Abstract
Context:
Restricted dorsiflexion (DF) at the ankle joint can cause acute and chronic injuries at the ankle and knee. Myofascial release and instrument-assisted soft tissue mobilization (IASTM) techniques have been used to increase range of motion (ROM); however, evidence directly comparing their effectiveness is limited.
Objective:
To compare the effects of a single session of compressive myofascial release (CMR) or IASTM using the Graston Technique (GT) on closed chain ankle-DF ROM.
Design:
Randomized controlled trial.
Setting:
Laboratory.
Patients or Other Participants:
Participants were 44 physically active people (53 limbs) with less than 30° of DF.
Intervention(s):
Limbs were randomly assigned to 1 of 3 groups: control, CMR, or GT. Both treatment groups received one 5-minute treatment that included scanning the area and treating specific restrictions. The control group sat for 5 minutes before measurements were retaken.
Main Outcome Measure(s):
Standing and kneeling ankle DF were measured before and immediately after treatment. Change scores were calculated for both positions, and two 1-way analyses of variance were conducted.
Results:
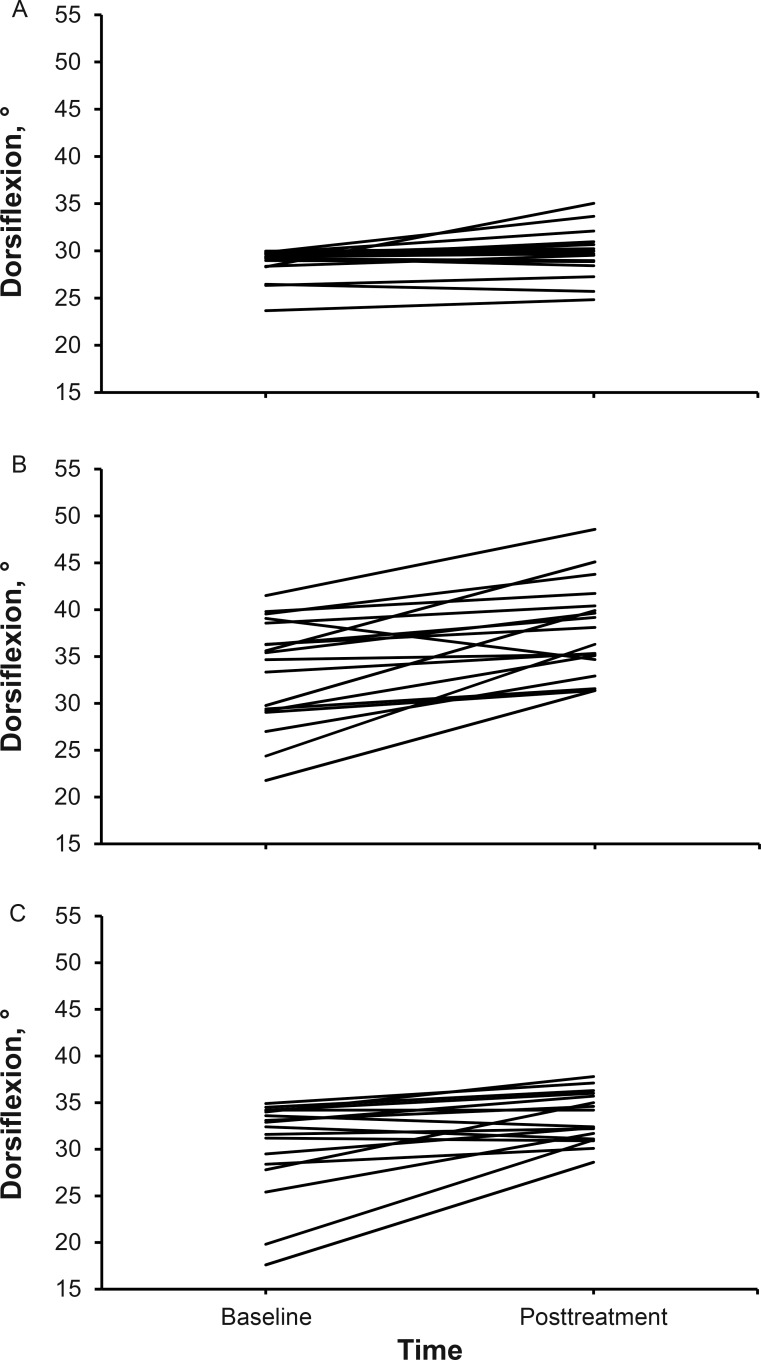
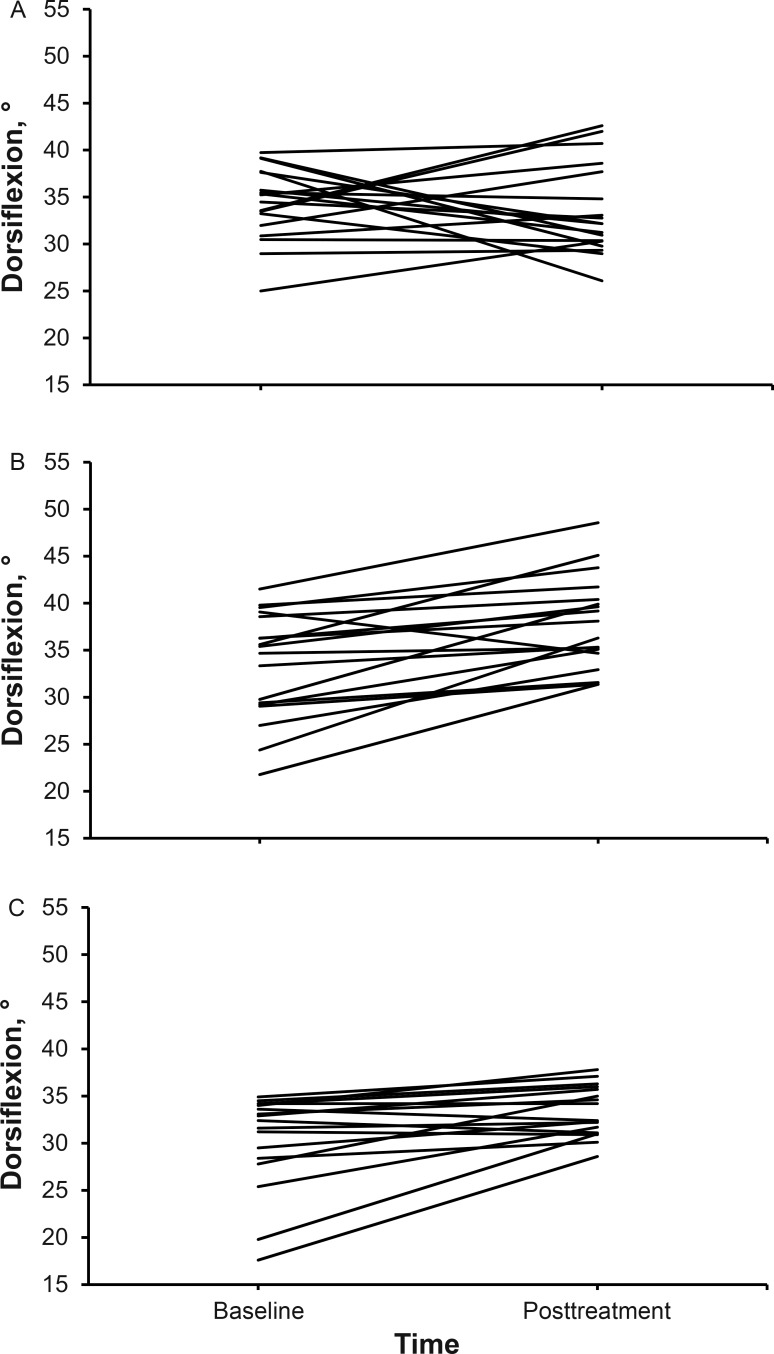
A difference between groups was found in the standing (F2,52 = 13.78, P = .001) and kneeling (F2,52 = 5.85, P = .01) positions. Post hoc testing showed DF improvements in the standing position after CMR compared with the GT and control groups (both P = .001). In the kneeling position, DF improved after CMR compared with the control group (P = .005).
Conclusions
Compressive myofascial release increased ankle DF after a single treatment in participants with DF ROM deficits. Clinicians should consider adding CMR as a treatment intervention for patients with DF deficits.
Key Words: muscle tightness, manual therapy, soft tissue mobilization
Key Points
Ankle dorsiflexion (DF) increased in participants with DF range-of-motion deficits after a single treatment of compressive myofascial release (CMR).
A single treatment of CMR may improve ankle DF more than a single treatment of the Graston Technique.
Clinicians should consider adding CMR to the treatment of patients with DF deficits.
Loss of joint range of motion (ROM) is a common dysfunction in physically active people and may be a predisposition to musculoskeletal injury.1,2 Numerous factors can contribute to loss of ROM, including poor flexibility,3,4 previous injury,5,6 and immobilization.7,8 Researchers9–11 have suggested that a lack of ankle-dorsiflexion (DF) ROM is a predisposing factor that increases the likelihood of a wide variety of lower extremity injuries. Specifically, having less than 20° to 30° of closed chain DF impedes normal gait and may cause compensatory gait patterns, leading to pathologic conditions throughout the foot and ankle and up the kinetic chain.12–15 A lack of ankle DF can predispose a healthy person to injuries and conditions such as genu recurvatum,15 early heel lift,15 excessive subtalar joint pronation,15 metatarsalgia,13,15 ankle sprains,13 medial tibial traction periostitis,13 medial tibial stress syndrome,16 Achilles tendinopathy,13,15,17,18 plantar fasciitis,13,15,17,18 anterior knee pain,15 gastrocnemius strains,13,15,18 and anterior cruciate ligament injuries.19 One cause of restricted DF is lack of flexibility within the triceps surae.3,4,20
Clinicians routinely use a variety of soft tissue mobilization techniques to address myofascial restrictions within the triceps surae to restore mobility and decrease pain. Several groups13,15,17,21 have examined the effects of manual therapy techniques on tissue extensibility and ROM in an open chain position. However, most activities involve closed chain ROM, and it may be more appropriate to evaluate the effectiveness of manual therapy on closed chain ROM. Two common forms of soft tissue mobilization are compressive myofascial release (CMR) and instrument-assisted soft tissue mobilization (IASTM). Compressive myofascial release is a form of soft tissue stretching that involves applying compression and sustained myofascial stretches to the target area to produce a release.22 Instrument-assisted soft tissue mobilization uses specifically designed instruments to identify and treat myofascial restrictions.23 Both techniques are intended to release scar tissue, fascial adhesions, or tightness within the musculotendinous unit.24,25 The Graston Technique (GT; Graston Technique, LLC, Indianapolis, IN) is a commonly used IASTM technique that involves applying 6 stainless-steel instruments to localize, treat, and release soft tissue restrictions. It is designed to reduce fatigue of the clinician's hands while helping him or her to detect lesions by amplifying the resonance felt through the instrument. Both techniques use similar principles aimed at localizing and treating specific areas of restriction within the fascial system; however, few researchers have compared the 2 techniques. Therefore, the purpose of our study was to examine the short-term effects of a single session of either GT or CMR on improving DF ROM. We hypothesized that we would observe no difference between interventions and that both interventions would effectively increase DF ROM compared with a control group.
METHODS
Participants
Based on a power analysis with power = 0.80 and α level = .05, the estimated sample size for the study was 31. A total of 82 participants (164 limbs) were initially screened for inclusion. Forty-four physically active participants (25 men, 19 women; 53 limbs) met the inclusion criteria and volunteered to participate between October 2014 and March 2015. Demographic data for all participants by group are presented in Table 1. Volunteers were enrolled in the study if they had less than 30° (27.3° ± 2.3°) of standing closed chain DF, had a Silfverskiold test26 result indicating soft tissue restriction, and engaged in at least 30 minutes of physical activity on 3 or more days per week. Volunteers were excluded if they had a history of lower extremity injury within the 6 months before the study, lower extremity surgery, or balance or vision impairments or were receiving any treatment for the triceps surae muscle at the time of the study. All participants provided written informed consent, and the study was approved by the Research, Ethics, & Compliance Institutional Review Board of Illinois State University.
Table 1. .
Demographic Data by Group
| Group |
n |
Mean ± SD |
||
| Age, y |
Height, cm |
Mass, kg |
||
| Control | 18 | 20.0 ± 1.8 | 168.4 ± 10.3 | 67.8 ± 10.9 |
| Compressive myofascial release | 18 | 20.6 ± 1.5 | 172.6 ± 9.4 | 68.4 ± 10.2 |
| Graston Techniquea | 17 | 20.1 ± 1.7 | 175.9 ± 8.5 | 69.9 ± 11.4 |
| All participants | 53 | 20.2 ± 1.7 | 172.3 ± 9.4 | 68.7 ± 10.8 |
Graston Technique, LLC, Indianapolis, IN.
Measurements
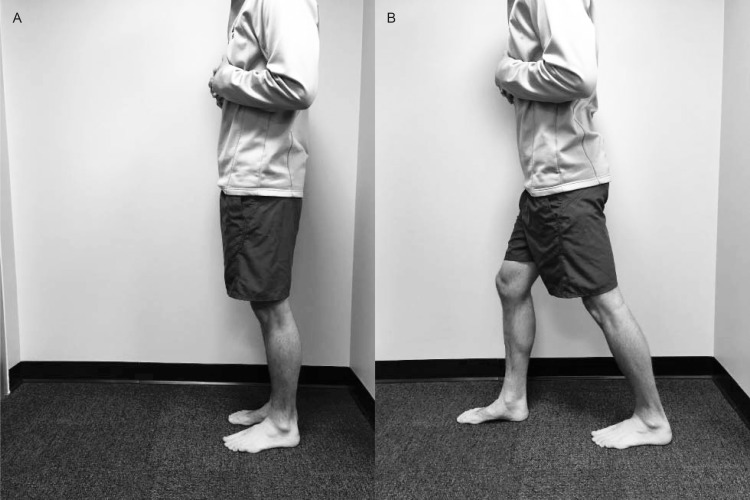
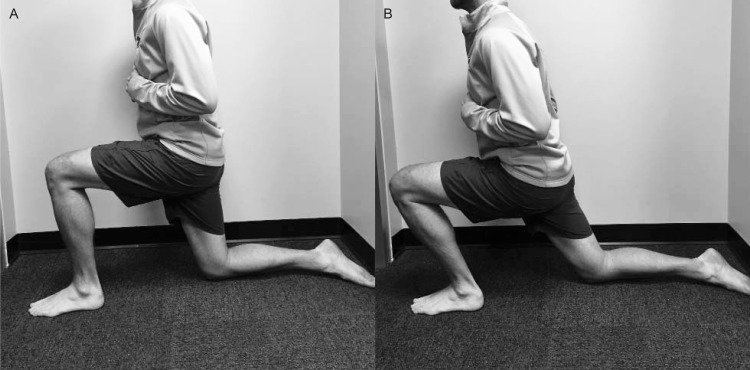
To determine study eligibility, we measured closed chain DF ROM using a digital inclinometer (model SmartTool Pro 3600; Swiss Precision Instruments, Inc, Garden Grove, CA) with the participant's knee straight and with the knee flexed to 90° at the starting position. Chisholm et al27 reported that the reliability of this measurement was high (interclass correlation coefficients range, 0.93 to 0.99) and the validity of closed chain measurements was greater than that of open chain goniometric measurements. The digital inclinometer was placed along the fibular shaft for all measurements. For the knee-extended measurement, we instructed participants to take a step forward with the nontest lower extremity, keeping the measurement limb straight with the heel on the ground and toes pointed directly forward (Figure 1). The stepping distance was not standardized across participants; instead, they were instructed to bend the front knee as needed to allow maximum DF of the test ankle. Participants were instructed to lunge forward as far as possible without allowing the heel to rise. For the kneeling measurement, participants knelt on the nontest lower extremity and initially flexed the test knee to 90°. They lunged forward as far as possible while maintaining alignment of the toes and not allowing the heel to rise (Figure 2). For both the standing and kneeling positions, participants were instructed to stop the movement at the first point when the heel began to rise, and this position was visually verified by the examiner (S.D.). Interrater reliability for the standing position was 0.91 (95% confidence interval [CI] = 0.81, 0.96) and for the kneeling position was 0.94 (95% CI = 0.87, 0.97). If volunteers demonstrated less than 30° of DF ROM, the Silfverskiold test was performed: we placed the participant's ankle in a neutral position, and the midtarsal joint was locked by supination of the forefoot. We passively placed the ankle in maximal DF with the knee in full extension and then in flexion. If DF was greater than 0° with the knee flexed but less than 0° with the knee extended, the test indicated a soft tissue restriction. Ankle DF that is less than 0° with the knee both flexed and extended can indicate a soft tissue or osseous restriction. At this point, the restriction is determined by end feel at end ROM, with a soft end feel indicating soft tissue restriction and a hard end feel indicating an osseous restriction.26 Only participants with soft tissue restriction were enrolled in the study and assigned to a group.
Figure 1. .
Standing weight-bearing dorsiflexion range of motion. A, Starting and, B, ending positions.
Figure 2. .
Kneeling weight-bearing dorsiflexion range of motion. A, Starting and, B, ending positions.
Procedures
Recruits met with investigators to complete preparticipation questionnaires before beginning their sessions. All procedures occurred during 1 visit to the same research clinic. First, baseline ROM and the Silfverskiold test were performed as described to determine enrollment status. A clinician (T.S.) who was blinded to treatment allocation took all baseline and posttreatment ROM measurements. All ROM measurements were completed 3 times in each position, and the average of the 3 measurements was used for analysis. Baseline and posttreatment data were first collected in the standing position and then in the kneeling position for all participants. Qualifying limbs were randomly assigned to 1 of 3 groups, control, CMR, or GT, using a parallel study design. We used block randomization to keep groups balanced with block sizes of 3 (1, 2, 3, 1, 2, 3, etc). When both the dominant and nondominant limbs qualified, the dominant limb was allocated to the next sequenced group, followed by the nondominant limb. Limb dominance was self-reported by the participant as the preferred kicking limb. We used a spreadsheet with the predetermined randomization and each qualifying limb was allocated sequentially. Regardless of group allocation, all participants completed a 5-minute bicycle warm-up before beginning their assigned intervention.
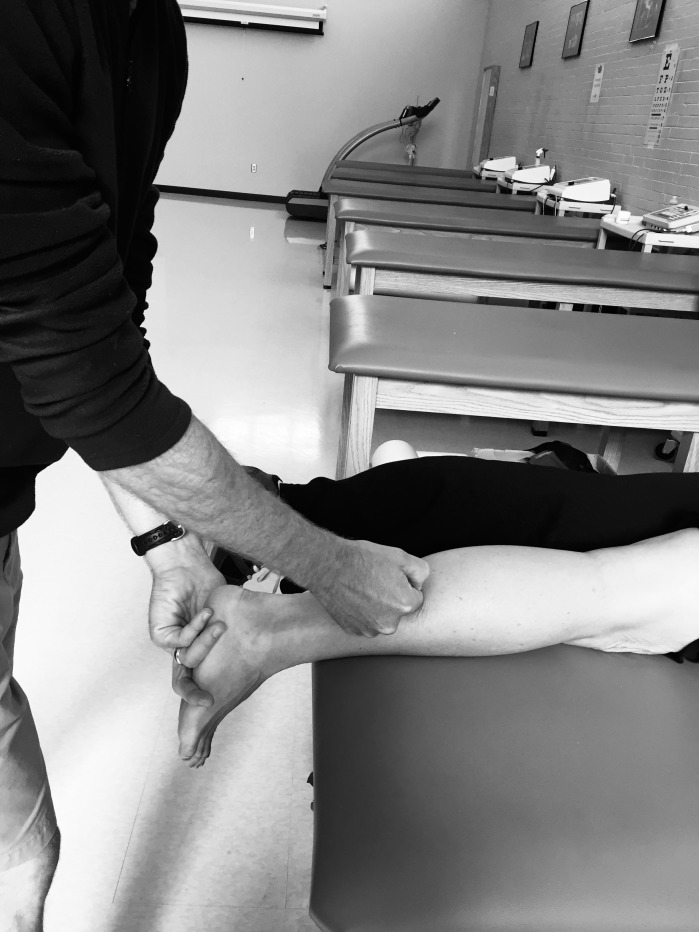
After the warm-up, participants in the control group were instructed to lie prone on a treatment table for 5 minutes before posttreatment measurements were taken. Our control procedures were consistent with those of previous investigators15,28 who assessed DF ROM using manual therapies. Participants in the CMR group were instructed to lie prone on the treatment table with their feet off the end of the table. The clinician (T.S.) began the treatment by bending the knee to 90° and shaking the muscle belly of the triceps surae group for 30 seconds. Next, the knee was fully extended, and CMR was performed on the medial and lateral sides of the Achilles tendon for 1 minute, followed by the musculotendinous junction for 2 minutes. Treatment consisted of broad strokes applied with the clinician's knuckles to release superficial restrictions, followed by more specific strokes applied with the clinician's thumb to any located restrictions (Figure 3). Strokes were applied at a contact point of 45° to the tissue, with pressure directed from distal to proximal. Participants were instructed to provide feedback so the clinician could monitor the treatment intensity and ensure their comfort. At the end of the intervention, the clinician shook the belly of the triceps surae complex for 30 seconds. The same examiner (T.S.) applied all CMR treatments.
Figure 3. .
Compressive myofascial release technique.
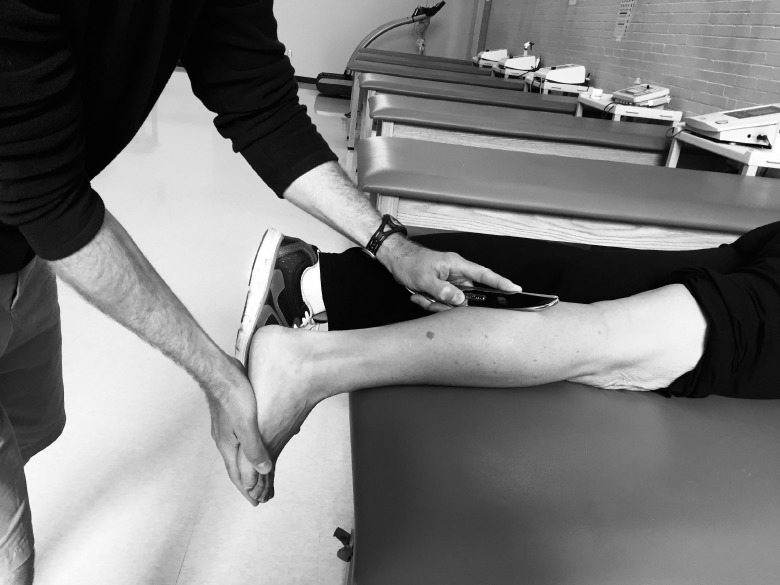
Participants assigned to the GT group were instructed to lie prone on a treatment table with their feet hanging off the end of the table. The clinician (S.D.) used her hands to apply a small amount of emollient to the triceps surae group. Participants were instructed to provide feedback to the clinician so she could monitor the treatment intensity and ensure patient comfort. The clinician began by scanning the triceps surae with the GT5 instrument using the sweep stroke for 1 minute. Areas of restriction were treated for 4 minutes based on the treatment guidelines suggested in the M1 Basic Training course (Graston Technique, LLC). Initially, localized restrictions were treated with the GT4 instrument using the sweep and fan strokes in multiple directions (Figure 4). Smaller restrictions were treated with the GT3 instrument using a strum stroke. The same clinician (S.D.) applied all GT treatments. After the intervention, ROM was remeasured using the same methods previously described.
Figure 4. .
Graston Technique (Graston Technique, LLC, Indianapolis, IN).
Statistical Analysis
To compare the effects of the interventions on DF ROM, change scores were calculated by subtracting the baseline measurement from the posttreatment measurement in the standing and kneeling positions. Two 1-way analyses of variance were used to compare change scores across the 3 interventions for the standing and kneeling conditions. Differences identified by the analyses of variance were assessed using Tukey post hoc tests. Effect sizes were calculated using the Cohen d and categorized as trivial (≤0.20), small (0.21–0.49), moderate (0.50–0.79), or large (≥0.80).29 The α level was set a priori at .05. We used SPSS (version 21; IBM Corp, Armonk, NY) for all analyses.
RESULTS
Means and standard deviations for all variables are reported in Tables 2 and 3. Changes in DF for each participant based on group allocation are presented in Figures 5 and 6. All limbs that were allocated to a group received the intended intervention and were analyzed posttreatment. Differences among groups were found in the standing (F2,52 = 13.78, P = .001) and kneeling (F2,52 = 5.85, P = .01) positions. Post hoc analysis showed improvements in DF in the standing position after CMR compared with the GT (P = .001; Cohen d = 1.23; 95% CI = 0.51, 1.95) and control (P = .001; Cohen d = 1.42; 95% CI = 0.69, 2.15) groups. In the kneeling position, DF improved after CMR compared with the control group (P = .005; Cohen d = 1.02; 95% CI = 0.33, 1.72). No adverse reactions or unintended effects were reported by any participant.
Table 2. .
Standing Dorsiflexion Range of Motion
| Group |
n |
Measurement (Mean ± SD)° |
95% Confidence Interval, ° |
||
| Baseline |
Posttreatment |
Change |
|||
| Control | 18 | 28.73 ± 1.65 | 29.79 ± 2.45 | 1.06 ± 1.82 | 0.13, 1.77 |
| Compressive myofascial release | 18 | 27.79 ± 2.53 | 32.62 ± 3.06 | 4.83 ± 3.28a,b | 2.78, 5.97 |
| Graston Technique | 17 | 29.13 ± 1.63 | 30.88 ± 1.55 | 1.75 ± 1.22 | 1.31, 2.38 |
Different from the control group (P = .001).
Different from the Graston Technique group (P = .001).
Table 3. .
Kneeling Dorsiflexion Range of Motion
| Group |
n |
Measurement (Mean ± SD)° |
95% Confidence Interval, ° |
||
| Baseline |
Posttreatment |
Change |
|||
| Control | 18 | 34.29 ± 3.87 | 33.53 ± 4.82 | −0.76 ± 5.92 | −3.63, 3.40 |
| Compressive myofascial release | 18 | 33.36 ± 5.71 | 37.79 ± 4.94 | 4.43 ± 4.08a | 2.59, 6.68 |
| Graston Technique | 17 | 30.30 ± 5.13 | 33.35 ± 2.69 | 3.05 ± 3.79 | 1.46, 5.81 |
Different from the control group (P = .005).
Figure 5. .
Change in standing dorsiflexion by participant and group. A, Control group. B, Compressive myofascial release group. C, Graston Technique group.
Figure 6. .
Change in kneeling dorsiflexion by participant and group. A, Control group. B, Compressive myofascial release group. C, Graston Technique group.
DISCUSSION
The purpose of our study was to compare the immediate effects of a single treatment of either CMR or GT on closed chain ankle-DF ROM. We observed that CMR produced immediate changes in both standing and kneeling DF compared with the control group. These findings contradict the findings reported in a previous study30 on manual interventions aimed at improving DF ROM. Vardiman et al30 used a method for the IASTM protocol similar to the one we used; however, unlike us, they did not recruit participants with a DF deficit. Specifically, in a systematic review, Young et al13 noted that evidence was insufficient to conclude that trigger-point therapy, ankle-joint mobilization, and manipulation were associated with changes in ankle-DF ROM. However, a common problem reported by many researchers who have examined manual therapy interventions is that they did not study a population with a known DF deficit. Investigators12,15,16 have shown that deficits in DF ROM can increase the risk of lower extremity injury; therefore, researchers need to study manual interventions in populations with a DF deficit to establish their effectiveness.
Investigators26 have generally accepted that clinically diagnosed ankle equinus exists with DF ROM of less than 10° to 15°; however, the method for obtaining this measurement and the threshold were inconsistent in the literature. We chose 30° of closed chain, straight-legged DF as the cutoff for inclusion in our study as a way to examine the effectiveness of these 2 interventions in a group of at-risk participants. Based on the findings reported by Willems et al2 and Pope et al,31 having less than 30° or 34°, respectively, is predictive of injury. We also measured closed chain ankle DF without regard to subtalar joint position. We acknowledge that foot posture and greater amounts of pronation specifically affect maximum DF27; however, 30° is on the low end of closed chain DF based on previous studies.2,10 Furthermore, our measurements showed excellent reliability in both the standing and kneeling positions.
Myofascial Release
Myofascial release is a rehabilitation technique commonly applied to restore optimal soft tissue length, decrease pain, and increase function.22,32 We used a slightly more aggressive form of myofascial release that localizes and treats restrictions as clinicians use their knuckles to apply compression. The wide variety in how myofascial release is used clinically makes direct comparisons across studies difficult. However, a systematic review33 on the effectiveness of myofascial release showed positive outcomes for increasing ROM and treating pain. These findings, specifically from the studies from the systematic review examining myofascial release for increasing hamstrings flexibility,34,35 shoulder ROM,36 and jaw mobility in patients with temporomandibular dysfunction,37 were consistent with our results. Together, these findings demonstrate the benefits of myofascial release for increasing ROM.
Graston Technique
The GT is similar to myofascial release except that clinicians use stainless-steel instruments instead of their hands. The instruments are designed to reduce hand fatigue and improve the detection and treatment of soft tissue lesions. We used the GT5 instrument to initially scan the triceps surae and identify localized lesions. Following the guidelines presented in the M1 Basic Training course, we treated localized lesions with the GT4 instrument using sweeping and fanning strokes and then with the GT3 instrument for smaller localized lesions using the strum stroke. Our methods were similar to those of Laudner et al38 except that we spent more time scanning and treating the affected area. Despite the greater treatment time, we did not observe increases in ROM compared with the control group. Our findings also conflicted with those of previous studies on ROM improvements after a GT treatment to improve knee-extension39 and hip-adduction40 angles. However, they supported the findings of Vardiman et al,30 who showed no change in DF ROM after a GT treatment to the plantar flexors. Interestingly, our methods and those of Vardiman et al30 involved a much longer treatment time (5–8 minutes) than those of Laudner et al38 (approximately 40 seconds). Our findings, combined with the previous literature on the GT, appear to show conflicting evidence for improving ROM. We believe many of the discrepancies among studies can be attributed to the populations studied. A number of investigators have studied a convenience sample of healthy people without soft tissue restrictions. Furthermore, it is difficult to quantify the pressures used during a GT application, which makes comparing the findings from individual studies difficult.
Improving ROM
Several theories exist for how myofascial techniques effect changes to the soft tissue. Twomey and Taylor41 proposed the concept of gel-to-solid transformation, called thixotrophy, but some researchers42 have questioned its ability to explain the immediate changes within the fascia. Similarly, the piezoelectricity theory43 presents an alternative hypothesis, but it also does not account for immediate changes due to manual therapy. Schleip44 presented an alternative hypothesis and suggested that immediate changes within the soft tissue may be due to a neurologic response that causes relaxation of the smooth or striated muscle fibers, which may also affect the metabolic ground substance within the immediate area. More recently, Bialosky et al45 further supported the neurologic response model by including a potential combined effect from biomechanical or neurologic mechanisms to explain the results of manual therapy. Based on this proposed theory, the mechanical properties, along with the proprioceptive input to the central nervous system, appear to immediately affect the tone of the tissue.
Both CMR and GT use similar treatment guidelines and are designed to affect the tone of the soft tissue while keeping the patient comfortable. It is unclear why our use of instruments did not produce the same magnitude of change in the soft tissue as the CMR. Using human touch may have produced a greater mechanical or neurologic response. Whereas this is plausible but purely speculative, several groups46–49 have reported that self-myofascial release achieved through foam rolling or other self-myofascial instruments routinely produced changes in ROM, which does not support our theory.
The most recent M1 Basic Training course for the GT emphasized using appropriate instrument pressure that is tolerable and should not cause severe pain or bruising. Vardiman et al30 indicated that GT applied using the correct pressure and treatment angle did not cause muscle damage or initiate an inflammatory response in healthy tissue. To our knowledge, no such data exist for CMR, and it is impossible to know for certain that the 2 techniques were applied using the same amount of force. Therefore, it is possible that the clinician was overly conservative when applying the GT in order to prevent discomfort to the patient, whereas the clinician using the CMR was applying pressure that was closer to the patient's tolerance threshold. Furthermore, the patient possibly had a greater perceived threat from the clinician using the GT instrument rather than the hands. This perceived threat could have affected the tolerable discomfort experienced by the patient. In both treatment scenarios, patients provided feedback on their comfort, but no other objective measures were available to examine the amount of force used.
The results of our study suggest that a single treatment of CMR was more beneficial in producing ROM changes than a single treatment of the GT. However, in the clinical environment, providing only 1 treatment of any manual intervention is rare; therefore, it is unclear how the 2 techniques would compare across multiple treatments. In fact, the GT recommends providing 4 to 12 treatments before reevaluating the patient for an alternative therapy. Many clinicians and patients have benefited from using these 2 manual techniques, and clinicians often continue to use the techniques that have been successful. Our findings may encourage clinicians to try CMR on a patient who has not had success with an alternative treatment; however, more research is needed.
Limitations
Our study had limitations. First, we applied only 1 treatment of CMR or GT to study the immediate changes in DF ROM. As mentioned, studying the effects after multiple treatments would be interesting. Furthermore, our control group received no treatment rather than a placebo intervention. Future investigators could use a sham treatment to explore potential neurologic contributions. Second, the same clinician did not apply both treatment techniques, which may have promoted or hindered the observed differences in treatment pressure. Instead, 1 clinician provided all CMR treatments, and another clinician applied all GT treatments to control any conscious or unconscious biases. Furthermore, we did not objectively measure the patients' comfort during the 2 interventions, which could be helpful for future research. Third, given that we focused on the effects of the manual intervention, we did not follow the recommendations from the M1 Basic Training course for static-stretching, high-repetition, low-load exercises and posttreatment cryotherapy. Fourth, we studied otherwise healthy people with an operationally defined DF deficit based on less than 30° of DF. We do not know if we would observe the same results in people after a lateral ankle sprain or with chronic ankle instability.
CONCLUSIONS
Compressive myofascial release increased ankle DF after a single treatment in participants with DF ROM deficits. These results suggest that a single CMR treatment was more beneficial than a single GT treatment for improving ankle DF. Clinicians should consider adding CMR as a treatment intervention for patients with DF deficits. Whereas additional research is needed, our study provides a direct comparison of 2 commonly used manual therapy techniques for DF ROM.
REFERENCES
- 1. You JY, Lee HM, Luo HJ, Lee CC, Cheng PG, Wu SK. . Gastrocnemius tightness on joint angle and work of lower extremity during gait. Clin Biomech (Bristol, Avon). 2009; 24 9: 744– 750. [DOI] [PubMed] [Google Scholar]
- 2. Willems TM, Witvrouw E, Delbaere K, De Bourdeaudhuij I, De Clercq D. . Intrinsic risk factors for inversion ankle sprains in male subjects: a prospective study. Am J Sports Med. 2005; 33 3: 415– 423. [DOI] [PubMed] [Google Scholar]
- 3. Denegar CR, Miller SJ III.. Can chronic ankle instability be prevented? Rethinking management of lateral ankle sprains. J Athl Train. 2002; 37 4: 430– 435. [PMC free article] [PubMed] [Google Scholar]
- 4. Hertel J. . Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002; 37 4: 364– 375. [PMC free article] [PubMed] [Google Scholar]
- 5. Reid A, Birmingham T, Alcock G. . Efficacy of mobilization with movement for patients with limited dorsiflexion after ankle sprain: a crossover trial. Physiother Can. 2007; 59 3: 166– 172. [Google Scholar]
- 6. Vicenzino B, Branjerdporn M, Teys P, Jordan K. . Initial changes in posterior talar glide and dorsiflexion of the ankle after mobilization with movement in individuals with recurrent ankle sprain. J Orthop Sports Phys Ther. 2006; 36 7: 464– 471. [DOI] [PubMed] [Google Scholar]
- 7. Chesworth BM, Vandervoot A. . Comparison of passive stiffness variables and range of motion in uninvolved and involved ankle joints of patients following ankle fractures. Phys Ther. 1995; 75 4: 253– 261. [DOI] [PubMed] [Google Scholar]
- 8. Kerkhoffs G, Rowe B, Assendelft W, Kelly K, Struijs P, van Dijk C. . Immobilisation for acute ankle sprain: a systematic review. Arch Orthop Trauma Surg. 2001; 121 8: 462– 471. [DOI] [PubMed] [Google Scholar]
- 9. Backman L, Danielson P. . Low range of ankle dorsiflexion predisposes for patellar tendinopathy in junior elite basketball players: a 1-year prospective study. Am J Sports Med. 2011; 39 12: 2626– 2633. [DOI] [PubMed] [Google Scholar]
- 10. Willems TM, Witvrouw E, Delbaere K, De Bourdeaudhuij I, De Clercq D. . Intrinsic risk factors for inversion ankle sprains in females: a prospective study. Scand J Med Sci Sports. 2005; 15 5: 336– 345. [DOI] [PubMed] [Google Scholar]
- 11. Tabrizi P, McIntyre WM, Quesnel MB, Howard AW. . Limited dorsiflexion predisposes to injuries of the ankle in children. J Bone Joint Surg Br. 2000; 82 8: 1103– 1106. [DOI] [PubMed] [Google Scholar]
- 12. Weir J, Chockalingam N. . Ankle joint dorsiflexion: assessment of true values necessary for normal gait. Int J Ther Rehabil. 2007; 14 2: 76– 82. [Google Scholar]
- 13. Young R, Nix S, Wholohan A, Bradhurst R, Bradhurst L. . Interventions for increasing ankle joint dorsiflexion: a systematic review and meta-analysis. J Foot Ankle Res. 2013; 6 1: 2– 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prentice W. . Arnheim's Principles of Athletic Training. Boston, MA: McGraw-Hill; 2006. [Google Scholar]
- 15. Grieve R, Clark J, Pearson E, Bullock S, Boyer C, Jarrett A. . The immediate effect of soleus trigger point pressure release on restricted ankle joint dorsiflexion: a pilot randomised controlled trial. J Bodyw Mov Ther. 2011; 15 1: 42– 49. [DOI] [PubMed] [Google Scholar]
- 16. Youdas JW, Krouse DA, Egan KS, Therneau TM, Laskowaki ER. . The effect of static stretching of the calf muscle-tendon unit on active ankle dorsiflexion range of motion. J Orthop Sports Phys Ther. 2003; 33 7: 408– 417. [DOI] [PubMed] [Google Scholar]
- 17. Miners AL, Bougie TL. . Chronic Achilles tendinopathy: a case study of treatment incorporating active and passive tissue warm-up, Graston Technique, ART, eccentric exercise, and cryotherapy. J Can Chiropr Assoc. 2011; 55 4: 269– 279. [PMC free article] [PubMed] [Google Scholar]
- 18. Muir IW, Chesworth BM, Vandervoot AA. . Effect of a static calf-stretching exercise on the resistive torque during passive ankle dorsiflexion in healthy subjects. J Orthop Sports Phys Ther. 1999; 29 2: 106– 113. [DOI] [PubMed] [Google Scholar]
- 19. Fong CM, Blackburn JT, Norcross MF, McGrath M, Padua DA. . Ankle dorsiflexion range of motion and landing biomechanics. J Athl Train. 2011; 46 1: 5– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riemann BL, DeMont RG, Ryu K, Lephart SM. . The effects of sex, joint angle, and the gastrocnemius muscle on passive ankle joint complex stiffness. J Athl Train. 2001; 36 4: 369– 375. [PMC free article] [PubMed] [Google Scholar]
- 21. George JW, Tunstall AC, Tepe RE, Skaggs CD. . The effects of active release technique on hamstring flexibility: a pilot study. J Manip Physiol Ther. 2006; 29 3: 224– 227. [DOI] [PubMed] [Google Scholar]
- 22. Manheim C. . The Myofascial Release Manual. 4th ed. Thorofare, NJ: SLACK Inc; 2008: 285. [Google Scholar]
- 23. Stow R. . Instrument-assisted soft tissue mobilization. Int J Athl Ther Train. 2011; 16 3: 5– 8. [Google Scholar]
- 24. Melham TJ, Sevier TL, Malnofski MJ, Wilson JK, Helfest RH Jr.. Chronic ankle pain and fibrosis successfully treated with a new noninvasive augmented soft tissue mobilization technique (ASTM): a case report. Med Sci Sports Exerc. 1998; 30 6: 801– 804. [DOI] [PubMed] [Google Scholar]
- 25. Gehlsen GM, Ganion LR, Helfest R. . Fibroblast responses to variation in soft tissue mobilization pressure. Med Sports Exerc. 1999; 31 4: 531– 535. [DOI] [PubMed] [Google Scholar]
- 26. Deheer PA. . Understanding equinus: this profound causal agent is commonly overlooked and under-treated. Podiatry Manage. 2012; 31 7: 157– 164. [Google Scholar]
- 27. Chisholm MD, Birmingham TB, Brown J, MacDermid J, Chesworth BM. . Reliability and validity of a weight-bearing measure of ankle dorsiflexion range of motion. Physiother Can. 2012; 64 4: 347– 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grieve R, Cranston A, Henderson A, John R, Malone G, Mayall C. . The immediate effect of triceps surae myofascial trigger point therapy on restricted active ankle joint dorsiflexion in recreational runners: a crossover randomised controlled trial. J Bodyw Mov Ther. 2013; 17 4: 453– 461. [DOI] [PubMed] [Google Scholar]
- 29. Cohen J. . Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 30. Vardiman JP, Sledlik J, Herda T, et al. Instrument-assisted soft tissue mobilization: effects on the properties of the human plantar flexors. Int J Sports Med. 2014; 36 3: 197– 203. [DOI] [PubMed] [Google Scholar]
- 31. Pope R, Herbert R, Kirwan J. . Effects of ankle dorsiflexion range and pre-exercise calf muscle stretching on injury risk in army recruits. Aust J Physiother. 1998; 44 3: 165– 172. [DOI] [PubMed] [Google Scholar]
- 32. Greenman P. . Principles of Manual Medicine. 2nd ed. Baltimore, MD: Lippincott Williams & Wilkins; 1996: 572. [Google Scholar]
- 33. Ajimsha MS, Al-Mudahka NR, Al-Madzhar JA. . Effectiveness of myofascial release: systematic review of randomized controlled trials. J Bodyw Mov Ther. 2015; 19 1: 102– 112. [DOI] [PubMed] [Google Scholar]
- 34. Hanten WP, Chandler SD. . Effects of myofascial release leg pull and sagittal plane isometric contract-relax techniques on passive straight-leg raise angle. J Orthop Sports Phys Ther. 1994; 20 3: 138– 144. [DOI] [PubMed] [Google Scholar]
- 35. Kuruma H, Takei H, Nitta O, et al. Effects of myofascial release and stretching technique on range of motion and reaction time. J Phys Ther Sci. 2013; 25 2: 169– 171. [Google Scholar]
- 36. Kain J, Martorello L, Swanson E, Sego S. . Comparison of an indirect tri-planar myofascial release (MFR) technique and a hot pack for increasing range of motion. J Bodyw Mov Ther. 2011; 15 1: 63– 67. [DOI] [PubMed] [Google Scholar]
- 37. Kalamir A, Bonello R, Graham P, Vitiello AL, Pollard H. . Intraoral myofascial therapy for chronic myogenous temporomandibular disorder: a randomized controlled trial. J Manipulative Physiol Ther. 2012; 35 1: 26– 37. [DOI] [PubMed] [Google Scholar]
- 38. Laudner K, Compton BD, McLoda TA, Walters CM. . Acute effects of instrument assisted soft tissue mobilization for improving posterior shoulder range of motion in collegiate baseball players. Int J Sports Phys Ther. 2014; 9 1: 1– 7. [PMC free article] [PubMed] [Google Scholar]
- 39. Toepper BW, Docherty CL, Donahue M, Kingma J, Schrader J. . The effects of the Graston Technique on knee extension angle [abstract]. J Athl Train. 2013; 48 suppl 3: S-128. [Google Scholar]
- 40. Heyer KM, Docherty CL, Donahue M, Schrader JW. . Effect of implement assisted soft tissue mobilization on iliotibial band tightness [abstract]. J Athl Train. 2012; 47 suppl 3: S-128. [Google Scholar]
- 41. Twomey L, Taylor J. . Flexion creep deformation and hysteresis in the lumbar vertebral column. Spine (Phila Pa 1976). 1982; 7 2: 116– 122. [DOI] [PubMed] [Google Scholar]
- 42. Schleip R. . Fascial plasticity: a new neurobiological explanation. Part 1. J Bodyw Mov Ther. 2003; 7 1: 11– 19. [Google Scholar]
- 43. Athenstaedt H. . Pyroelectric and piezoelectric properties of vertebrates. Ann N Y Acad Sci. 1974; 238: 68– 94. [DOI] [PubMed] [Google Scholar]
- 44. Schleip R. . Fascial plasticity: a new neurobiological explanation. Part 2. J Bodyw Mov Ther. 2003; 7 2: 104– 116. [Google Scholar]
- 45. Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. . The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009; 14 5: 531– 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. MacDonald GZ, Penney MD, Mullaley ME, et al. An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J Strength Cond Res. 2013; 27 3: 812– 821. [DOI] [PubMed] [Google Scholar]
- 47. Bradbury-Squires DJ, Noftall JC, Sullivan KM, Behm DG, Power KE, Button DC. . Roller-massager application to the quadriceps and knee-joint range of motion and neuromuscular efficiency during a lunge. J Athl Train. 2015; 50 2: 133– 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sullivan KM, Silvey DB, Button DC, Behm DG. . Roller massager application to the hamstrings increases sit-and-reach range of motion within five to ten seconds without performance impairments. Int J Sports Phys Ther. 2013; 8 3: 228– 236. [PMC free article] [PubMed] [Google Scholar]
- 49. Halperin I, Aboodarda SJ, Button DC, Andersen LL, Behm DG. . Roller massager improves range of motion of plantar flexor muscles without subsequent decreases in force parameters. Int J Sports Phys Ther. 2014; 9 1: 92– 102. [PMC free article] [PubMed] [Google Scholar]