Abstract
Gene-engineered T-cell therapies have the potential to revolutionize the treatment of cancer. These therapies have shown exceptional clinical efficacy specifically in the field of B-cell malignancies and the first products (Kymriah™ and Yescarta™) have recently been approved in the United States for specific indications. The power of these treatments is also linked with a distinct set of toxicities both predicted and unpredicted, including off-tumor activity, cytokine release syndromes, and neurotoxicity, occasionally with fatal consequences. As these therapies begin to reach more patients, it is critical to develop the nonclinical tools to adequately determine the mechanisms driving these toxicities, to assess the safety risks of candidate products, and to develop strategies for safety management.
Keywords: immunotherapy, CAR T cells, TCR T cells, cancer, safety, cytokine release syndrome, neurotoxicity, gene modification, on-target toxicity, off-target toxicity
Until recently, for most advanced cancers, the primary treatment modality had remained cytotoxic chemotherapies, radiotherapy, and where applicable surgery. However, it had long been postulated that the host immune system could be manipulated to target and destroy cancer cells. The clinical validation of several immunotherapeutic regimes, such as monoclonal antibody blocking of cytotoxic T lymphocyte-associated protein 4 (Ipilimumab; Mansh 2011) and programed cell death protein 1 (Postow, Callahan, and Wolchok 2015), showed that such strategies were feasible. The growth of the immunotherapy field continues at a pace with strategies being developed, from oncolytic vaccines to checkpoint inhibitors, targeting different aspects of the immune system. T cells that play a central role in cell-mediated immunity can facilitate long-lived, antigen-specific, effector and immune memory responses. One highly promising immunotherapy area is gene-engineered or adoptive T-cell therapy (ACT), which harnesses the power of the T cell with selective tumor antigen targeting, exploiting the antitumor properties of lymphocytes to eradicate tumor cells (Vonderheide and June 2014).
Tumor cells can generate neoantigens that have the potential to be immunogenic, since mutated proteins or proteins with altered translational processing can be seen as foreign by the immune system. However, in many cases, the tumor-associated antigens are upregulated or overexpressed antigens from the normal cell antigen repertoire. These so-called self-antigens will likely not induce a functional immune response against the tumor due to the process of central tolerance, in which T cells expressing T-cell receptors (TCRs) that are highly reactive to self-antigens will have been negatively selected within the thymus (Xing and Hogquist 2012; Ruella and Kalos 2014). Therefore, only T cells with low-affinity TCRs for self-antigens remain in circulation. Tumor cells have also developed strategies such as modulation of the local environment, induction of peripheral tolerance, and systemic disruption of T-cell signaling to escape and suppress the immune system to overcome immune control, enabling them to survive and progress (Pinzon-Charry, Maxwell, and Lopez 2005; Blankenstein et al. 2012). The resulting immune suppressive state is key to tumors survival. The goal of gene-engineered T-cell therapies is to shift the balance of power back to the immune system (Sharpe and Mount 2015).
Within tumors, there can be found rare populations of tumor antigen–specific T cells, known as tumor-infiltrating lymphocytes (TILs), which have the potential to target and destroy the tumor cells (Kvistborg et al. 2014; Robbins et al. 2013). Through the development of cell isolation and expansion technologies, it has been shown that injection of large numbers of these activated tumor-specific TILs can induce complete and durable regression particularly in the treatment of melanoma (Yee et al. 2002; Kvistborg et al. 2014; Dudley et al. 2013), highlighting the therapeutic potential of tumor-specific T cells.
To widen the clinical applicability of ACT gene engineering, predominantly using γ-retroviral and lentiviral transduction approaches has been employed to alter the target antigen specificity of T cells (Eshhar et al. 1993; Sadelain, Riviere, and Brentjens 2003). Gene editing theoretically allows targeting of any tumor for which a tumor-specific antigen can be identified. Autologous (patient-specific) T cells can be engineered to express modified TCRs (so-called TCR therapies) or protein fusion–derived chimeric antigen receptors (CAR) that have enhanced antigen specificity and are composed of an antigen-binding region, typically derived from the single-chain variable fragment (scFv) of an antibody (Figure 1). These gene-engineered T cells can be expanded ex vivo and then returned to the patient (Figure 2; Levine et al. 2017). These approaches are generating compelling clinical data, especially in B-cell cancers for CAR T-cell therapies and more recently in multiple myeloma and synovial sarcoma for gene-modified TCR T-cell therapies (Table 1), indicating that the therapies can overcome the fundamental limitations associated with central and peripheral tolerance and generate T cells that are more efficient at targeting tumors without the requirement for de novo T-cell activation in the patient. In September and October 2017, the first products, tisagenlecleucel (Kymriah™ Novartis, East Hanover, NJ) and axicabtagene ciloleucel (Yescarta™ Kite Pharma, Santa Monica, CA), were approved by the Federal Drug Agency, respectively (Kaiser 2017).
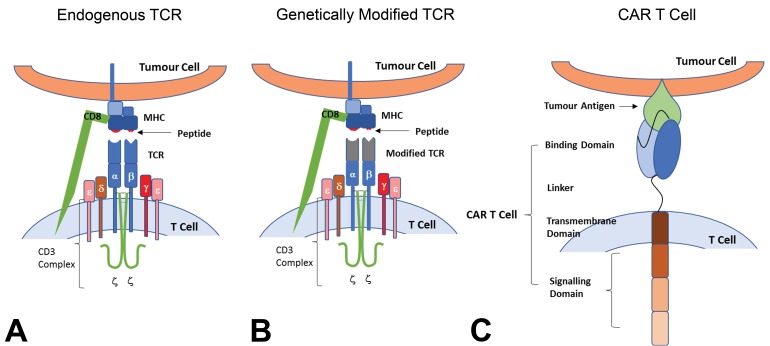
Figure 1.
Genetically modified T cells for cancer immunotherapy. T cells are distinguished from other lymphocytes by the presence of the T-cell receptor (TCR) on the cell surface (A). The TCR is a multisubunit transmembrane complex that mediates the antigen-specific activation of T cells. The TCR is composed of 2 different polypeptide chains, the TCR α and β chains. Both chains have an amino-terminal variable region and a constant region. The chains are linked by a disulfide bond with each receptor providing a single antigen-binding site. The TCR confers antigenic specificity on the T cell, by recognizing an antigen ligand comprising a short contiguous amino acid sequence of a protein that is presented on the target cell by a major histocompatibility complex (MHC) molecule. Accessory adhesion molecules such as CD4 for MHC class II and CD8 for MHC class I are also involved. The TCR interacts with this ligand by making contacts with both the MHC molecule and the antigen peptide. Signal transduction is through the associated invariant CD3 complex, which is composed of 4 different CD3 proteins that form 2 heterodimers (CD3δ∊ and CD3γ∊) and 1 homodimer (CD3ζζ). Genetically modified TCR T-cell therapies are based on altering T-cell specificity through the expression of tumor antigen–specific TCR α and β chains, which mediate the antigen-recognition process (Figure B). The tumor-specific TCR α and β chains are identified, isolated, and cloned into transduction vectors and transduction of T cells creates tumor antigen–specific T cells. Chimeric antigen receptors (CARs) combine both antibody-like recognition with T-cell activating function (C). They are composed of an antigen-binding region (typically derived from an antibody single-chain variable fragment but other receptors may be used), a transmembrane domain to anchor the CAR into the T cell (e.g., the transmembrane and endodomain of the CD3ζ coreceptor), and 1 (first-generation CAR) or more (second and later generation CARs) intracellular signaling domains (e, g, CD28, OX40, and CD40L), which induce persistence, trafficking, and effector functions in transduced T cells (Sharpe and Mount 2015). CD = cluster of differentiation.
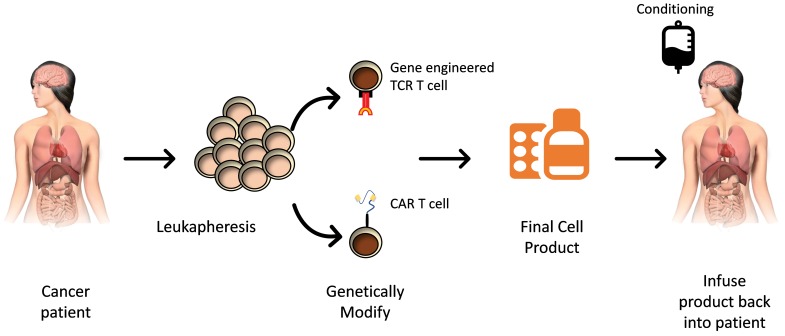
Figure 2.
Manufacturing of and treatment using gene-engineered T cells. T cells are harvested from a cancer patient and sent to good manufacturing practices facility. Cells are genetically engineered with either a new T-cell receptor or a receptor based on a recognition sequence of an antibody (chimeric antigen receptor). After a brief period of in vitro expansion and passing of product-specific release criteria, the T-cell product must be returned to the correct patient. The patient may undergo conditioning regimens prior to infusion of the genetically modified T-cell product.
Table 1.
Impressive Clinical Responses to Gene-modified T-cell Therapies.
| Disease | Product | Number of patients | Response rate | Reference |
|---|---|---|---|---|
| ALL (adult) | CD19 CAR T | 16 | 88% CR | Davila et al. (2014) |
| ALL (pediatric) | CD19 CAR T | 25 | 90% CR | Maude, Frey, et al. (2014) |
| ALL (pediatric) | CD19 CAR T | 21 | 68% CR | Lee et al. (2015) |
| ALL (adult) | CD19 CAR T | 29 | 100% CR | Turtle, Hanafi, Berger, Gooley, et al. (2016) |
| NHL/CLL | CD19 CAR T | 15 | 53% CR | Kochenderfer et al. (2015) |
| NHL | CD19 CAR T | 32 | 79% CR | Turtle, Hanafi, Berger, Hudecek, et al. (2016) |
| MM | NY-ESO-1c259 TCR T | 20 | 70% (CR or nCR) | Rapoport et al. (2015) |
| SS | NY-ESO-1c259 TCR T | 24 | 50% ORR (1CR and 5PR) | Mackall et al. (2017) |
Note: ALL = acute lymphoblastic leukemia; CAR = chimeric antigen receptor; CD = cluster of differentiation; CLL = chronic lymphocytic leukemia; CR = complete response; MM = multiple myeloma; nCR = near complete response; NHL = non-Hodgkin’s lymphoma; ORR = objective response rate; PR = partial response; SS = synovial sarcoma; TCR = T-cell receptor.
However, treatment with gene-engineered T-cell therapies has been associated with a number of toxicities, in some cases with severe to fatal side effects. To support the wider clinical application of gene-engineered T-cell therapies, it is important that these risks can be identified and mitigated, specifically in the preclinical phase of product development. These risks are associated with the nature of the cytotoxic T-cell reaction, the effect of rapid tumor lysis, and off-target activities. This review aims to address these safety aspects and provide points to consider when designing a safety strategy for gene-modified T-cell programs.
Use of Animal Models for Target Specificity Assessment of Adoptive T-cell Therapies
Gene-modified TCR T-cell Therapies
For gene-engineered TCR T-cell therapies, animal models have considerable limitations for target specificity assessment. The TCR is a multisubunit transmembrane complex that mediates the antigen-specific activation of T cells (Figure 1A). The TCR confers antigenic specificity on the T cell by recognizing an antigen ligand comprising a short contiguous amino acid sequence of a protein that is presented on the target cell by a major histocompatibility complex (MHC) molecule (Figure 1A). All proteins, including intracellular ones, are processed and presented as MHC-peptide complexes, which are recognized by the TCRs, including the cancer antigens. However, due to the MHC and proteome mismatches between the animal model and the human, differences in gene expression and the critical differences in the amino acid sequences of peptides derived from homologous proteins, in vivo studies may have limited utility for testing target specificity of gene-engineered TCR T cells. Where animal models are used, confirmation of the presence of the epitopes and tissue expression pattern of the epitopes is essential. Even when using human leukocyte antigen (HLA)-transgenic mice, although the peptide target of choice may be expressed, the degree of overlap for all peptides may be incomplete and it is essential that limitations in the models are considered when assessing the safety aspects of a gene-engineered TCR T-cell therapy. This is particularly pertinent when considering off-target specificity.
Where animal models are not relevant or where the use is severely limited, it is essential that a comprehensive in vitro panel of tests is designed to assess target specificity (A. Gerry et al. 2015; A. B. Gerry 2016). Assessments may include T-cell reactivity against an extensive panel of human cell types relating to all the major organ systems of the body, the use of organotypic or Induced Pluripotent Stem Cell (iPSC)-derived models for key tissues, and robust molecular analysis, peptide screening, and other predictive models to address concerns of target specificity (A. B. Gerry 2016). Gene-engineered TCR T-cell therapies, especially when the TCR has been affinity matured, require the use of the more biologically relevant culture systems that are now available, as it is essential to assess for the risks of off-target activity due to TCR plasticity (Attaf et al. 2015).
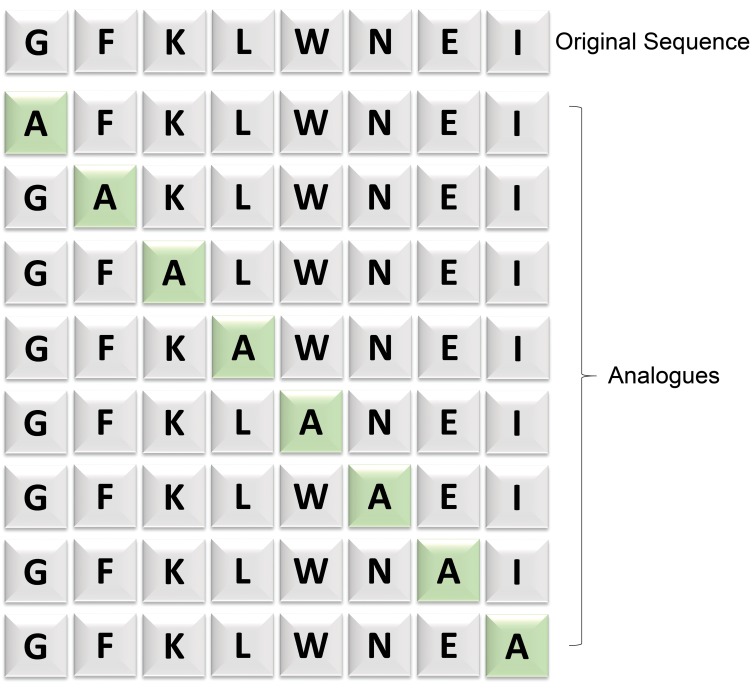
A further approach is the in silico strategy of peptide scanning (Cameron et al. 2013). The principle is that each amino acid within a peptide does not contribute equally to TCR binding. In these initial studies, each amino acid within the peptide was replaced systematically by alanine or glycine, using a combinatorial approach to amino acid substitutions (Figure 3). This technique has subsequently been further refined such that each amino acid in a given peptide sequence is substituted for all other possible amino acids to generate a binding motif, and these sequences searched against the human genome to establish the fine specificity of each TCR (A. B. Gerry 2016). The activity of the gene-engineered TCR T cells against these mutated peptide sequences is compared to the target sequence in an in vitro system monitoring for interferon (IFN)-γ release against target cells expressing each peptide/MHC complex. Where there is a significant decrease in activity for a given peptide that residue is considered essential. From this, direct in silico searches (using, e.g., ScanProsite) can be performed to identify protein sequences that contain the essential peptide motif. This allows putative off-target peptides to be identified and in vitro studies can then be designed to assess the ability of the genetically modified TCR T cells to recognize these and hence identify potential off-target tissues.
Figure 3.
Principle of peptide scanning. In this example, an Alanine scanning library is designed to identify the essential amino acid residues critical for T-cell receptor reactivity. Alanine, the smallest chiral amino acid, is sequentially substituted for each nonalanine residue 1 at a time. Subsequently, corresponding change in epitope activity can be measured.
CAR T-cell Therapies
CAR T-cell therapies combine both antibody-like recognition with T-cell activating function (Figure 1c; Maher 2012). Sequences used to define the antigen-targeting motif for a CAR are typically derived from a monoclonal antibody, but ligands (Muniappan et al. 2000) and other receptors (Zhang, Wu, and Sentman 2012) may also be used. The target for a CAR T-cell therapy is proteins expressed on the surface of tumor cells. For any CAR T-cell therapy, as a key part of the nonclinical program, it is essential to understand the distribution and expression patterns of the target antigen in both human tumor and healthy and other disease tissues. In addition, it is critical to show similar distribution patterns in the planned safety species and reactivity of the CAR to the animal equivalent antigen in in vitro testing. Where there is a difference in antigen expression patterns or a lack of reactivity with the animal equivalent antigen, the suitability of the test species for safety assessment is in question, and the in vitro analyses have even greater significance.
The choice of target and associated expression patterns is a key primary consideration for all these therapies (see above for TCR T-cell therapies), and product design and/or mitigation strategies must be designed accordingly. It is essential, therefore, for the CAR T cell to describe in detail any unintentional reactivity and/or cytotoxicity toward human tissues distinct from the intended tumor target. Such tissue expression studies should be carried out by appropriate immunohistochemical (IHC) procedures, with the relevant parental monoclonal antibody or scFv, on a range of human tissues covering all major organs. Most studies focus on healthy and tumor tissue, but consideration also needs to be given to other diseased tissues that may also result in the target antigen having enhanced expression. How the tissue samples have been collected and prepared is also important as antigen expression patterns may be very different between samples that have been promptly excised and fixed and those that may have been collected many hours postdeath at autopsy (Gillio-Meina, Zielke, and Fraser 2016). In addition, information from online gene and protein expression databases may be supportive of the tissue expression patterns of the target antigen.
Tissue binding characteristics can also be affected by the process of tissue fixation, and this needs to be considered when interpreting the data. The CE7 monoclonal antibody (mAb), for example, recognizes a tumor-specific glycosylated epitope on cluster of differentiation (CD) 171 not present on normal tissues. However, tissue fixation for IHC strips carbohydrates and unmasks the CE7 epitope resulting in normal tissues showing positive staining (Kunkele et al. 2017). This was the challenge for developers of a CD171 CAR T-cell product (CE7). Since expression of CD171 was observed on critical tissues and organs in the human healthy tissue arrays, to derisk the CE7 CAR T-cell program, it was important to identify a relevant animal model. The extracellular domain of CD171 in rhesus macaques was 100% homologous to that of humans and the rhesus tissue array IHC showed the same distribution of CD171 expression to that of humans, identifying the rhesus macaques as the pivotal toxicology study test system. Subsequently, no toxicities were observed in animals dosed with CE7-CAR T-cell products at doses 10× and 100× higher than the planned starting dose for the phase-I trial (Kunkele et al. 2017). Where expression of the target antigen (or potential cross-reactive protein) is seen in human tissue panels, identifying potential target tissues, it is important to confirm that the animal models being employed for the nonclinical studies not only have exhibit similar staining patterns but that the ACT will target the animal homologue.
Use of Immunodeficient Animals in Efficacy and Safety Assessments
The majority of reported nonclinical studies utilize severe combined immunodeficient mice engrafted with human tumors and treated with human gene-engineered T-cell therapies cells (Milone et al. 2009; Kowolik et al. 2006; Alcantar-Orozco et al. 2013; Whilding et al. 2017). The use of human T cells in immune deficient mice may have several drawbacks (Kochenderfer et al. 2010). The transferred human T cells may recognize murine xeno-antigens, which can lead to graft-versus-host disease. This may limit the use of these animal models for therapies targeting slow growing tumors where it will be important to understand the usable therapeutic window for the model (Alcantar-Orozco et al. 2013). Another challenge is that immunodeficient mice have low levels of endogenous lymphocytes and do not replicate the clinical situation where host lymphocytes, for example, T-regulatory cells, can reduce the antitumor efficacy of transferred T cells (North 1982; Gattinoni et al. 2005). Although patients are subject to preparatory lymphodepletive regimens, lymphocyte recovery will occur, a situation that is not replicated in the immunodeficient animals. Therefore, although the models confirm the T cells can target the tumors, the challenges, in particular of the solid tumor microenvironment, may be underestimated. The use of syngeneic tumor models and animal equivalent products (e.g., CAR expressed on animal T cells) may have utility for assessing both the efficacy and safety of CAR T cells (Kochenderfer et al. 2010; Davila et al. 2013) allowing more extensive and rationale analyses than are possible with immunodeficient mouse models or assessment that are feasible in patients (Davila et al. 2013).
For gene-engineered TCR T cells, a further challenge is the TCR/peptide MHC interaction. Murine MHC molecules, murine cytokines, and murine costimulation molecules do not interact efficiently with transferred gene-engineered human T cells, and therefore the effects of antigen driven persistence cannot be accurately modeled. For a gene-engineered TCR T-cell product, the murine host would ideally need to express the human MHC molecule that restricts the TCR under investigation, for example, the HLA-A201 transgenic mouse model. In the majority of cases, for gene-engineered TCR T-cell therapies, the nonclinical studies focus solely on efficacy against target tumor cells. There is a significant need for relevant and physiologic preclinical models to serve as a platform for understanding the function, efficacy, and safety of gene-engineered TCR T-cell therapies. While studies in immunodeficient mice and in HLA transgenic mice provide evidence for efficacy, persistence, and toxicity, there is unfortunately no experimental model to accurately mimic the function of human TCR T cells in vivo.
Gene-engineered T-cell Therapies in the Clinical Setting
In the clinical setting, the patient is administered with high numbers of gene-modified autologous T cells (typically 106–108cells/kg) that express receptors capable of recognizing, often with high affinity, the target tumor antigen. The patients are usually pretreated with lymphoconditioning chemotherapy and, therefore, have few circulating leukocytes, few regulatory immune cells, and higher than normal amounts of cytokines that promote T-cell survival at the time of product administration (Gattinoni et al. 2005; Muranski et al. 2006). One of the specific characteristics of gene-engineered T-cell therapies is that the administered dose does not reflect the maximum dose the patient will receive as the T cells, in response to target antigen activation, will proliferate, with 100- to 100,000-fold expansion observed clinically (Teachey et al. 2016). Antitumor responses can be very impressive with complete response rates of up to 90% reported (Davila et al. 2014; Maude, Frey, et al. 2014). However, treatment with gene-engineered T-cell therapies is also associated with unique acute toxicities, which can be severe or even fatal (Tables 2 –5). Within clinical trials, the severity of side effects after administration is characterized by the Common Terminology Criteria for Adverse Events Scale. Unspecific- and organ-related adverse events (AEs) are graded into different categories according to their severity: 1 = mild, 2 = moderate, 3 = severe, 4 = life-threatening or disabling, and 5 = fatal. Of the AEs seen within gene-engineered T-cell therapy clinical trials, the most critical were related to on-target, off-tumor activity, off-target reactivity, neurotoxicity, and cytokine release syndromes (Casucci et al. 2015; Bedoya, Frigault, and Maus 2017).
Table 2.
On-target, Off-tumor Toxicities Associated with CAR T-cell Therapies.
| Disease | Target | Toxicity | Reference |
|---|---|---|---|
| B-cell malignancies | CD19 | B-cell aplasia, which can be maintained long term with reported cases up to 4 years | Grupp et al. (2013); Maude, Frey, et al. (2014); Porter et al. (2015) |
| Colon cancer | HER2/ERBB2 | Lethal pulmonary failure Suspected cytokine release following the recognition by the CAR T cells of low levels of ERBB2 on lung epithelial cells | Morgan et al. (2010) |
| Renal cancer | Carbonic anhydrase-IX | Liver enzyme disturbances in subjects, reaching National Cancer Institute Common Toxicity Criteria grades 2 to 4 The development of cholestasis due to expression of carboxy anhydrase-IX on bile duct epithelium. Liver biopsies showed T-cell infiltration around the bile ducts | Lamers et al. (2006); Lamers et al. (2013) |
| Non-Hodgkin’s lymphoma/multiple myeloma | κ light chain | Elimination of κ-expressing B and plasma cells However, spares the normal B cells expressing the nontargeted λ light chain, thus potentially minimizing humoral immunity impairment | Ramos et al. (2016) |
Note: CAR = chimeric antigen receptor; CD = cluster of differentiation.
Table 3.
Grade 5 Toxicities Associated with CD19 CAR T Cells.
| Disease | Target | Toxicity | Reference |
|---|---|---|---|
| Chronic lymphocytic leukemia (NCT00466531) | CD19 | One death related to CAR T cells Low-grade sepsis was the most likely trigger in this heavily pretreated immunosuppressed patient but also considered the possibility that a cyclophosphamide-induced cytokine storm may have enhanced the in vivo activation of modified T cells | R. Brentjens et al. (2010) |
| Lymphoma (NCT02631044) | CD19 | One death related to CAR T cells Diffuse alveolar damage | Plieth (2017) |
| Adult acute lymphoblastic leukemia (NCT01044069) | CD19 | Three deaths related to CAR T cells Severe hypotension and CRS Status epilepticus and CRS Sepsis and multi-organ failure | Plieth (2017) |
| Adult acute lymphoblastic leukemia (NCT02535364) | CD19 | Five deaths related to CAR T cells Cerebral edema | Plieth (2017) |
| Relapsed or refractory chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, or acute lymphoblastic leukemia (NCT01865617) | CD19 | Six deaths One CRS One cerebral edema Two CRS or neurotoxicity One CRS, cerebral edema One encephalopathy and pontine hemorrhage | Plieth (2017) |
| Lymphoma (NCT02348216) | CD19 | Three deaths Hemophagocytic lymphohistiocytosis Cardiac arrest in the setting of CRS, later specified as anoxic brain injury CRS, multi-organ failure leading to cerebral edema | Plieth (2017) |
| Adult acute lymphoblastic leukemia (NCT02614066) | CD19 | One death CRS | Plieth (2017) |
| Lymphoma (NCT02030834) | CD19 | One death Encephalitis possibly related to the CAR product | Plieth (2017) |
| Adult acute lymphoblastic leukemia and lymphoma (NCT02030847 and NCT01029366) | CD19 | Three deaths CRS and infection (Influenza B, pseudomonas infection, or stenotrophomonas infection) | Teachey et al. (2016); Plieth (2017) |
| Pediatric acute lymphoblastic leukemia (NCT02435849) | CD19 | One death Cerebral hemorrhage | Plieth (2017) |
Note: CAR = chimeric antigen receptor; CD = cluster of differentiation; CRS = cytokine release syndrome.
Table 4.
On-target, Off-tumor Toxicities Associated with Genetically Modified TCR T-cell Therapies.
| Disease | Target | Toxicity | Reference |
|---|---|---|---|
| Melanoma | MAGE-A3 peptide (KVAELVHFL) | On-target, off-tumor toxicity due to previously undetected MAGE-A expression in the human brain. TCR also recognizes peptides: MAGE-A12 (KMAELVHFL) MAGE-A2 (KMVELVHFL) MAGE-A6 (KVAKLVHFL). Three subjects developed neurological toxicity. Two subjects died and 1 subject made a full neurological recovery | Morgan et al. (2013) |
| Melanoma | TCR T-cell therapy TCR recognizing melanoma antigen MART-1 (amino acids 27–35 epitope) TCR recognizing the HLA-A*02-restricted melanoma antigen gp100 (amino acids 154–162 epitope) | On-target, off-tumor reactivity, destruction of normal melanocytes in the skin, eye, and ear | Johnson et al. (2009) |
| Metastatic colorectal cancer | TCR recognizing the carcinoembryonic antigen (CEA) peptide: (IMIGVLVGV) | On-target, off-tumor reactivity resulting in severe transient inflammatory colitis caused by T-cell reactivity to CEA expression on normal colonic mucosa | Parkhurst et al. (2011) |
Note: HLA = human leukocyte antigen; TCR = T-cell receptor.
Table 5.
Off-target Toxicities Associated with Genetically Modified TCR T-cell Therapies.
| Disease | Target | Tumor Toxicity | Reference |
|---|---|---|---|
| Myeloma and melanoma | An affinity-enhanced TCR recognizing MAGE-A3 (EVDPIGHLY) | Off-target reactivity. Lethal cardiac toxicity. Two subjects died approximately 5 days’ postdosing Following adverse events, in vitro investigations revealed cross-recognition of an off-target peptide | Linette et al., (2013); Cameron et al. (2013) |
Note: TCR = T-cell receptor.
The Risks of On-target, Off-tumor Activity and Off-target, Off-tumor Activity
The optimal gene-engineered T-cell therapy target antigen is one that is only present on the tumor cell and is absent in healthy cells. However, in most cases, the selected tumor target antigens are overexpressed or abnormally expressed proteins that are also present to some extent in normal cells. Gene-engineered T-cell therapies may, therefore, trigger a potent cellular immune response against these normal cells, even those that express the target antigens at low levels (Johnson et al. 2009) in a type of toxicity known as on-target, off-tumor activity.
On-target, off-tumor activity has been observed in numerous clinical trials (Tables 2 and 5), and it is widely associated with the most successful ACT therapy to date, the CD19 CAR T-cell therapies (Table 2). Clinical trials where CD19 CAR T cells have reported significant clinical efficacy have near universally reported that participants in these studies have shown sustained B-cell aplasia (Grupp et al. 2013). This is not unexpected as CD19 expression is restricted to normal mature B cells, B-cell precursors, as well as malignant B cells. Due to normal mature B-cell depletion, antibodies are not produced as readily, and this can lead to an elevated infection risk of the patient. The B-cell aplasia must therefore be managed with monthly intravenous immunoglobulin infusions. Data from clinical trials have shown that functional CD19 CAR T cells can persist in a patient for >1 year after infusion. While this effect can augment prolonged remissions, it will also lead to prolonged B-cell depletion. Indeed, long-term data from early clinical trial patients suggest that B-cell aplasia may persist for >3 years (Novartis 2017). It is not yet known if B-cell aplasia will be an ongoing or reversible effect over time. Although in this case, the on-target, off-tumor toxicity can be clinically managed by immunoglobulin transfusion, it highlights the challenges of identifying tumor-specific targets.
Many antigens being targeted for the treatment of solid tumors have high levels of expression in tumor tissues but are not tumor-specific and have a low level of expression in normal tissues (Morgan et al. 2010; Berger et al. 2015; Kunkele et al. 2017). Indeed, the risk from on-target, off-tumor toxicities is patient fatalities (Tables 2 and 4; Morgan et al. 2010; Morgan et al. 2013). In a clinical study testing, a Her2/neu-specific CAR T cell as a treatment for patients with colon cancer, fatal respiratory failure was triggered by CAR T-cell recognition of low levels of antigen on lung epithelial cells. The resulting T-cell activation lead to the release of inflammatory cytokines (including tumor necrosis factor-α and IFN-γ) that caused pulmonary toxicity and edema followed by a cascading cytokine storm resulting in multi-organ failure (Morgan et al. 2010). This CAR was designed based on the widely used humanized mAb trastuzumab (Herceptin). Safety considerations that preceded this trial included the use of trastuzumab in thousands of cancer patients. This clinical study finding shows the potential enhanced potency of linking antibody specificity with the cytotoxic potential of a T cell.
Another specific concern is previously undetected expression of an antigen as was the case in a clinical trial with a gene-engineered anti-MAGE-A3 TCR T-cell therapy (Morgan 2013; Morgan et al. 2013). MAGE-A3 is a member of the cancer-testes class of tumor-associated antigens and is widely expressed in common epithelial malignancies. The MAGE-A3 TCR (epitope KVAELVHFL) was known to also recognize MAGE-A12 (epitope KMAELVHFL) and, to a lesser extent, MAGE-A2 (KMVELVHFL) and MAGE-A6 (KVAKLVHFL). No risk of off-tumor activity was predicted by the completed nonclinical program. In the subsequent clinical trial, 3 of the 9 treated subjects suffered neurological toxicity (including mental status changes, grand mal seizures, generalized tonic-clonic seizure and loss of fine motor coordination, and vacuolation of white matter). Two subjects died and following consent, autopsy showed that damage was confined mainly to the white matter of the brain. There was a spectrum of myelin/axonal damage with morphologic changes including white matter spongy vacuolation, myelin pallor, and axonal injury characterized by thickening and axonal spheroids; frankly necrotic areas with myelin and axonal loss and mineralization were also present (Morgan et al. 2013). Subsequent antigen expression analysis both by immunohistochemistry (of patient and control tissues) and by polymerase chain reaction and RNA deep sequencing (control tissues) suggested that MAGE-A12 may be present at low level in normal human neurological tissues. Therefore, the neurological toxicity resulted from the recognition of MAGE-A12 by the gene-engineered TCR T-cell therapy in a small subset of neurons in the brain, which initiated a destructive immune response resulting in severe damage to the white matter. This study highlighted the importance of target specificity for TCR T-cell therapies.
Off-target reactivity has also been reported in clinical trials (Table 5). This cross-reactivity is particularly a risk of gene-engineered TCR T cells, which may react against related peptides in proteins other than the one targeted. This is a result of the plasticity of the TCR receptor, which can lead to potentially, extensive TCR cross-reactivity (Attaf et al. 2015). In addition, many of the TCR sequences that are used to construct the gene-engineered TCR T-cell therapies are affinity-enhanced by phage display, yeast display, and computational design (Attaf et al. 2015). Such affinity-enhanced TCRs are attractive as the active moiety for gene-engineered TCR T-cell therapies, as within the normal TCR repertoire, thymic selection during development will have destroyed T cells whose TCRs strongly recognize self-antigens. However, since T cells baring the gene-engineered TCRs have not undergone the process of thymic selection, there is a risk of autoreactivity due to the inherent plasticity of the TCR. This was observed in a clinical trial for patients with myeloma and melanoma for treatment with gene-engineered T cells expressing an affinity-enhanced TCR against HLA-A*01-restricted MAGE-A3 (Linette et al. 2013). The first 2 treated patients developed cardiogenic shock and died within a few days of T-cell infusion, events that had not been predicted by preclinical studies of the high-affinity TCRs. The gross findings at autopsy revealed severe myocardial damage and subsequent histopathological analysis of cardiac tissue revealed T-cell infiltration. The data suggested that T-cell-mediated acute cardiac injury contributed to acute cardiac failure in both patients (Linette et al. 2013).
Subsequently, elegant studies by Cameron et al. (2013) identified the target as a peptide from the protein titin. Standard in vitro cell culture assessments failed to induce the expression of titin in cardiac myocytes. Following coculture of the gene-engineered T cells with a set of 38 normal cardiac-derived primary cells, no T-cell activation activity (IFN-γ ELISpot [Enzyme-Linked ImmunoSpot] assay) was observed against any of the cardiac cells. Indeed, the ability of the genetically modified T cell to target cardiac cells was only able to be confirmed when the team used a more biologically relevant cell culture, the iCell® cardiomyocytes, which contained a mixture of spontaneously electrically active atrial, nodal, and ventricular-like myocytes with typical cardiac biochemical, electrophysiological, and mechanical characteristics. Coculture of the genetically modified T cells with the iCell cardiomyocytes resulted in cell killing (Cameron et al. 2013). Indeed, when considering the preclinical safety assessment of genetically modified TCR T cells, 3-D cultures of differentiated human cells may represent a more physiological target with respect to target specificity. The team also retrospectively employed the peptide scanning approach (described above) and were subsequently able to identify the epitope within the protein titan as the target for the affinity-enhanced TCR against HLA-A*01-restricted MAGE-A3 (Cameron et al. 2013; Linette et al. 2013). Interestingly, when the team performed a similar in vitro study using the equivalent mouse titin peptide, no activation of engineered T cells was observed indicating that for this product, preclinical toxicity testing in an HLA-A1 transgenic mouse model would not have revealed potential off-target binding of the engineered T cells. Recently, nonhuman primate (NHP) models have been described for specific toxicities relating to CD19 CAR T cells (Taraseviciute, Kean, and Jensen 2016). It is important to note that although the NHP model is relevant for the specific safety aspect described (see detail below), the use of NHP by default is not warranted and specifically is not expected as a routine model of choice. For example, on- or off-target gene-modified TCR specificity is unlikely to be evaluable in the NHP due to proteomic differences. NHP models, like all in vivo models, need to be carefully considered for ethical and scientific merit, including the risk of potential false positives and negatives.
Safety Related to Genetic Modification of T Cells
Although a number of different gene editing technologies could be employed, retroviral and lentiviral gene transfer systems are the most commonly used in the genetic modification of T-cell therapies. These vectors are capable of sustained high levels of expression and the ability to package large inserts. Vector systems derived from these Retroviridae family of viruses come with 2 generally accepted risks: the production of replication-competent viruses (RCV) and insertional mutagenesis, specifically oncogenic activation. The issue of reducing the probability of RCV has been systematically addressed over the years during the ongoing development of next generation retroviral vectors, with each new generation aimed at minimizing and reducing the risk. This is supported by the experience in the clinic. The results of replication-competent retrovirus testing from clinical trials of genetically modified T-cell therapies using methodology developed in the National Gene Vector Biorepository showed that all 460 transduced cell products tested were negative for replication-competent retroviruses indicating that there is negligible risk (Cornetta et al. 2017).
Oncogenic activation has been observed in the clinic following the administration of γ-retrovirally modified hematopoietic stem cells (HSC), with leukemia’s or preleukemia’s reported in the context of gene therapy of HSC for X-linked severe combined immunodeficiency (Hacein-Bey-Abina et al. 2003, 2008). In general, the risk of insertional mutagenesis, while poorly defined, is considered to be related to disease background, cell type to be transduced, and vector characteristics (Persons and Baum 2011). Numerous clinical trials with γ-retroviral and lentiviral modified T cells have not yielded evidence for insertional AEs despite long-term persistence of transduced cells and there is a decadelong safety profile without evidence of vector-induced immortalization, clonal expansion, or enrichment for integration sites near genes implicated in growth control or transformation (Persons and Baum 2011; Scholler et al. 2012). While the risk remains very low, strict monitoring is part of current clinical trial protocols, and vector integration analysis is part of product testing.
More recently, alternative nonviral molecular methods are being explored for genetically modifying T cells including the use of the RNA-guided DNA targeting technology such as Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/CRISPR-associated protein 9 (CRISPR/Cas9) system (Ren and Zhao 2017) and transcription activator-like effector nucleases (TALEN) gene editing (Qasim et al. 2017). The use of such systems has the potential to achieve site specificity of the inserted gene and limit the potential for disruption of essential genetic elements. The risk of off-target mutagenesis remains, but studies suggest this can be mitigated based on the small guide RNA design and target sequence as well as the doses administered (Ren and Zhao 2017). Indeed, the first molecular edited T-cell therapies have recently gone into the clinic (Qasim et al. 2017).
Cytokine Release Syndrome
The most commonly reported toxicity following the infusion of gene-engineered T-cell therapies to patients is cytokine release syndrome (CRS; Tables 2 and 3), which can be associated with significant morbidity and mortality (Figure 4). CRS is a nonantigen-specific toxicity that occurs following high-level immune activation and is not unique to gene-engineered T-cell therapies but has also been reported following the administration of other biological products such as monoclonal antibodies (Attarwala 2010), as well being associated with conditions such as severe infections. CRS following treatment with gene-engineered T-cell therapies is associated with the T-cell activation and response to the T-cell target antigen. The magnitude of immune activation achieved by efficacious gene-engineered T-cell therapies can result in 100- to 100,000-fold expansion of the administered T-cell product (Teachey et al. 2016). However, a clear administered gene-engineered T-cell dose–response relationship for CRS has been difficult to define, and outcome analysis has differed between studies (Hay et al. 2017; Teachey et al. 2016). Other factors identified as potential predictors of CRS include high tumor burden, lymphodepletion protocols, and thrombocytopenia before lymphodepletion, suggesting that other factors associated with the resulting immune response could also be having an effect (Hay et al. 2017). This shows the fine balance that needs to be achieved with these therapies because the more potent the subsequent response, the greater the risk of severe CRS.
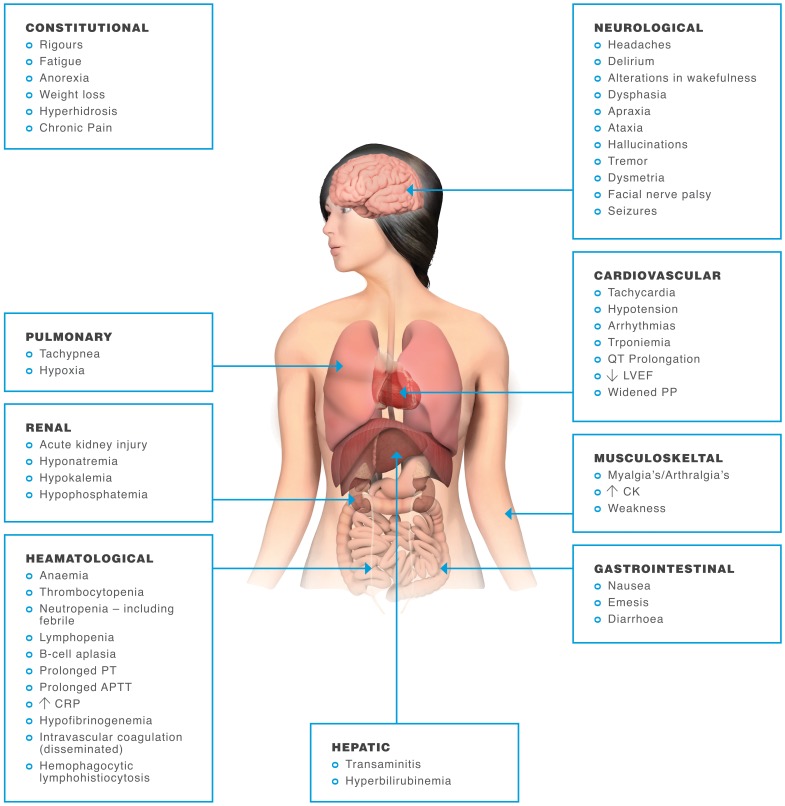
Figure 4.
Symptoms of cytokine release syndrome. In most adoptive T-cell therapy patients, cytokine release syndrome (CRS) symptoms are mild and flu-like, with fevers and myalgias. However, some patients experience a severe inflammatory syndrome with potentially life-threatening complications of CRS, including cardiac dysfunction, adult respiratory distress syndrome, neurological toxicity, renal and/or hepatic failure, vascular leak, hypotension, pulmonary edema, and coagulopathy, resulting in multi-organ system failure (Brudno and Kochenderfer 2016).
Severe CRS is a potentially life-threatening toxicity. Indeed, fulminant CRS may be life-threatening (Table 3); however, some degree of cytokine release is likely a necessary consequence of gene-engineered T-cell therapy efficacy. Therefore, the ability to predict which patients may develop severe CRS prior to its development could mitigate toxicity, as cytokine-directed therapy could be instituted before a patient becomes critically ill. CRS clinically manifests when large numbers of lymphocytes (B cells, T cells, and/or natural killer cells) and/or myeloid cells (macrophages, dendritic cells, and monocytes) become activated and release inflammatory cytokines (Lee et al. 2014). Following the administration of a gene-engineered T-cell therapy, the expansion and activation of a large numbers of T cells can lead to excessive secretion of inflammatory cytokines and the recruitment and activation of other immune cells. Affected patients mostly develop a mild syndrome requiring minimally invasive supportive care. Symptoms include fever, chills, hypotension, and tachycardia generally occurring days to occasionally weeks after gene-engineered T-cell therapy infusion but typically peaking around 8 days coinciding with maximal in vivo T-cell expansion. In some cases, the CRS may cause a broad spectrum of constitutional and organ-related disorders as well as blood test abnormalities (Figure 4) necessitating intensive interventions such as vasopressor support and mechanical ventilation (Rouce and Heslop 2016).
Unlike all other therapies, a unique characteristic of the gene-engineered T-cell therapy is the expansion of the delivered therapy; therefore, the dose administered will not reflect the maximum dose received by the patient and the level of expansion will be patient-specific. Reports of CRS following gene-engineered T-cell therapies for cancer (Kochenderfer et al. 2012; R. J. Brentjens et al. 2013; Grupp et al. 2013; Kalos et al. 2011; Maude, Barrett, et al. 2014; Davila et al. 2014; Lee et al. 2015) suggest that the incidence and severity may be greater when patients have large tumor burdens (Hay et al. 2017), presumably because this leads to higher levels of T-cell activation. However, from studies that focus on identifying characteristics and biomarkers that accurately predict whether patients will develop severe CRS, disease burden alone is not predictive of risk of severe CRS (Teachey et al. 2016). The management of CRS can be challenging but recently recommendations for monitoring, grading, and managing the acute toxicities that can occur in patients treated with CAR T-cell therapy have been published (Neelapu et al. 2017; Hay et al. 2017).
The most significantly elevated cytokines in the CRS associated with gene-engineered T-cell therapies appear to be those released after T-cell engagement (IFN-γ, interleukin [IL]-6, serum soluble interleukin (sIL)-2Rα, sIL-6R, and granulocyte-macrophage colony-stimulating factor [GM-CSF]), and therefore, elevations are both anticipated and likely required for efficacy (Teachey et al. 2016). There have also been reports of increases in cytokines associated with activated monocytes/macrophages (IL1-receptor antagonist, IL-10, IL-6, IP-10, monokine induced by gamma interferon, INF-α, macrophage inflammatory protein [MIP]-1α, MIP-1β, and sIL6R) or that are chemotactic for them (monocyte chemoattractant protein-1 and MIP-1β). In fact, CRS associated with T-cell therapies may represent a spectrum of symptoms, with a subset of patients developing symptoms indistinguishable from hemophagocytic lymphohistiocytosis and macrophage-activation syndrome (Rouce and Heslop 2016; Teachey et al. 2016). Finally, cytokines associated with tissue damage and inflammation (IL-8, granulocyte colony-stimulating factor, GM-CSF, vascular endothelial growth factor, IL-6) have also been shown to increase (Teachey et al. 2016).
The role of standard clinical laboratory tests has provided conflicting data in the ability to predict CRS risk. This is particularly the case for C-reactive protein (CRP); in 1 study (Davila et al. 2014), these were found to be predictive of CRS risk, whereas in another (Teachey et al. 2016), this was found along with many other clinical laboratory tests not helpful in predicting CRS severity as many (ferritin, CRP, lactate dehydrogenase [LDH], aspartate aminotransferase, alanine aminotransferase, and blood urea nitrogen) had peaked as the patients became ill.
Emerging evidence has implicated IL-6 as the central mediator of CRS toxicity (Lee et al. 2014; Mackall et al. 2016). IL-6 is a pleiotropic cytokine with anti-inflammatory and proinflammatory properties (Lee et al. 2014), and it is proposed that high levels of IL-6, present in the context of CRS, likely initiate a proinflammatory IL-6-mediated signaling cascade. Indeed, CRS can be successfully treated with the IL6-receptor inhibitor tocilizumab, and its use is becoming commonplace in gene-engineered T-cell therapy trials (Grupp et al. 2013; Maude, Barrett, et al. 2014; Davila et al. 2014; Lee et al. 2014; Mackall et al. 2016). Tocilizumab has predominantly been used only in the case of severe symptoms, and it is not known if its use could be preventative or whether early use could limit ACT therapeutic efficacy. Corticosteroids have also been used therapeutically for CRS treatment. Corticosteroids indirectly reduce cytokine levels through the reduction of transduced T cells that are respectively causing the CRS (Davila et al. 2014; Lee et al. 2015), but there is the concomitant risk if loss of efficacy of the therapy (Davila et al. 2014). Although in most cases of severe CRS treatment, regimens can resolve symptoms, unfortunately some patients still die indicating the complex biology behind the syndrome.
Despite the frequency of CRS following gene-engineered T-cell therapy treatment, the underlying biology remains largely unknown. Preclinically, the majority of efficacy and safety models do not replicate the symptoms observed in the clinical trials. One preclinical study where the risk of CRS was identified was in a severe combined immunodeficiency (SCID) beige murine model for an ErbB CAR T cell (van der Stegen et al. 2013). In this study, following intravenous and intratumoral administration of the ErbB CAR T cells, partial tumor regression was observed without toxicity. However, when the ErbB CAR T-cell therapy was administered intraperitoneally, toxicity was reproducibly observed. At low cell doses, animals exhibited weight loss, while administration of larger numbers of cells to mice with advanced tumor burdens resulted in lethal CRS, indicated by the presence of both human and murine cytokines in the circulation (van der Stegen et al. 2013). These studies suggest that SCID beige mice may be more susceptible to cytokine release syndrome and be suitable for assessing the risk, but such models may also lead to progressive loss of CAR T cells following adoptive transfer, which may limit their wider utility (Whilding et al. 2017).
Recently an NHP, Macaca mulatta, animal model of B-cell-directed CAR T-cell therapy targeting CD20 has been developed (Taraseviciute, Kean, and Jensen 2016). The NHP was chosen because it closely recapitulates the human immune system. The animals received cyclophosphamide conditioning prior to infusion of 1 × 107 CD20 CAR T cells/kg (n = 3). There was significant expansion of the CAR T cells concomitant with B-cell aplasia, and in addition, the animals developed clinical signs and symptoms of CRS (as well as neurologic toxicity—see section below) mirroring the clinical situation. The primate clinical syndrome was accompanied by elevations in CRP, ferritin, LDH, and serum cytokines, including IL-6 and IL-8, as has been seen from clinical trials using CD19 CAR T cells. These data demonstrate NHP will likely permit a detailed interrogation of the mechanisms driving these toxicities as well as the preclinical evaluation of therapies designed to prevent or abort CRS after gene-engineered T-cell therapy infusion (Taraseviciute, Kean, and Jensen 2016).
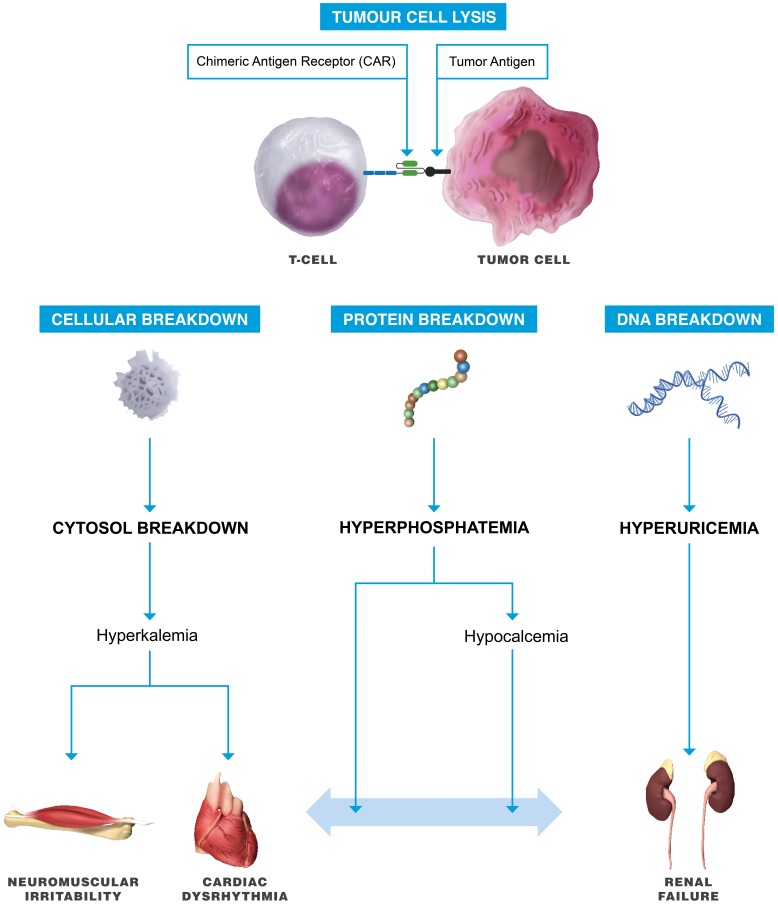
Tumor Lysis Syndrome
Tumor lysis syndrome (Figure 5) may also occur coincident with CRS, as the gene-engineered T-cell therapy activation and expansion correlates with enhanced tumor antitumor efficacy (Porter et al. 2014). Tumor lysis syndrome, a potentially life-threatening emergency, is the result of extreme tumor cell lysis with the release of intracellular potassium, nucleic acids, and phosphorus into the systemic circulation. Clinically, the syndrome is characterized by rapid development of hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia, and acute kidney injury (Sarno 2013). Traditionally, the syndrome most commonly occurs in patients with high-grade lymphomas and acute leukemia’s and is typically related to treatment responsive tumors. The potential rapid efficacy of gene-engineered T-cell therapies means that tumor lysis syndrome may be evidenced for a wider variety of tumors. It is therefore essential to recognize the risk of tumor lysis syndrome coincident with CRS as concomitant therapies for appropriate management of tumor lysis will be essential for optimal clinical outcome (Lee et al. 2014). Tumor lysis syndrome can occur in laboratory animals (Treuting, Albertson, and Preston 2010) but has not been reported in the preclinical setting following administration of gene-engineered T-cell therapies. However, it should remain a consideration if signs of toxicity are reported following treatment with gene-engineered T-cell therapies, particularly in animals with a large tumor burden and disseminated disease.
Figure 5.
Tumor lysis syndrome. Rapid tumor cell destruction will result in the release of intracellular contents into the circulation. The release can inundate renal elimination and cellular buffering mechanisms, leading to numerous metabolic derangements, that characterize this syndrome, specifically hyperuricemia, hyperkalemia, and hyperphosphatemia.
Neurological Toxicities
Neurological toxicities have been reported in patients following CD19 CAR T-cell therapy treatment (Table 3) with symptoms including confusion, delirium, expressive aphasia, obtundation, myoclonus, seizure, and cerebral edema (Gust et al. 2017; Maude, Frey, et al. 2014; Lee et al. 2015; Santomasso et al. 2017; Park et al. 2017). It is not yet known if the neurological toxicity is specific to CD19 CAR T cells or will be exhibited by CAR T cells targeting other tumor antigens. Neurotoxicity appears to occur in 2 phases, those patients affected in the first wave tend to show symptoms rapidly within the first 5 days of treatment. Those patients affected in the second phase do not show symptoms until beyond 5 days and typically around 2 to 4 weeks postadministration (Neelapu et al. 2017).
The pathophysiology of the neurotoxicity remains to be determined, but 2 explanations have been postulated (Neelapu et al. 2017). Firstly, the toxicity is the clinical sequelae of passive diffusion of cytokines such as IL-6 and IL-15, which can be found at high levels in the blood following CAR T-cell administration and as found following the induction of CRS (see above). Secondly, neurotoxicity may be associated with trafficking of the CAR T cell in the central nervous system, and this tends to be seen beyond 5 days of infusion (Grupp et al. 2013; Maude, Barrett, et al. 2014; Lee et al. 2015; Garfell et al. 2015). Disruption of the blood–brain barrier may also be a contributory factor (Gust et al. 2017), as it has been reported that protein levels in the CSF are elevated in patients with exhibiting neurotoxicity, compared with baseline measurements (Santomasso et al. 2017). Although to date most cases of neurological toxicity are reversible, rare life-threatening cerebral edema, in patients treated with CD19 CAR T cells, has led to a very rapid disease course with brain death within 24 hr with up to 8 patient deaths reported to date (Gust et al. 2017; Turtle et al. 2017; Reuters 2017; Harris 2017; Plieth 2017). Some of the deaths were initially attributed to the use of the chemotherapy drug fludarabine in combination with cyclophosphamide, part of a preconditioning treatment given to patients before CD19 CAR T-cell infusions. This was subsequently proven wrong when fludarabine was removed from preconditioning regimens, but there were still patient deaths. It is likely that the cause of neurological toxicity is multifactorial with disease state, levels of CD19+ cells in bone marrow, high CAR T-cell dose, CRS, and preexisting neurologic comorbidities all reported as associated with increased risk of neurologic AEs (Gust et al. 2017). There are also differences between the CD19 CAR T cells used on the different trials such as scFv affinities and costimulatory domains as well as differences in manufacturing and the impact, if any, on toxicity risk is as yet unclear.
Currently, the mechanisms leading to neurotoxicity are poorly understood and active research is ongoing to unravel the mediators and develop preventive treatments. This includes utilization of an NHP model that has been developed (Taraseviciute, Kean, and Jensen 2016). In this model, the animals in receipt of the CAR T cells but not the control T cells developed not only clinical signs and symptoms of CRS (see above) but also neurological toxicity that was manifested as behavioral abnormalities and extremity tremors with the onset of clinical symptoms coinciding with maximum CAR T-cell expansion and activation. In addition, the team reported detection, by flow cytometry, of CAR T cells in multiple regions of the brain including the frontal, parietal, and occipital lobes, as well as the cerebellum. The establishment of this model should allow a detailed analysis of the mechanisms driving these toxicities associated with CD19 CAR T cells allowing management plans to be established.
Managing Toxicity in the Clinical Setting
Understanding and developing the tools to limit potential toxicities is key to a wider adoption. Some toxicities (CRS) are a consequence of the efficacy of the therapies and detailed clinical management plans have now been published (Neelapu et al. 2017). Significant research is ongoing into methods to limit off-tumor reactivity. A number of these are based on combinatorial antigen recognition in which T-cell activation and hence specificity are the result of the activity of 2 CARs (Wilkie et al. 2012; Roybal et al. 2016). An alternative approach is the introduction of suicide genes into ACT through the introduction of herpes simplex virus thymidine kinase or inducible caspase-9 (iCasp9) genes (Hoyos et al. 2010; Oliveira et al. 2012). In these circumstances, the use of corresponding small molecule ganciclovir or the chemical inducer of dimerization AP20187, respectively, can result in the elimination of T cells when there are clinical signs of significant toxicity. An alternative approach is the use of messenger ribonucleic acid (mRNA) CAR T cells where the dose is a series of repeat administrations given over a 3-week period (Barrett et al. 2013; Beatty et al. 2014). Where the target cell surface protein is also found at low levels in normal cells, CAR T cells need to be used with caution. The advantage of the RNA CAR approach is that if AEs were noted, T-cell infusions can be terminated with the expectation that toxicity would rapidly abate because mRNA CAR expression is limited to a few days, thus rendering adverse effects self-limiting. In addition, treatments such as corticosteroids could be employed to ablate T cells when clinically predicated. Such an approach has been used to test the safety of a mesothelin CAR product where low levels of expression were known on a several normal tissues (Beatty et al. 2014).
Other research activities are focused on precision-controlled ACT in which promoters are designed to enable condition-specific activation. Thereby controlling when, for example, a CAR is expressed on the surface of the T cell. The broad range of antigen receptor models and concepts in development has the potential to enable highly specific antigens to be targeted, off-tumor effects to be minimized, and safety to be enhanced in the clinic (Fesnak, June, and Levine 2016).
Conclusions
Although this review focuses on the safety aspects of ACT, it is important to emphasize the significant and impressive clinical advances that have been achieved. However, clinically, the majority of trials to date have focused on hematological malignancies. As we move into the solid tumor arena, combinatorial approaches with, for example, cytokine-modulating therapies will be of interest in difficult to treat populations. Dissecting the role of different components in the case of safety signals may be challenging. The wider clinical use of gene-engineered T-cell therapies may also depend on the development of universal sources of allogeneic T cells, as cost will be a factor for health-care systems and indeed such treatments are now progressing into clinical trial and early indications are that toxicity management will be as important for these therapies, with the risks of graft-versus-host disease as well as the potential for even greater cell potency to be considered. It will be important to have a repertoire of nonclinical tools to understand both the benefits and the safety of these new strategies. The risks and an understanding of the biological mechanisms underpinning toxicities should be a major part of the nonclinical program for any therapy including ACTs. Due to the nature of the products, this will require the development of extensive in vitro tools as well as in vivo models where available. The continued development in our understanding of the immunological response will also support and enhance clinical management of patients receiving these therapies.
Footnotes
Authors’ Note: The author is an employee of the Cell and Gene Therapy Catapult, which is a not-for-profit organization supported by the UK Government through Innovate UK.
Author Contribution: All authors (MS) contributed to conception or design; data acquisition, analysis, or interpretation; drafting the manuscript; and critically revising the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michaela E. Sharpe ![]() http://orcid.org/0000-0002-0850-4639
http://orcid.org/0000-0002-0850-4639
References
- Alcantar-Orozco E. M., Gornall H., Baldan V., Hawkins R. E., Gilham D. E. (2013). Potential limitations of the NSG humanized mouse as a model system to optimize engineered human T cell therapy for cancer. Hum Gene Ther Methods 24, 310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaf M., Legut M., Cole D. K., Sewell A. K. (2015). The T cell antigen receptor: The Swiss army knife of the immune system. Clin Exp Immunol 181, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attarwala H. (2010). TGN1412: From discovery to disaster. J Young Pharm 2, 332–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D. M., Liu X., Jiang S., June C. H., Grupp S. A., Zhao Y. (2013). Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Hum Gene Ther 24, 717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty G. L., Haas A. R., Maus M. V., Torigian D. A., Soulen M. C., Plesa G., Chew A., et al. (2014). Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res 2, 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya F., Frigault M. J., Maus M. V. (2017). The flipside of the power of engineered T cells: Observed and potential toxicities of genetically modified T cells as therapy. Mol Ther 25, 314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C., Sommermeyer D., Hudecek M., Berger M., Balakrishnan A., Paszkiewicz P. J., Kosasih P. L., Rader C., Riddell S. R. (2015). Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol Res 3, 206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenstein T., Coulie P. G., Gilboa E., Jaffee E. M. (2012). The determinants of tumour immunogenicity. Nat Rev Cancer 12, 307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens R., Yeh R., Bernal Y., Riviere I., Sadelain M. (2010). Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: Case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther 18, 666–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens R. J., Davila M. L., Riviere I., Park J., Wang X., Cowell L. G., Bartido S., et al. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5, 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudno J. N., Kochenderfer J. N. (2016). Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 127, 3321–30.27207799 [Google Scholar]
- Cameron B. J., Gerry A. B., Dukes J., Harper J. V., Kannan V., Bianchi F. C., Grand F., et al. (2013). Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 5, 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casucci M., Hawkins R. E., Dotti G., Bondanza A. (2015). Overcoming the toxicity hurdles of genetically targeted T cells. Cancer Immunol Immunother 64, 123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornetta K., Duffy L., Turtle C. J., Jensen M., Forman S., Binder-Scholl G., Fry T., et al. (2018). Absence of replication competent lentivirus in the clinic: Analysis of infused T cell products. Mol Ther 26, 280–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila M. L., Kloss C. C., Gunset G., Sadelain M. (2013). CD19 CAR-targeted T cells induce long-term remission and B cell aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS One 8, e61338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila M. L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S. S., et al. (2014). Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6, 224ra25–ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M. E., Gross C. A., Somerville R. P., Hong Y., Schaub N. P., Rosati S. F., White D. E., et al. (2013). Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol 31, 2152–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z., Waks T., Gross G., Schindler D. G. (1993). Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 90, 720–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesnak A. D., June C. H., Levine B. L. (2016). Engineered T cells: The promise and challenges of cancer immunotherapy. Nat Rev Cancer 16, 566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfall A. L., Maus M. V., Hwang W. T., Lacey S. F., Mahnke Y. D., Melenhorst J. J., Zheng Z., et al. (2015). Chimeric antigen receptor T cells against CD19 for multiple myeloma. N Engl J Med 373, 1040–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Finkelstein S. E., Klebanoff C. A., Antony P. A., Palmer D. C., Spiess P. J., Hwang L. N., et al. (2005). Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202, 907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerry A., Sanderson J., Saini M., Tavano B., Docta R., Pumphrey N., Maroto M., et al. (2015). Preclinical safety testing of an affinity-optimised MAGE-A10T cell receptor for adoptive T cell therapy. J Immunother Cancer 3, P14–14. [Google Scholar]
- Gerry A. B. (2016). Preclinical safety testing of enhanced-affinity TCRs. European Medicines Agency. Accessed October 14, 2017 http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2016/12/WC500217500.pdf.
- Gillio-Meina C., Zielke H. R., Fraser D. D. (2016). Translational research in pediatrics IV: Solid tissue collection and processing. Pediatrics 137 doi:10.1542/peds.2015-0490. [DOI] [PubMed] [Google Scholar]
- Grupp S. A., Kalos M., Barrett D., Aplenc R., Porter D. L., Rheingold S. R., Teachey D. T., et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368, 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust J., Hay K. A., Hanafi L.-A., Li D., Myerson D., Gonzalez-Cuyar L. F., Yeung C., et al. (2017). Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 7, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Garrigue A., Wang G. P., Soulier J., Lim A., Morillon E., Clappier E., et al. (2008). Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 118, 3132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S., Von Kalle C., Schmidt M., Mccormack M. P., Wulffraat N., Leboulch P., Lim A., et al. (2003). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–19. [DOI] [PubMed] [Google Scholar]
- Harris J. (2017). Kite reports cerebral edema death in ZUMA-1 CAR T-cell trial. Onc Live. Accessed December 23, 2017 http://www.onclive.com/web-exclusives/kite-reports-cerebral-edema-death-in-zuma1-car-tcell-trial.
- Hay K. A., Hanafi L. A., Li D., Gust J., Liles W. C., Wurfel M. M., Lopez J. A., et al. (2017). Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T cell therapy. Blood 130, 2295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos V., Savoldo B., Quintarelli C., Mahendravada A., Zhang M., Vera J., Heslop H. E., et al. (2010). Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 24, 1160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. A., Morgan R. A., Dudley M. E., Cassard L., Yang J. C., Hughes M. S., Kammula U. S., et al. (2009). Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J. (2017). Modified T cells that attack leukemia become first gene therapy approved in the United States. Science. Accessed October 31, 2017 http://www.sciencemag.org/news/2017/08/modified-t-cells-attack-leukemia-become-first-gene-therapy-approved-united-states.
- Kalos M., Levine B. L., Porter D. L., Katz S., Grupp S. A., Bagg A., June C. H. (2011). T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3, 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J. N., Dudley M. E., Feldman S. A., Wilson W. H., Spaner D. E., Maric I., Stetler-Stevenson M., et al. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J. N., Dudley M. E., Kassim S. H., Somerville R. P., Carpenter R. O., Stetler-Stevenson M., Yang J. C., et al. (2015). Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33, 540–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer J. N., Yu Z., Frasheri D., Restifo N. P., Rosenberg S. A. (2010). Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116, 3875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowolik C. M., Topp M. S., Gonzalez S., Pfeiffer T., Olivares S., Gonzalez N., Smith D. D., et al. (2006). CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res 66, 10995–1004. [DOI] [PubMed] [Google Scholar]
- Kunkele A., Taraseviciute A., Finn L. S., Johnson A. J., Berger C., Finney O., Chang C. A., et al. (2017). Preclinical assessment of CD171-directed CAR T-cell adoptive therapy for childhood neuroblastoma: CE7 epitope target safety and product manufacturing feasibility. Clin Cancer Res 23, 466–77. [DOI] [PubMed] [Google Scholar]
- Kvistborg P., Shu C. J., Heemskerk B., Fankhauser M., Thrue C. A., Toebes M., Van Rooij N., et al. (2014). TIL therapy broadens the tumor-reactive CD8+T cell compartment in melanoma patients. Oncoimmunology 1, 409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers C. H. J., Sleijfer S., Van Steenbergen S., Van Elzakker P., Van Krimpen B., Groot C., Vulto A., et al. (2013). Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: Clinical evaluation and management of on-target toxicity. Mol Ther 21, 904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers C. H. J., Sleijfer S., Vulto A. G., Kruit W. H., Kliffen M., Debets R., Gratama J. W., Stoter G., Oosterwijk E. (2006). Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: First clinical experience. J Clin Oncol 24, e20–22. [DOI] [PubMed] [Google Scholar]
- Lee D. W., Gardner R., Porter D. L., Louis C. U., Ahmed N., Jensen M., Grupp S. A., Mackall C. L. (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. W., Kochenderfer J. N., Stetler-Stevenson M., Cui Y. K., Delbrook C., Feldman S. A., Fry T. J., et al. (2015). T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385, 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B. L., Miskin J., Wonnacott K., Keir C. (2017). Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev 4, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linette G. P., Stadtmauer E. A., Maus M. V., Rapoport A. P., Levine B. L., Emery L., Litzky L., et al. (2013). Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall C., D’angelo S. P., Cristea M. C., Odunsi K., Norry E., Pandite L., Holdich T., et al. (2016). Cytokine release syndrome (CRS) in patients treated with NY-ESO-1c259 TCR. J Clin Oncol 34, 3040–40. [Google Scholar]
- Mackall C., Tap W. D., Glod J., Druta M., Chow W. A., Araujo D. M., Grupp S. A., et al. (2017). Open label, non-randomized, multi-cohort pilot study of genetically engineered NY-ESO-1 specific NY-ESO-1c259t in HLA-A2+ patients with synovial sarcoma (NCT01343043). J Clin Oncol 35, 3000. [Google Scholar]
- Maher J. (2012). Immunotherapy of malignant disease using chimeric antigen receptor engrafted T cells. ISRN Oncol 2012, 278093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansh M. (2011). Ipilimumab and cancer immunotherapy: A new hope for advanced stage melanoma. Yale J Biol Med 84, 381–89. [PMC free article] [PubMed] [Google Scholar]
- Maude S. L., Barrett D., Teachey D. T., Grupp S. A. (2014). Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J 20, 119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude S. L., Frey N., Shaw P. A., Aplenc R., Barrett D. M., Bunin N. J., Chew A., et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371, 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone M. C., Fish J. D., Carpenito C., Carroll R. G., Binder G. K., Teachey D., Samanta M., et al. (2009). Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 17, 1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A. (2013). Risky business: Target choice in adoptive cell therapy. Blood 122, 3392–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P. F., Zheng Z., Dudley M. E., et al. (2013). Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36, 133–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Yang J. C., Kitano M., Dudley M. E., Laurencot C. M., Rosenberg S. A. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18, 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniappan A., Banapour B., Lebkowski J., Talib S. (2000). Ligand-mediated cytolysis of tumor cells: Use of heregulin-zeta chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther 7, 128–34. [DOI] [PubMed] [Google Scholar]
- Muranski P., Boni A., Wrzesinski C., Citrin D. E., Rosenberg S. A., Childs R., Restifo N. P. (2006). Increased intensity lymphodepletion and adoptive immunotherapy—How far can we go? Nat Clin Pract Oncol 3, 668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelapu S. S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F. L., Komanduri K. V., et al. (2017). Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat Rev Clin Oncol 15, 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. (1982). Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 155, 1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novartis. (2017). CTL019 (tisagenlecleucel): In pediatric and young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Accessed October 31, 2017 https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/oncologicdrugsadvisorycommittee/ucm567385.pdf.
- Oliveira G., Greco R., Lupo-Stanghellini M. T., Vago L., Bonini C. (2012). Use of TK-cells in haploidentical hematopoietic stem cell transplantation. Curr Opin Hematol 19, 427–33. [DOI] [PubMed] [Google Scholar]
- Park J. H., Santomasso B., Riviere I., Senechal B., Wang X., Purdon T., Wang Y., et al. (2017). Baseline and early post-treatment clinical and laboratory factors associated with severe neurotoxicity following 19-28z CAR T cells in adult patients with relapsed B-ALL. J Clin Oncol 35, 7024–24. [Google Scholar]
- Parkhurst M. R., Yang J. C., Langan R. C., Dudley M. E., Nathan D. A., Feldman S. A., Davis J. L., et al. (2011). T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 19, 620–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons D. A., Baum C. (2011). Solving the problem of gamma-retroviral vectors containing long terminal repeats. Mol Ther 19, 229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon-Charry A., Maxwell T., Lopez J. A. (2005). Dendritic cell dysfunction in cancer: A mechanism for immunosuppression. Immunol Cell Biol 83, 451–61. [DOI] [PubMed] [Google Scholar]
- Plieth J. (2017). Spotlight—Putting a number on CAR-T deaths. EPVantage. Accessed October 31, 2017 http://epvantage.com/Universal/View.aspx?type=Story&id=716231&isEPVantage=yes.
- Porter D. L., Frey N. V., Melenhorst J. J., Hwang W.-T., Lacey S. F., Shaw P., Chew A., et al. (2014). Randomized, phase II dose optimization study of chimeric antigen receptor modified T cells directed against CD19 (CTL019) in patients with relapsed, refractory CLL. Blood 124, 1982–82. [Google Scholar]
- Porter D. L., Hwang W. T., Frey N. V., Lacey S. F., Shaw P. A., Loren A. W., Bagg A., et al. (2015). Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7, 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M. A., Callahan M. K., Wolchok J. D. (2015). Immune checkpoint blockade in cancer therapy. J Clin Oncol 33, 1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., et al. (2017). Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med 9 doi:10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- Ramos C. A., Savoldo B., Torrano V., Ballard B., Zhang H., Dakhova O., Liu E., et al. (2016). Clinical responses with T lymphocytes targeting malignancy-associated kappa light chains. J Clin Invest 126, 2588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport A. P., Stadtmauer E. A., Binder-Scholl G. K., Goloubeva O., Vogl D. T., Lacey S. F., Badros A. Z., et al. (2015). NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 21, 914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Zhao Y. (2017). Advancing chimeric antigen receptor T cell therapy with CRISPR/Cas9. Protein Cell 8, 634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuters. (2017). Juno ends development of high-profile leukemia drug after deaths. Accessed December 23, 2017 https://www.reuters.com/article/us-juno-leukemia/juno-ends-development-of-high-profile-leukemia-drug-after-deaths-idUSKBN1685QQ.
- Robbins P. F., Lu Y. C., El-Gamil M., Li Y. F., Gross C., Gartner J., Lin J. C., et al. (2013). Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 19, 747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouce R. H., Heslop H. E. (2016). Forecasting cytokine storms with new predictive biomarkers. Cancer Discov 6, 579–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal K. T., Rupp L. J., Morsut L., Walker W. J., Mcnally K. A., Park J. S., Lim W. A. (2016). Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 164, 770–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruella M., Kalos M. (2014). Adoptive immunotherapy for cancer. Immunol Rev 257, 14–38. [DOI] [PubMed] [Google Scholar]
- Sadelain M., Riviere I., Brentjens R. (2003). Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 3, 35–45. [DOI] [PubMed] [Google Scholar]
- Santomasso B., Park J. H., Riviere I., Mead E., Halton E., Diamonte C., Purdon T., et al. (2017). Biomarkers associated with neurotoxicity in adult patients with relapsed or refractory B-ALL (R/R B-ALL) treated with CD19 CAR T cells. J Clin Oncol 35, 3019–19. [Google Scholar]
- Sarno J. (2013). Prevention and management of tumor lysis syndrome in adults with malignancy. J Adv Pract Oncol 4, 101–06. [PMC free article] [PubMed] [Google Scholar]
- Scholler J., Brady T. L., Binder-Scholl G., Hwang W. T., Plesa G., Hege K. M., Vogel A. N., et al. (2012). Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 4, 132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M., Mount N. (2015). Genetically modified T cells in cancer therapy: opportunities and challenges. Dis Model Mech 8, 337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraseviciute A., Kean L., Jensen M. C. (2016). Creation of the first non-human primate model that faithfully recapitulates chimeric antigen receptor (CAR) T cell-mediated cytokine release syndrome (CRS) and neurologic toxicity following B cell-directed CAR-T cell therapy. Blood 128, 651. [Google Scholar]
- Teachey D. T., Lacey S. F., Shaw P. A., Melenhorst J. J., Maude S. L., Frey N., Pequignot E., et al. (2016). Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 6, 664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuting P. M., Albertson T. M., Preston B. D. (2010). Case series: Acute tumor lysis syndrome in mutator mice with disseminated lymphoblastic lymphoma. Toxicol Pathol 38, 476–85. [DOI] [PubMed] [Google Scholar]
- Turtle C. J., Hanafi L. A., Berger C., Gooley T. A., Cherian S., Hudecek M., Sommermeyer D., et al. (2016). CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest 126, 2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle C. J., Hanafi L. A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., et al. (2016). Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8, 355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle C. J., Hay K. A., Hanafi L. A., Li D., Cherian S., Chen X., Wood B., et al. (2017). Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 35, 3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stegen S. J., Davies D. M., Wilkie S., Foster J., Sosabowski J. K., Burnet J., Whilding L. M., et al. (2013). Preclinical in vivo modeling of cytokine release syndrome induced by ErbB-retargeted human T cells: Identifying a window of therapeutic opportunity? J Immunol 191, 4589–98. [DOI] [PubMed] [Google Scholar]
- Vonderheide R. H., June C. H. (2014). Engineering T cells for cancer: Our synthetic future. Immunol Rev 257, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whilding L. M., Parente-Pereira A. C., Zabinski T., Davies D. M., Petrovic R. M. G., Kao Y. V., Saxena S. A., et al. (2017). Targeting of aberrant αvβ6 integrin expression in solid tumors using chimeric antigen receptor-engineered T cells. Mol Ther 25, 259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie S., Van Schalkwyk M. C., Hobbs S., Davies D. M., Van Der Stegen S. J., Pereira A. C., Burbridge S. E., et al. (2012). Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol 32, 1059–70. [DOI] [PubMed] [Google Scholar]
- Xing Y., Hogquist A. (2012). T-cell tolerance: Central and peripheral. Cold Spring Harb Perspect Biol 4, a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C., Thompson J. A., Byrd D., Riddell S. R., Roche P., Celis E., Greenberg P. D. (2002). Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A 99, 16168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Wu M. R., Sentman C. L. (2012). An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J Immunol 189, 2290–99. [DOI] [PMC free article] [PubMed] [Google Scholar]