Abstract
Objectives
Children requiring cortisol replacement therapy are often prescribed hydrocortisone doses of 2.5 mg, but as this is commercially unavailable 10 mg tablets, with functional break lines, are split commonly in an attempt to deliver the correct dose. This study aimed to determine the dose variation obtained from quartered hydrocortisone tablets when different operators performed the splitting procedure and to ascertain whether better uniformity could be attained from mini-tablets as an alternative formulation.
Methods
Hydrocortisone 10 mg tablets were quartered by four different operators using a standard pill splitter. Hydrocortisone 2.5 mg mini-tablets (3 mm diameter) were formulated using a wet granulation method and manufactured using a high-speed rotary press simulator. The weight and content uniformity of the quartered tablets and mini-tablets were assessed according to pharmacopoeial standards. The physical strength and dissolution profiles of the mini-tablets were also determined.
Results
More than half of all quartered 10 mg tablets were outside of the ±10% of the stated US Pharmacopoeia hydrocortisone content (mean 2.34 mg, SD 0.36, coefficient of variation (CV) 15.18%) and more than 40% of the quartered tablets were outside the European Pharmacopoeia weight variation. Robust mini-tablets (tensile strengths of >4 MPa) were produced successfully. The mini-tablets passed the pharmacopoeial weight and content uniformity requirements (mean 2.54 mg, SD 0.04, CV 1.72%) and drug release criteria during in vitro dissolution testing.
Conclusion
This study confirmed that quartering 10 mg hydrocortisone tablets produces unacceptable dose variations and that it is feasible to produce 3 mm mini-tablets containing more accurate doses for paediatric patients.
Keywords: endocrinology, congenital abnorm, general paediatrics, basic science
What is already known on this topic?
Children often require a hydrocortisone dose of 2.5 mg but there is no suitable, licensed, formulation available.
Hydrocortisone 10 mg tablets, with functional break lines, are split commonly in an attempt to deliver the correct dose.
Mini-tablets are an alternative and acceptable oral dosage form for children as young as 6 months old.
What this study hopes to add?
Quartering 10 mg hydrocortisone tablets produces unacceptable dose variations.
Additional variability in dosing could be introduced by different operators.
It is feasible to produce 3 mm hydrocortisone mini-tablets that meet pharmacopoeial requirements of weight and dose uniformity.
Introduction
Hydrocortisone is the preferred cortisol replacement therapy in childhood, because it is of lower potency than the synthetic glucocorticoids and may be associated with fewer side effects.1 Hydrocortisone doses of 8 mg/m2/day,2 given in three to four divided doses, are thought to be adequate for cortisol replacement therapy in childhood. Higher, supraphysiological doses of 10–15 mg/m2/day are used to treat patients with congenital adrenal hyperplasia (CAH),3 in whom the goal of treatment is to achieve suppression of adrenocorticotropic hormone (ACTH) drive to the adrenal gland, while avoiding the adverse effects of glucocorticoid excess.
Liquid hydrocortisone formulations, such as a 1 mg/mL oral suspension, are only available as unlicensed specials and in addition to the general limitations of transport, storage and stability there are potential concerns relating to the bioavailability of liquid hydrocortisone formulations.4 In paediatric practice, 2.5 mg hydrocortisone doses are prescribed frequently and to achieve these doses, it is recommended that tablets are divided or crushed.3 Crushing and dissolving tablets may result in unacceptably high variability of dosing2 3 and it may be preferable to quarter 10 mg tablets. The hydrocortisone 10 mg tablets licensed for oral administration are quarter scored, allowing them to be divided into equal halves or quarters.5 However, despite the presence of functional break lines, splitting may result in unequal parts thereby producing unequal doses and loss of mass due to crumbling.6
To date, most data relating to the medium-term/long-term outcomes of children with CAH report features more likely to represent over, rather than under, dosing: obesity, insulin resistance, elevated leptin levels, dyslipidaemia and impaired glucose metabolism.7–10 However, working memory performance is lower in children with CAH than in unaffected relatives,11 and health-related quality of life is also reported to be reduced, with boys and girls equally affected, suggesting that this is not simply related to androgen excess in girls and associated disorders of sex development.12 Erratic and inadequate doses of hydrocortisone may contribute to these adverse effects.
No current licensed oral hydrocortisone formulation adequately meets the dosing requirements of children. Mini-tablets provide an alternate to standard tablets and oral liquids mainly for paediatric patients, ≥4 years of age. Variations exist in the defined size of mini-tablets in literature, but a diameter of ≤3 mm is commonly compatible with paediatric patients.13 Mini-tablets can be administered to paediatric patients as young as 6 months old with their food/beverages and recent studies have demonstrated mini-tablets to be more acceptable than oral syrups.14–16
Aims and objectives
The aim of this study was to manufacture 3 mm mini-tablets to provide a 2.5 mg dose of hydrocortisone and to compare the content uniformity of the mini-tablets against quartered hydrocortisone tablets. The content uniformity of hydrocortisone mini-tablets and the quarters of commercial hydrocortisone tablets was also determined. The tensile strength of the mini-tablets and their compliance with the pharmacopoeial tests for dissolution and uniformity of mass were also assessed.
Materials and methods
Materials
Hydrocortisone (10 mg) tablets (Auden Mckenzie Pharma Division, UK) were used for the dose uniformity study. Analytical grades of hydrocortisone (Sigma Aldrich, UK); water (Liverpool John Moores University, Liverpool, UK); Methanol (Sigma Aldrich) and Acetonitrile (Fischer Scientific UK, Loughborough, UK) were used for high-performance liquid chromatography (HPLC) analysis.
Mini-tablets were manufactured using hydrocortisone European Pharmacopoeia (Ph. Eur.; Courtin and Warner, UK), Microcrystalline cellulose (Avicel PH101; FMC, Brussels, Belgium), lactose (Pharmatose 200M; DFE Pharma, Goch, Germany), hydroxypropyl methyl cellulose 603 (Shin-Etsu Chemicals, Tokyo, Japan), croscarmellose sodium (Ac-Di-Sol; FMC Europe NV, Brussels, Belgium), silicon dioxide (Aerosil 200; Degussa-Hüls AG, Frankfurt, Germany) and magnesium stearate (BDH Laboratory Supplies, Poole, UK).
Formulation and manufacture of mini-tablets
The mini-tablet formulation was prepared using a wet granulation technique to ensure uniformity of die fill, since adequate flowability of a formulation is essential for the manufacture of mini-tablets due to the small size of the die orifice.17 All of the excipients listed in table 1 (with the exception of the glidant (Aerosil) and the lubricant (magnesium stearate) were blended with hydrocortisone for 5 min using a Turbula Shaker Mixer type 2C (Willy A. Bachofen, Basel, Switzerland) at 42 rpm. The blended powder mixture was then transferred to a Model KM330 series planetary mixer (Kenwood, UK). Water (0.48 mL per g of powder) was added uniformly during mixing by spraying with an atomiser from a distance of 10–15 cm from the powder bed over a period of 15 min. The wet powder mass was screened evenly onto a flat, stainless steel tray and oven-dried overnight at 40°C. Dry granules were screened using a 1 mm aperture sieve prior to separation into size fractions using a laboratory shaker (Endecotts, UK). Granules in the size range 125–355 µm were subsequently blended with glidant (5 min) and lubricant (2 min) using the Turbula Shaker Mixer. The bulk density (ρB) of the granules in the size range of 125–355 µm was determined by filling a measuring cylinder with a known weight of granules. The tapped density (ρT) was determined by dropping the volumetric cylinder 250 times from a height of 2.5 cm using a dropbox. The tapping procedure was repeated until there was no change in the volume of the granule giving the tapped density. The % Carr’s Compressibility Index (equation 1) and Hausner ratio (equation 2), both indicators of flowability18 in fluencing key tablet parameters such as mechanical strength and weight uniformity, were calculated.
Table 1.
Composition of the mini-tablets
| Component | % per batch |
| Hydrocortisone | 16.67 |
| Microcrystalline cellulose | 22.40 |
| Lactose monohydrate | 51.60 |
| Hydroxypropyl methyl cellulose | 3.00 |
| Croscarmellose sodium | 5.00 |
| Colloidal silicon dioxide | 0.33 |
| Magnesium stearate | 1.00 |
| (1) |
| (2) |
The mini-tablets were produced using a Stylcam 100R rotary press simulator (Medel’Pharm, Beynost, France) fitted with 3 mm flat-faced, single-tip tooling at a speed of 20 rpm. A compression force of 1–2 kN (compression pressure of 140–280 MPa) was maintained with a fill height of 7.10 mm.
Tensile strength
The dimensions of 10 mini-tablets were measured using a digital micrometer (Mitutoyo, Tokyo, Japan) and their crushing strengths were determined with a 6D Tablet Tester (Schleuniger, Germany). Values were used to calculate the tensile strength, σ, using equation 3,19 where P is the crushing strength (N), D is the diameter (mm) and T is the tablet thickness (mm).
| (3) |
Content uniformity analysis of hydrocortisone tablets and mini-tablets
An Agilent 1200 series with Variable Wavelength Detector (Agilent Technologies, UK) set to 254 nm was used with a 4.6 mmx15 cm column containing 5 µm packing of octadecyl silane chemically bonded to porous silica (Phenomenex, HyperClone 5µ ODS C18). The mobile phase (degassed 50:25:25 mixture of water, acetonitrile and methanol) flow rate was 1 mL/min and an injection volume of 10 µL was used. Chemstation open lab CDS software for LC and LC-MS Rev C.01.05 (Agilent Technologies) were used for all data analysis.
Standard solutions of hydrocortisone (0%–0.2% w/v) were prepared and filtered into HPLC vials using a 0.45 µm PTFE filter (Agilent Technologies) and 5 mL syringe. The peak area–concentrations response was acceptably linear (R2=0.9985), and thus a 0.01% w/v was used as a single-point calibration for the assays. The retention time of hydrocortisone was 4.0 (±0.2) min. The active content of whole hydrocortisone 10 mg tablets was determined by weighing and dispersing individual tablets into 100 mL mobile phase by sonification. Each tablet was analysed in duplicate. The hydrocortisone content of quartered tablets was determined, each individual tablet was weighed and, using a Deluxe Pill Splitter (W+W Medsystems, UK), cut into quarters. Each quarter was weighed and dispersed into 25 mL mobile phase by sonification. Each tablet quarter was analysed in duplicate. Four individuals each quartered five tablets to account for interoperator variability. The operators, comprising two students and two academics, had no previous experience of tablet splitting. The quarters were then weighed and assayed.
The active content of the 2.5 mg hydrocortisone mini-tablets was determined by weighing and dispersed individual mini-tablets into 25 mL mobile phase by sonification. As per US Pharmacopoeia (USP) 905 (uniformity of dosage units), 10 whole mini-tablets were analysed per batch.20 Each mini-tablet was analysed in duplicate. All solutions were filtered into HPLC vials using a 0.45 µm PTFE filter (Agilent Technologies) and 5 mL syringe prior to analysis.
According to USP (905) uniformity of dosage units, the acceptance value (AV) is calculated using equation 4, where X is the sample mean as a % of label claim, k is 2.4 for L1 criteria and s is the SD of the sample.20 M is dependent on the sample mean and if X ≥98.5% and ≤101.5% of the label claim then M=X and, as in the present study, (M–X) becomes zero. The L1 criteria states that 10 samples should be tested and the AV should be ±15%.20
| (4) |
Weight uniformity analysis of hydrocortisone tablets and mini-tablets
The Ph. Eur. monograph ‘Uniformity of mass of single-dose preparations’ method21 was employed to determine the uniformity of weight of the 2.5 mg mini-tablets, and quartered 10 mg hydrocortisone tablets. Twenty tablets and mini-tablets were weighed individually and their mean weights calculated. The products failed if >2 of the individual tablets’ weight deviated by more than 10% (mini-tablets) or 7.5% (tablets) from the average weight and if one tablet weight deviated by >20% (mini-tablets) or 15% (tablets) from the average weight.21 For quartered tablets, the mean weight was calculated and the percentage of samples that failed to meet the same weight variation criteria as mini-tablets was calculated.
Drug release from hydrocortisone mini-tablets
The dissolution method from USP monograph for hydrocortisone tablets was used,22 using a Varian VK 7010 dissolution apparatus (Agilent Technologies) attached to UV spectrophotometer (Cary 50 UV spectrophotometer) at 248 nm. USP apparatus 2 (paddle apparatus), with a paddle speed of 50 rpm and 900 mL of water as the dissolution media (at 37°C) were used for the test.23
Statistical analysis
The weight and content uniformity of tablet quarters (data were normally distributed) were compared by one-way analysis of variance using the Minitab V.17.1.0 statistical software package (Minitab, USA). A P value of less than 0.05 was considered significant. Regression analysis was used to determine the correlation coefficient (R2 value) between weight and content of quartered hydrocortisone tablets.
Results
Splitting of hydrocortisone tablets
The tablets used for the study were convex, diamond shaped and quarter scored allowing them to be divided into equal halves or quarters.5 The assay of the whole hydrocortisone tablets gave >98% recovery, which is within the specified 90%–110% limits of the USP monograph for hydrocortisone tablets.22 The mean weight of whole hydrocortisone tablets was 244.18 mg (SD 1.70 mg, coefficient of variation (CV) 0.7%).
The recovered weights of the quartered tablets indicated that approximately 2% mass was lost during the subdivision process. The expected weight for each tablet quarter was 61.05 mg, based on the mean whole tablet weight. The obtained weight was 59.83 mg (SD 8.45 mg). However, 33 of the 80 (41%) quartered tablets failed to meet the Ph. Eur. monograph specification.21 Based on the mean mass of 59.83 mg, the criteria would allow quartered tablets to have a mass in range 53.85–65.81 mg.
The mean content of hydrocortisone in all of the quartered tablets (n=80) was 2.34 mg (94% of 2.5 mg target dose), with a CV of 15%, range 1.28 (51%) to 3.39 mg (136%). Of the 80, 43 quartered tablets (54%) failed to achieve ±10% (2.25–2.75 mg) of the target 2.5 mg dose.
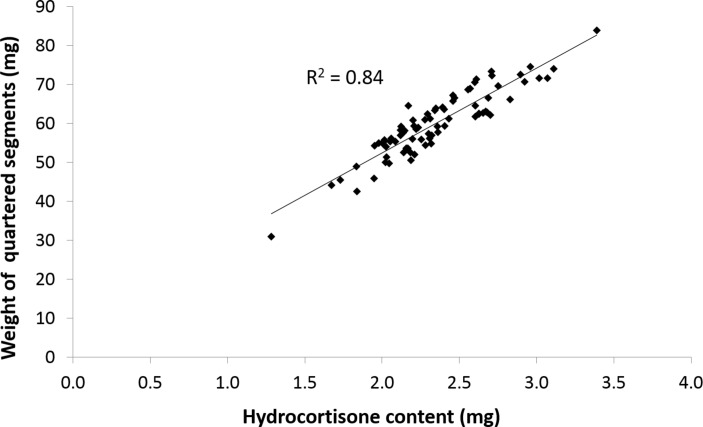
Figure 1 shows the correlation between quartered tablet weight and hydrocortisone content, which explains the large number of substandard hydrocortisone doses in the quarters, as content is directly related to weight.
Figure 1.
Correlation between weight and content of quartered hydrocortisone tablets.
Operator bias
The data obtained by the four individual operators when performing the tablet quartering is summarised in table 2. Although there was no significant difference in the mean weights (P=0.206) or mean hydrocortisone content (P=0.253) of the quartered tablets produced by the different operators, there were marked differences in the ranges obtained. All four operators produced quartered tablets outside of the weight variation limits specified. Splitting by operator A resulted in a mean hydrocortisone content of 2.40 mg (96% of the target dose) but had the largest variation between the quantities; 51%–136% of the target 2.5 mg dose per quarter. Operators B and C obtained a mean quarter content of 2.23 and 2.22 mg (89% of 2.5 mg target dose), respectively, and operator C had a much narrower range for the hydrocortisone content in quartered tablets at 78%–103%. Operator D on the other hand obtained a mean hydrocortisone content of 2.51 mg (100.4% of target) but the range was relatively high at 73%–124% of the target dose. The mean hydrocortisone contents for operators B and C were outside of the ±10% limit stated in the USP.20 In addition to this, each of the operators had individual quarters that had hydrocortisone contents outside of the ±10% limit.
Table 2.
Weight and content uniformity of hydrocortisone tablet quarters (n=20 for each operator)
| Operator | Mean weight, mg (SD) | CV (%) | Range (mg) | Mean hydrocortisone content, mg (SD) | CV (%) | Range (mg) |
| A | 59.67 (11.49) | 19.26 | 31.00–83.90 | 2.40 (0.46) | 19.06 | 1.28–3.39 |
| B | 60.18 (8.63) | 14.34 | 44.20–73.40 | 2.23 (0.31) | 13.92 | 1.67–2.71 |
| C | 60.14 (4.39) | 7.30 | 54.30–69.00 | 2.22 (0.18) | 7.95 | 1.95–2.57 |
| D | 59.33 (8.38) | 14.13 | 42.50–74.00 | 2.51 (0.36) | 14.23 | 1.84–3.11 |
CV%, coefficient of variation.
Mini-tablets
Hydrocortisone mini-tablets were manufactured successfully under simulated rotary press production conditions. The tapped density of granules used for the manufacture of the mini-tablets was determined as 0.48 g/mL and the Carr’s Index value of 16% and Hausner ratio of 1.19 indicated a fair flowability. The Stylcam is a high precision, single station press capable of producing up to 2400 tablets per hour using an automatic feeder operates using a mechanical cam, which produces a biaxial compaction profile analogous to that of a rotary tablet press.
Although mini-tablets were produced at a relatively high compression speed of 20 rpm (equivalent to a rotary press production rate of approximately 80 000 tablets per hour24), the flow of the granules from the hopper into the narrow die orifice during manufacture was satisfactory and all mini-tablets met the pharmacopoeial specification for uniformity of mass.21 Consistent and high tensile strengths were also achieved throughout the batch as shown in table 3, indicating a good compactibility of the granules. Figure 2 illustrates that hydrocortisone was released rapidly and consistently from mini-tablets under in vitro dissolution conditions. The full dose was released within 10 min from all mini-tablets, thus passing the dissolution specification for immediate release dosage forms.23
Table 3.
Weight (n=20), strength and content (n=10) of 3 mm hydrocortisone mini-tablets
| Mean weight, mg (SD) | CV (%) | Mean tensile strength, MPa (SD) | Mean content, mg (SD) | Mean content as a percentage of 2.5 mg target dose (%) | CV (%) |
| 16.40 (0.64) | 3.93 | 4.45 (0.50) | 2.54 (0.04) | 101.68 | 1.72 |
CV%, coefficient of variation.
Figure 2.

Hydrocortisone release from mini-tablets in water at 37°C (mean±SD, n=6).
Content uniformity analysis carried out on 10 mini-tablets gave an AV of 4.37% when using equation 4, thus meeting the USP (905) Uniformity of Dosage L1 criteria. Furthermore, mini-tablets obtained a mean hydrocortisone content of 2.54 mg (101.68% of target dose) which is compliant to the USP.22 These weight and content uniformity data for hydrocortisone mini-tablets demonstrate clear superiority over quartered 10 mg hydrocortisone tablets and, unlike manipulated tablets, mini-tablets did not fail compliance with any of the USP requirements.
Discussion
The availability of age-appropriate medicines for children as solid dosage forms remains a pressing need. The European Medicines Agency (EMA) Paediatric Committee’s Formulation Working Group recommends that for younger patients, those aged 6–8 years, tablets of 6–7 mm with appropriate shape are acceptable.25 Growing evidence suggests that some children may have already acquired the ability to swallow tablets from an earlier age or can be taught using behavioural training interventions, especially those with severe diseases,26 such as children with HIV as young as 3 years who were prescribed stavudine as a solid dosage form.27
There is limited evidence to support the use of dosage from manipulation to obtain an intended dose in paediatric practice.28 A study in UK hospitals reported that in paediatric practice, 42%–62% of manipulations involved tablets and 6% of total manipulations were steroid drugs.29 In the absence of an age-appropriate solid dosage form of hydrocortisone, parents/carers, young people and healthcare professionals are required to manipulate 10 mg tablets to derive an intended dose for use in paediatric practice. The data presented in this report clearly demonstrate that children treated with 2.5 mg doses of hydrocortisone derived from quartered 10 mg tablets are subject to unacceptable variability in hydrocortisone doses.
While minor fluctuations in doses may be of little significance, as hydrocortisone pharmacokinetics are influenced by a number of factors, including fasting status30 puberty31 and the time of day,32 the lowest doses obtained from quartered tablets may be associated with symptoms of cortisol deficiency, and in patients with CAH, loss of ACTH suppression.
As hydrocortisone doses are reduced in a drive to address the long-term morbidity associated with glucocorticoid excess, the margin for error in dosing is lower, and patients are at increased risk for the adverse effects of underdosing due to formulation issues.
Clinical markers of glucocorticoid excess, such as slow growth or excess weight gain, require observation over an extended period, while features of cortisol insufficiency, such as tiredness, nausea or poor concentration may be subjective and difficult to assess in the young child. For this reason, some clinicians advocate the use of 24-hour profiles of cortisol and, in patients with CAH, 17α-hydroxyprogesterone (17-OHP) as a tool to determine the adequacy of treatment, and to titrated doses. However, the application of the data obtained from these studies relies on the assumption that hydrocortisone doses are reliable and reproducible over time. Clearly, this is not the case during treatment with quartered 10 mg tablets and this unpredictability makes interpretation of clinical symptoms or biochemical measures unreliable and dose titration and optimisation extremely difficult.
The Ph. Eur. monograph21 states that a 10% deviation from the mean mass is allowed for tablets weighing ≤80 mg is allowed with no more than 2 out of the 20 individual masses deviating from the mean mass by 10%. Based on the mean mass of 59.83 mg, the criteria would allow quartered tablets to have a mass in range 53.85–65.81 mg. However, 33 of the 80 (41.25%) quartered tablets failed to meet this specification.
This signifies the importance of the technique employed during tablet splitting, as there will be inevitably variation from person to person during the operation. Given the relationship between quartered tablet weight and hydrocortisone content (figure 1), it is unsurprising that the coefficients of variation for these data are very similar (table 2).
In a recent study,33 8 mm tablets were halved and their weight variation was compliant with pharmacopoeial standards, but the tablets were split by an experienced pharmacist while in reality the process may not always be performed by a qualified healthcare professional. For example, parents or carers may be required to split tablets on a regular basis and the data in this study highlight the variations, which could be obtained if an untrained operator performs the subdivision of doses. Other previous studies have also highlighted the potential interoperator variation obtained when splitting scored tablets.34 35
It is not possible to replicate the physiological, diurnal pattern of cortisol secretion using standard formulations of hydrocortisone, and patients experience highly non-physiological cortisol profiles, with periods when cortisol concentrations are excessively high, shortly after a dose of hydrocortisone and prolonged periods of hypocortisolaemia between doses.36 37The half-life of hydrocortisone is short, requiring three to four doses a day, and concordance with treatment can be particularly difficult during adolescence. A future aim should be to produce alternative modified-release dosage forms to provide more consistent and tailored hydrocortisone release profiles, and a reduced frequency of dosing.
Conclusions
This study confirms that quartering of 10 mg hydrocortisone tablets by untrained operators produces an unacceptable variation in the weight of the quartered segments with 41% of the quartered tablets failing to meet the weight variation limits. In addition, 54% of the quartered tablets were outside of the ±10% stated active pharmaceutical ingredient (API) content (2.5 mg for the quartered tablets) proving that under and over dosing is a major risk in formulations manipulated in this way. The feasibility of industrial production of 3 mm mini-tablets with allowing delivery of more accurate doses of hydrocortisone than quartered tablets has been demonstrated.
Supplementary Material
Footnotes
Contributors: All authors planned the study, were involved in study design and critically revised the manuscript. MR, RP, JM and MP collected the data. All authors participated in the analysis and interpretation of the data. MR, MP, JLF and JB drafted the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not- for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Whitaker MJ, Spielmann S, Digweed D, et al. . Development and testing in healthy adults of oral hydrocortisone granules with taste masking for the treatment of neonates and infants with adrenal insufficiency. J Clin Endocrinol Metab 2015;100:1681–8. doi:10.1210/jc.2014-4060 [DOI] [PubMed] [Google Scholar]
- 2.Peters CJ, Hill N, Dattani MT, et al. . Deconvolution analysis of 24-h serum cortisol profiles informs the amount and distribution of hydrocortisone replacement therapy. Clin Endocrinol 2013;78:347–51. doi:10.1111/j.1365-2265.2012.04502.x [DOI] [PubMed] [Google Scholar]
- 3.Joint LWPES/ESPE CAH Working Group. Consensus statement on 21-hydroxylase deficiency from the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. J Clin Endocrinol Metab 2002;87:4048–53. doi:10.1210/jc.2002-020611 [DOI] [PubMed] [Google Scholar]
- 4.Merke DP, Cho D, Calis KA, et al. . Hydrocortisone suspension and hydrocortisone tablets are not bioequivalent in the treatment of children with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2001;86:441–5. doi:10.1210/jcem.86.1.7275 [DOI] [PubMed] [Google Scholar]
- 5. The electronic Medicines Compendium (eMC): hydrocortisone 10mg tablets summary of product characteristics. https://www.medicines.org.uk/emc/medicine/31179 (accessed 1 Jul 2017).
- 6.van Santen E, Barends DM, Frijlink HW. Breaking of scored tablets: a review. Eur J Pharm Biopharm 2002;53:139–45. doi:10.1016/S0939-6411(01)00228-4 [DOI] [PubMed] [Google Scholar]
- 7.Charmandari E, Weise M, Bornstein SR, et al. . Children with classic congenital adrenal hyperplasia have elevated serum leptin concentrations and insulin resistance: potential clinical implications. J Clin Endocrinol Metab 2002;87:2114–20. doi:10.1210/jcem.87.5.8456 [DOI] [PubMed] [Google Scholar]
- 8.Völkl TM, Simm D, Beier C, et al. . Obesity among children and adolescents with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 2006;117:e98–e105. doi:10.1542/peds.2005-1005 [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann A, Grigorescu-Sido P, AlKhzouz C, et al. . Alterations in lipid and carbohydrate metabolism in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr 2010;74:41–9. doi:10.1159/000313368 [DOI] [PubMed] [Google Scholar]
- 10.Williams RM, Deeb A, Ong KK, et al. . Insulin sensitivity and body composition in children with classical and nonclassical congenital adrenal hyperplasia. Clin Endocrinol 2010;72:155–60. doi:10.1111/j.1365-2265.2009.03587.x [DOI] [PubMed] [Google Scholar]
- 11.Browne WV, Hindmarsh PC, Pasterski V, et al. . Working memory performance is reduced in children with congenital adrenal hyperplasia. Horm Behav 2015;67:83–8. doi:10.1016/j.yhbeh.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilban DL, Alves Junior PA, Beserra IC. Health related quality of life of children and adolescents with congenital adrenal hyperplasia in Brazil. Health Qual Life Outcomes 2014;12:107 doi:10.1186/s12955-014-0107-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts M, Vellucci D, Mostafa S, et al. . Development and evaluation of sustained-release Compritol888 ATO matrix mini-tablets. Drug Dev Ind Pharm 2012;38:1068–76. doi:10.3109/03639045.2011.638302 [DOI] [PubMed] [Google Scholar]
- 14.Klingmann V, Spomer N, Lerch C, et al. . Favorable acceptance of mini-tablets compared with syrup: a randomized controlled trial in infants and preschool children. J Pediatr 2013;163:1728–32. doi:10.1016/j.jpeds.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 15.Spomer N, Klingmann V, Stoltenberg I, et al. . Acceptance of uncoated mini-tablets in young children: results from a prospective exploratory cross-over study. Arch Dis Child 2012;97:283–6. doi:10.1136/archdischild-2011-300958 [DOI] [PubMed] [Google Scholar]
- 16.Klingmann V, Seitz A, Meissner T, et al. . Acceptability of uncoated mini-tablets in neonates-a randomized controlled trial. J Pediatr 2015;167:893–6. doi:10.1016/j.jpeds.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 17.Kachrimanis K, Petrides M, Malamataris S. Flow rate of some pharmaceutical diluents through die-orifices relevant to mini-tableting. Int J Pharm 2005;303:72–80. doi:10.1016/j.ijpharm.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 18.Carr RL. Evaluating flow properties of solids. Chem Eng 1965;72:163–8. [Google Scholar]
- 19.Fell JT, Newton JM. The tensile strength of lactose tablets. J Pharm Pharmacol 1968;20:657–9. doi:10.1111/j.2042-7158.1968.tb09832.x [DOI] [PubMed] [Google Scholar]
- 20.2016 U.S. Pharmacopoeia-national formulary [USP 39 NF 34]. 1 Rockville, Md: United States Pharmacopeial Convention, Inc, 2016:736–40. [905] Uniformity of dosage Units. [Google Scholar]
- 21.European Pharmacopoeia 9.0, Vol 1. Ph. Eur. 2.9.5 Uniformity of mass of single-dose preparations. [Google Scholar]
- 22.2016 U.S. Pharmacopoeia-national formulary [USP 39 NF 34]. 1 Rockville, Md: United States Pharmacopeial Convention, Inc, 2016:4222–3. [Google Scholar]
- 23.2016 U.S. Pharmacopoeia-national formulary [USP 39 NF 34]. 1 Rockville, Md: United States Pharmacopeial Convention, Inc, 2016:540–51. [711] Dissolution. [Google Scholar]
- 24.Mohamed FAA, Roberts M, Seton L, et al. . Production of extended release mini-tablets using directly compressible grades of HPMC. Drug Dev Ind Pharm 2013;39:1690–7. doi:10.3109/03639045.2012.730524 [DOI] [PubMed] [Google Scholar]
- 25.European Medicines Agency. List of criteria for screening PIPs with regard to paediatric specific quality issues and referring them to the PDCO FWG for discussion. http://wwwemaeuropaeu/docs/en_GB/document_library/Other/2014/01/WC500159380pdf (accessed 01 Jul 2017).
- 26.Nahirya-Ntege P, Cook A, Vhembo T, et al. . Young HIV-infected children and their adult caregivers prefer tablets to syrup antiretroviral medications in Africa. PLoS One 2012;7:e36186 doi:10.1371/journal.pone.0036186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung VW, Wong IC. When do children convert from liquid antiretroviral to solid formulations? Pharm World Sci 2005;27:399–402. doi:10.1007/s11096-005-7911-z [DOI] [PubMed] [Google Scholar]
- 28.Richey RH, Hughes C, Craig JV, et al. . A systematic review of the use of dosage form manipulation to obtain required doses to inform use of manipulation in paediatric practice. Int J Pharm 2017;518:155–66. doi:10.1016/j.ijpharm.2016.12.032 [DOI] [PubMed] [Google Scholar]
- 29.Richey RH, Shah UU, Peak M, et al. . Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr 2013;13:81 doi:10.1186/1471-2431-13-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen BL, Brøndsted H, Lennernäs H, et al. . Dissolution of hydrocortisone in human and simulated intestinal fluids. Pharm Res 2000;17:183–9. doi:10.1023/A:1007517414200 [DOI] [PubMed] [Google Scholar]
- 31.Charmandari E, Hindmarsh PC, Johnston A, et al. . Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: alterations in cortisol pharmacokinetics at puberty. J Clin Endocrinol Metab 2001;86:2701–8. doi:10.1210/jcem.86.6.7522 [DOI] [PubMed] [Google Scholar]
- 32.Charmandari E, et al. . Bioavailability of oral hydrocortisone in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Endocrinol 2001;169:65–70. doi:10.1677/joe.0.1690065 [DOI] [PubMed] [Google Scholar]
- 33.C Andersson Åsa, Lindemalm S, Eksborg S. Dividing the tablets for children – good or bad? Pharm Methods 2016;7:23–7. doi:10.5530/phm.2016.7.4 [Google Scholar]
- 34.Footitt AR. Dose accuracy in paediatric medicine. Brit J Pharm Prac 1983;5:16–27. [Google Scholar]
- 35.Van Vooren L, De Spriegeleer B, Thonissen T, et al. . Statistical analysis of tablet breakability methods. J Pharm Pharmaceut Sci 2002;5:190–8. [PubMed] [Google Scholar]
- 36.Maguire AM, Ambler GR, Moore B, et al. . Prolonged hypocortisolemia in hydrocortisone replacement regimens in adrenocorticotrophic hormone deficiency. Pediatrics 2007;120:e164–e171. doi:10.1542/peds.2006-2558 [DOI] [PubMed] [Google Scholar]
- 37.Bleicken B, Hahner S, Loeffler M, et al. . Influence of hydrocortisone dosage scheme on health-related quality of life in patients with adrenal insufficiency. Clin Endocrinol 2010;72:297–304. doi:10.1111/j.1365-2265.2009.03596.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.