Abstract
Background
Hereditary spherocytosis is the most common form of inherited hemolytic anemia and is characterized by a structural defect in the RBC membrane. The disorder is commonly inherited in an autosomal dominant fashion and leads to a mild to moderate anemia. The autosomal recessive form of hereditary spherocytosis is rarely reported in association with fetal anemia and hydrops fetalis.
Case
A 25 year old G5 P2112 at 25 2/7 weeks gestation presents with severe fetal anemia and nonimmune hydrops fetalis requiring multiple fetal intrauterine transfusions. After delivery, the neonate required several double volume exchange transfusions and ultimately was diagnosed with autosomal recessive hereditary spherocytosis weeks after birth. The neonate was identified to have a rare homozygous genetic mutation, SPTA1c.6154delG, which leads to absent production of normal α-spectrin.
Conclusion
The case highlights the importance of considering less common genetic mutations involving the RBC structural proteins when patients present with severe fetal anemia and nonimmune hydrops fetalis.
Keywords: Hereditary spherocytosis, Fetal anemia, Nonimmune hydrops fetalis, Autosomal recessive, α-Spectrin, SPTA1
Highlights
-
•
A case of severe fetal hydrops due to hereditary spherocytosis is presented.
-
•
A rare homozygous genetic mutation of the SPTA1 gene was identified.
-
•
Genetic mutations of the RBC structural proteins can lead to severe fetal hydrops.
1. Introduction
Hereditary spherocytosis is the most common form of inherited hemolytic anemia, affecting approximately 1 in 5000 patients [1], [2]. Most cases of hereditary spherocytosis are inherited as an autosomal dominant disorder, but approximately 25% of individuals will have either a de novo mutation or autosomal recessive inheritance [2]. The disorder causes abnormalities in the red blood cell (RBC) membrane due to a genetic defect in one of the five following proteins, ankyrin-1, band 3, β spectrin, α spectrin, or protein 4.2 [2]. The clinical presentation of patients with hereditary spherocytosis is extremely variable and ranges from mild to severe depending on the genetic mutation and deficient protein. There have been several reported cases in the literature where severe hemolytic anemia from hereditary spherocytosis has led to nonimmune hydrops fetalis [3], [4], [5].
Nonimmune hydrops fetalis (NIH) is a condition that can be diagnosed by prenatal ultrasound with findings that include fetal ascites, pleural effusions, pericardial effusions, and skin edema. There are a multitude of pathologic causes for NIH including chromosomal abnormalities, maternal infections, cardiovascular malformations of the fetus, genetic metabolic diseases and fetal anemia, amongst others [6]. Fetal anemia resulting from RBC enzyme deficiencies, such as hereditary spherocytosis, must be considered in the evaluation for nonimmune fetal hydrops. The following case illustrates a fetus with NIH resulting from severe fetal anemia due to autosomal recessive hereditary spherocytosis.
2. Case
A 25 year old G5 P2112 presents at 25 2/7 weeks gestation for polyhydramnios, fetal abdominal ascites and pericardial effusion on outside ultrasound. Initial MFM ultrasound reveals severe hydrops fetalis with abdominal ascites, pericardial effusion, skin edema, and significant cardiomegaly with thickened myocardium (Fig. 1, Fig. 2). Middle cerebral artery (MCA) Doppler was found to be elevated with a peak systolic velocity of 57.1 cm/s, consistent with moderate to severe fetal anemia at the current gestational age.
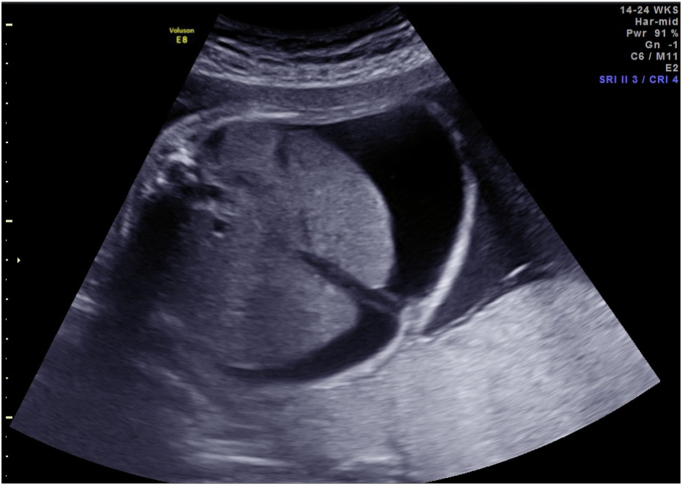
Fig. 1.
Abdominal ascites prior to first PUBS procedure.
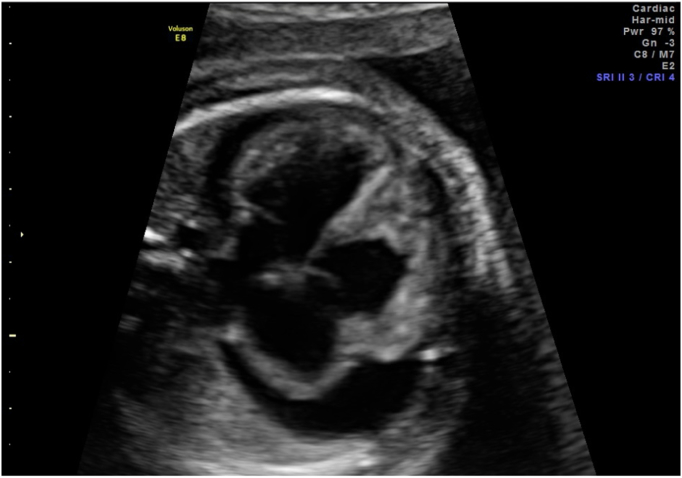
Fig. 2.
Pericardial effusion prior to first PUBS procedure.
Maternal obstetrical history was significant for a previous neonatal demise. The couple's second child was born at 28 weeks gestation and lived for 90 min. Prenatally, the child was noted to have hydrops fetalis but there was no evaluation or interventions other than a possible diagnosis of trachea-esophageal fistula after birth. The couple has two other living children with no known medical problems. The couple is Mennonite and consanguineous third cousins without significant family history of anemia, thalassemia, or family members requiring blood transfusion. However, several cousins on the maternal side of the family required splenectomies.
Maternal prenatal labs were unremarkable with a positive Rh status and negative antibody screen. The patient denied any sick contacts. Maternal Parvovirus B19 IgG and IgM were found to be negative at presentation. Further infectious evaluation was deferred due to the patient's uninsured status.
The decision was made to proceed with amniocentesis for fetal karyotype and percutaneous umbilical cord blood sampling (PUBS) with possible fetal intrauterine transfusion (IUT) if indicated at 25 3/7 weeks gestation. MCA Doppler prior to procedure was 60.52 cm/s. Amniocentesis resulted in a normal XX female. A successful PUBS was performed and fetal blood was sent for evaluation with an initial hemoglobin and hematocrit of 2.5 g/dL and 8.2%. Fetal IUT was then performed and a post-transfusion hemoglobin and hematocrit resulted 11 g/dL and 31.9%. Furthermore, the post-transfusion MCA Doppler was 26.2 cm/s, which was within normal limits.
Within two weeks of the initial IUT, fetal skin edema resolved and there was significant improvement in fetal ascities and pericardial effusion; however, cardiomegaly persisted throughout the remainder of the pregnancy (Fig. 3, Fig. 4). The fetus required four additional PUBS procedures, occurring at two to three week intervals, based on worsening MCA Doppler studies. Of note, the patient received antenatal steroid administration prior to the first PUBS procedure at 25 2/7 weeks gestation and rescue steroid administration at 34 1/7 weeks gestation. Labor induction was started at 34 3/7 weeks gestation, 48 h after the last IUT, as the patient was 4 cm dilated after the fifth procedure.
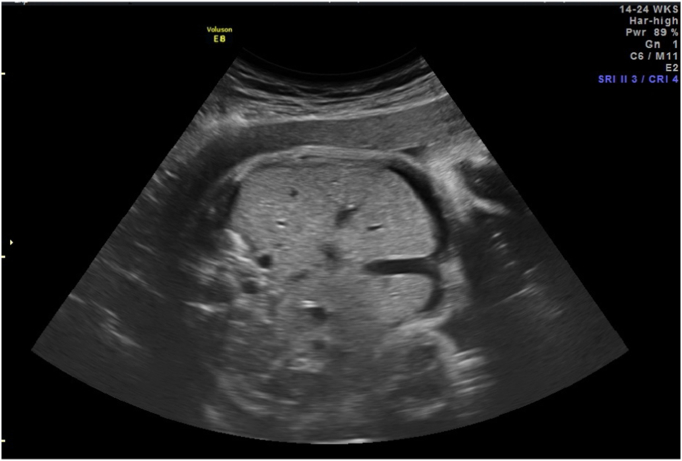
Fig. 3.
Improvement in abdominal ascites status post initial PUBS procedure.
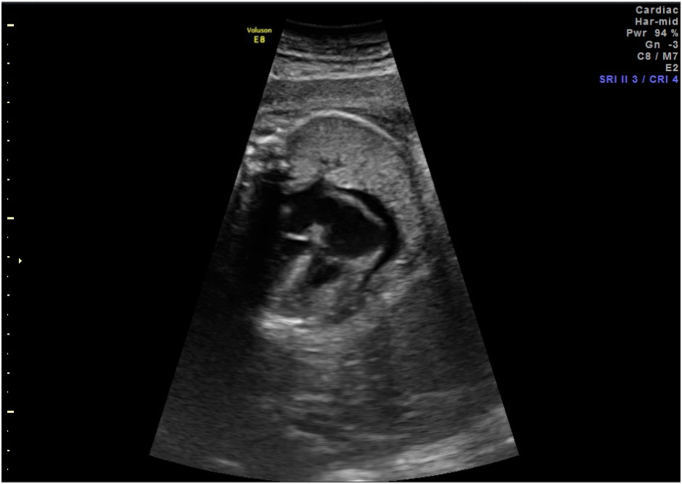
Fig. 4.
Improvement in pericardial effusion status post initial PUBS procedure.
The patient underwent a spontaneous vaginal delivery of a viable female infant weighing 2220 g with Apgars of 7 and 8 at one and 5 min, respectively. Initial laboratory evaluation revealed a hemoglobin and hematocrit of 17.2 g/dL and 51.5%. Neonatal echocardiogram showed significant biventricular hypertrophy but good left ventricular function. The baby was found to have hepatosplenomegaly on physical exam with the liver edge 3 cm below the right costal margin. Liver function panel revealed a total bilirubin of 13.5 mg/dL and direct bilirubin of 1.6 mg/dL, which led to initiation of intensive phototherapy, intravenous immunoglobulin and a double volume exchange transfusion. The total bilirubin peaked at 21.4 mg/dL and the baby required three double volume exchange transfusions during her neonatal course.
A complete genetic, metabolic and infectious workup has been negative for the infant since birth. Parental RBC cross-matching did not reveal any alloimmune causes of RBC destruction. The Cincinnati Hemolytic Anemia next generation sequencing panel was performed and found the baby to be homozygous for the SPTA1c.6154delG genetic mutation, which leads to absence of production of normal alpha-spectrin, consistent with autosomal recessive hereditary spherocytosis.
The neonate went on to require a transfusion of packed red blood cells (PRBC) at 23 days old when she was found to have a hemoglobin level of 7 g/dL. Within two weeks of her initial postnatal PRBC transfusion, the infant's hemoglobin dropped below 8 g/dL and she received a second transfusion. The infant has been reticulocytopenic since birth, which is due to suppression of erythrocyte production from multiple fetal and neonatal transfusions and likely hemolysis of her own new RBCs. It is anticipated that the infant will require PRBC transfusions every three to four weeks, in order to maintain her hemoglobin level above 8 g/dL. She currently remains under the care of a pediatric hematologist and further spectrin studies are planned when the infant begins producing reticulocytes.
3. Discussion
This case report represents a rare genetic mutation of the SPTA1 gene that has not previously been documented in the literature. There is a previous case report where autosomal recessive hereditary spherocytosis due to α-spectrin deficiency resulted in Coombs-negative hemolytic jaundice in a neonate [7]. Ultimately, α-spectrin deficiency leads to less than 5% of hereditary spherocytosis cases; however, it does result in severe hemolytic anemia that can be life threatening in the fetus and/or neonate [2], [7].
It is important to consider autosomal recessive hereditary spherocytosis in the differential diagnosis for a fetus that presents with severe NIH due to fetal anemia. Typically, hereditary spherocytosis is an autosomal dominant disorder resulting in mild to moderate anemia and would only be considered as a differential diagnosis if there were a significant family history [2]. Regarding our case, anemia had not been previously diagnosed in either the mother or father. Parental complete blood cell counts revealed normal hemoglobin, hematocrit, RBC indices and platelet count. Furthermore, both parental peripheral blood smears showed normal RBC, platelet and WBC morphology and no spherocytes were noted. Thus, autosomal dominant hereditary spherocytosis was ruled out as a potential etiology. Due to the consanguinity of the parents, autosomal recessive disorders that may lead to severe fetal anemia should always be evaluated no matter how rare.
In the heterozygous state, individuals with the SPTA1c.6154delG genetic mutation will produce an adequate amount of α-spectrin and are asymptomatic with normal RBC indices and no spherocytosis on peripheral blood smear [7], [8]. After identification of our infant's genetic mutation, both the mother and father are obligate carriers of the SPTA1 mutation. Future offspring have a 25% risk of inheriting hereditary spherocytosis and they may also present with severe fetal anemia and NIH requiring fetal intrauterine transfusion. It is suspected that the couple's previous neonatal demise also suffered from hereditary spherocytosis.
In conclusion, our case highlights a genetic mutation of the SPTA1 gene that has not been previously reported in literature. This specific mutation in the homozygous state leads to severe fetal anemia and NIH that requires fetal intrauterine transfusion for fetal survival. Rare autosomal recessive disorders of the RBC membrane should be included in the differential diagnosis for NIH, especially in patients with consanguinity.
Disclosure Statements
There are no financial disclosures or conflicts of interest.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Acknowledgements
None.
References
- 1.Kilpatrick S.J. Anemia and Pregnancy. In: Creasy R.K., Resnik R., Iams J.D., Lockwood C.J., Moore T.R., Greene M.F., editors. 7th ed. Elsevier; 2014. pp. 918–931. (Maternal Fetal Medicine). [Google Scholar]
- 2.Segel G.B., Casey D. Hereditary Spherocytosis . In: 20th ed. Kliegman R.M., Stanton B.F., St Geme J.W., Schor N.F., editors. Elsevier; 2016. (Nelson Textbook of Pediatrics). P. 2330-2330.e1. [Google Scholar]
- 3.Yetgin S., Aytac S., Gurukan F., Yurdakok M. Nonimmune hydrops fetalis in two cases of consanguineous parents and associated with hereditary spherocytosis and hemophagocytic hystiocytosis. J. Perinatol. 2007;27:252–254. doi: 10.1038/sj.jp.7211657. [DOI] [PubMed] [Google Scholar]
- 4.Whitfield C.F., Follweiler J.B., Lopresti-Morrow L., Miller B.A. Deficiency of α-spectrin synthesis in burst forming units-erythroid in lethal hereditary spherocytosis. Blood. 1991;78:3043–3051. [PubMed] [Google Scholar]
- 5.Gallagher P.G., Weed S.A., Tse W.T., Benoit L., Morrow J.S., Marchesi S.L. Recurrent fatal hydrops fetalis associated with a nucleotide substitution in the erythrocyte beta-spectrin gene. J. Clin. Invest. 1995;95:1174–1182. doi: 10.1172/JCI117766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkins I. Nonimmune hydrops. In: Creasy R.K., Resnik R., Iams J.D., Lockwood C.J., Moore T.R., Greene M.F., editors. 7th ed. Elsevier; 2014. pp. 569–577. (Maternal Fetal Medicine). [Google Scholar]
- 7.Yaish H.M., Christensen R.D., Agarwal A. A neonate with Coombs-negative hemolytic jaundice with spherocytes but normal erythrocyte indices: a rare case of autosomal-recessive hereditary spherocytosis due to alpha-spectrin deficiency. J. Perinatol. 2013;33:404–406. doi: 10.1038/jp.2012.67. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher P.G., Glader B. Hereditary spherocytosis, hereditary elliptocytosis, and other disorders associated with abnormalities of the erythrocyte membrane. In: Greer J.P., Foerster J., Rodgers G.M., Paraskevas F., Glader B., Arber D.A., Means R.T. Jr., editors. 12th ed. Wolters Kluwer/Lippincott/Williams & Wilkins; Philadelphia: 2009. pp. 913–920. (Wintrobe's Clinical Hematology). [Google Scholar]