Abstract
Background
Obstructive sleep apnea (OSA) associates with increased risk of cardiovascular diseases (CVD). Immune abnormalities and surges in sympathetic activity accompany OSA and CVD. We hypothesized that OSA associates with leukocytosis partially by abnormalities in autonomic nervous system (ANS) function that would suggest a pathway linking OSA and CVD.
Methods
Participants from the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective cohort of individuals initially without overt CVD, underwent polysomnography and assays for white blood cells (WBC) and subsets. Heart rate (HR) and heart rate variability (HRV), indirect measurements of ANS, were obtained from overnight electrocardiography. A formal statistical mediation analysis tested the indirect effect that mean HR and HRV measures contribute to associations between OSA and leukocytosis.
Results
The analytical sample consisted of 1,298 participants (54% female), ages 54–93years, 14% with severe OSA (apnea-hypopnea-index, AHI≥30). Severe OSA associated with a higher prevalence of obesity, diabetes, and increased levels of WBC total and subsets. Neutrophil count associated with severe OSA after adjusting for confounders (p=0.017). Mean HR positively associated with OSA indices and neutrophils. A mediation analysis revealed an “indirect” effect of mean HR that explained an estimated 11% of the association between AHI and neutrophils. Overnight hypoxia also associated with neutrophil count (p=0.009), and mean HR explained 14% of the association between neutrophils and hypoxia.
Conclusions
In the MESA cohort, OSA measures associate with elevated neutrophil counts and increases in overnight mean HR. These data link innate immune dysregulation with OSA and provide a potential pathophysiologic pathway between CVD and OSA.
Keywords: neutrophils, granulocytes, polymorphonuclear leukocytes, sleep apnea, hypoxia, heart rate
1. INTRODUCTION
Obstructive sleep apnea (OSA) occurs in over 50% of patients with hypertension1,2 and over 30% of patients with coronary artery disease.3,4 Longitudinal studies demonstrate that OSA independently predicts an increased incidence of cardiovascular disease (CVD).5,6 OSA might contribute to CVD through the consequences of airway occlusion, which include negative intrathoracic pressure swings, intermittent hypoxia, and sleep fragmentation.7 Preliminary observations also suggest that OSA contributes to abnormal interactions of leukocytes with endothelial cells, a process that promotes atherogenesis.8,9 Yet, the specific pathways by which OSA operates to increase CVD risk remain uncertain.
OSA involves inflammation.10–12 A meta-analysis highlighted a role for circulating inflammatory mediators in the pathogenesis of atherosclerosis in OSA patients.11 In contrast to the literature on serum markers of inflammation,10–12 few reports have explored how leukocyte counts, which reflect inflammatory processes, vary with OSA severity in large samples of individuals.13 Inflammation drives atherothrombosis and leukocytes participate pivotally in lesion formation and complications.14 Since the sympathetic and parasympathetic arms of the autonomic nervous system (ANS) influence immune interactions,15 and as abnormalities of the ANS occur with OSA,16,17 immune dysregulation associated with ANS function might link OSA and CVD. Prior research has not evaluated the inter-relationships among leukocytes, ANS function, and OSA. Therefore, we tested the hypothesis that leukocyte counts rise relative to OSA severity, and that increased overnight heart rate and altered heart rate variability (HRV) mediate in part these associations.
2. METHODS
2.1. Study Population
The sample was a subset of participants in the Multi-Ethnic Study of Atherosclerosis (MESA). As previously described,18 MESA is a multi-site prospective study designed to investigate the prevalence and progression of subclinical CVD in a racially/ethnically sample. Participants, ages 45 to 84 years, were enrolled from six US-communities (2000–2002). At Exam 5 (2010–2013), all MESA participants other than those reporting regular use of oral appliance (n=4), oxygen use (n=4), and PAP device use (n=95) were invited to participate in the MESA Sleep Ancillary Study, which included polysomnography. After exclusions, 2,261 participants were recruited and technically acceptable polysomnography data were available for 2,057 participants, with 1,964 records meeting quality standards for HRV. Of these, 1,298 individuals also had blood assayed for white blood cells (WBC).
Subject characteristics, body mass index (BMI), hypertension, diabetes mellitus (DM), smoking status, alcohol use, and medications were ascertained at Exam 5 using standardized questionnaires, blood assays, and relevant measurements. Smoking was defined as never, former (no smoking within the past 30 days), or current. Alcohol drinking was defined as current use (yes/no), as part of a history questionnaire (“Do you presently drink alcoholic beverages?”). The estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD equation) indexing per 1.73m2 for body surface area. Type 2 DM was classified by the 2003 American Diabetes Association, defined as fasting glucose ≥126mg/dL or use of hypoglycemic agents.19 Resting blood pressure was measured three times in the seated position and the average of the second and the third served as systolic blood pressure (SBP) and diastolic blood pressure (DBP). Hypertension was defined as SBP>140mmHg, DBP>90mmHg or use of anti-hypertension medication.20 Institutional Review Board approval was obtained at each study site and written informed consent was obtained from all participants.
2.2. Sleep measurements
Polysomnography was conducted using a 15-channel monitor (Compumedics Somte System; Compumedics Ltd., Abbotsville, AU). The recording included electroencephalography, bilateral electrooculograms, chin electromyography, bipolar electrocardiography, thoracic and abdominal respiratory inductance plethysmography, airflow measured by thermocouple and nasal pressure cannula, finger pulse oximetry, and bilateral limb movements. Sleep stages, arousals and respiratory events were scored according to published guidelines.21,22 Apneas were scored when the thermocouple signal flattened for >10 seconds. Hypopneas were scored when nasal pressure flow signal decreased by 30% or more for a period of at least 10 seconds. OSA severity was assessed with the apnea hypopnea index (AHI), which included all apneas regardless of desaturation and hypopneas accompanied by at least a 4% drop in oxygen saturation.21,22 OSA severity was defined by conventional categories: none (AHI<5), mild (AHI≥5 to <15), moderate (AHI≥15 to <30), and severe (AHI≥30).23 Related indices, including oxygen desaturation (percentage of time less than 92%: Pc time SpO2<92%) and arousal index (number of arousals per hour of sleep) were also analyzed.
2.3. Leukocyte Measurement
Leukocytes were assessed in blood samples collected at Exam 5 at a central laboratory. Assays included total WBC count and leukocyte subsets (eosinophils, neutrophils, lymphocytes, and monocytes) were determined as complete blood count with differential analysis.18
2.4. Overnight Heart Rate and Heart Rate Variability
As part of polysomnography, continuous electrocardiography signals were recorded at 256Hz. QRS complexes (R-points) were detected using Compumedics Somte software version 2.10. The R-points were manually reviewed by trained technicians and classified as normal sinus, supraventricular or ventricular premature complex. HRV indices were calculated in consecutive 5-minute windows across the sleep period. Studies with <2h of sleep or with fewer than 1,000 normal-to-normal (NN) intervals were excluded (n=17 individuals). The beat annotations were reviewed by an expert technician who manually annotated each QRS complex. From these files, we analyzed only NN intervals for both time and frequency domain indices. The latter were computed using the Lomb periodogram,24 which is ideal for analysis of time series with missing data. To minimize the influence of respiratory events, we further performed a sensitivity analysis that compared results with and without respiratory events. The restricted analysis represents the 47%of windows with no respiratory events.
We analyzed time and frequency domain variables averaged over all sleep stages. Following the recommendations of the HRV Task Force,25 we computed the time domain measures that included: mean heart rate derived from the average of instantaneous heart rate derived from beat-to-beat analysis; the standard deviation of NN intervals (SDNN); the square root of the mean of the squares of the successive differences between adjacent NN intervals (rMSSD); the percentage of absolute differences in successive NN values >50ms (pNN50). Frequency domain measures included: low frequency (LF) spectral power of the NN interval time series between 0.04 to 0.15 Hz; high frequency (HF) spectral power of the NN intervals between 0.15 and 0.4Hz; and LF/HF ratio. HF and LF spectral power indices were shown in absolute and normalized values. Normalized were calculated as follows: HFnu= (HF/(HF+LF)) and LFnu= (LF/(HF+LF)).25 We explored other time domain variable (pNN20), since there is evidence that thresholds as low as 20ms or less rather than the standard 50ms threshold enhanced discrimination between a variety of normal and pathological conditions.26
2.5. Statistical Analysis
Categorical variables were presented as frequencies and percentages and compared by chi-squared statistic. Continuous variables were compared using the independent t-test or the Mann-Whitney non-parametric test. Given that severe OSA associates more consistently with CVD outcomes than milder levels,27 logistic regression analysis was conducted considering the dichotomous outcome, severe OSA (AHI ≥30). Linear regression analysis was used to assess the associations between indices of OSA (by AHI category, oxygen desaturation, and arousal index) as exposures with leukocyte numbers (WBC total and subsets), modeled as outcomes. Covariates were age, sex, race/ethnicity, smoking status, BMI, hypertension, DM, and medications that associate with heart rate, such as beta adrenergic blockers and any calcium channel blockers. Similarly, linear regression was used to assess the association of overnight mean heart rate and HRV indices as predictors, with OSA indices as the outcome, adjusting for the same covariates listed before. Natural log transformations were applied to WBC total, leukocytes subsets, and AHI to achieve approximate normality. Heart rate and HRV measures that associated significantly with AHI were further evaluated as potential mediators in the association between OSA and leukocytes, using a methodology described by Baron and Kenny.28,29 Point estimates and 95% confidence intervals for mediated effects were calculated using resampling methods described by Mackinnon et al.30 Analyses were conducted with SPSS (V.18.0, SPSS, Chicago, Ill) and SAS (V.9.4, Cary, NC). All probability values were two-sided; a P-value less than 0.05 was considered significant.
3. RESULTS
Characteristics of the analytic sample according to OSA severity are shown in Table 1. The characteristics of the full MESA sleep sample and analytical sample are shown in supplemental material; the analytical sample is similar to the larger MESA sleep exam sample in regards to demographic and health-related factors (Table 1S). OSA associated with older age, male sex, measures of adiposity, and hypertension. Univariate analysis showed significant associations of WBC, neutrophils, and monocytes with OSA severity (Table 2S-supplemental material). After adjusting for traditional confounders, only neutrophil counts remained associated with severe OSA (Table 2). Similarly, an increasing neutrophil count associated significantly with higher AHI level (continuously measured) in an adjusted multiple linear regression model (Table 3S-supplemental material). Since neutrophil count associated most strongly with OSA in the foregoing analyses, we selected neutrophil count as our outcome for testing whether the association between OSA indices and leukocytes is mediated by ANS measures. Then, we tested whether indices of OSA were predictors in a multivariate linear regression analysis with neutrophil count as the outcome (Table 4S-supplemental material). Neutrophil count was significantly associated with AHI, hypoxia, and severe OSA (AHI ≥30), but not arousal index.
Table 1.
Participants Characteristics by OSA Severity (AHI Category)
| Overall (n=1,298) |
AHI < 30 (n=1,120) |
AHI ≥ 30 (n=178) |
p-value | |
|---|---|---|---|---|
|
| ||||
| Age, y | 68 ±9 | 68 ±9 | 69 ±9 | 0.722 |
| Male sex, % | 46 | 43 | 68 | <0.001 |
| BMI, kg/m2 | 29 ±5 | 29± 5 | 33 ±6 | <0.001 |
| Waist circumference, cm | 100 ±14 | 100 ±14 | 110 ±14 | <0.001 |
| Hypertension, % | 58 | 58 | 63 | 0.180 |
| Diabetes Mellitus, % | 21 | 20 | 31 | <0.001 |
| eGFR, ml/min Race, % | 82 ±21 | 81 ±20 | 84 ±22 | 0.194 |
| White | 38 | 39 | 29 | |
| Chinese | 1 | 1 | 1 | 0.009 |
| African American | 29 | 29 | 27 | |
| Hispanic | 32 | 31 | 43 | |
| Smoking status, % | ||||
| Never | 42 | 43 | 35 | |
| Former | 51 | 50 | 58 | 0.125 |
| Current | 7 | 7 | 7 | |
| Alcohol, % | 45 | 45 | 52 | 0.056 |
| Any hypertension medication, % | 54 | 54 | 60 | 0.143 |
| Beta-blocker, % | 16 | 16 | 15 | 0.838 |
| Calcium channel blockers, % | 17.5 | 17 | 18 | 0.691 |
| Digitalis, % | 0.1 | 0.1 | 0 | 0.690 |
| Statin, % | 37 | 36 | 39 | 0.518 |
Data are shown in mean ±SD for continuous variables and percentages for categorical. P-value by independentt-test for continuous and by Chi-Square for categorical variables. AHI= apnea hypopnea index; BMI= body mass index; eGFR= estimated glomerular filtration rate indexing per 1.73m2 for body surface area.
Table 2.
Multivariate Logistic Regression Analysis Relating severe OSA (AHI ≥ 30, outcome) to leukocytes/subsets (n=1,298)
| OR | 95% CI lower, upper | P-value | |
|---|---|---|---|
|
| |||
| Immune cells, ×103/μL | |||
|
| |||
| WBC total | 1.037 | 0.984, 1.093 | 0.178 |
| Neutrophils | 1.152 | 1.026, 1.284 | 0.017 |
| Monocytes | 1.387 | 0.752, 2.557 | 0.294 |
| Lymphocytes | 0.995 | 0.906, 1.094 | 0.923 |
| Eosinophils | 0.698 | 0.201, 2.422 | 0.571 |
Analyses adjusted by age, sex, race/ethnicity, smoking status, BMI, HTN, DM, bb, and any ccb.
OSA= obstructive sleep apnea; AHI= apnea-hypopnea index; AHI ≥30= severe OSA; OR= odds ratio; CI= confidence interval; WBC= white blood cells; BMI= body mass index; HTN= hypertension; DM= Diabetes Mellitus, bb= beta-blocker; ccb= calcium channel blocker;
Given that neutrophil count associated significantly with several indices of OSA severity, we further evaluated their relationships with mean heart rate and HRV. Adjusted analyses showed that higher overnight mean heart rate, higher SDNN, higher LFnu and lower HFnu associated with a higher adjusted odds of severe OSA (Table 3) Of these indices, only mean heart rate also significantly associated with neutrophils (Table 5S-supplemental material).
Table 3.
Multivariate Logistic Regression Analysis Relating Severe OSA (AHI ≥30, outcome) to heart rate/HRV measures (n=1,298)
| OR | 95% CI lower, upper |
P-value | |
|---|---|---|---|
|
| |||
| HRV (time domain) | |||
|
| |||
| Mean heart rate (bpm) | 1.023 | 1.004, 1.043 | 0.019 |
| SDNN (ms) | 1.008 | 1.003, 1.013 | 0.002 |
| rMSSD (ms) | 1.003 | 0.999, 1.006 | 0.189 |
| pNN20, % | 0.999 | 0.990, 1.007 | 0.734 |
| pNN50, % | 1.000 | 0.989, 1.012 | 0.984 |
|
| |||
| HRV (frequency domain) | |||
|
| |||
| LF nu | 5.210 | 1.433, 18.942 | 0.012 |
| LF (ms2) | 1.000 | 0.999, 1.000 | 0.126 |
| HF nu | 0.192 | 0.053, 0.698 | 0.012 |
| HF (ms2) | 1.000 | 0.999, 1.000 | 0.301 |
| LF/HF ratio | 1.083 | 0.996, 1.177 | 0.062 |
Analysis adjusted by age, sex, race/ethnicity, smoking status; BMI; HTN, DM, bb, and any ccb. OSA= obstructive sleep apnea; OR= odds ratio; CI= confidence interval; HRV= heart rate variability; NN= normal-to-normal intervals; SDNN= the standard deviation of NN intervals; rMSSD= the square root of the mean of the squares of the successive differences between adjacent NN intervals; pNN20= the percentage of absolute differences in successive NN values >20ms; pNN50= the percentage of absolute differences in successive NN values >50ms; LF= low frequency power (between 0.04 to 0.15 Hz); HF= high frequency power (between 0.15 to 0.4 Hz); nu= normalized units; ms= milliseconds. BMI= body mass index; HTN= hypertension; DM= Diabetes Mellitus, bb= beta-blocker; ccb= calcium channel blocker;
Given the significant associations between OSA and neutrophils, and between neutrophils and mean heart rate, we then conducted formal mediation tests. Presentation of the results use a visual depiction model proposed by Mackinnon et al30 (Figure 1), with each step of the analysis shown in the supplemental material. These analyses, adjusted for potential confounders, yielded an estimate that mean heart rateexplains11% of the association between AHI level and neutrophils. Similar analyses evaluated overnight hypoxia and severe OSA (AHI ≥ 30) as markers of OSA. Adjusted analyses showed similar associations for hypoxia as for AHI (Figure 1), with mean heart rate explaining 14% of the association between hypoxia and neutrophils (Tables 6S and 7S-supplemental material). Adjusted models, testing associations between severe OSA (AHI≥30) and neutrophils, however, did not provide evidence for a significant mediation effect by mean heart rate, possibly due to reduced power (Table 8S-supplemental material). We did not test arousal index in the model, since it did not associate with the outcome (neutrophils).
Figure 1.
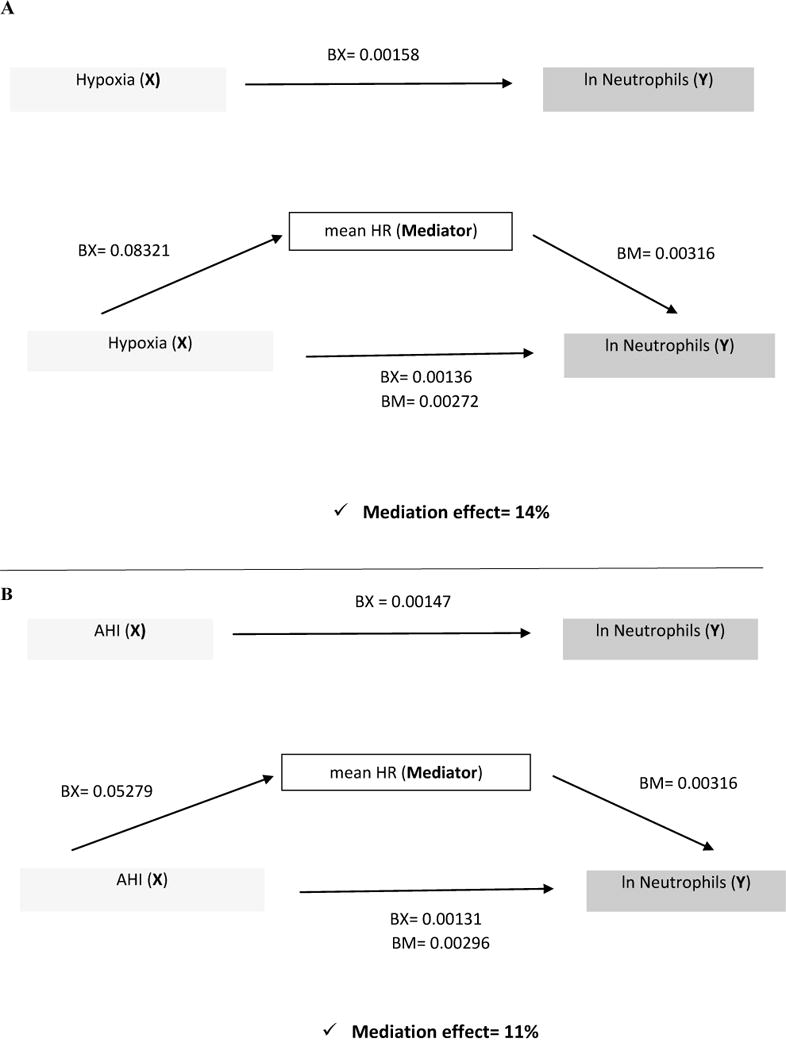
Schematic Depiction Showing how mean HR Mediates the Relationship between OSA measures and Neutrophil counts.
Sensitivity analyses using HRV measurements derived only from 5-min windows of the polysomnogram without respiratory events did not yield appreciable differences in associations based on HRV data derived from the full polysomnogram (Tables 9S and 10S-supplemental material).
4. DISCUSSION
Analysis of a large and well-characterized community sample identified a novel association between OSA and elevations in neutrophil counts, but not in total or other WBC subsets, in models adjusted for multiple confounders. Furthermore, overnight indices of heart rate provided the opportunity to test whether markers of cardiac sympathovagal balance might influence this association. Several indices of HRV time and frequency domains associated significantly with OSA severity. Of these variables, only mean heart rate significantly associated with neutrophils. A formal mediation analysis yielded the estimates that mean heart rate explained 14% of the association between hypoxia and neutrophils and 11% of the association between AHI and neutrophils. Together, these findings: a) identify elevations in neutrophil count as a more specific marker of OSA-associated inflammation than total WBC; b) suggest that mean overnight heart rate, a proxy of sympathetic activity, associates with neutrophils and OSA; c) and indicate that sympathovagal balance, as measured by overnight heart rate, partially mediates the association between neutrophils and OSA measures.
Overall, these findings support a growing literature that implicates inflammatory processes with OSA,9–13 and provide new mechanistic insight of the “inflammatory reflex.” This concept posits that vagal activity exerts anti-inflammatory actions.31,32 Thus, a net increase in sympathetic activity, or vagal withdrawal, should augment inflammation. The links shown here between elevations in overnight mean heart rate, a readily derived electrocardiographic index of integrated cardiac autonomic status, support this novel mechanistic connection between OSA and inflammation.
The stronger associations observed for neutrophils compared to total or other WBC subsets with OSA has interest given enhanced appreciation of in the participation of granulocytes in innate immunity in atheroclerosis.33,34 Neutrophils, as part of the innate immune response, participate in the first line defense in acute inflammatory conditions. Recent evidence also supports their role in chronic inflammatory conditions such as atherosclerosis, as direct mediators or by “second-wave” modulation of mononuclear cell functions.34 Recent studies of neutrophil-driven atherogenesis and thrombotic complications suggest that these inflammatory cells provoke endothelial dysfunction, by: 1) generation of reactive oxygen species and increasing vascular permeability; 2)activating macrophages and promoting foam cell formation, thus aggravating plaques; or 3) through neutrophil-derived mediator release promoting endothelial damage and contribution to weakening of the fibrous cap.34–36 An emerging understanding of leukocyte arterial infiltration in atherosclerotic lesions has stimulated interest in controlling hyperlipidemia-dependent neutrophilia.34,35 Furthermore, neutrophil counts, even in the normal range, associate strongly with the incidence of myocardial infarction,37 heart failure,37 and total mortality.38 Our findings showing that OSA is associated with elevated neutrophils, provide a potential explanation for the higher CVD risk in this patient population, and suggest that targeting inflammatory pathways in OSA patients could reduce CVD burden in these patients.
Prior experimental studies identified inflammatory cell-activation and endothelial dysfunction in OSA patients,8, 39 a process that may augment atherogenesis. Yet few studies have focused on neutrophils and OSA.40–45 Most prior studies relating OSA to neutrophils have limited sample size40–42, 45 or overlap with asthma syndrome43 or only focus on significant associations between neutrophil-to-lymphocyte ratio and OSA.44,45 Our work adds novel aspects to this literature by examining the associations of leukocyte total and subsets in a large, well-characterized, diverse cohort with a wide spectrum of OSA severity, and further, in relation to measures of cardiac ANS function. It is possible that associations between neutrophils and OSA may relate to subclinical airway infection or to unidentified systematic inflammatory disease. However, participants were recruited from community settings were studied when in stable health. The prevalence of chronic diseases other than heart disease and diabetes was low. While infection is implicated in adenotonsillary hypertrophy occurring in children with OSA, its role in adult OSA is not clear.
The HRV Task Force describes the bases for variation of HRV parameters, noting that LF reflects both sympathetic and vagal modulations; LF/HF ratio reflects sympathovagal balance; HF reflects vagal modulation; and rMSSD and pNN50 (time domain measures) correlate with HF (frequency domain measure)25. However, HRV parameters are increasingly appreciated to have complex and as yet incompletely resolved physiological significance, mainly because of the influence of respiration-related changes of heart rate, cardiorespiratory synchronization, and cyclical variation of heart rate with sleep apnea.46 Our study did not address new methodologies for overcoming these limitations but analyzed standardized parameters across a range of models, including sensitivity analyses that restricted to epochs without respiratory events. Both time and frequency domain variables associated significantly with severe OSA in adjusted analyses, suggesting altered sympathovagal balance. A formal mediation analysis identified that mean heart rate explained a significant portion of the variation in the association between OSA and neutrophil elevations.
The finding that mean overnight heart rate best explained the association between OSA and neutrophils is consistent with a growing literature supporting the prognostic value of mean resting heart rate. Overnight heart rate has been shown to be a significant independent predictor of increased mortality and cardiovascular risk in a population based study.47 Similarly, resting heart rate has been shown to have predict major cardiovascular events in patients with asymptomatic aortic stenosis.48 Our data further highlights that use of a relatively simple measurement of autonomic activation may further inform studies of OSA-related cardiovascular risk. This interpretation is consistent with prior reports of augmented sympathetic activation occurring in OSA as measured using elegant techniques such as microneurography49 or via circulating catecholamine levels,50 and thought to be due to intermittent hypoxia with cycles of hypoxia/reoxygenation, promoting vascular oxidative stress and inflammation.7–11, 17 Restoration of ventilation after airway occlusion also can trigger peripheral chemoreflex activation and subsequent sympathetic activation.17
In summary, our findings indicate that readily measured indices such as elevations in neutrophils and overnight mean heart rate may aid in the characterization of the pathophysiology of OSA.
4.1. Strengths and Limitations
The study has several strengths, including its large and diverse sample, and rigorous analysis of overnight heart rate and HRV and use of standardized polysomnography. Analyses were adjusted for potential confounders that could influence both inflammatory pathway and the autonomic nervous system and employed a rigorous mediation analysis. However, important limitations merit consideration. The observational nature of the study limits causal inference. While inflammation is most commonly considered a response to OSA-related stressors, inflammation might contribute to the pathogenesis of OSA. The heart rate and HRV measures only indirectly assess the ANS, and several factors may influence these indices. The limitations of traditional HRV measurements (time and frequency domain) are documented in older adults and those with heart disease,51 and may relate to subtle anomalies caused by non-vagal mediated sinus arrhythmia. This influence is more likely to affect specific indices of HRV than average heart rate. While multiple measures of HRV associated with OSA, only mean overnight heart rate associated with neutrophil counts. This finding may be related to the supposition that changes in heart rate, while non-specific, provide a more useful assessment of autonomic status in general population than specific indexes of components of HRV.52 Given that aging may change HRV,53,54 the older age of our study population may have mitigated the ability of the available parameters to measure cardiac ANS. Measurement of HRV also depends on the effects of sleep-disordered breathing. Yet, a sensitivity analysis did not indicate that the observed relationships varied substantively when data were restricted to areas of the polysomnogram without respiratory events. Furthermore, a full panel of inflammatory markers was not available to explore “downstream” pathways. Residual confounding due to unknown confounders may result in incomplete adjustment and overestimation of these associations.
5. CONCLUSIONS
In summary, analyses of a large community sample demonstrate that OSA independently associates with elevations in neutrophil counts. Mediation analysis identified autonomic activation, as assessed by subtle, but significant increases overnight heart rate, as a potential contributor to inflammation in OSA. These data point to the value of studies aimed at further elucidating the role of autonomic/immune mechanisms in OSA. Use of immune markers may provide strategies for improve risk stratification and identify mechanistic targets for attenuating OSA-related atherogenesis.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Disclosures
This was not an industry supported study. Peter Libby has support from the R01HL08047 and from the RRM Charitable Fund; Ary L. Goldberger support from: 2R01GM104987 and from The G. Harold and Leila Y. Mathers Charitable Foundation; Madalena D. Costa support from: The James S. McDonnell Foundation; Glaucylara R. Geovanini has support from the Lemann Foundation.
Funding Sources: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, and HL080472 from the National Heart, Lung, and Blood Institute, the RRM charitable fund, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS.
ABBREVIATIONS
- AHI
Apnea Hypopnea Index
- ANS
Autonomic Nervous System
- BMI
Body Mass Index
- CVD
Cardiovascular Diseases
- DM
Diabetes Mellitus
- HRV
Heart Rate Variability
- OSA
Obstructive Sleep Apnea
- WBC
White Blood Cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no relationships that could be construed as a conflict of interest.
Author’s contributions
GRG, RW, and SR contributed substantially to the study design,
GRG, JW, ALG, MDC, RT, NSJ, and YL contributed to data acquisition,
GRG, JW, RW, SR contributed to data analysis and interpretation,
and GRG, SR, PL, ALG, MDC, and NSJ contributed to the writing of the manuscript.
Data sharing agreement: Data can be obtained, with the appropriate permissions, through the Multi-Ethnic Study of Atherosclerosis: http://www.mesanhlbi.org/
References
- 1.Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, Lorenzi-Filho G. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105(8):1135–1139. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–7. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 3.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118:955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 4.Geovanini GR, Gowdak LH, Pereira AC, Danzi-Soares Nde J, Dourado LO, Poppi NT, Cesar LA, Drager LF, Lorenzi-Filho G. OSA and depression are common and independently associated with refractory angina in patients with coronary artery disease. Chest. 2014;146(1):73–80. doi: 10.1378/chest.13-2885. [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kylintireas I, Craig S, Nethononda R, Kohler M, Francis J, Choudhury R, Stradling J, Neubauer S. Atherosclerosis and arterial stiffness in obstructive sleep apnea-a cardiovascular magnetic resonance study. Atherosclerosis. 2012;222(2):483–489. doi: 10.1016/j.atherosclerosis.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94:179–184. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- 10.Lavie L, Dyugovskaya L, Polyakov A. Biology of peripheral blood cells in obstructive sleep apnea-the tip of the iceberg. Arch Physiol Biochem. 2008;114(4):244–54. doi: 10.1080/13813450802306701. [DOI] [PubMed] [Google Scholar]
- 11.Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Naseem J, Loomba R. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013 Oct 15;9(10):1003–12. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li K, Wei P, Qin Y, Wei Y. Is C-reactive protein a marker of obstructive sleep apnea? A meta-analysis. Medicine (Baltimore) 2017 May;96(19):e6850. doi: 10.1097/MD.0000000000006850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisele HJ, Markart P, Schulz R. Obstructive Sleep Apnea, Oxidative Stress, and Cardiovascular Disease: Evidence from Human Studies. Oxid Med Cell Longev. 2015;2015:608438. doi: 10.1155/2015/608438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libby P, Nahrendorf M, Swirski FK. Leukocytes Link Local and Systemic Inflammation in Ischemic Cardiovascular Disease: An Expanded “Cardiovascular Continuum”. J Am Coll Cardiol. 2016 Mar 8;67(9):1091–103. doi: 10.1016/j.jacc.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Physiol. 2014 Jul;4(3):1177–200. doi: 10.1002/cphy.c130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansukhani MP, Kara T, Caples SM, Somers VK. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr Hypertens Rep. 2014;16(9):476. doi: 10.1007/s11906-014-0476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gammoudi N, Ben Cheikh R, Saafi MA, Sakly G, Dogui M. Cardiac autonomic control in the obstructive sleep apnea. Libyan J Med. 2015;10:269–89. doi: 10.3402/ljm.v10.26989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P, Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 20.Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997 Nov 24;157(21):2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 21.Redline S, Budhiraja R, Kapur V, Marcus CL, Mateika JH, Mehra R, Parthasarthy S, Somers VK, Strohl KP, Sulit LG, Gozal D, Wise MS, Quan SF. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 22.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–58. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 23.Ruehland WR, Rochford PD, O’Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009 Feb;32(2):150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moody GB. Computers in Cardiology. IEEE Computer Society Press; 1993. Spectral analysis of heart rate without resampling; pp. 715–718. 1993). http://www.physionet.org/physiotools/lomb/lomb.html. [Google Scholar]
- 25.Marek Malik, Thomas Bigger J, John Camm A, Kleiger Robert E, Malliani Alberto, Moss Arthur J, Schwartz Peter J. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996 Mar;17(3):354–81. [PubMed] [Google Scholar]
- 26.Mietus JE, Peng CK, Henry I, Goldsmith RL, Goldberger AL. The pNNx files: reexamining a widely used heart rate variability measure. Heart. 2002 Oct;88(4):378–80. doi: 10.1136/heart.88.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CH, Khoo SM, Chan MY, Wong HB, Low AF, Phua QH, Richards AM, Tan HC, Yeo TC. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7:616–621. doi: 10.5664/jcsm.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 30.MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods. 2007;39:384–9. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007 Mar-Apr;13(3–4):178–84. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005 Nov;19(6):493–9. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Döring Y, Drechsler M, Soehnlein O, Weber C. Neutrophils in atherosclerosis: from mice to man. Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):288–95. doi: 10.1161/ATVBAHA.114.303564. [DOI] [PubMed] [Google Scholar]
- 34.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 35.Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment – implications for superficial erosion. Eur Heart J. 2015;36(22):1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franck G, Mawson T, Sausen G, Salinas M, Masson GS, Cole A, Beltrami-Moreira M, Chatzizisis Y, Quillard T, Tesmenitsky Y, Shvartz E, Sukhova GK, Swirski FK, Nahrendorf M, Aikawa E, Croce KJ, Libby P. Flow Perturbation Mediates Neutrophil Recruitment and Potentiates Endothelial Injury via TLR2 in Mice: Implications for Superficial Erosion. Circ Res. 2017 Jun 23;121(1):31–42. doi: 10.1161/CIRCRESAHA.117.310694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil Counts and Initial Presentation of 12 Cardiovascular Diseases: A CALIBER Cohort Study. J Am Coll Cardiol. 2017 Mar 7;69(9):1160–1169. doi: 10.1016/j.jacc.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah AD, Thornley S, Chung SC, Denaxas S, Jackson R, Hemingway H. White cell count in the normal range and short-term and long-term mortality: international comparisons of electronic health record cohorts in England and New Zealand. BMJ Open. 2017 Feb 17;7(2):e013100. doi: 10.1136/bmjopen-2016-013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–128. [PubMed] [Google Scholar]
- 40.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 41.Dyugovskaya L, Polyakov A, Lavie P, Lavie L. Delayed neutrophil apoptosis in sleep apnea patients. Am J Respir Crit Care Med. 2008;177:544–54. doi: 10.1164/rccm.200705-675OC. [DOI] [PubMed] [Google Scholar]
- 42.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–70. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 43.Taillé C, Rouvel-Tallec A, Stoica M, Danel C, Dehoux M, Marin-Esteban V, Pretolani M, Aubier M, d’Ortho MP. Obstructive Sleep Apnoea Modulates Airway Inflammation and Remodelling in Severe Asthma. PLoS One. 2016 Mar 2;11(3):e0150042. doi: 10.1371/journal.pone.0150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uygur F, Tanriverdi H, Aktop Z, Erboy F, Altinsoy B, Damar M, Atalay F. The neutrophil-to-lymphocyte ratio in patients with obstructive sleep apnoea syndrome and its relationship with cardiovascular disease. Heart Lung. 2016 Mar-Apr;45(2):121–5. doi: 10.1016/j.hrtlng.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Oyama J, Nagatomo D, Yoshioka G, Yamasaki A, Kodama K, Sato M, Komoda H, Nishikido T, Shiraki A, Node K. The relationship between neutrophil to lymphocyte ratio, endothelial function, and severity in patients with obstructive sleep apnea. J Cardiol. 2016 Mar;67(3):295–302. doi: 10.1016/j.jjcc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Penzel T, Kantelhardt JW, Bartsch RP, Riedl M, Kraemer JF, Wessel N, Garcia C, Glos M, Fietze I, Schöbel C. Modulations of Heart Rate, ECG, and Cardio-Respiratory Coupling Observed in Polysomnography. Front Physiol. 2016 Oct 25;7:460. doi: 10.3389/fphys.2016.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johansen CD, Olsen RH, Pedersen LR, Kumarathurai P, Mouridsen MR, Binici Z, Intzilakis T, Køber L, Sajadieh A. Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur Heart J. 2013 Jun;34(23):1732–9. doi: 10.1093/eurheartj/ehs449. [DOI] [PubMed] [Google Scholar]
- 48.Greve AM, Bang CN, Berg RM, Egstrup K, Rossebø AB, Boman K, Nienaber CA, Ray S, Gohlke-Baerwolf C, Nielsen OW, Okin PM, Devereux RB, Køber L, Wachtell K. Resting heart rate and risk of adverse cardiovascular outcomes in asymptomatic aortic stenosis: the SEAS study. Int J Cardiol. 2015 Feb 1;180:122–8. doi: 10.1016/j.ijcard.2014.11.181. [DOI] [PubMed] [Google Scholar]
- 49.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR. Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest. 1993;103:722–727. doi: 10.1378/chest.103.3.722. [DOI] [PubMed] [Google Scholar]
- 51.Stein PK, Domitrovich PP, Hui N, Rautaharju P, Gottdiener J. Sometimes higher heart rate variability is not better heart rate variability: results of graphical and nonlinear analyses. J Cardiovasc Electrophysiol. 2005 Sep;16(9):954–9. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]
- 52.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005 Jan;10(1):88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stein PK, Barzilay JI, Chaves PH, Domitrovich PP, Gottdiener JS. Heart rate variability and its changes over 5 years in older adults. Age Ageing. 2009 Mar;38(2):212–8. doi: 10.1093/ageing/afn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kara T, Narkiewicz K, Somers VK. Chemoreflexes–physiology and clinical implications. Acta Physiol Scand. 2003 Mar;177(3):377–84. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


