Abstract
Purpose
For patients with a high likelihood of having metastatic disease (high-risk prostate cancer), bone scan is the standard, guideline-recommended test to look for bony metastasis. We quantified the use of bone scans and downstream procedures, along with associated costs, in patients with high-risk prostate cancer, and their use in low- and intermediate-risk patients for whom these tests are not recommended.
Methods and Materials
Patients in the Surveillance, Epidemiology, and End Results (SEER)-Medicare database diagnosed with prostate cancer from 2004 to 2007 were included. Prostate specific antigen (PSA), Gleason score, and clinical T stage were used to define D’Amico risk categories. We report use of bone scans from the date of diagnosis to the earlier of treatment or 6 months. In patients who underwent bone scans, we report use of bone-specific x-ray, computed tomography (CT), and magnetic resonance imaging (MRI) scans, and bone biopsy within 3 months after bone scan. Costs were estimated using 2012 Medicare reimbursement rates.
Results
In all, 31% and 48% of patients with apparent low- and intermediate-risk prostate cancer underwent a bone scan; of these patients, 21% underwent subsequent x-rays, 7% CT, and 3% MRI scans. Bone biopsies were uncommon. Overall, <1% of low- and intermediate-risk patients were found to have metastatic disease. The annual estimated Medicare cost for bone scans and downstream procedures was $11,300,000 for low- and intermediate-risk patients. For patients with apparent high-risk disease, only 62% received a bone scan, of whom 14% were found to have metastasis.
Conclusions
There is overuse of bone scans in patients with low- and intermediate-risk prostate cancers, which is unlikely to yield clinically actionable information and results in a potential Medicare waste. However, there is underuse of bone scans in high-risk patients for whom metastasis is likely.
Introduction
More than 238,000 men will be diagnosed with prostate cancer in 2013 (1). Management of this common disease is very costly to the US health care system. A recent study showed that adoption of new technologies in prostate cancer management increased the annual cost of care in the US by $350 million (2). These figures for prostate cancer highlight the importance for research to examine health care use in this disease for potential opportunities in savings without compromising patient care.
Patients with newly diagnosed prostate cancer are stratified into low-, intermediate-, and high-risk groups based on clinical stage, prostate specific antigen (PSA) levels, and Gleason score. This stratification (staging) system serves not only to guide treatment decisions but also to help determine the extent of workup required. For patients with high-risk prostate cancer, a bone scan is the guideline-recommended imaging study to look for bony metastasis. On the other hand, for patients with low- and intermediate-risk disease, guidelines do not recommend the routine use of bone scans because metastatic disease is unlikely in these relatively indolent types of prostate cancer (3). These unnecessary scans are costly to the health care system, give unnecessary radiation dose to the patients, and can also lead to downstream consequences in terms of additional procedures to further work up “findings” from the bone scans.
Therefore, the goal of this study was to examine the prevalence of bone scan use in patients with low-, intermediate-, and high-risk prostate cancers; to quantify the use of additional imaging (such as dedicated, bone-specific computed tomography [CT] and magnetic resonance imaging [MRI] scans and x-rays [XR], as well as biopsies after bone scans); and to quantify costs to Medicare associated with the bone scans and these downstream procedures. We further quantify the proportion of patients with apparent low-, intermediate-, and high-risk cancers who are ultimately diagnosed with metastatic disease to determine whether these procedures led to clinically useful information.
Methods and Materials
Data source
We used the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. This is a commonly used, population-based data source to examine patterns of care in older cancer patients. SEER is a National Cancer Institute-supported collection of cancer registries that covers approximately 28% of the US population (4). SEER provides details regarding age, race/ethnicity, marital status, census tract measure of education (a proxy for socioeconomic status), population density of patient residence, and geographic region. Medicare is the primary health insurance program for Americans 65 years and older; its claims data provide information regarding procedures and treatments that patients receive. Medicare data from 12 months before prostate cancer diagnosis were used to calculate a validated comorbidity score specific for claims data (5). This study was exempted by the University of North Carolina at Chapel Hill Institutional Review Board.
Patient selection
Men diagnosed with prostate cancer between 2004 and 2007 were included. Patients were excluded if they were diagnosed with cancer at autopsy, if they had prior cancers, if the month and year of diagnosis were unknown, or if unknown diagnostic information precluded risk group stratification (including all patients diagnosed before 2004). To ensure that all claims related to initial diagnosis and management were available for analysis, we included only patients who had continuous Medicare Part A and B coverage with no enrollment in a health maintenance organization (HMO) for 12 months after diagnosis. These criteria resulted in 47,224 patients in the analytic cohort.
Risk group stratification
Patients were stratified into risk groups using standard criteria defined by D’Amico et al: low risk (clinical stage T1-T2a, Gleason score ≤6, and PSA ≤10 ng/mL), intermediate risk (T2b, or Gleason score 7, or PSA >10 ng/mL and ≤20 ng/mL), and high risk (T2c or T3a, or PSA >20 ng/mL, or Gleason score ≥8) (6). We grouped patients into “apparent” risk groups based on these criteria, which represents the information that was available at the time of the initial diagnosis and that was used for clinical decision making about whether to pursue a staging bone scan to look for metastasis. As one of the outcomes in this study, we examined the proportion of patients in each of these apparent risk groups who was diagnosed with metastatic (M1) disease.
Bone scan and imaging studies
The Current Procedural Technology/Healthcare Common Procedure Coding System (CPT/HCPCS) codes in Medicare data provided information regarding imaging, procedures, and treatments. Bone scans performed after date of diagnosis and before the earlier of 180 days or date of first treatment were considered part of the initial staging workup. Because SEER provides only the month and year of diagnosis, we assigned each patient’s diagnosis to the first of each month and deemed this reasonable because bone scans are unlikely to be used for purposes other than cancer staging.
For patients who received a bone scan, we then examined uses of additional imaging and procedures: x-ray, CT, and MRI specific to bone evaluation, and bone biopsy. We defined imaging and procedures performed within 90 days after bone scan and before treatment as potential downstream studies to further work-up abnormalities identified on bone scan. A complete list of HCPCS codes is shown in the Appendix.
Statistical and cost analysis
We report the proportions of patients in each risk group who received a bone scan and subsequently underwent imaging and procedures. We further explore regional variation in use, as well as variation by primary treatment modality (conservative management, external beam radiation, non-external beam treatment). The reason for the latter is that external beam radiation often includes a CT and/or MRI scan for treatment planning, which can be a source of confounding in this analysis for these 2 specific categories of imaging. Multivariate logistic regression models assessed for covariates associated with bone scan use in each risk group. All analysis was performed with SAS 9.2 (Cary, NC), and a P value of <.05 was considered statistically significant.
Direct cost to Medicare was calculated based on the cost of a bone scan from the CPT code 78306, which accounted for the majority of Medicare’s bone scan claims among patients with prostate cancer. The 2012 Medicare national average reimbursement rate of $257.66 was multiplied by the estimated annual incidence of prostate cancer in patients older than 65. This figure was then multiplied by the proportion of patients receiving bone scans as reported in this study. Similarly, the costs of downstream imaging and procedures were estimated using 2012 national reimbursement rates multiplied by the relative frequency of each procedure.
Results
Baseline cohort characteristics are shown in Table 1. Overall, 83% of patients were of white ethnicity, and 42% had apparent high-risk cancer at diagnosis.
Table 1.
Characteristics of the analytic cohort (N=47,224)
| n | (%) | |
|---|---|---|
| Age at diagnosis (y) | ||
| 66–69 | 12,697 | 26.9 |
| 70–74 | 14,383 | 30.5 |
| 75–79 | 10,874 | 23.0 |
| 80–84 | 6141 | 13.0 |
| 85+ | 3129 | 6.6 |
| Race | ||
| Nonwhite | 8074 | 17.1 |
| White | 39,150 | 82.9 |
| Marital Status | ||
| Married | 32,466 | 68.7 |
| Not married/unknown | 14,758 | 31.3 |
| NCI Comorbidity Index | ||
| 0 | 30,326 | 64.2 |
| >0 | 16,898 | 35.8 |
| Year of diagnosis | ||
| 2004 | 12,015 | 25.4 |
| 2005 | 11,364 | 24.1 |
| 2006 | 12,011 | 25.4 |
| 2007 | 11,834 | 25.1 |
| % Non-high school graduate in census tract | ||
| Quartile 1 (lowest) | 12,179 | 25.8 |
| Quartile 2 | 11,861 | 25.1 |
| Quartile 3 | 11,773 | 24.9 |
| Quartile 4 (highest) | 11,411 | 24.2 |
| Median household income in census tract | ||
| Quartile 1 (lowest) | 11,591 | 24.5 |
| Quartile 2 | 11,755 | 24.9 |
| Quartile 3 | 11,817 | 25.0 |
| Quartile 4 (highest) | 12,061 | 25.5 |
| Geographic region | ||
| Central | 8824 | 18.7 |
| Northeast | 10,317 | 21.8 |
| South | 8628 | 18.3 |
| West | 19,455 | 41.2 |
| Population Density | ||
| Not Urban/missing | 7364 | 15.6 |
| Urban | 39,860 | 84.4 |
| Apparent risk group | ||
| High | 19,885 | 42.1 |
| Intermediate | 16,235 | 34.4 |
| Low | 11,104 | 23.5 |
Abbreviation: NCI = National Cancer Institute.
Geographic regions are as follows: Northeast (Connecticut, New Jersey), South (Atlanta, Rural Georgia, Kentucky, Louisiana), Central (Detroit, Iowa, Utah, New Mexico), and West (California, Seattle, Hawaii).
Table 2 and Figure 1 show the frequency of bone scans during initial workup in each apparent risk group, and the proportion within each group with diagnosed metastatic disease. Although metastatic disease is rare (<1% overall) in patients with apparent low- and intermediate-risk disease, 31% and 48% of patients in these groups underwent bone scans, respectively. Among high-risk patients, 62% received a bone scan and 12% overall had metastatic disease. There was regional variation in the use of bone scans in low- and intermediate-risk patients, with the highest use in the Northeast and lowest in the West. For high-risk cancer, regional variation in bone scan use was less dramatic.
Table 2.
Frequency of bone scans and metastatic disease in men with prostate cancer, stratified by apparent risk group at initial diagnosis
| Low risk | Intermediate risk | High risk | |
|---|---|---|---|
| Total no. of patients | 11,104 | 16,235 | 19,885 |
| Patients who received a bone scan (%) | |||
| Overall (all regions) | 31 | 48 | 62 |
| Central | 26 | 46 | 61 |
| Northeast | 46 | 63 | 68 |
| South | 31 | 48 | 59 |
| West | 24 | 43 | 60 |
| Patients with metastatic disease (among all patients in risk group), n (%) | 14 (0.1) | 113 (0.7) | 2432 (12) |
| Patients with metastatic disease (among those who had a bone scan), n (%) | 11 (0.3) | 85 (1.1) | 1736 (14) |
Fig. 1.
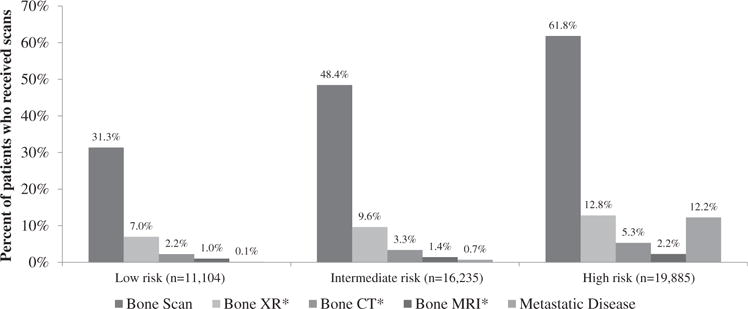
Percentage of patients in each apparent risk group who underwent bone scans or downstream imaging studies, and percentage with metastatic disease. *For illustration purposes, percentages of patients who underwent x-ray (XR), computed tomography (CT), and magnetic resonance imaging (MRI) after bone scan were normalized to the total number of patients in each risk group (denominator) rather than the total number of patients who underwent a bone scan.
Among patients who received a bone scan, the proportion who underwent additional scans within 90 days after the bone scan and before start of treatment is shown in Table 3. These data are stratified by risk group, first treatment, and SEER region. Overall, 20.7% of patients who received a bone scan received downstream bone-specific x-rays (22.2% low-risk, 19.9% intermediate-risk, and 20.7% high-risk); 7.8% received bone-specific CT scans (7.1%, 6.9%, and 8.6%), and 3.3% bone-specific MRIs (3.2%, 2.9%, and 3.6%). There was no statistically significant increase in CT or MRI use in external beam radiation versus that in other patients. The use of bone biopsies after bone scan was rare: only 76 patients (0.3%) underwent this procedure.
Table 3.
Percentage of patients with downstream bone-specific imaging performed within 90 days after bone scan and before first treatment
| Risk | First treatment |
Bone x-ray
|
Bone CT
|
Bone MRI
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Central | South | West | Northeast | Total | P | Central | South | West | Northeast | Total | P | Central | South | West | Northeast | Total | P | ||
| Low | EBRT | 18.4% | 19.5% | 21.3% | 24.2% | 21.8% | .066 | 5.6% | 6.2% | 6.2% | 7.6% | 6.7% | .415 | 1.9% | 3.0% | 3.5% | 3.7% | 3.3% | .753 |
| NON-EBRT | 24.8% | 16.7% | 17.5% | 27.7% | 21.2% | 8.3% | 6.1% | 9.8% | 7.4% | 8.0% | 0.9% | 1.9% | 3.2% | 4.1% | 2.9% | ||||
| CM | 16.7% | 13.8% | 29.0% | 31.9% | 26.1% | 7.6% | 9.2% | 8.6% | 4.3% | 6.9% | 1.5% | 2.3% | 2.5% | 5.7% | 3.6% | ||||
| Intermediate | EBRT | 20.3% | 16.5% | 19.1% | 21.6% | 19.6% | .360 | 7.0% | 6.5% | 6.2% | 6.5% | 6.5% | .233 | 3.0% | 3.1% | 2.6% | 2.4% | 2.7% | .431 |
| NON-EBRT | 24.9% | 14.9% | 17.6% | 23.8% | 19.6% | 2.9% | 5.5% | 10.3% | 6.0% | 7.3% | 2.9% | 2.3% | 3.6% | 3.8% | 3.3% | ||||
| CM | 17.6% | 18.1% | 24.0% | 23.3% | 21.3% | 5.8% | 12.5% | 6.5% | 7.9% | 7.6% | 2.6% | 2.4% | 3.7% | 3.1% | 3.1% | ||||
| High | EBRT | 19.3% | 17.3% | 19.3% | 23.5% | 20.1% | .052 | 8.0% | 6.6% | 6.9% | 7.4% | 7.2% | <.001 | 3.3% | 3.6% | 3.8% | 3.4% | 3.6% | .026 |
| NON-EBRT | 24.8% | 13.4% | 19.5% | 23.2% | 20.4% | 4.8% | 7.5% | 7.4% | 10.3% | 7.5% | 1.6% | 3.0% | 2.8% | 2.2% | 2.5% | ||||
| CM | 22.3% | 15.7% | 22.2% | 27.4% | 22.0% | 9.7% | 9.5% | 11.4% | 15.6% | 11.5% | 3.3% | 3.3% | 4.5% | 5.0% | 4.1% | ||||
Abbreviations: CM = conservative management (includes hormone therapy only or no treatment); CT = computed tomography; EBRT = external beam radiation therapy; MRI = magnetic resonance imaging; NON-EBRT = non-external beam radiation therapy (includes prostatectomy and brachytherapy).
In multivariate analysis, Northeast region and higher comorbidity score were associated with increased bone scan use in all risk groups (Table 4). For low- and intermediate-risk patients, year of diagnosis was not associated with bone scan; for high-risk patients, later years were associated with increased bone scan use. Increasing age was associated with increased bone scan use for intermediate-risk patients. Further analysis demonstrated that older patients were more likely to have Gleason 4+3 disease: ages 66 to 69 (21.1%), ages 70 to 74 (24.7%), ages 75 to 79 (26.0%), and ages 80 to 84 (27.5%, P<.001). This difference in Gleason 4+3 disease by age may explain the increased use of bone scans in older patients with intermediate-risk prostate cancer.
Table 4.
Multivariate logistic regression models for receipt of bone scan
| Low risk
|
Intermediate risk
|
High risk
|
||||
|---|---|---|---|---|---|---|
| Characteristic | Odds ratio | P value | Odds ratio | P value | Odds ratio | P value |
| Age, y, at diagnosis (Ref: 66–69) | ||||||
| 70–74 | 1.096 | .065 | 1.138 | .001 | 1.037 | .386 |
| 75–79 | 1.058 | .328 | 1.125 | .007 | 1.051 | .254 |
| 80–84 | 1.104 | .254 | 1.203 | .001 | 0.883 | .007 |
| 85+ | 0.714 | .064 | 1.002 | .977 | 0.547 | <.001 |
| Race (Ref: white) | ||||||
| Nonwhite | 1.135 | .033 | 1.231 | <.001 | 1.004 | .923 |
| Marital status (Ref: Married) | ||||||
| Not married/unknown | 0.889 | .015 | 0.980 | .569 | 0.927 | .013 |
| NCI Comorbidity Index (Ref: 0) | ||||||
| >0 | 1.128 | .007 | 1.136 | <.001 | 1.065 | .035 |
| Year of diagnosis (Ref: 2004) | ||||||
| 2005 | 1.044 | .471 | 1.037 | .419 | 1.123 | .003 |
| 2006 | 1.032 | .584 | 1.065 | .154 | 1.197 | <.001 |
| 2007 | 0.969 | .592 | 1.071 | .116 | 1.247 | <.001 |
| % Non-high school graduate in census tract (Ref: Quartile 1 (lowest)) | ||||||
| Quartile 2 | 0.987 | .845 | 1.083 | .113 | 0.964 | .441 |
| Quartile 3 | 1.118 | .145 | 1.105 | .082 | 0.994 | .911 |
| Quartile 4 (highest) | 1.156 | .106 | 1.182 | .011 | 0.940 | .310 |
| Median household income in census tract (Ref: Quartile 1 (lowest)) | ||||||
| Quartile 2 | 1.077 | .278 | 1.155 | .004 | 1.027 | .538 |
| Quartile 3 | 1.051 | .518 | 1.246 | <.001 | 1.112 | .038 |
| Quartile 4 (highest) | 1.209 | .037 | 1.304 | <.001 | 1.123 | .061 |
| Geographic region (Ref: Northeast) | ||||||
| Central | 0.414 | <.001 | 0.501 | <.001 | 0.736 | <.001 |
| South | 0.514 | <.001 | 0.520 | <.001 | 0.647 | <.001 |
| West | 0.360 | <.001 | 0.462 | <.001 | 0.707 | <.001 |
| Population density (Ref: urban) | ||||||
| Not urban | 1.075 | .287 | 1.112 | .028 | 0.944 | .535 |
Abbreviations: NCI = National Cancer Institute; Ref = reference.
The annual estimated cost to Medicare of bone scans for all patients is $19,300,000, including $9,300,000 for low- and intermediate-risk patients. An additional $2,000,000 is spent annually on downstream imaging after bone scan for low- and intermediate-risk patients (Fig. 2).
Fig. 2.
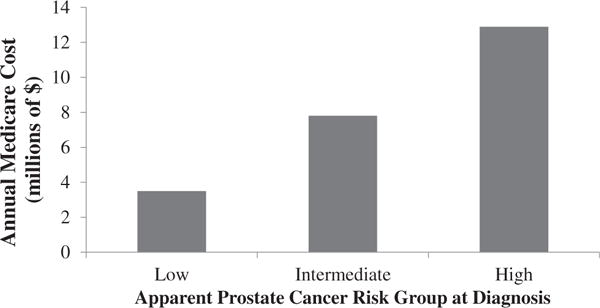
Annual cost to Medicare of bone scans and downstream imaging tests.
Discussion
In this analysis of SEER-Medicare data, we found that almost one-third of low-risk and almost one-half of intermediate-risk prostate cancer patients received a staging bone scan. Among these patients who had a bone scan, 21%, 7%, and 3% received further scans including bone-specific x-rays, CT, and MRI scans, respectively. We found that these patients had almost no chance of having metastatic disease—indicating that metastatic work-up for patients with apparent low- and intermediate-risk cancer, which leads to $11.3 million in annual Medicare costs, is unlikely to yield useful clinical information. On the other hand, bone scan use was only 62% in patients with apparent high-risk prostate cancer, for whom staging studies are recommended by published guidelines (3). Among high-risk patients who received a bone scan, 14% were diagnosed with metastatic disease.
Early-stage prostate cancer has recently come under increased scrutiny, with an awareness of the overtreatment in patients with indolent disease and a pervasive use of new and expensive treatments with unproven clinical benefit (2). A recent study estimated that the use of new technologies in prostate cancer was associated with a $350 million increase in health care costs in 2005 alone (2). Although recent studies have focused on treatments, the total annual cost of imaging performed as part of staging workup is unknown. It is important for physicians to be judicious when ordering tests and procedures to spare patients from unnecessary procedures and to help curb rising health care costs. One way to accomplish this is to consider the test characteristics of imaging studies when deciding whether or not to perform a scan. Because patients with apparent low- and intermediate-risk prostate cancer almost never have metastatic disease at the time of diagnosis, the positive predictive value of a bone scan is low, and the risk of false-positive results is high. However, as we show in this study, bone scans are commonly performed in these patients, and these are frequently followed by additional bone imaging studies.
Three recent studies also using the SEER-Medicare dataset have examined the rate of bone scan use in prostate cancer (7–9). The most important difference between the prior studies and ours is that we examined rates of imaging by “apparent” risk group, which includes the following information available at the time of diagnosis: PSA, Gleason score (from diagnostic prostate biopsy), and clinical stage (rectal examination). Patients and physicians base their decisions on whether to obtain a bone scan from this information. In contrast, prior studies excluded men with metastatic disease, that is, a positive result of the bone scan, which may have led to biased results (7, 8). We have used a more recent cohort of patients than prior studies, and our examination of downstream imaging and procedures after bone scan is also unique. We believe that the $11.3 million annual Medicare cost from bone scans and downstream tests for patients with low- and intermediate-risk prostate cancer represents a “waste” because the risk of these patients having metastatic disease is close to zero. Future studies should examine the potential overuse of staging scans in patients less than 65 years of age, and associated costs to private insurers, to fully assess the overall impact of this practice on health care expenditures.
On the other hand, our study also demonstrates an underuse of bone scans in high-risk prostate cancer patients, for whom this scan is recommended by published guidelines (3). As this study shows, among patients who had an apparent high-risk cancer and received a bone scan, 14% were found to have metastatic disease. Patients who did not have a bone scan may harbor metastatic disease but may pursue aggressive surgical or radiation treatment for an incurable cancer. This can lead not only to patient harm from unnecessary treatment but also to significant health care costs. We are currently undertaking a follow-up study of patient treatments and outcomes in this group of patients.
There are several potential limitations to this study. The SEER-Medicare database does not include individuals younger than 65 years. However, as the median age of diagnosis for prostate cancer is 67 years, this disease and its associated costs are highly relevant to Medicare. Although we attempted to examine x-rays, CT scans, and MRI scans specific to the assessment of bones, it is possible that some scans included may have been performed for another purpose. We limited the time window for capturing these downstream scans (within 90 days of bone scans) in an attempt to minimize this concern. Our examination of downstream imaging by treatment did not yield higher rates for patients who received external beam radiation (which commonly involves a pelvic CT and/or MRI for treatment planning) versus other treatments, suggesting that our bone-specific imaging codes were unlikely to have been confounded by other types of CT and MRI scans.
Conclusions
In summary, one-third to one-half of patients with apparent low- and intermediate-risk prostate cancer received staging bone scans, which have almost no chance of finding metastatic disease. However, only 62% of patients with apparent high-risk disease received a bone scan. These results demonstrate a pervasive lack of adherence to guidelines, and a common overuse and underuse of this test in prostate cancer.
Supplementary Material
Summary.
This SEER-Medicare study examines the use of bone scans and downstream tests during initial prostate cancer workup. One-third to one-half of apparent low- and intermediate-risk prostate cancer patients received bone scans—a proportion of whom also received further downstream tests—despite almost 0% risk of metastatic disease, and resulting in an $11 million annual cost to Medicare. We also report underuse of bone scans in apparent high-risk patients, for whom metastatic disease is likely.
Footnotes
Presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL. May 30-June 3, 2013.
Note—An online CME test for this article can be taken at http://astro.org/MOC.
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.American Cancer Society. Cancer facts & figures, 2013. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. Accessed November 19, 2013.
- 2.Nguyen PL, Gu XM, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Guidelines. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed November 19, 2013.
- 4.National Cancer Institute. Overview of the SEER program. Available at: http://seer.cancer.gov/about/overview.html. Accessed November 19, 2013.
- 5.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Am Med Assoc. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 7.Choi WW, Williams SB, Gu XM, et al. Overuse of imaging for staging low risk prostate cancer. J Urol. 2011;185:1645–1649. doi: 10.1016/j.juro.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 8.Prasad SM, Gu XM, Lipsitz SR, et al. Inappropriate utilization of radiographic imaging in men with newly diagnosed prostate cancer in the United States. Cancer. 2012;118:1260–1267. doi: 10.1002/cncr.26416. [DOI] [PubMed] [Google Scholar]
- 9.Makarov DV, Desai RA, Yu JB, et al. The population level prevalence and correlates of appropriate and inappropriate imaging to stage incident prostate cancer in the Medicare population. J Urol. 2012;187:97–102. doi: 10.1016/j.juro.2011.09.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


