypes of PD using an unsupervised clustering methodology and evaluate subtype changes with disease duration.
Methods
The Parkinson's Progression Markers Initiative database of 423 newly diagnosed PD patients was utilized to retrospectively identify unique motor subtypes through a data-driven, hierarchical correlational clustering approach. For each patient, we assigned a subtype to each motor assessment at each follow-up visit (time points) and by using published criteria. We examined changes in PD subtype with disease duration using both qualitative and quantitative methods.
Results
Five distinct motor subtypes were identified based on the motor assessment items and these included: Tremor Dominant (TD), Axial Dominant, Appendicular Dominant, Rigidity Dominant, and Postural and Instability Gait Disorder Dominant. About half of the patients had consistent subtypes at all time points. Most patients met criteria for TD subtype soon after diagnosis. For patients with inconsistent subtypes, there was an overall trend to shift away from a TD phenotype with disease duration, as shown by chi-squared test, p < 0.001, and linear regression analysis, p < 0.05.
Conclusion
These results strongly suggest that classification of motor subtypes in PD can shift with increasing disease duration. Shifting subtypes is a factor that should be accounted for in clinical practice or in clinical trials.
Keywords: Parkinson's disease, clustering, subtypes
Introduction
It is widely accepted that Parkinson's disease (PD) is a clinically heterogeneous disease with several different motor phenotypes [1,2]. Accurately characterizing the heterogeneity that occurs in PD is important for assessing prognosis, enrolling in clinical trials, and for screening for possible co-morbidities [3]. Properly subtyping patients is also a critical step for determining inclusion in both preclinical [4,5] and clinical studies, particularly those involving individualized treatment plans based on motor symptoms [6-8].
PD subtype categories were originally based on clinical observation of motor symptoms [1,9]. Commonly recognized subtypes of PD include a tremor dominant (TD) group and a postural instability and gait disorder/difficulty/dominant (PIGD) group [2]. Some clinicians and researchers also recognize an ill-defined intermediate or indeterminate type (IT) classification for patients that do not fit the TD or PIGD phenotype. Recently, studies have proposed additional subtypes based on data-driven cluster analysis approaches using other clinical characteristics including age of onset, speed of disease progression, cognitive measures, and behavioral measures [10-14]. Aside from establishing subtypes, several studies have attempted to clarify whether subtypes are fixed or whether they represent different stages of disease progression [15,16].
In this study, our goal was to use a data-driven approach to discover distinct motor subtypes in PD, and to apply these subtypes to longitudinal motor assessment data to look for shift in category over time. To accomplish this task, we used hierarchical correlational clustering on motor assessment data from 423 early PD patients included in the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data).
Methods
Data Source
Data used in this study were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. Further details on the PPMI project have been previously published [17]. The study was approved by the Institutional Review Board (IRB) at all participating institutions. The PPMI database offers unique access to longitudinal data from 423 early PD patients. Participants were required to enroll within two years of PD diagnosis and be untreated for at least the first year of participation. Participants were also required to have abnormal 123-I Ioflupane dopamine transporter (DaTscan™) results, a Hoehn and Yahr stage of I or II at baseline, and be at least 30 years old. Exclusion criteria were in part used to exclude patients with atypical parkinsonism disorders, and included previous MRI with clinically significant neurological disorder. Patient follow-up, including motor assessment, occurred at various times after enrollment. All 423 PD patients and all follow-up visits were included in the present study.
Motor Scores
The PPMI schedule of events includes motor assessments every 3 months after enrollment up to month 12, followed by motor assessments every 6 months up to month 60, followed by motor assessments every 12 months up to month 96. However, exact time points varied across patients (see Results). We compiled together all OFF and ON-medication scores from the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) Part III assessment as well as items 2.10, 2.11, 2.12, and 2.13 from MDS-UPDRS Part II. Per the PPMI protocol, OFF-medication scores were obtained after patients withheld dopaminergic medication for up to 12 hours and ON-medication scores were obtained at least 1 hour after dosing. MDS-UPDRS items with a “left” and “right” component were summed into a single score, and different body parts were scored separately (e.g., rigidity scores for neck, right upper extremity, left upper extremity, right lower extremity, and left lower extremity were analyzed as three scores: neck rigidity, upper extremity rigidity, and lower extremity rigidity). This approach resulted in 22 individual scores for each motor assessment time point.
Clustering
Cluster analysis is a widely-used machine learning technique for uncovering groups of data points that are similar [18,19]. It is a form of unsupervised learning, meaning that the underlying structure of the data is initially unknown. While several types of clustering algorithms are used in practice, we chose to use hierarchical clustering because this type of clustering approach does not require the number of clusters as input [18]. In a hierarchical approach, data points may belong to a cluster, which in turn, might be part of a larger cluster.
All ON and OFF-medication scores from each motor assessment time point were used as input to a hierarchical clustering using squared spearman correlation coefficients as similarity measures [20]. This procedure treated each motor score from the same patient at different time points as separate entries into the algorithm. This ensured that a representative range of possible entries for MDS-UPDRS scores were included in the algorithm. No a priori models were used. We named each identified cluster based on the MDS-UPDRS items that the cluster included.
Subtyping
For each patient, we assigned each OFF and ON-medication motor assessment time point to a subtype from the identified clusters. To do this, we summed together the values from the MDS-UPDRS items included in each cluster to obtain subtype scores. Final subtype designation was determined based on ratios of subtype scores. We followed previous guidelines for assigning these subtypes based on motor scores [21]. That is, we summed the MDS-UPDRS items within each subtype to obtain a subscore for each subtype. If TDsubscore/PIGDsubscore was greater than 1.15, we assigned the subtype TD, and if it was less than 0.9, we assigned the subtype PIGD [21]. If neither of these criteria were met, we then checked for IT subtypes. Since Appendicular-Dominant (ApD) and Rigidity-Dominant (RD) categories were within a distinct subgroup within the overall IT group (Figure 1), separate from the Axial-Dominant (AxD) group, we computed the ratio AxDsubscore/(ApDsubscore+RDsubscore). If this value was greater than 1, we assigned the subtype AxD. Otherwise, if ApDsubscore/RDsubscore was greater than 1, we assigned the subtype ApD, and if it was less than 1, we assigned the subtype RD. Note that in this protocol, it is possible that a motor assessment would result in an unassigned subtype, usually in the case of two subtype scores being equivalent, and hence leading to a ratio of 1.0.
Figure 1.
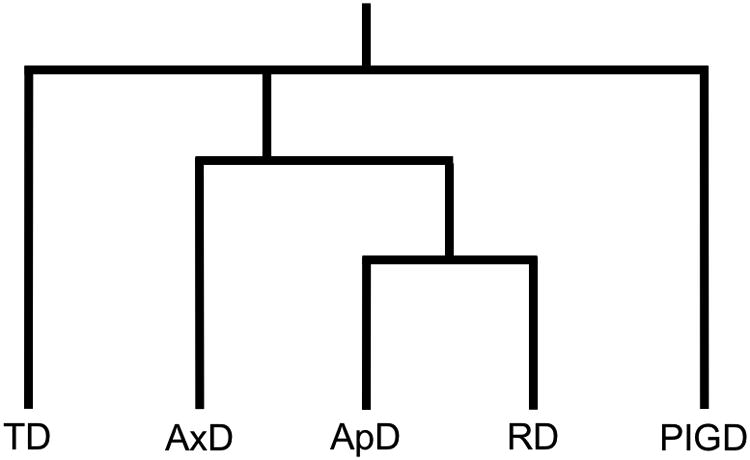
Schematic representation of the output from the hierarchical clustering algorithm. Taken together, we categorized Axial Dominant (AxD), Appendicular Dominant (ApD), and Rigidity Dominant (RD) as Intermediate or Indeterminate Type (IT).
Next, we examined changes in OFF-medication motor subtype over time within subjects and across subjects. We assigned the categorical subtypes ordinal values for quantitative analysis.
Statistical Analysis
All data analyses for the present study were completed in R software (www.cran.r-project.org). Subtype frequency changes over time were analyzed using Chi-square and linear regression tests.
Results
The number of total motor score assessments available in the PPMI database varies between 2 and 14 for each patient (M = 6.81, SD = 2.40). Follow-up visits for the sample as a whole occurred between 0 and 7.84 years after diagnosis (M = 1.63, SD = 1.46). The vast majority (96.4%) of time points were within 5 years of diagnosis. A total of 2866 motor score assessments were used for clustering, which resulted in three overall clusters: TD, IT, and PIGD. Within IT, three specific clusters emerged:AxD,ApD, and RD (Figure 1). Subtypes were named based on the MDS-UPDRS items they included (Table 1).
Table 1. Motor Subtypes.
| Subtype Name | MDS-UPDRS Itemsa |
|---|---|
| Tremor Dominant (TD) | 2.10, 3.15 (R, L), 3.16 (R, L), 3.17 (RUE, LUE, RLE, LLE, Lip/Jaw), 3.18 |
| Intermediate Type (IT) | |
| Axial Dominant (AxD) | 3.1, 3.2, 3.13, 3.14 |
| Appendicular Dominant (ApD) | 3.4 (R, L), 3.5 (R, L), 3.6 (R, L), 3.7 (R, L), 3.8 (R, L) |
| Rigidity Dominant (RD) | 3.3 (Neck, RUE, LUE, RLE, LLE) |
| Postural Instability and Gait Disorder (PIGD) | 2.11, 2.12, 2.13, 3.9, 3.10, 3.11, 3.12 |
R: Right, L: Left, UE: Upper Extremity, LE: Lower Extremity
The column on the right indicates the MDS-UPDRS items that were included in each cluster. The left column indicates the name we assigned to these clusters.
Using all available motor score assessment data (OFF and ON-medication) at all time points, the following frequencies of subtypes were observed: 70.4% TD, 1.1% AxD, 4.7% ApD, 20.3% PIGD. 0.7% of cases were not assigned a subtype. However, these values are irrespective of disease duration.
Next we evaluated for any changes in subtypes during the duration of data collection. We examined OFF-medication subtype changes over time for each patient to study natural disease progression. We found that 232 patients (54.8%) maintained a stable motor subtype designation at all follow-up visits. Of these patients, 91.4% were classified as TD and 8.6% were classified as PIGD. The remaining 191 (45.2%) patients met criteria for least two different motor subtype designations throughout the duration of the study. We were interested in whether or not there were trends in the subtype designation within this group specifically.
All patients with inconsistent subtypes were subsequently examined together to produce sample-level summary data. Using 6-month bins between 0 and 5 years (yrs) after diagnosis, we totaled the number of time points assigned to each subtype. For analysis, we dropped bins that had less than 25% of patients included (2.5-3.0 yrs, 3.5-4.0 yrs, and 4.5-5.0 yrs). Chi-square test between 0-0.5 yrs and 4-4.5 yrs suggested that subtype frequencies change over time, Χ2(4) = 18.34, p < 0.01 (Figure 2).
Figure 2.
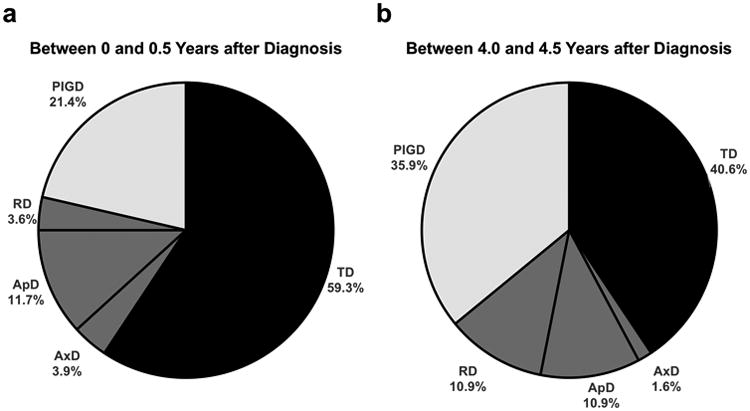
Frequencies of each subtype for patients with inconsistent subtypes between 0 and 0.5 years after diagnosis (a) and between 4.0 and 4.5 years after diagnosis (b). There is a significant shift away from a TD phenotype.
To better understand this change over time, we examined the proportion of subtypes continuously with increasing disease duration (Figure 3). Using a stacked area plot, for visualization we ordered the subtypes from TD to PIGD, with intermediate subtypes in between. We chose this designation based on previous work suggesting that TD could represent the earliest stages of PD, whereas PIGD may represent later stages [22,23]. We chose to place AxD following TD since it has an overall decreased prevalence in the time range we considered (Figure 2). And while ApD and RD both have an overall increased prevalence with disease duration (Figure 2), we noticed that RD increased relatively more, hence we placed RD after ApD and before PIGD. When considering all time points, there remained a significant change in subtype frequencies over time, Χ2(24) = 54.80, p < 0.001. Post-hoc comparisons revealed no differences within the first 2 years of diagnosis: 0.5 vs 1 yr after diagnosis, Χ2(4) = 7.17, p = 0.13, 0.5 vs1.5 yrs after diagnosis, Χ2(4) = 4.66, p = 0.32, and 0.5 vs 2 yrs after diagnosis, Χ2(4) = 2.57, p = 0.63. However, beginning at 2 yrs after diagnosis, we observed significant changes in subtype frequencies for the remaining follow-up periods: 2 vs 2.5 yrs, Χ2(4) = 12.19, p < 0.05, 2 vs 3.5 yrs, Χ2(4) = 10.78, p < 0.05, and 2 vs 4.5 yrs, Χ2(4) = 20.48, p < 0.001.
Figure 3.
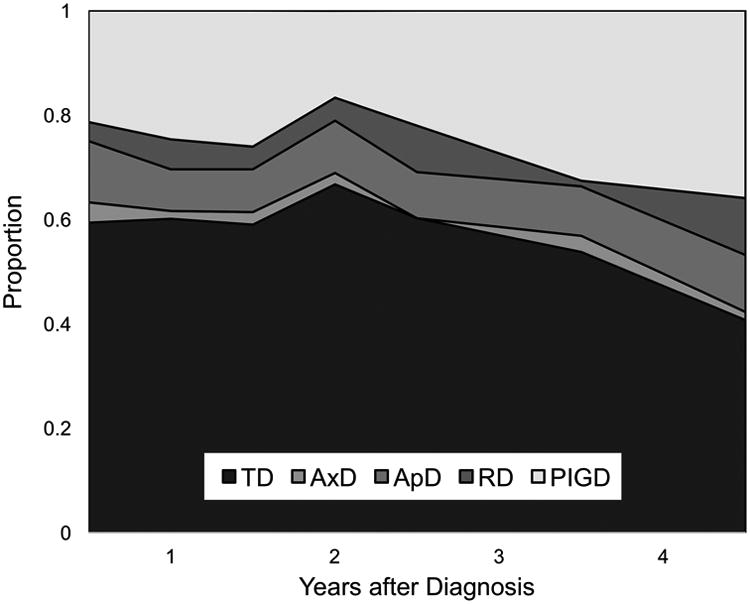
Proportions of motor subtypes for patients with changing motor subtypes up to 4.5 years following diagnosis. Data were grouped into 6-month bins. For instance, the proportions of different subtypes between 1.5 and 2.0 years is plotted on the x-axis at the value 2.
Given the significant differences observed beginning at 2 years after diagnosis, we used a linear regression to quantify the stepwise change in subtype frequencies between 2 and 4.5 years after diagnosis. In this time range, TD frequency decreased by 0.086 per year (p < 0.01) and PIGD frequency increased by 0.097 per year (p< 0.01).
Discussion
Our results identified an additional AxD, ApD, and RD subtypes of PD, in addition to the previously reported TD, IT, and PIGD subtypes [2]. Our methods employed a novel, unsupervised machine learning approach to isolate different motor subtypes in newly diagnosed PD by using MDS-UPDRS motor assessment data. These results also suggested that early PD sub-classification was stable for some patients, but quite unstable for others. Around 60% of patients are classified as TD and 20% as PIGD within 6 months of diagnosis. By five 5 after diagnosis, these values shifted to 40% and 35%, respectively. That is, there was an overall shift away from TD. These results strongly suggest that motor subtype designation may be influenced by disease duration.
Previous studies using data-driven techniques differ from the present study in several ways. This was the first clustering-based investigation to specifically utilize the MDS-UPDRS assessment, which therefore resulted in subtypes that have direct motor clinical correlation. In contrast, several previous clustering studies have resulted in groups such as “good motor control”[13], which may be difficult to interpret or difficult to apply to patients in the clinical setting. Some of the prior clustering routines relied on previously defined models [24] or variables such as a tremor score [25], while our methodology used no a priori information. Also, our study uses a particularly large sample size (n = 423). For comparison, a recent systematic review of cluster analysis in PD noted studies with sample sizes ranging from 44 to 176 [26]. Finally, our approach utilized longitudinal data, distinguishing it from most data-driven clustering studies, with relatively low sample sizes [26],that usually rely on single time points, allowing us to identify subtype changes over time and more precisely categorize subtype shifting.
Our results align well with prior studies and general clinical observation regarding identification of the most commonly recognized motor subtypes. Stebbins and colleagues used a non-data-driven approach to determine which MDS-UPDRS Part II and III items to sum when computing a TD or PIGD score in practice [21]. In our study, the MDS-UPDRS items assigned to the PD and PIGD subtype respectively were identical to those listed in Stebbins's report, with the exception of the addition of Item 2.11 (getting out of a bed, a car, or a deep chair) and 3.9 (arising from a chair) within the PIGD score. Interestingly, since we elected to use a hierarchical technique, we were able to discern a potential solution to the highly controversial IT group. That is, there appeared to be distinct AxD, ApD, and RD groups. However, these subtypes were not as prevalent, particularly the AxD and RD, though there were more cases of RD with longer disease duration. Previous authors suggested the presence of these dominant symptoms in certain PD patients [3,15]. It is unclear whether these intermediate subtypes in particular are distinct from others with regards to comorbidities and non-motor measures. Longer follow-up may elucidate whether these patients progress differently than others.
For patients with inconsistent subtypes, we discovered an overall trend to shift away from a TD phenotype and toward a PIGD phenotype. It is possible that patients with consistent subtypes may have slower disease progression (for TD patients) or faster progression (for PIGD patients), hence this may have masked changes in subtype for some patients in our cohort [15]. These findings contribute to the collective body of evidence showing that with disease duration, the prevalence of TD subtype designation decreases and that of PIGD increases [3,27-29].
Our study has several important limitations. First, the motor subtypes we identified were limited by the items included in the MDS-UPDRS assessment. Only those motor symptoms captured by this assessment could be used to arbitrarily classify motor subtype categories. Second, our ratio-based approach to setting cutoff criteria for assigning subtypes, while also based on previous research [21], may not have been stringent enough for categorizing patients into one of several subtypes. Finally, it is important to note the number of time points for each patient and the disease duration at each time point varied considerably in this large sample. Longer and consistent follow-up will be needed to ascertain that this sample does not include any atypical parkinsonism patients.
Our findings overall suggest that there exist at least 5 distinct motor subtypes in early PD patients: TD, AxD, ApD, RD, and PIGD. Subtypes were mutable throughout disease duration with a trend that was detectable within the first 5 years. Further studies should investigate the differences between patients with consistent and inconsistent subtypes with respect to variables such as demographics, genetics, environmental, and other disease attributes. Longer patient follow-up periods may overcome some of the limitations of the present study.
Acknowledgments
The authors thank the Michael J. Fox Foundation for Parkinson's Research for driving the PPMI development efforts, and all investigators that contributed to the database. We also thank Jackson Cagle, Stephanie Cernera, Enrico Opri, and Rene Molina for helpful discussion and feedback.
Funding Sources: This work was supported by the UF Pruitt Family Endowed Faculty Fellowship. PPMI – a public private partnership – is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen, Bristol-Myers Squibb, Covance, GE Healthcare, Genentech, GlaxoSmithKline, Lilly, Lindbeck, Merck, Meso Scale Discovery, Pfizer, Piramal, Roche, Sanofi Genzyme, Servier, Teva, UCB, and Golub Capital.
C.W.H. receives grant support from the University of Florida Clinical and Translational Research Institute, which is supported in part by NIH award KL2 TR001429. He has served as a research committee member for the Michael J. Fox Foundation and as a speaker for the National Parkinson Foundation, the Parkinson's Disease Foundation, and the Davis Phinney Foundation. Dr. Hess has participated in CME and educational activities on movement disorders sponsored by Allergan, Ipsen, Mertz Pharmaceuticals, Peerview Online, and QuantiaMD.
D.M.R. serves as a consultant for the National Parkinson Foundation and has received honoraria from UCB and the International Parkinson and Movement Disorders Society.
K.D.F.has received research grant support from Medtronic, St. Jude (now Abbott), Boston Scientific, Neuropace, and Functional Neuromodulation. He has also received fellowship support from Medtronic. He has not received any personal renumeration from any industrial source in the past 12 months.
M.S.O. serves as a consultant for the National Parkinson Foundation and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. M.S.O.'s DBS research is supported by: R01 NR014852. M.S.O. has previously received honoraria, but in the past >60 months has received no support from industry. M.S.O. has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). M.S.O. is an associate editor for New England Journal of Medicine Journal Watch Neurology. M.S.O. has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, QuantiaMD, WebMD, MedNet, Henry Stewart, and by Vanderbilt University. The institution and not M.S.O. receives grants from Medtronic, AbbVie, Allergan, and ANS/St. Jude, and the Principal Investigator (PI) has no financial interest in these grants. M.S.O. has participated as a site PI and/or Co-Investigator for several NIH, foundation, and industry sponsored trials over the years, but has not received honoraria. M.S.O. and A.G.receive device donations from Medtronic.
Footnotes
Author Roles: Research Project: Conception – RSE, KDF; Organization – RSE, CWH, DM-R; Execution – RSE
Statistical Analysis: Design – RSE; Execution – RSE; Review and Critique – RSE, CWH, DM-R, LA, MSO, AG
Manuscript: Writing of the First Draft – RSE; Review and Critique – RSE, CWH, DM-R, LA, MSO, AG
Disclosures: R.S.E has no financial disclosures to report.
L.A. has no disclosures to report.
References
- 1.Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson's disease Clinical and prognostic implications. Neurology. 1985;35:522–522. doi: 10.1212/WNL.35.4.522. [DOI] [PubMed] [Google Scholar]
- 2.Foltynie T, Brayne C, Barker RA. The heterogeneity of idiopathic Parkinson's disease. J Neurol. 2002;249:138–145. doi: 10.1007/PL00007856. [DOI] [PubMed] [Google Scholar]
- 3.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson's disease. Brain. 2009;132:2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 4.Clarimón J, Pagonabarraga J, Paisán-Ruíz C, Campolongo A, Pascual-Sedano B, Martí-Massó JF, et al. Tremor dominant parkinsonism: Clinical description and LRRK2 mutation screening. Mov Disord. 2008;23:518–523. doi: 10.1002/mds.21771. [DOI] [PubMed] [Google Scholar]
- 5.Corti O, Lesage S, Brice A. What Genetics Tells us About the Causes and Mechanisms of Parkinson's Disease. Physiological Reviews. 2011;91:1161–1218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- 6.Giladi N, Mirelman A, Thaler A, Orr-Urtreger A. A Personalized Approach to Parkinson's Disease Patients Based on Founder Mutation Analysis. Front Neurol. 2016;7:71. doi: 10.3389/fneur.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marjama-Lyons J, Koller W. Tremor-Predominant Parkinson's Disease. Drugs & Aging. 2000;16:273–278. doi: 10.2165/00002512-200016040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Katz M, Luciano MS, Carlson K, Luo P, Marks WJ, Larson PS, et al. Differential effects of deep brain stimulation target on motor subtypes in Parkinson's disease. Ann Neurol. 2015;77:710–719. doi: 10.1002/ana.24374. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 10.Reijnders JSAM, Ehrt U, Lousberg R, Aarsland D, Leentjens AFG. The association between motor subtypes and psychopathology in Parkinson's disease. Parkinsonism & Related Disorders. 2009;15:379–382. doi: 10.1016/j.parkreldis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Post B, Speelman JD, De Haan RJ O.B.O.T.C.-S. group. Clinical heterogeneity in newly diagnosed Parkinson's disease. J Neurol. 2008;255:716–722. doi: 10.1007/s00415-008-0782-1. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SJG, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach, Journal of Neurology. Neurosurgery & Psychiatry. 2005;76:343–348. doi: 10.1136/jnnp.2003.033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham JM, Sagar HJ. A data-driven approach to the study of heterogeneity in idiopathic Parkinson's disease: identification of three distinct subtypes. Mov Disord. 1999;14:10–20. doi: 10.1002/1531-8257. [DOI] [PubMed] [Google Scholar]
- 14.Dujardin K, Defebvre L, Duhamel A, Lecouffe P, Rogelet P, Steinling M, et al. Cognitive and SPECT characteristics predict progression of Parkinson's disease in newly diagnosed patients. J Neurol. 2004;251:1383–1392. doi: 10.1007/s00415-004-0549-2. [DOI] [PubMed] [Google Scholar]
- 15.Nutt JG. Motor subtype in Parkinson's disease: Different disorders or different stages of disease? Mov Disord. 2016;31:957–961. doi: 10.1002/mds.26657. [DOI] [PubMed] [Google Scholar]
- 16.Calne DB. Is “Parkinson's disease” one disease? Journal of Neurology. Neurosurgery & Psychiatry. 1989;52:18–21. doi: 10.1136/jnnp.52.Suppl.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkinson Progression Marker Initiative, The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everitt B, Landau S, Leese M, Stahl D. Cluster Analysis. fifth. West Sussix, United Kingdom: 2011. [Google Scholar]
- 19.Dilts D, Khamalah J, Plotkin A. Using Cluster Analysis for Medical Resource Decision Making. Med Decis Making. 1995;15:333–346. doi: 10.1177/0272989×9501500404. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman L, Rousseeuw PJ. Finding groups in data: an introduction to cluster analysis. first. Hoboken, New Jersey: 2009. [Google Scholar]
- 21.Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson's disease rating scale: Comparison with the unified Parkinson's disease rating scale. Mov Disord. 2013;28:668–670. doi: 10.1002/mds.25383. [DOI] [PubMed] [Google Scholar]
- 22.Deuschl G. Benign tremulous Parkinson's disease: A misnomer? Mov Disord. 2013;28:117–119. doi: 10.1002/mds.25317. [DOI] [PubMed] [Google Scholar]
- 23.Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Tanner C, Marek K, et al. How stable are Parkinson's disease subtypes in de novo patients: Analysis of the PPMI cohort? Parkinsonism & Related Disorders. 2016;28:62–67. doi: 10.1016/j.parkreldis.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 24.Stochl J, Boomsma A, Ruzicka E, Brozova H, Blahus P. On the structure of motor symptoms of Parkinson's disease. Mov Disord. 2008;23:1307–1312. doi: 10.1002/mds.22029. [DOI] [PubMed] [Google Scholar]
- 25.Erro R, Vitale C, Amboni M, Picillo M, Moccia M, Longo K, et al. The Heterogeneity of Early Parkinson's Disease: A Cluster Analysis on Newly Diagnosed Untreated Patients. PLoS ONE. 2013;8:e70244. doi: 10.1371/journal.pone.0070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Rooden SM, Heiser WJ, Kok JN, Verbaan D, van Hilten JJ, Marinus J. The identification of Parkinson's disease subtypes using cluster analysis: A systematic review. Mov Disord. 2010;25:969–978. doi: 10.1002/mds.23116. [DOI] [PubMed] [Google Scholar]
- 27.Josephs KA, Matsumoto JY, Ahlskog E. Benign Tremulous Parkinsonism. Arch Neurol. 2006;63:354–357. doi: 10.1001/archneur.63.3.354. [DOI] [PubMed] [Google Scholar]
- 28.Simuni T, Long JD, Caspell-Garcia C, Coffey CS, Lasch S, Tanner CM, et al. Predictors of time to initiation of symptomatic therapy in early Parkinson's disease. Ann Clin Transl Neurol. 2016;3:482–494. doi: 10.1002/acn3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selikhova M, Kempster PA, Revesz T, Holton JL, Lees AJ. Neuropathological findings in benign tremulous Parkinsonism. Mov Disord. 2013;28:145–152. doi: 10.1002/mds.25220. [DOI] [PubMed] [Google Scholar]


