Abstract
Products containing psychoactive synthetic cathinones, such as mephedrone and 3,4-methylenedioxypyrovalerone (MDPV) are prevalent in our society. Synthetic cathinones are structurally similar to methamphetamine, and numerous synthetics have biological activity at dopamine, serotonin, and norepinephrine transporters. Importantly, monoamine transporters co-transport sodium ions along with their substrate, and movement of substrates and ions through the transporter can generate measurable ionic currents. Here we review how electrophysiological information has enabled us to determine how synthetic cathinones affect transporter-mediated currents in cells that express these transporters. Specifically, drugs that act as transporter substrates induce inward depolarizing currents when cells are held near their resting membrane potential, whereas drugs that act as transporter blockers induce apparent outward currents by blocking an inherent inward leak current. We have employed the two-electrode voltage-clamp technique in Xenopus laevis oocytes overexpressing monoamine transporters to determine whether synthetic cathinones found in the so-called bath salts products behave as blockers or substrates. We also examined the structure-activity relationships for synthetic cathinone analogs related to the widely abused compound MDPV, a common constituent in “bath salts” possessing potent actions at the dopamine transporter.
Keywords: Bath salts, Dopamine transporter, Serotonin Transporter, Two-electrode voltage-clamp
1 Synthetic Cathinones Hit the Streets
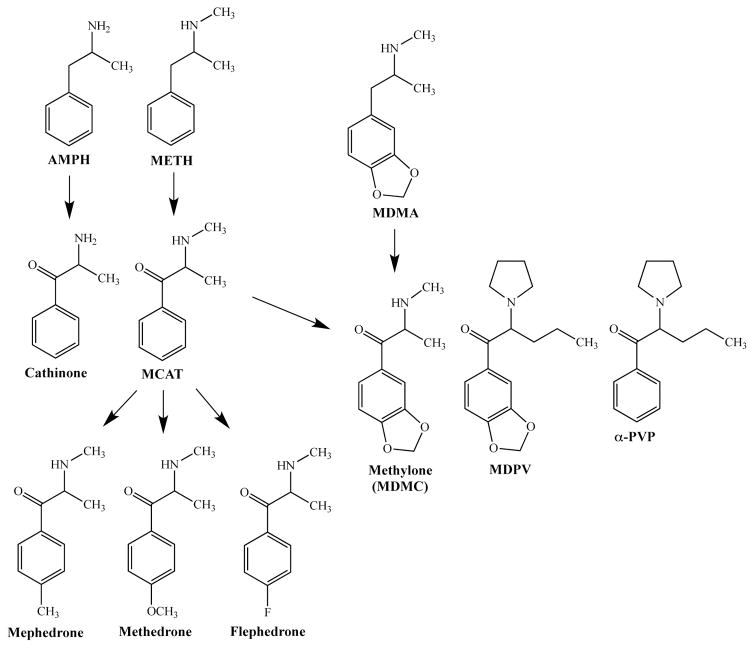
Not long ago, legal amphetamine-related drugs suddenly emerged in Western Europe and the United States. Many human drug users were sent to the emergency room with severe neurological, psychiatric, and cardiovascular effects after being exposed to products marketed as “legal high”, “bath salts”, “insect repellant”, “plant food”, etc. that were purchased legally in convenience stores or on-line. To avoid regulatory control, these drugs were labeled “not for human consumption”. American poison centers assessed drug content in blood and urine samples from patients who had ingested bath salts and discovered that the common ingredients were β-keto amphetamine (cathinone) derivatives (i.e., synthetic cathinones) (1). In 2011, three synthetic cathinones commonly found in bath salts were identified and classified by the DEA as Schedule I controlled substances. Cathinone consumption can be traced back for at least hundreds of years to people ranging from South Africa to the Arabian Peninsula who have chewed on the plant Catha edulis (or khat) for its mild central stimulant effects (2, 3). The main stimulant effects in khat derive from cathinone, a β-keto amphetamine (AMPH) analog (see Fig. 1 for common synthetic cathinone structures). The β-keto methamphetamine (METH) analog (called methcathinone or MCAT) was highly abused in the former Soviet Union and Eastern Europe dating back to the early 1980s. Clandestine chemists realized that by making simple chemical modifications to cathinone and MCAT, a vast number of completely legal ‘designer’ synthetic cathinones could be synthesized that would elicit a range of behavioral effects in people. In 2009, synthetic cathinones surfaced as an abuse problem in the United Kingdom (4) where primarily the para-methyl analog of MCAT, 4-methylmethcathinone (4MMC or mephedrone) appeared. Shortly thereafter (as mentioned above) synthetic cathinones found their way to the US disguised as home products with ulterior uses. The most notorious product containing synthetic cathinones was bath salts, and the prevalent synthetic cathinones identified in bath salts were mephedrone, methylone (the β-keto analog of 3,4-methylenedioxymethamphetamine or MDMA) and a more complex synthetic cathinone called 3,4-methylenedioxypyrovalerone (MDPV), which possesses a pyrrolidine ring and was ubiquitous in bath salts concoctions taken by people who presented to the ER. Following drug scheduling of these synthetic cathinones by the DEA, an MDPV analog recently emerged called α-pyrrolidinovalerophenone (α-PVP or flakka), which has become an abuse problem in Florida and other states (5).
Fig. 1. Structural relationship between AMPH (and related drugs) and synthetic cathinones.
Cathinone is the beta-keto analog of AMPH and similarly MCAT is the beta-keto analog of METH. Substitutions to the para position of MCAT yields additional synthetic analogs, including mephedrone, methedrone, and flephedrone. The beta-keto analog of MDMA is methylone (or MDMC). Further modifications to methylone results in the potent substituted cathinones MDPV and α-PVP. Abbreviations: AMPH, amphetamine; METH, methamphetamine; MCAT, methcathinone; MDMA, methylenedioxymethamphetamine; MDMC, methylenedioxymethcathinone; MDPV, methylenedioxypyrovalerone; α-pyrrolidinovalerophenone, α-PVP.
2 Neurotransmitters at the Synapse
In the central nervous system (CNS), the neurotransmitters norepinephrine (NE), dopamine (DA), and serotonin (5HT) are ordinarily released into the synaptic cleft via vesicular fusion in response to presynaptic depolarization. After release, neurotransmitters diffuse and activate postsynaptic and presynaptic neurons, then neurotransmission is terminated by reuptake of the transmitter into the presynaptic terminal via transporters, or in some cases hyperpolarization of the presynaptic terminal via transmitter auto-receptors. The respective reuptake transporters for NE, DA and 5HT (i.e., NET, DAT, and SERT) are located at perisynaptic sites (6, 7), whence monoamines are re-packaged into synaptic vesicles via vesicular monoamine transporters (VMATs). In particular, the vesicular monoamine transporter 2 (VMAT2), which is primarily found in the CNS, is responsible for neurotransmitter reuptake into synaptic vesicles that are poised for docking and release in dopaminergic, serotonergic and noradrenergic neurons (8).
3 Monoamine Neurotransmitters and Behavior
5HT plays a role in regulating many behaviors, such as mood, sleep, appetite, temperature, sexual behavior, and aggression (9, 10). Disturbances in the serotonergic system are implicated in mental diseases, including depression, bipolar disorder, autism, and a spectrum of psychiatric disorders, such as anorexia nervosa, bulimia, and obsessive-compulsive disorder (OCD) (11–14). Behaviors that are regulated by DA include cognition, attention, working memory, motivation, and voluntary movement. Disturbances in the dopaminergic system have been implicated in Huntington’s chorea, Parkinson’s disease, schizophrenia, attention-deficit/hyperactivity-disorder (ADHD), depression, and addiction (15–18). NE plays a role in attention, emotion, learning and memory, and dysregulation of the noradrenergic system can lead to severe physiological effects (19). In addition, dysfunction of the adrenergic system is linked to medical conditions, such as depression, post-traumatic stress disorder, and hypertension (20). Behaviors modulated by monoamine neurotransmitter systems have been linked to synthetic cathinone use; for instance the components in bath salts can cause intense euphoria, alertness, increased concentration, heightened libido, as well as anorexia, anxiety, increased heart rate and memory problems (21–23). Whereas cardiovascular symptoms and stimulant effects caused by MDPV can be linked to the dopaminergic and adrenergic systems (23, 24), the emphathogenic symptoms induced by mephedrone and methylone are associated with the serotonergic system (22).
4 Therapeutic and Abused Drugs Target Monoamine Transporters
To treat medical conditions associated with disturbances in the serotoninergic system, several classes of drugs targeting monoamine transporters have been developed, such as tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) that inhibit reuptake of 5HT into presynaptic terminals and prolong neurotransmitter action at the synapse (25). In addition to increasing the extracellular levels of 5HT in the brain, a TCA can exert effects as a NE reuptake inhibitor, an anticholinergic-antimuscarinic agent, an alpha1-adrenergic antagonist, an antihistamine, and a Na+ channel inhibitor, which can potentially cause lethal cardiac arrhythmias and seizures (9). The adverse side effects of TCAs have led to the development of SSRIs targeting SERT. Fluoxetine (FLX, Prozac) was the first drug of this kind approved as a therapeutic agent by regulatory authorities in the United States. Other SSRIs have been synthesized to lessen the adverse side effects of FLX; these include citalopram, escitalopram, fluvoxamine, and sertraline that lack adverse side effects, such as insomnia, anxiety, and tremors and display fewer gastrointestinal side effects, such as nausea, diarrhea, anorexia, and vomiting (26). Presently, SSRIs are the most widely prescribed drugs for the treatment of depression, OCD, and bipolar disorder, as well as anxiety, anorexia, and panic disorders (27). In addition, the recreational drug MDMA (i.e., ecstasy) has been effectively used to treat anxiety disorders, including post-traumatic stress disorder (PTSD) (28, 29). MDMA targets all monoamine transporters but possesses greater potency for SERT. It is thought that MDMA increases monoamine transmitter levels in the brain by reverse transport via SERT, DAT, and NET of the corresponding endogenous transmitters (10).
The human dopamine transporter (hDAT) is a major molecular target for therapeutic agents, such as methylphenidate hydrochloride (MPH, Ritalin) and AMPH (Adderall). Both compounds, which are often prescribed to treat attention-deficit/hyperactivity disorder (ADHD), act directly on hDAT to increase extracellular DA levels, but they do so via different actions on hDAT. Whereas MPH is a DAT reuptake inhibitor, AMPH is a DAT substrate thought to stimulate DA release through non-vesicular reverse DA transport through DAT (30, 31). Certain drugs of abuse, such as cocaine (COC) and METH increase DA levels via the same mechanisms. Like MPH, COC inhibits DA transport and thus increases extracellular DA. On the other hand, METH behaves like AMPH and is transported by DAT to release DA by reversing DAT transport. Although abnormal increases in DA may underlie psychiatric disorders, drug abuse liability (32), and might cause adverse reactions such as psychosis, some of these compounds that elevate transmitter levels are effective treatments for certain mental illnesses (33). In particular, since the psychostimulants AMPH and METH lead to the release of catecholamines (DA and NE) in the frontal lobe and limbic system (by transmitter reuptake inhibition at DAT and NET and transmitter efflux by DAT and NET), they have been used clinically to treat medical conditions such as ADHD and narcolepsy (33, 34). The improved potency to release DA through hDAT by the dextrorotary AMPH isomer (S(+)AMPH) over the levorotary isomer (R(−)AMPH) (35–37) underlies the therapeutic efficacy of agents composed primarily of S(+)AMPH. For example, Adderall is composed of 3:1 S(+):R(−)AMPH (38), and Vyvanse (lisdexamphetamine) is a pro-drug composed of S(+)AMPH conjugated to L-lysine, which is metabolized to S(+)AMPH (39, 40). The dextrorotary isomer of METH is marketed as Desoxyn for the treatment of ADHD and narcolepsy (41). Clinical manifestations associated with the abuse of AMPH or its precursors or derivatives, such as phenethylamine or METH, are well documented (42–44). In an attempt to bypass the reward system, the selective NE reuptake inhibitor (NRI) atomoxetine (Strattera) is used to treat ADHD (39). Lastly, bupropion (Wellbutrin) is used to treat depression (31, 45) through its action as a dual DA and NE reuptake inhibitor (46).
5 Functional Mechanisms of Monoamine Transporters
5.1 Human Serotonin, Dopamine, and Norepinephrine Transporters
Biogenic amine transporters, such as human SERT (hSERT) and human NET (hNET), are classified as Na+/Cl−-coupled co-transporters since they require both Na+ and Cl− to transport substrates; however, the role of Cl− as a transported ion is less established (47). hSERT, hNET and related proteins belonging to the SLC6 gene family that includes GABA, glycine, and taurine transporters are also termed neurotransmitter sodium symporters (48), reflecting the limitation of knowledge about the ionic contribution for substrate transport (49). Co-transporters use existing ion gradients to concentrate their substrate against their own concentration gradient, e.g., Na+ levels are ten times higher outside than inside cells (50–53). Historically, alternating access models describe transport in which ions (Na+ and Cl−) and substrate (5HT or NE) bind to the transporter in its outward-facing conformation, catalyze an inward-facing conformational change and transport the neurotransmitter from outside to inside the cell. In some cases a counter-ion, either a proton (H+) or a K+, is transported from inside to outside of the plasma membrane returning the transporter to the outward-facing conformation. This model is supported by biochemical and radiolabeled neurotransmitter uptake data (54, 55) and is consistent with recent structural data for co-transporters (56–61). In particular, the human DAT (hDAT) is described by the alternating access model in which DA transport is coupled with fixed stoichiometry to the downhill movement of two Na+ and one Cl− coupling to each DA in the outward-facing hDAT conformation, and either a K+ or H+ binds to the inward-facing conformation to return hDAT to the outward-facing conformation (52, 53, 62–64).
5.2 Reverse Transport (Efflux) Mechanism of Monoamine Transporters
The reward and addiction properties of AMPH, METH, and MDMA rely on their ability to increase extracellular DA, NE, and 5HT levels by mechanisms as yet only partially understood. These agents competitively inhibit monoamine transporters, leading to diminished uptake and increased neurotransmitters levels at the synaptic cleft. Additionally, AMPH and related compounds may increase neurotransmitter levels via ‘reverse transport’ or efflux (65). DAT is the predominant transporter studied presumably due to its implications for addiction. The principal proposed mechanisms for AMPH-induced DAT-mediated DA efflux are: 1) facilitated exchange diffusion (34), 2) channel-in-transporter DA efflux model (66), 3) oligomer-based counter-transport (67), and 4) vesicular depletion (or weak-base model), in which interaction of the releasing substrate (AMPH) with the vesicular monoamine transporter disrupts vesicular storage leading to an increase in free cytoplasmic transmitter levels (68, 69). Regulation of DAT-mediated DA efflux includes protein kinase C (PKC)-activated DA efflux (70) and Ca++/calmodulin-dependent protein kinase II (CaMKII) facilitated phosphorylation (71).
5.3 Transport-Associated Currents of Monoamine Transporters
Early biochemical and radiolabeled flux data led to the alternating access model for SERT, NET and DAT transport; however, subsequent studies uncovered uncoupled currents and channel-like activity in transporters (47, 72–74). Since the early 1990s, currents associated with substrate transport were found to be larger than alternating access models predicted (50, 64, 75–84). Uncoupled currents in transporters are largely unexplained structurally and their function is speculative; one possibility is that these currents may depolarize or hyperpolarize neurons to a sufficient extent to produce changes in neuronal excitability (72, 85–89). Most evidence for channels in transporters comes from heterologous expression systems; however, large 5HT-induced currents are generated in SERT at native serotonergic synapses (90, 91). In two studies, Cl− is reported to contribute to the ionic composition of DAT substrate-induced currents (86, 88); however, Na+ seems to be a major contributor to these DAT currents (92).
5.4 The Leak Current
Mager and colleagues established the existence of endogenous leak currents at monoamine transporters as revealed with use of transporter inhibitors (75). For SERT, studies that employed the two-electrode voltage-clamp (TEVC) technique in SERT-expressing Xenopus laevis oocytes demonstrated that a variety of inhibitors could uncover the SERT leak current. This response is seen as an outward current relative to baseline but is actually the inhibition of a constitutive inward current that is thought to be mediated primarily by Na+. Compounds that helped uncover the SERT leak current include FLX (93), citalopram (both R and S isomers) (94), and the tricyclic antidepressants desipramine (DES) (95) and imipramine (IMI) (96). The majority of SERT inhibitors elicit long-lasting electrophysiological effects after their removal, a distinct action on SERT compared to natural substrates that are easily washed out. Wang and colleagues showed that exposure to FLX leads to a greatly diminished 5HT-induced hSERT-mediated current response, measured by the peak current time constant: “the time constant for 5HT-induced current became much greater than that for the first 5HT perfusion”. In other words, after exposure to FLX, there is a much weaker inward current produced by 5HT (or other substrates) at hSERT as compared to an initial 5HT response at hSERT. Lastly, under physiologically relevant experimental concentrations, FLX-induced outward currents supersede inward 5HT-induced currents when 5HT and FLX are simultaneously applied – even at high 5HT concentrations. This result suggests FLX inhibits the endogenous leak current and disables substrate-induced currents at SERT. These results are consistent with other SERT inhibitors, including DES (95), IMI (96), and paroxetine (unpublished data). Storustovu and colleagues demonstrate that applying either citalopram enantiomer (especially the S- isoform) during the 5HT-induced SERT current results in an outward current (94). Cocaine and cocaine analogs also reveal leak currents in DAT-expressing, voltage-clamped Xenopus laevis oocytes (64). However, the DAT-mediated outward current elicited by cocaine washes out more slowly than the substrate (DA)-induced inward current, which is attributed to its action as an inhibitor – rather than a substrate – at DAT. Inhibitors with much higher affinity, such as the cocaine analog (1R)-2beta-Carbomethoxy-3beta-(4-iodophenyl)tropane (β-CIT), are also more difficult to wash out, similar to FLX on SERT. The leak current has been observed in NET overexpressed in HEK cells by desipramine (76), but the technical limitation to overexpress NET in oocytes has precluded extensive NET research (20).
5.5 DAT and SERT Display an Induced Persistent Current
Recent studies have uncovered a novel mechanism of the action of AMPH on DAT based on electrophysiological data. In this model, AMPH is transported by DAT and concentrated inside the cell where the drug persists and is available to bind to the transporter at an internal site. The binding of AMPH at this internal site may maintain the transporter in a conductive state even when the external substrate is removed, leading to a persistent leak or “shelf” inward current; furthermore, it is proposed that external DA and other substrates can hold DAT in a constitutively-active state once internal AMPH is present (92). The induced persistent current can be elicited with additional select releasing substrates and in different monoamine transporters; in particular S(+)METH can produce a persistent leak current in hDAT, and para-chloroamphetamine can produce the same response in hSERT (97). This mechanism could have consequences on synaptic transmission, as the persistent current would depolarize neurons long after exposure to the drug. More work needs to be done to address the importance and implications of this novel monoamine transporter mechanism (98).
6 Mechanism of Action of Synthetic Cathinones
6.1 Synthetic Cathinones Target Monoamine Transporters
Using different techniques, several groups have sought to understand the pharmacology and action of synthetic cathinones; particularly, ones found in bath salts. Since amphetamine and related drugs act on the monoamine transporters by inhibiting reuptake of endogenous neurotransmitters and by releasing endogenous transmitter, studies of structurally-related synthetic cathinones are being carried out to determine their precise mechanisms of action. Experiments using rat brain synaptosomes pre-loaded with radiolabeled transmitter show that mephedrone exhibits similar releasing properties as MDMA at NET and DAT but weaker at SERT. Furthermore, methylone exhibits similar albeit weaker behavior (99). In rat brain synaptosomes, MDPV was much stronger as an uptake inhibitor than AMPH, COC, or MEPH through DAT and NET, whereas MEPH and COC were more potent at inhibiting SERT uptake than MDPV or AMPH (100). AMPH and MEPH, but not COC or MDPV, behave as releasers at DAT, NET, and SERT (100). Further uptake inhibition assays in rat brain synaptosomes employing MDPV analogs confirmed that the α-alkyl chain is essential for hDAT affinity, whereas the methylenedioxy group does not affect affinity (101). Substituting a trifluoromethyl on the 3 or 4 position of MCAT’s ring increases the compound’s selectivity towards SERT over NET and DAT (102). Second generation MEPH analogs also elicit release through SERT and DAT (5). Studies employing HEK293 cells expressing monoamine transporters confirmed MDPV is an uptake blocker without release properties at hDAT, hSERT, and hNET, whereas MEPH (along with methylone and 4-fluoromethcathinone) behave as uptake inhibitors and METH-like releasers at all three transporters, but with highest potency at hNET (103). Another study employing a number of synthetic cathinones classified the compounds based on their actions on monoamine transporters, including compounds that: 1) exhibit relatively non-selective actions as uptake inhibitors and display “MDMA-like” 5HT release through SERT, 2) show preferential catecholamine transporter actions as DAT and NET uptake inhibitors and induce DA release (like METH), and 3) are potent and selective catecholamine transporter uptake inhibitors but do not induce release (MDPV) (104). A similar study assessing synthetic cathinones on monoamine transporters showed that: 1) most of the compounds were more potent inhibitors of NET uptake, 2) addition of a β-keto group tends to enhance the DAT uptake inhibition over SERT uptake inhibition, 3) ring substitutions enhance serotonergic uptake inhibition, 4) some synthetic cathinones behave as pure uptake inhibitors, whereas others act as substrate releasers, and 5) there is weak binding of synthetic cathinones to a panel of receptors (105).
6.2 Electrophysiological Actions of Synthetic Cathinones on hDAT
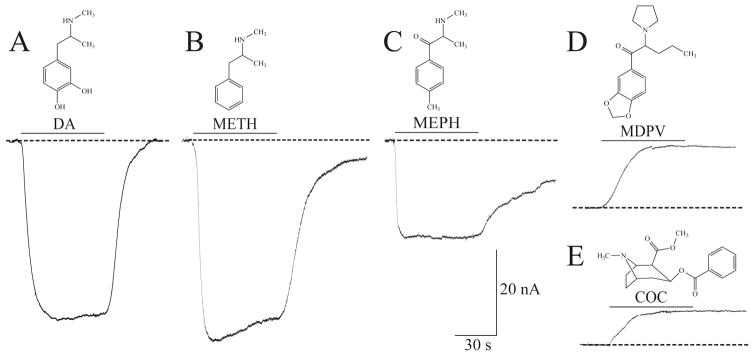
The electrophysiological effects of a drug can provide a molecular signature of the specific interaction between a drug and the transporter. In a cell voltage-clamped to −60 mV, compounds that produce transporter-mediated inward currents are considered transported substrates (or releasers), and compounds that produce outward currents (interpreted as a block of an endogenous leak current) are considered as non-transported inhibitors. To illustrate this, currents from hDAT overexpressed in Xenopus laevis oocytes were recorded in response to DA, METH, the synthetic cathinones MEPH and MDPV, and cocaine (COC) (Fig. 2). DA, METH and MEPH induced hDAT-mediated inward currents, in agreement with previous studies that determined that METH and MEPH act as releasers (99, 100). On the other hand, MDPV and COC possess the signature of a non-transported blocker at hDAT, which qualitatively is seen as an upward deflection (Fig. 2) (106). The mixture of mephedrone and MDPV commonly found in ‘bath salts’, in combination with these findings indicate that bath salts may contain a DA releasing agent and a DA reuptake inhibitor. The two drugs have different kinetics and rather than cancel each other they would exacerbate the effect of either drug taken alone. Further recordings showed that MDPV produces a long-lasting effect at hDAT, that is, washout fails to return the outward current to baseline in contrast to COC, which is more easily washed out (106). In congruence, MDPV proved to be 10–35 times more potent than COC as an uptake inhibitor for DAT (100, 106).
Fig. 2. Electrophysiological signature of DA, METH, MEPH, MDPV, and cocaine at hDAT.
(A–E) By employing the two-electrode voltage-clamp technique (TEVC), currents through hDAT elicited by external drug application (60 s duration, 10 μM) are measured in hDAT-expressing Xenopus laevis oocytes voltage clamped to −60 mV. (A) DA induces a large inward peak current that returns to baseline when DA is removed. (B) METH and (C) MEPH elicit inward peak currents and induced persistent currents (enhanced current in the absence of drug). (D) MDPV exposure produces an outward, hyperpolarizing current similar to the current induced by (E) COC, which is a known reuptake inhibitor. The responses induced by MDPV and COC reveal the presence of an endogenous inward leak current typically uncovered with inhibitors. Figure adapted from Cameron et al. 2013.
6.3 Electrophysiological Actions of Synthetic Cathinones on hSERT
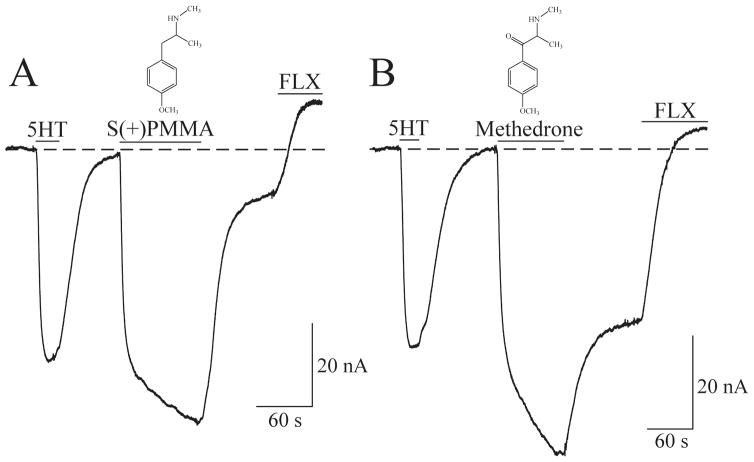
There are limited studies on the electrophysiological actions of synthetic cathinones on hSERT, which require more elaboration. Mephedrone analogs (second-generation cathinones) are stimulants that induce euphoria and elicit inward currents (5). The METH analog, para-methoxymetheamphetamine (PMMA), which induces behavioral effects similar to MDMA without stimulant effects, and the β-keto analog methedrone, found in bath salts, display potent effects at SERT and NET (105). In particular, methedrone is the synthetic cathinone with the highest selectivity for SERT, and it behaves as a substrate that induces monoamine efflux (105). In agreement, employing the TEVC technique in voltage-clamped (−60 mV) oocytes overexpressing hSERT, show that the S(+) isomer of PMMA or methedrone elicit inward currents through hSERT comparable to the 5HT-induced inward current (Fig. 3). This is the signature of substrates (or releasers) and, interestingly, methedrone produces a large persistent inward current after washout, which confirms its potent effect at hSERT.
Fig. 3. Electrophysiological signature of S(+)PMMA and the synthetic cathinone methedrone at hSERT.
By utilizing the TEVC technique currents are measured in Xenopus laevis oocytes overexpressing hSERT in response to 5 μM 5HT followed by 10 μM of either the S(+) isomer of para-methoxy-N-methylamphetamine (S(+)PMMA) (A) or methedrone (B). While the 5HT-induced hSERT response returns to baseline after washout, the washout following exposure to S(+)PMMA or methedrone for 100 s results in a persistent inward current. The hSERT inhibitor fluoxetine (FLX) reveals the endogenous leak current.
6.4 Structural Determinants for Potency of MDPV on hDAT
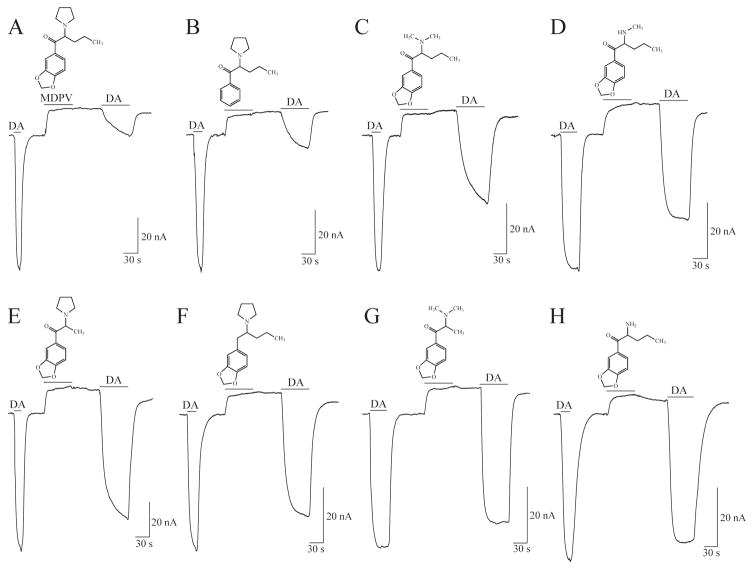
Deconstruction of MDPV into analogs allowed the determination of the moieties in MDPV responsible for its potency. In hDAT-expressing Xenopus laevis oocytes clamped to −60 mV, MDPV and its analogs induced comparable outward currents (block of an endogenous inward leak) that, after drug washout, did not return to the baseline before drug application. Furthermore, DA-induced currents obtained following application of either MDPV or its analogs displayed amplitude recovery profiles relative to the initial DA-induced currents (see Fig. 4) that highly correlated with the compounds’ potency to inhibit DA uptake via hDAT. In this study, the combination of uptake inhibition assays and an electrophysiological protocol, revealed the major contributor for MDPV’s potency at hDAT to be the extended α-alkyl group, followed by the carbonyl group and a tertiary amine, whereas the methylenedioxy group made a minimal contribution (107).
Fig. 4. Currents induced by MDPV and its analogs in voltage-clamped (−60 mV) Xenopus laevis oocytes expressing hDAT.
(A–H) Initial exposure to DA (5 μM) yields an hDAT-mediated inward current. Subsequent exposure to MDPV (A) or any of its analogs (B–H) (10 μM, 1 min) produces the typical response associated with the block of the endogenous current at hDAT. The upward deflection does not return to baseline when any of the compounds are washed out for 1 min. (A) Following exposure to MDPV a 5 μM DA application induces a diminished hDAT-mediated inward current (as compared to the current produced in response to the initial DA exposure). The protocol is repeated for the MDPV analogs (B–H). Exposure to the different MDPV analogs elicits variable DA current recovery (compare second DA exposure to first DA exposure). For example, in contrast to the diminished DA-induced hDAT-mediated inward current following MDPV exposure (A), after exposing hDAT to the last two compounds (G–H) application of DA results in large inward currents that fully recover to the level of the current elicited by the first DA exposure. Figure adapted from Kolanos et al. 2013.
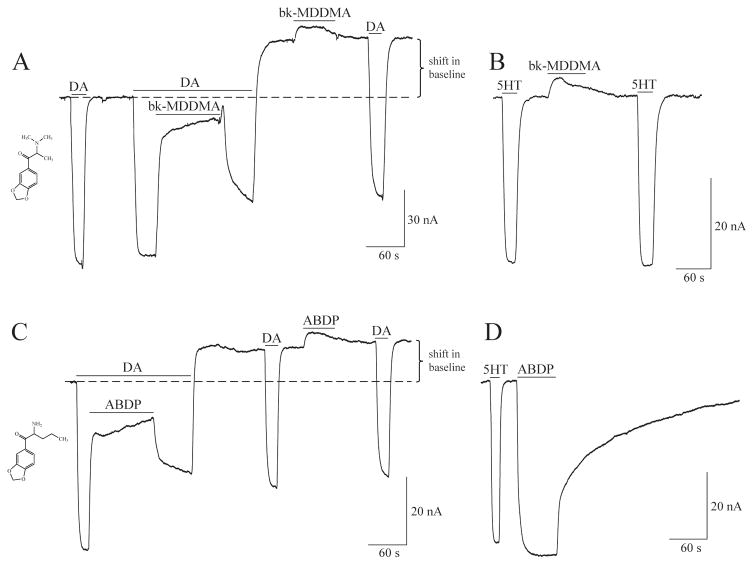
6.5 A Distinct Site for Action of MDPV Analogs on hDAT
All compounds used in the Kolanos et al. study of the electrophysiological effects of MDPV analogs at hDAT, shifted the baseline after washout to more positive values, indicating hyperpolarization (see Fig. 4). Interestingly, for the MDPV analogs 1-(benzo[d][1,3]dioxol-5-yl)-2-(dimethylamino)propan-1-one (bk-MDDMA) (Fig. 4G) and 2-amino-1-(benzo[d]-1,3-dioxol-5-yl)pentan-1-one (ABDP) (Fig. 4H), the second DA response recovered to 100% of the first DA relative to the new baseline. The baseline shift following 10 μM of either bk-MDDMA or ABDP application cannot be washed out and is unaffected by a second exposure to DA. In subsequent recordings from hDAT oocytes, bk-MDDMA was perfused and removed while extracellular DA was present (Fig. 5A); however, a shift in baseline still occurred. Similarly, ABDP produces a shift in baseline even in the presence of constant extracellular DA (Fig. 5C). The shift in baseline produced by these analogs was not impeded by the presence of a high concentration of dopamine (data not shown). At hSERT, bk-MDDMA elicits a weak, reversible block of the inward leak current (Fig. 5B), and ABDP produces an inward, substrate-like current and a persistent inward current (Fig. 5D). These results suggest two distinct sites of action for MDPV analogs targeting DAT, and the distinct effect of the compounds at hSERT differentiates these compounds at the two transporters. There are only a few reports of a secondary site of action on monoamine transporters (108), but none employing electrophysiology. This secondary site of action of drugs targeting hDAT could have important implications for drug development to treat addiction and other disorders.
Fig. 5. Currents induced by MDPV analogs at hDAT and hSERT in voltage-clamped (−60 mV) Xenopus laevis oocytes.
(A) Application of bk-MDDMA (20 μM) during constant perfusion of DA counteracts the inward DA-induced current. After removal of bk-MDDMA, a DA-induced inward current is observed; however, after DA is washed out, the current goes above baseline (above dashed line). A second exposure to bk-MDDMA induces a small outward current that returns to the level the baseline was shifted to. A subsequent DA-induced hDAT current is similar to the initial DA response, and after the last DA application is washed out, the baseline remains shifted. (B) At hSERT, bk-MDDMA (20 μM) induces a small block of the endogenous leak current, but in contrast to what happens at hDAT, after washing out bk-MDDMA the holding current at hSERT returns to its original level. (C) Application of ABDP shifts the baseline even in the presence of DA. ABDP application (20 μM) is applied to hDAT in the presence of DA, which elicits a counteracting hDAT-mediated current. After removal of ABDP application, the DA present induces an hDAT-mediated inward current; however, after DA is washed out, the current goes above baseline. A second exposure to ABDP application induces a small outward current. A subsequent DA-induced hDAT current is similar to the initial DA response, and after the last DA application is washed out, the baseline remains shifted. (D) ABDP (20 μM) induces an hSERT-mediated inward current that does not return to baseline. Note: All DA and 5HT challenges are 5 μM.
7. Conclusion
Electrophysiological methods can characterize the actions of drugs on the monoamine transporters, determine whether they act as releasers or inhibitors, can quantify potency and efficacy, and evaluate structure-activity relationships of new compounds. Correlative studies with neurotransmitter uptake/release and fluorescent microscopy will enhance our understanding of drug action. Lastly, structure-function analysis of monoamine transporter protein structures as they become available can be combined with functional information to uncover the molecular mechanisms underlying drug-transporter interactions.
Acknowledgments
I would like to acknowledge Louis J. De Felice and Michael H. Baumann for valuable input in the writing of the chapter. The work described in this chapter was supported by NIH/NIDA R01DA033930 and R01DA033930-S2.
References
- 1.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49(6):499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 2.De Felice LJ, Glennon RA, Negus SS. Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci. 2014;97(1):20–26. doi: 10.1016/j.lfs.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schechter MD, Glennon RA. Cathinone, cocaine and methamphetamine: similarity of behavioral effects. Pharmacol Biochem Behav. 1985;22(6):913–916. doi: 10.1016/0091-3057(85)90295-3. [DOI] [PubMed] [Google Scholar]
- 4.Iversen L, White M, Treble R. Designer psychostimulants: pharmacology and differences. Neuropharmacology. 2014;87:59–65. doi: 10.1016/j.neuropharm.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Saha K, et al. ‘Second-generation’ mephedrone analogs, 4-MEC and 4-MePPP, differentially affect monoamine transporter function. Neuropsychopharmacology. 2015;40(6):1321–1331. doi: 10.1038/npp.2014.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffman BJ, Hansson SR, Mezey E, Palkovits M. Localization and dynamic regulation of biogenic amine transporters in the mammalian central nervous system. Front Neuroendocrinol. 1998;19(3):187–231. doi: 10.1006/frne.1998.0168. [DOI] [PubMed] [Google Scholar]
- 7.Tao-Cheng JH, Zhou FC. Differential polarization of serotonin transporters in axons versus soma-dendrites: an immunogold electron microscopy study. Neuroscience. 1999;94(3):821–830. doi: 10.1016/s0306-4522(99)00373-5. [DOI] [PubMed] [Google Scholar]
- 8.Wimalasena K. Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry. Med Res Rev. 2011;31(4):483–519. doi: 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl SM. Basic psychopharmacology of antidepressants, part 1: Antidepressants have seven distinct mechanisms of action. J Clin Psychiatry. 1998;59(Suppl 4):5–14. [PubMed] [Google Scholar]
- 10.Schloss P, Williams DC. The serotonin transporter: a primary target for antidepressant drugs. J Psychopharmacol. 1998;12(2):115–121. doi: 10.1177/026988119801200201. [DOI] [PubMed] [Google Scholar]
- 11.Coppen A, Shaw DM, Herzberg B, Maggs R. Tryptophan in the treatment of depression. Lancet. 1967;2(7527):1178–1180. doi: 10.1016/s0140-6736(67)91894-6. [DOI] [PubMed] [Google Scholar]
- 12.Vaswani M, Kalra H. Selective serotonin re-uptake inhibitors in anorexia nervosa. Expert Opin Investig Drugs. 2004;13(4):349–357. doi: 10.1517/13543784.13.4.349. [DOI] [PubMed] [Google Scholar]
- 13.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 14.Feighner JP. Clinical effects of serotonin reuptake inhibitors--a review. Fortschr Neurol Psychiatr. 1994;62(Suppl 1):9–15. [PubMed] [Google Scholar]
- 15.Barbeau A. Dopamine and disease. Can Med Assoc J. 1970;103(8):824–832. [PMC free article] [PubMed] [Google Scholar]
- 16.Javitch JA, Snyder SH. Uptake of MPP(+) by dopamine neurons explains selectivity of parkinsonism-inducing neurotoxin, MPTP. Eur J Pharmacol. 1984;106(2):455–456. doi: 10.1016/0014-2999(84)90740-4. [DOI] [PubMed] [Google Scholar]
- 17.Gainetdinov RR. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(4–6):301–313. doi: 10.1007/s00210-007-0216-0. [DOI] [PubMed] [Google Scholar]
- 18.Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 19.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77(5):3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz JW, Piston D, DeFelice LJ. Molecular microfluorometry: converting arbitrary fluorescence units into absolute molecular concentrations to study binding kinetics and stoichiometry in transporters. Handb Exp Pharmacol. 2006;(175):23–57. doi: 10.1007/3-540-29784-7_2. [DOI] [PubMed] [Google Scholar]
- 21.Banks ML, Worst TJ, Rusyniak DE, Sprague JE. Synthetic cathinones (“bath salts”) J Emerg Med. 2014;46(5):632–642. doi: 10.1016/j.jemermed.2013.11.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97(1):2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miotto K, Striebel J, Cho AK, Wang C. Clinical and pharmacological aspects of bath salt use: a review of the literature and case reports. Drug Alcohol Depend. 2013;132(1–2):1–12. doi: 10.1016/j.drugalcdep.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Valente MJ, Guedes de Pinho P, de Lourdes Bastos M, Carvalho F, Carvalho M. Khat and synthetic cathinones: a review. Arch Toxicol. 2014;88(1):15–45. doi: 10.1007/s00204-013-1163-9. [DOI] [PubMed] [Google Scholar]
- 25.White KJ, Walline CC, Barker EL. Serotonin transporters: implications for antidepressant drug development. AAPS J. 2005;7(2):E421–433. doi: 10.1208/aapsj070242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sghendo L, Mifsud J. Understanding the molecular pharmacology of the serotonergic system: using fluoxetine as a model. J Pharm Pharmacol. 2012;64(3):317–325. doi: 10.1111/j.2042-7158.2011.01384.x. [DOI] [PubMed] [Google Scholar]
- 27.Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51(3):215–235. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- 28.Johansen PO, Krebs TS. How could MDMA (ecstasy) help anxiety disorders? A neurobiological rationale. J Psychopharmacol. 2009;23(4):389–391. doi: 10.1177/0269881109102787. [DOI] [PubMed] [Google Scholar]
- 29.Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of {+/−}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. 2011;25(4):439–452. doi: 10.1177/0269881110378371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wall SC, Gu H, Rudnick G. Biogenic amine flux mediated by cloned transporters stably expressed in cultured cell lines: amphetamine specificity for inhibition and efflux. Mol Pharmacol. 1995;47(3):544–550. [PubMed] [Google Scholar]
- 31.Wu X, Gu HH. Molecular cloning of the mouse dopamine transporter and pharmacological comparison with the human homologue. Gene. 1999;233(1–2):163–170. doi: 10.1016/s0378-1119(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 32.Henry LK, Blakely RD. Distinctions between dopamine transporter antagonists could be just around the bend. Mol Pharmacol. 2008;73(3):616–618. doi: 10.1124/mol.107.044586. [DOI] [PubMed] [Google Scholar]
- 33.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 34.Burnette WB, et al. Human norepinephrine transporter kinetics using rotating disk electrode voltammetry. Anal Chem. 1996;68(17):2932–2938. doi: 10.1021/ac960022x. [DOI] [PubMed] [Google Scholar]
- 35.Phillips AG, Brooke SM, Fibiger HC. Effects of amphetamine isomers and neuroleptics on self-stimulation from the nucleus accumbens and dorsal noradrenergic bundle. Brain Res. 1975;85(1):13–22. doi: 10.1016/0006-8993(75)90998-1. [DOI] [PubMed] [Google Scholar]
- 36.Holmes JC, Rutledge CO. Effects of the d- and l-isomers of amphetamine on uptake, release and catabolism of norepinephrine, dopamine and 5-hydroxytryptamine in several regions of rat brain. Biochem Pharmacol. 1976;25(4):447–451. doi: 10.1016/0006-2952(76)90348-8. [DOI] [PubMed] [Google Scholar]
- 37.Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15(2):1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cody JT, Valtier S, Nelson SL. Amphetamine enantiomer excretion profile following administration of Adderall. J Anal Toxicol. 2003;27(7):485–492. doi: 10.1093/jat/27.7.485. [DOI] [PubMed] [Google Scholar]
- 39.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57(7–8):608–618. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Najib J. The efficacy and safety profile of lisdexamfetamine dimesylate, a prodrug of d-amphetamine, for the treatment of attention-deficit/hyperactivity disorder in children and adults. Clin Ther. 2009;31(1):142–176. doi: 10.1016/j.clinthera.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Mendelson J, et al. Human pharmacology of the methamphetamine stereoisomers. Clin Pharmacol Ther. 2006;80(4):403–420. doi: 10.1016/j.clpt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Potkin SG, et al. Phenylethylamine in paranoid chronic schizophrenia. Science. 1979;206(4417):470–471. doi: 10.1126/science.504988. [DOI] [PubMed] [Google Scholar]
- 43.Romanelli F, Smith KM. Clinical effects and management of methamphetamine abuse. Pharmacotherapy. 2006;26(8):1148–1156. doi: 10.1592/phco.26.8.1148. [DOI] [PubMed] [Google Scholar]
- 44.Winslow BT, Voorhees KI, Pehl KA. Methamphetamine abuse. Am Fam Physician. 2007;76(8):1169–1174. [PubMed] [Google Scholar]
- 45.Mazei-Robinson MS, Blakely RD. ADHD and the dopamine transporter: are there reasons to pay attention? Handb Exp Pharmacol. 2006;(175):373–415. doi: 10.1007/3-540-29784-7_17. [DOI] [PubMed] [Google Scholar]
- 46.Arias HR. Is the inhibition of nicotinic acetylcholine receptors by bupropion involved in its clinical actions? Int J Biochem Cell Biol. 2009;41(11):2098–2108. doi: 10.1016/j.biocel.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 47.De Felice LJ. Chloride requirement for monoamine transporters. Pflugers Arch. 2016;468(3):503–511. doi: 10.1007/s00424-015-1783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh SK. LeuT: A prokaryotic stepping stone on the way to a eukaryotic neurotransmitter transporter structure. Channels (Austin) 2008;2(5) doi: 10.4161/chan.2.5.6904. [DOI] [PubMed] [Google Scholar]
- 49.Ramamoorthy S, et al. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90(6):2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galli A, Blakely RD, DeFelice LJ. Norepinephrine transporters have channel modes of conduction. Proc Natl Acad Sci U S A. 1996;93(16):8671–8676. doi: 10.1073/pnas.93.16.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeFelice LJ, Blakely RD. Pore models for transporters? Biophys J. 1996;70(2):579–580. doi: 10.1016/S0006-3495(96)79604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudnick G. Ion-coupled neurotransmitter transport: thermodynamic vs. kinetic determinations of stoichiometry. Methods Enzymol. 1998;296:233–247. doi: 10.1016/s0076-6879(98)96018-9. [DOI] [PubMed] [Google Scholar]
- 53.Rudnick G. Bioenergetics of neurotransmitter transport. J Bioenerg Biomembr. 1998;30(2):173–185. doi: 10.1023/a:1020573325823. [DOI] [PubMed] [Google Scholar]
- 54.Naftalin RJ. The thermostatics and thermodynamics of cotransport. Biochim Biophys Acta. 1984;778(1):155–175. doi: 10.1016/0005-2736(84)90459-0. [DOI] [PubMed] [Google Scholar]
- 55.Stein WD, Lieb WR. Transport and diffusion across cell membranes. Academic Press; Orlando: 1986. p. xvii.p. 685. [Google Scholar]
- 56.Abramson J, et al. The lactose permease of Escherichia coli: overall structure, the sugar-binding site and the alternating access model for transport. FEBS Lett. 2003;555(1):96–101. doi: 10.1016/s0014-5793(03)01087-1. [DOI] [PubMed] [Google Scholar]
- 57.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301(5633):610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437(7056):215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 59.Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431(7010):811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 60.Penmatsa A, Wang KH, Gouaux E. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat Struct Mol Biol. 2015;22(6):506–508. doi: 10.1038/nsmb.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang KH, Penmatsa A, Gouaux E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature. 2015;521(7552):322–327. doi: 10.1038/nature14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994;269(10):7124–7130. [PubMed] [Google Scholar]
- 63.McElvain JS, Schenk JO. A multisubstrate mechanism of striatal dopamine uptake and its inhibition by cocaine. Biochem Pharmacol. 1992;43(10):2189–2199. doi: 10.1016/0006-2952(92)90178-l. [DOI] [PubMed] [Google Scholar]
- 64.Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17(3):960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem. 2003;278(14):12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- 66.Kahlig KM, et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005;102(9):3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seidel S, et al. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores the amphetamine action. Mol Pharmacol. 2005;67(1):140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- 68.Sulzer D, et al. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15(5 Pt 2):4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sulzer D, Maidment NT, Rayport S. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem. 1993;60(2):527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 70.Khoshbouei H, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2(3):E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fog JU, et al. Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron. 2006;51(4):417–429. doi: 10.1016/j.neuron.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 72.Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40(3):537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- 73.DeFelice LJ, Goswami T. Transporters as channels. Annu Rev Physiol. 2007;69:87–112. doi: 10.1146/annurev.physiol.69.031905.164816. [DOI] [PubMed] [Google Scholar]
- 74.DeFelice LJ. Going against the flow. Nature. 2004;432(7015):279. doi: 10.1038/432279a. [DOI] [PubMed] [Google Scholar]
- 75.Mager S, et al. Conducting states of a mammalian serotonin transporter. Neuron. 1994;12(4):845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 76.Galli A, DeFelice LJ, Duke BJ, Moore KR, Blakely RD. Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. J Exp Biol. 1995;198(Pt 10):2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- 77.Galli A, Blakely RD, DeFelice LJ. Patch-clamp and amperometric recordings from norepinephrine transporters: channel activity and voltage-dependent uptake. Proc Natl Acad Sci U S A. 1998;95(22):13260–13265. doi: 10.1073/pnas.95.22.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeFelice LJ, Galli A. Fluctuation analysis of norepinephrine and serotonin transporter currents. Methods Enzymol. 1998;296:578–593. doi: 10.1016/s0076-6879(98)96041-4. [DOI] [PubMed] [Google Scholar]
- 79.DeFelice LJ, Galli A. Electrophysiological analysis of transporter function. Adv Pharmacol. 1998;42:186–190. doi: 10.1016/s1054-3589(08)60724-3. [DOI] [PubMed] [Google Scholar]
- 80.Adams SV, DeFelice LJ. Flux coupling in the human serotonin transporter. Biophys J. 2002;83(6):3268–3282. doi: 10.1016/S0006-3495(02)75328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petersen CI, DeFelice LJ. Ionic interactions in the Drosophila serotonin transporter identify it as a serotonin channel. Nat Neurosci. 1999;2(7):605–610. doi: 10.1038/10158. [DOI] [PubMed] [Google Scholar]
- 82.Adams SV, DeFelice LJ. Ionic currents in the human serotonin transporter reveal inconsistencies in the alternating access hypothesis. Biophys J. 2003;85(3):1548–1559. doi: 10.1016/S0006-3495(03)74587-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramsey IS, DeFelice LJ. Serotonin transporter function and pharmacology are sensitive to expression level: evidence for an endogenous regulatory factor. J Biol Chem. 2002;277(17):14475–14482. doi: 10.1074/jbc.M110783200. [DOI] [PubMed] [Google Scholar]
- 84.Li C, et al. Voltage and ionic regulation of human serotonin transporter in Xenopus oocytes. Clin Exp Pharmacol Physiol. 2006;33(11):1088–1092. doi: 10.1111/j.1440-1681.2006.04491.x. [DOI] [PubMed] [Google Scholar]
- 85.Sonders MS, Amara SG. Channels in transporters. Curr Opin Neurobiol. 1996;6(3):294–302. doi: 10.1016/s0959-4388(96)80111-5. [DOI] [PubMed] [Google Scholar]
- 86.Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5(10):971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- 87.Quick MW. Role of syntaxin 1A on serotonin transporter expression in developing thalamocortical neurons. Int J Dev Neurosci. 2002;20(3–5):219–224. doi: 10.1016/s0736-5748(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 88.Carvelli L, McDonald PW, Blakely RD, Defelice LJ. Dopamine transporters depolarize neurons by a channel mechanism. Proc Natl Acad Sci U S A. 2004;101(45):16046–16051. doi: 10.1073/pnas.0403299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ryan RM, Mindell JA. The uncoupled chloride conductance of a bacterial glutamate transporter homolog. Nat Struct Mol Biol. 2007;14(5):365–371. doi: 10.1038/nsmb1230. [DOI] [PubMed] [Google Scholar]
- 90.Bruns D. Serotonin transport in cultured leech neurons. Methods Enzymol. 1998;296:593–607. doi: 10.1016/s0076-6879(98)96042-6. [DOI] [PubMed] [Google Scholar]
- 91.Bruns D, Engert F, Lux HD. A fast activating presynaptic reuptake current during serotonergic transmission in identified neurons of Hirudo. Neuron. 1993;10(4):559–572. doi: 10.1016/0896-6273(93)90159-o. [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez-Menchaca AA, Solis E, Jr, Cameron K, De Felice LJ. S(+)amphetamine induces a persistent leak in the human dopamine transporter: molecular stent hypothesis. Br J Pharmacol. 2012;165(8):2749–2757. doi: 10.1111/j.1476-5381.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang HW, et al. Electrophysiological effect of fluoxetine on Xenopus oocytes heterologously expressing human serotonin transporter. Acta Pharmacol Sin. 2006;27(3):289–293. doi: 10.1111/j.1745-7254.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- 94.Storustovu S, et al. R-citalopram functionally antagonises escitalopram in vivo and in vitro: evidence for kinetic interaction at the serotonin transporter. Br J Pharmacol. 2004;142(1):172–180. doi: 10.1038/sj.bjp.0705738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin F, Lester HA, Mager S. Single-channel currents produced by the serotonin transporter and analysis of a mutation affecting ion permeation. Biophys J. 1996;71(6):3126–3135. doi: 10.1016/S0006-3495(96)79506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barker EL, Moore KR, Rakhshan F, Blakely RD. Transmembrane domain I contributes to the permeation pathway for serotonin and ions in the serotonin transporter. J Neurosci. 1999;19(12):4705–4717. doi: 10.1523/JNEUROSCI.19-12-04705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandtner W, et al. A quantitative model of amphetamine action on the 5-HT transporter. Br J Pharmacol. 2014;171(4):1007–1018. doi: 10.1111/bph.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Felice LJ, Cameron KN. Comments on ‘A quantitative model of amphetamine action on the serotonin transporter’, by Sandtner et al. Br J Pharmacol 171: 1007–1018. Br J Pharmacol. 2015;172(19):4772–4774. doi: 10.1111/bph.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baumann MH, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baumann MH, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marusich JA, et al. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cozzi NV, et al. Pharmacological examination of trifluoromethyl ring-substituted methcathinone analogs. Eur J Pharmacol. 2013;699(1–3):180–187. doi: 10.1016/j.ejphar.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eshleman AJ, et al. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85(12):1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simmler LD, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014;79:152–160. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 106.Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013;168(7):1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolanos R, Solis E, Jr, Sakloth F, De Felice LJ, Glennon RA. “Deconstruction” of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. ACS Chem Neurosci. 2013;4(12):1524–1529. doi: 10.1021/cn4001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bulling S, et al. The mechanistic basis for noncompetitive ibogaine inhibition of serotonin and dopamine transporters. J Biol Chem. 2012;287(22):18524–18534. doi: 10.1074/jbc.M112.343681. [DOI] [PMC free article] [PubMed] [Google Scholar]