Abstract
Introduction
Limited information is available on the usefulness of the PIMA™ analyser in predicting antiretroviral treatment eligibility and outcome in a primary healthcare clinic setting in disadvantaged communities in KwaZulu-Natal, South Africa.
Materials and methods
The study was conducted under the eThekwini Health Unit, Durban, KwaZulu-Natal. Comparison of the enumeration of CD4+ T-cells in 268 patients using the PIMA™ analyser and the predicate National Health Laboratory Services (NHLS) was undertaken during January to July 2013. Bland-Altman analysis to calculate bias and limits of agreement, precision and levels of clinical misclassification at various CD4+ T-cell count thresholds was performed.
Results
There was high precision of the PIMA™ control bead cartridges with low and normal CD4+ T-cell counts using three different PIMA™ analysers (%CV < 5). Under World Health Organization (WHO) guidelines (≤ 500 cells/mm3), the sensitivity of the PIMA™ analyser was 94%, specificity 78% and positive predictive value (PPV) 95%. There were 24 (9%) misclassifications, of which 13 were false-negative in whom the mean bias was 149 CD4+ T-cells/mm3. Most (87%) patients returned for their CD4 test result but only 67% (110/164) of those eligible (≤ 350 cells/mm3) were initiated on antiretroviral therapy (ART) with a time to treatment of 49 days (interquartile range [IQR], 42–64 days).
Conclusion
There was adequate agreement between PIMA™ analyser and predicate NHLS CD4+ T-cell count enumeration (≤ 500 cells/mm3) in adult HIV-positive individuals. The high PPV, sensitivity and acceptable specificity of the PIMA™ analyser technology lend it as a reliable tool in predicting eligibility and rapid linkage to care in ART programmes.
Introduction
Poor rates of linkage to care for those with low CD4+ T-cell counts, eligible for antiretroviral therapy (ART), have been reported in several African cohort studies.1,2,3,4,5
Several attrition steps exist in the continuum of care pathway: patients lost to care between testing HIV-positive and going for a CD4 test6; CD4 test result not available and/or lost7,8; patient not returning for their CD4 test result; and lack of uptake of care from eligibility to initiation of ART even in those who return for test results.9 These challenges may be overcome by point-of-care (POC) testing10, resulting in less attrition over time.2,4 It has been suggested that POC CD4 testing in those who do not return for their results would potentially increase enrolment pre-ART.9 POC CD4 testing was shown to modestly increase linkage to care and reduce pre-treatment loss to follow-up in fixed and mobile clinics.2,11,12,13 Factors contributing to pre-treatment loss to follow-up have been previously documented.8,14,15,16,17
The Alere PIMA™ POC has been evaluated against the ‘gold standard’ flow cytometry platforms, for example, Beckman Coulter using panleucogating (PLG)18; BD FACS count19,20,21; PARTEC Cytoflow™19,20; Guava and BD FACS Calibur20,21,22,23,24 for the enumeration of CD4+ T-cells in HIV-1-infected adults and in HIV-1-infected pregnant women.22
This study assessed the accuracy, sensitivity and specificity of the Alere PIMA™ POC analyser in CD4+ T-cell count enumeration compared to the predicate South African National Health Laboratory Services (NHLS) flow cytometry test (Beckman Coulter) and its potential operational role as a predictor of ART eligibility in a primary healthcare clinic (PHC) in Durban, South Africa.
Materials and methods
The study occurred at Lancers Road PHC, a facility under the eThekwini Health Unit, situated in the centre of the convergence of the taxi rank in the city centre of Durban. This PHC offers HIV Counselling and Testing (HCT) – approximately 900 per month to walk-in patients who receive pre- and post-test counselling and CD4 testing for the staging of HIV-1-infected disease to determine eligibility for ART. Patients are advised to return after 7 days for their CD4 results. As per the SA HIV and AIDS guidelines25 operating at the time of this study, patients with a CD4+ T-cell count ≤ 350 cells/mm3, upon their return, were medically assessed, and education and counselling undertaken prior to ART initiation. Those ineligible for ART, viz CD4+ count > 350 cells/mm3, were counselled to return 6 monthly for CD4+ T-cell count testing and for further medical assessment. Eligible patients, who did not return for results, were contacted telephonically to ascertain whether they had been initiated on ART elsewhere, and if not, they were encouraged to return for further care.
Testing of venous blood samples
Routine CD4+ T-cell enumeration is conducted at the NHLS one day after the blood draw via Beckman Coulter flow cytometry using PLG methodology, the standard of care in this setting as described previously.26 During January 2013 to July 2013, in 268 patients, an extra 2 mL of venous blood was drawn from the same blood draw as the routine NHLS test into another ethylenediaminetetraacetic acid (EDTA) tube for the comparison of the enumeration of CD4+ T-cells using the Alere PIMA™ technology (Alere Health Care, Waltham, Massachusetts). PIMA™ POC CD4+ T-cell enumeration was conducted by a laboratory technician who pipette-filled the PIMA™ cartridges. Three PIMA™ analysers were used in this study. CD4+ T-cell count enumeration was performed in a subset of 100 samples using the FACS Calibur.
Quality control and/or precision of PIMA™ analysers
Quality control and routine PIMA™ analyser maintenance were performed daily as per manufacturer’s guidelines: one control has low CD4+ T-cell counts (115 cells/mm3 – 235 cells/mm3) and the other has normal CD4+ T-cell counts (719 cells/mm3 – 1355 cells/mm3). Daily quality control was conducted on all 3 analysers for the first 10 measurements when a new cartridge was used and over a period of 165 days (23 January – 25 March 2014). Accuracy and precision of the NHLS PLG testing was established in the NHLS laboratories by daily monitoring of instrument stability (Flow check TM, Beckman Coulter Miami, FL) and system performance verification using normal (394 cells/mm3 – 754 cells/mm3) and low (62 cells/mm3 – 206 cells/mm3) Immunotrol™ controls (Beckman Coulter, Miami, FL). The Addington NHLS laboratory participates in the NHLS proficiency testing panels and is accredited by the South African National Accreditation System.27
Reproducibility of CD4+ T-cell enumeration across flow cytometry instruments
Comparisons of CD4+ T-cell enumeration was undertaken between flow cytometry instruments (PIMA™ POC analysers and the predicate NHLS) on 268 blood samples. Due to transport logistics, the NHLS laboratory performs testing the day after the blood draw. Therefore, a subset of 100 blood samples were tested by the PIMA™ analyser, FACS Calibur and the NHLS to ensure that differences observed between the PIMA™ analyser versus NHLS were not due to CD4 testing performed on the next day in the NHLS laboratory. CD4+ T-cell enumeration using the FACS Calibur reference method28 was undertaken on the same blood sample tube as the PIMA™ POC analyser at the Medical Research Council Central laboratory, which participates in the United Kingdom National External Quality Assessment Scheme (UK NEQAS) quality assessment programme.
Predictions of benefit of PIMA™ POC CD4 test results for ART eligibility and linkage to care
Prediction of the benefits of the PIMA™ POC CD4 testing in terms of ART eligibility and decision making was undertaken. Additionally, an assessment was undertaken to determine whether HIV-infected individuals return for their CD4+ test result and how many are lost to follow-up between ART eligibility and initiation.
The protocol was approved by the Biomedical Research Ethics Committee, University of KwaZulu-Natal (BE 212/11) and the eThekwini Research Ethics committee (28 November 2011). Written informed consent was obtained from patients > 18 years of age enrolled in the study.
Statistical analysis
It was determined that a sample size of 254 HIV-positive patients would be required to detect a difference of 15 cells/mm3 between the results of the PIMA™ POC analyser and the conventional test with 95% probability and 80% power assuming the standard deviation of difference in means is 85. In order to allow for potential problems with samples, the sample size was increased by 14 patients giving a sample size of 268.
Statistical methods
Pairwise comparison of the PIMA™ analysers was conducted using t-tests. To assess the precision of the control cartridge within each of the three PIMA™ analysers, the %CV was calculated for the 10 observations (intra-day reproducibility) and over a period of 165 days (inter-day reproducibility) at low and normal beads.
The percentage similarity (% SIM) model, Bland-Altman (BA) plots, limits of agreement (LOA) and Lin’s concordance correlation coefficient were used to assess agreement between PIMA™ analysers, FACS Calibur and NHLS.29
To assess the diagnostic accuracy of CD4+ T-cell counts by the PIMA™ POC analysers in identifying ART eligibility, sensitivity, specificity, false-negative (FN) and false-positive (FP) rates, positive predictive value (PPV) and negative predictive value (NPV) were computed for the ART initiation thresholds of ≤ 200 cells/mm3, ≤ 350 cells/mm3 and ≤ 500 cells/mm3 CD4+ T-cells. All analyses were performed using STATA (Statacorp, College Station, TX, USA) statistical version 13.
Results
Reproducibility of results of PIMA™ machines used in this study
There was high reproducibility and instrument precision (%CVs < 5%) within PIMA™ analysers 1, 2, 3 of the control cartridges over a replicate set of 10 bead analyses and over time (n = 165 days; 23 January – 25 March 2014). The bead quality control (QC) count for low and normal bead cartridges showed median %CV results for the 10 same-day observations of 2.13%, 1.28%, and 1.41% and 0.86%, 1.36%, and 0.96% for analysers 1, 2, and 3, respectively. Bead QC counts for low and normal bead cartridges showed median %CV results over the 165 days of 1.75%, 1.70%, and 1.86% and 1.14%, 1.67%, and 1.30% for PIMA™ analysers 1, 2, and 3, respectively.
System performance verification using normal (394 cells/ mm3 – 754 cells/mm3) and low (62 cells/mm3 – 206 cells/mm3) Immunotrol controls for the NHLS PLG testing was < 6%.
The majority (218/268) of HIV-1-positive individuals undergoing CD4+ T-cell count testing were women of whom 25% were 25–29 years, whereas the majority of the men were older than 30 years (Table 1). There was no significant difference in the median CD4+ T-cell count between men and women performed by the NHLS versus the PIMA™ POC analyser, although the median CD4+ T-cell count was higher in the latter. According to the NHLS versus PIMA™ POC, 81% versus 80% of HIV-positive individuals were eligible for ART initiation (≤ 500 cells/mm3), of whom 82% versus 84% were males and 81% versus 79% were females, respectively.
TABLE 1.
Characteristics of HIV-1-positive individuals undergoing CD4+ T-cell count enumeration.
| Patient characteristics | Female† | Male‡ | Total§ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | Range | n | % | Range | n | % | Range | |
| Median age (IQR), years | 32 | - | 26–37 | 33 | - | 30–40 | 32 | - | 27–38 |
| 18–24 | 39 | 17.90 | - | 4 | 8 | - | 43 | 16.00 | - |
| 25–29 | 54 | 24.77 | - | 8 | 16 | - | 62 | 23.10 | - |
| 30–34 | 46 | 21.10 | - | 18 | 36 | - | 64 | 23.90 | - |
| 35–39 | 44 | 20.20 | - | 7 | 14 | - | 51 | 19.00 | - |
| > 40 | 35 | 16.10 | - | 13 | 26 | - | 48 | 17.90 | - |
| Median (IQR) NHLS CD4 count cells/mm3 | 292 | - | 184–453 | 254 | - | 151–387 | 286 | - | 176.5–444.5 |
| Number (%) NHLS ≤ 350 cells/mm3 | 130 | 60 | - | 34 | 68 | - | 164 | 61.19 | - |
| Number (%) NHLS ≤ 500 cells/mm3 | 176 | 81 | - | 42 | 82 | - | 218 | 81 | - |
| Median (IQR) PIMA™ CD4 count cells/mm3 | 328 | - | 204–451 | 308 | - | 179–419 | 322 | - | 204–449 |
| Number (%) PIMA™ ≤ 350 cells/mm3 | 114 | 52.30 | - | 31 | 62 | - | 145 | 54.10 | - |
| Number (%) PIMA™ ≤ 500 cells/mm3 | 173 | 79 | - | 42 | 84 | - | 215 | 80 | - |
IQR, interquartile range; NHLS, National Health Laboratory Services.
Female, n = 218;
Male, n = 50;
Total, n = 268.
In a subset of 100 samples, the highest agreement was observed between PIMA™ analysers and FACS Calibur as evidenced by smaller mean bias of 7.52 and narrower BA limits of agreement from -111 to 126 and a correlation of 0.97 (Table 2). Wider BA limits of agreement (from -216 to 176 mean bias -20.3) were observed between the FACS Calibur versus NHLS with a correlation of 0.92 compared to PIMA™ analysers versus NHLS (BA limits of agreement from -226 to 200 mean bias -12.78) with a correlation of 0.90.
TABLE 2.
Bland–Altman comparison of PIMA™ analysers versus National Health Laboratory Services versus FACS Calibur.
| Measure of agreement | PIMA analysers – NHLS† | PIMA analysers – FACS Calibur† | FACS Calibur – NHLS† |
|---|---|---|---|
| Mean bias (± 1 s.d.) | -12.78 ± 106.63 | 7.52 ± 59.26 | -20.3 ± 97.97 |
| 95% CI bias | -33.94–8.38 | -4.24–19.28 | -39.74 – -0.86 |
| BA 95% LOA | -226.04–200.48 | -111.01–126.05 | -216.23–175.63 |
| % Similarity to predicate (%SIM Mean ± s.d.) | 101.3 ± 15 | 103.1 ± 12.7 | 98.7 ± 11.9 |
NHLS, National Health Laboratory Services; BA, Bland-Altman; LOA, limits of agreement.
n = 100.
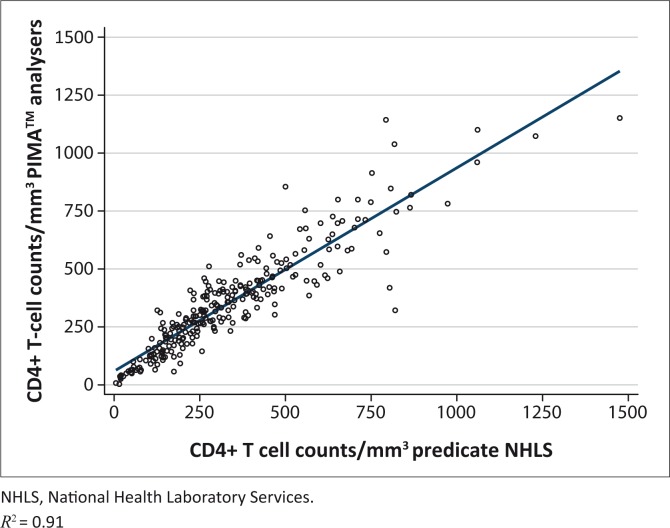
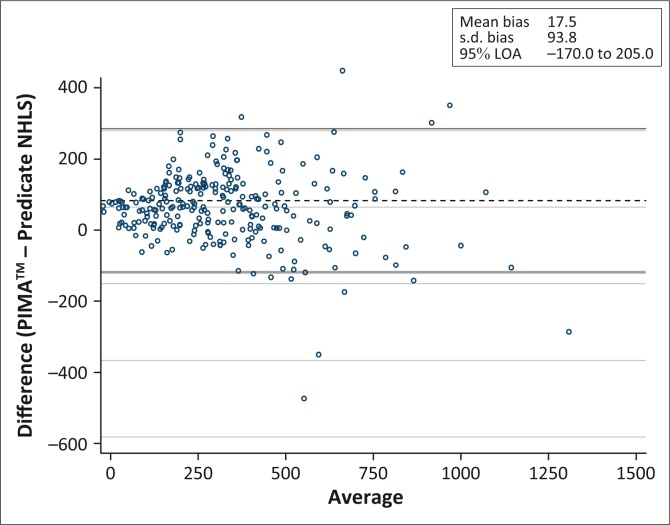
An overall correlation of 0.91 in CD4+ T-cell counts between the PIMA™ analysers and NHLS was observed (Figure 1). The overall mean difference of PIMA™ analysers NHLS was 17.5 cells/mm3 (95% confidence interval [CI] 6.2–28.8) (Table 3; Figure 2). When stratified by the following CD4+ T-cell counts: ≤ 350 cells/mm3, 351 cells/mm3 – 500 cells/mm3, ≤ 500 cells/mm3 and > 500 cells/mm3, the mean difference of PIMA™ analysers – NHLS was 33 cells/mm3 (95% CI 23–42), 22 cells/mm3 (95% CI -3.5–47), 30 cells/mm3 (95% CI 21–39) and -36 cells/mm3 (95% CI -78– 6.1), respectively. Acceptable mean percentage similarity in the range of 95% – 110%, with %SIM CVs < 15%, was observed at all CD4+ T-cell count ranges.
FIGURE 1.
Comparison of CD4+ T-cell counts obtained by the PIMA™ analysers and the National Health Laboratory Services in whole-blood samples.
TABLE 3.
Comparison of PIMA™ analysers versus National Health Laboratory Services as categorised by CD4+ T-cell counts.
| Measure of agreement | ≤ 350 cells/mm3† | 351 cells/mm – 500 cells/mm3‡ | ≤ 500 cells/mm3§ | > 500 cells/mm3¶ | All CD4+ T-cell counts†† |
|---|---|---|---|---|---|
| Median bias | 21 | 13 | 21 | -23 | 18 |
| BA bias (± 1 s.d.) | 32.9 ± 61.0 | 21.5 ± 90.8 | 30.1 ± 69.4 | -36.1±150.04 | 17.5 ±93.8 |
| 95% CI bias | 23.4–42.3 | -3.5–46.6 | 20.8–39.4 | -78.3–6.1 | 6.2–28.8 |
| BA 95% LOA | -89.2 –154.9 | -160.0–203.0 | -108.7–168.9 | -336.2–263.9 | -170.0–205.0 |
| % Similarity to predicate (% SIM mean ± s.d.) | 107.4 ± 15.2 | 102.7 ± 10.5 | 106.2 ± 14.35 | 97.97 ± 10 | 106 ± 15.5 |
| %SIM CV | 14.20% | 10.20% | 13.50% | 10.20% | 14.60% |
BA, Bland-Altman; LOA, limits of agreement.
n = 164;
n = 53;
n = 217;
n = 51;
n = 268.
FIGURE 2.
Bland–Altman plot PIMA™ point-of-care analyser – National Health Laboratory Services versus the average of PIMA™ point-of-care analyser and National Health Laboratory Services.
Under previous SA ART guidelines of ≤ 200 cells/mm3 and ≤ 350 cells/mm3, the PIMA™ POC analysers displayed a sensitivity and specificity of 73.5%/98.4% and 83.5%/92.3%, respectively (Table 4). Under the current SA guidelines of ≤ 500 CD4+ T-cells/mm3, a high sensitivity of 94% and PPV of 95% was observed at the sacrifice of lower specificity of 78%. In the 13 FNs with ≤ 500 cells/mm3, the mean bias was 149 CD4+ T-cells/mm3.
TABLE 4.
Performance of PIMA™ analysers compared to National Health Laboratory Services at different CD4+ T-cell thresholds.
| CD4+ T-cells/mm3 | Sensitivity (%) | Specificity (%) | Number misclassified | Number misclassified (%) | Correctly classified (%) | FP Rate | FN Rate | Negative predictive value (%) | Positive predictive value (%) |
|---|---|---|---|---|---|---|---|---|---|
| ≤ 200 | 73.50 | 98.40 | 25 | 9.3 | 90.7 | 3/25 | 22/25 | 85.20 | 95.30 |
| ≤ 350 | 83.50 | 92.30 | 35 | 13.0 | 87.0 | 8/35 | 27/35 | 78.10 | 94.50 |
| ≤ 500 | 94.00 | 78.40 | 24 | 9.0 | 91.0 | 11/24 | 13/24 | 75.50 | 94.90 |
Note: PIMA™ point-of-care [POC] testing for CD4 counts in predicting antiretroviral initiation in HIV-infected individuals in KwaZulu-Natal, Durban, South Africa.
FP, false-positive; FN, false-negative.
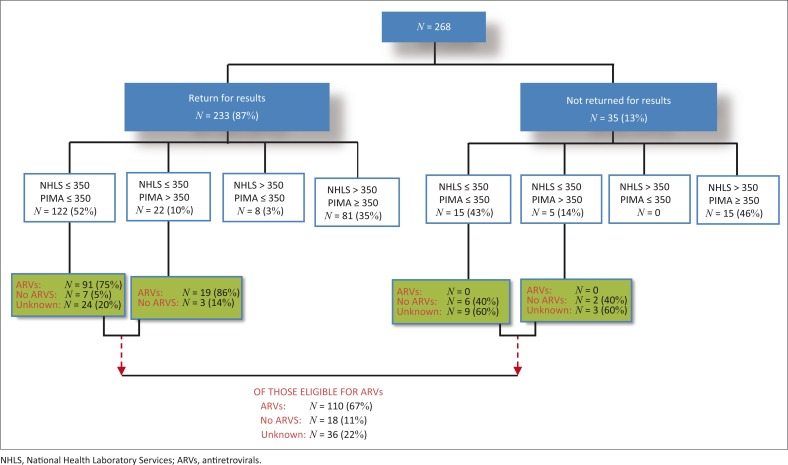
As the study was conducted during 2013, linkage to care data is presented according to the NHLS laboratory CD4 test result of ≤ 350 cells/mm3,25 164/268 (61%) of patients were eligible for ART on the day of HCT compared to 145/268 (54%) with the PIMA™ analyser POC CD4 test (Figure 3). The majority of patients (87%) returned to the Lancers Road PHC for their CD4 test result. However, according to the ART register at Lancers Road PHC, 110/164 (67%) of eligible patients were initiated on ART. Of the 35 individuals who did not return to the clinic for their CD4 test result, 20 were eligible (according to the NHLS CD4 result), and not initiated on ART. The median time taken for patients to return for CD4 results was 8 days (IQR 7–14 days) and 7 days (IQR 7–11 days) in those with ≤ 200 cells/mm3.
FIGURE 3.
Comparison of CD4+ T-cell counts with respect to antiretroviral therapy eligibility by PIMA™ point-of-care analyser versus National Health Laboratory Services in those HIV-1-infected patients who returned and did not return for their results.
The median time to ART initiation from date of CD4 test was: 49 days (IQR 42–64 regardless of CD4+ T-cell count; 36–63 days in those with ≤ 200 cells/mm3).
Discussion
Conventional flow cytometry to determine CD4 counts usually requires that samples be sent to a central laboratory, which may be off-site. Although the turn-around time for a CD4 test result by the NHLS is 24–72 hours, HIV-1-infected patients are counselled to return to the PHC within 1 week for receipt of these results. POC technologies can reduce these delays resulting in rapid linkage to care. This study demonstrated a high PPV and sensitivity and acceptable specificity in predicting ART eligibility (≤ 500 cells/mm3) using the PIMA™ POC analyser as compared to the NHLS CD4 test.
The majority of HIV-1-positive individuals undergoing CD4 testing were women, of whom 25% were 25–29 years old, whereas the majority of men were older than 30 years of age. There were no significant differences in the median CD4+ T-cell count in men versus women performed by the NHLS versus the PIMA™ POC analyser, although the median count was higher in the latter. Overall, according to NHLS versus PIMA™ POC, 81% versus 80% of individuals were eligible for ART initiation (≤ 500 cells/mm3), of whom 82% versus 84% were men and 81% versus 79% were women, respectively.
There was high reproducibility in all three PIMA™ POC analysers using normal and low beads with coefficient of variation < 5% over time (10 and 165 days). The PIMA™ POC analyser slightly overestimates NHLS flow cytometry in CD4+ T-cell enumeration in this study, which corroborates most studies using capillary or venous blood.26,30,31,32 This overestimation is minimal (mean bias 17 cells/mm3) and is not clinically significant. Differences have been reported on conventional CD4 testing platforms between the BD FACS count versus the BD FACS Calibur23 where the mean bias between the two platforms was -76 cells/mm3 (95% CI LOA -316.0–163.0).
The adequate correlation between the PIMA™ POC analyser and FACS Calibur (0.97) corroborates similar findings in another study.21 Although a correlation of > 0.90 was observed between the three platforms, these differences are due to variability of instrument settings, antibodies and fluorochromes used, gating strategies and sample volume input.
The overall sensitivity of the PIMA™ POC CD4 test in HIV-1-infected adults and pregnant mothers to determine their eligibility for ART has been reported at 96.3% in individuals with a CD4+ T-cell count of ≤ 250 cells/mm3,24 and 92% and 91% in those with ≤ 350 cells/mm3,20,22. The total misclassifications have been documented in several studies using the PIMA™ POC analyser: 31%,18 17%,12 5.2%,33 6.7% – 14%,31 10%,22 11.4%34 and 9%.19 This study found 13% misclassifications, of which 27/35 were FNs at ≤ 350 CD4+ T-cells/mm3. At a CD4+ T-cell threshold of ≤ 500 cells/mm3, 91% of patients were correctly classified as either eligible or ineligible for ART. In the 13 FNs, the mean bias observed was 149 cells/mm3. The PPV of 95% indicates that only 5% of those who are diagnosed as eligible for ART according to the PIMA™ POC analyser would not be needing treatment according to the NHLS CD4 test result. A high sensitivity of 94% was observed at the sacrifice of lower specificity of 78%. This high sensitivity corroborates recent findings21,22,34 and fits in well with current SA HIV and AIDS guidelines35 where all those eligible for treatment will be initiated but it will come at a cost of low specificity, whereby individuals not needing treatment will be commenced on ART. However, in light of ART-lowering viral loads and reducing horizontal transmission,36,37 this downside is minimised. A recent study has demonstrated that as household ART coverage is increased, there is a decrease in HIV acquisition.38 The agreement in these data between the PIMA™ POC analyser and NHLS laboratory-based flow cytometry appears to decline with increasing CD4+ T-cell count ≥ 500 cells/mm3. This is not of concern as these HIV-1-infected individuals are ineligible for ART under current guidelines.
From the operational perspective in the use of the PIMA™ POC analyser, similar to other studies using venous or capillary blood,18,20,21,22,33 we also experienced reading errors (8%) mostly because of movement and vibration.34 The ‘operator’ used in our study was a trained laboratory technician compared to health professionals, for example, nurse or counsellor. Several studies have reported that the PIMA™ POC is interchangeable with conventional platforms,18,20,22,30,31,33,39 although a study in Kenya23 found it to be unreliable due to the high coefficient of repeatability and misclassification in favour of undertreatment compared to the FACS Calibur.
Under the standard SA HIV and AIDS guidelines operating at the time of the study, we observed that the median time for patients to return for their CD4 results was 8 days and 7 days in those with ≤ 200 cells/mm3, with a median of 49 days regardless of CD4+ T-cell count from CD4 testing to ART initiation. The use of the PIMA™ POC analysers could facilitate the fast tracking of patients with CD4+ T-cell count ≤ 200 cells/mm3 onto ART within 7 days. In this study, the provision of immediate CD4 test results to patients would have prevented the 35/268 not having access to their results (through them not returning), in whom over half (57%) were eligible for ART.
The high rates (61%) of ‘walk–in’ patients found in this study who were eligible (≤ 350 cells/mm3) for immediate ART at the time of the HIV test, half of whom had CD4+ T-cell counts ≤ 248 cells/mm3, and the time lapse to ART initiation undergirds the urgent need for the use of the rapid PIMA™ POC technology. At a threshold of ≤ 500 cell/mm3, 75% of patients had a median CD4+ T-cell count of 444 cells/mm3 at the time of the HIV test. A recent study reported that providing same-day POC CD4 testing that is not rapid has no benefit in health outcomes.9 As suggested by others,11,18,40,41 we agree that using existing infrastructure and based on demand, the integration of a PHC POC mini-laboratory run by dedicated personnel (laboratory technician) is possible, offering tests for staging and pathology that assess ART eligibility.42,43 However, as suggested in a recent systematic review,44 this needs to be supported by streamlining services through minimising patient clinic visits,22 addressing psychosocial issues and barriers to healthcare,45 optimising the opportunity for patient empowerment through counselling and peer support,46 emphasising the importance of starting and adhering to ART if eligible,6 positive health-seeking behaviours and encouragement for patient ownership of their health. A family-centred model of integrated healthcare incorporating most of the above-mentioned health system changes has previously been shown, in a similar population, to yield high adherence (94%) and retention in the care and management of HIV-1-positive individuals.47,48 In this study, similar reasons for not linking into care were given as found previously45; of those eligible for ART who did not access treatment (33%), the reasons given upon telephonic communication were economic (no money to cover transport costs), social (too busy to come to the clinic), structural (cannot take time off work) and emotional (were not ready to take ART and they were still feeling well). We would anticipate that there would be an increase in loss of uptake of care at the higher CD4+ T-cell count threshold of ≤ 500 cells/mm3 because of the reasons re-iterated. Previous studies have shown that provision of immediate CD4+ T-cell count results increased the number of patients linking into care.2,4,6,11,13
Conclusion
In summary, the overall agreement between PIMA™ POC analyser and NHLS CD4+ T-cell count enumeration in adult HIV-1-positive individuals was acceptable with clinically insignificant mean bias. Together with high PPV and sensitivity and acceptable specificity, the PIMA™ POC CD4 test has the potential role for CD4+ T-cell enumeration in PHC settings and lends itself to be an excellent facilitator in rapid linkage to care in ART programmes, particularly that it has been demonstrated in simulated cohort models of HIV-1-infected adults and pregnant women, to result in not only better clinical outcomes but also to cost savings in the long term.49,50 Even in the era of ‘test and treat’,51 PIMA™ POC CD4 testing would facilitate the fast tracking of patients with low CD4+ T-cell counts (< 200 cells/mm3) for the administration of cotrimoxazole prophylaxis as well as in screening for cryptococcal infection in patients with < 100 cells/mm3. The operational role of the PIMA™ POC CD4 test in provision of immediate CD4+ T-cell count results combined with integrated health system changes and interventions such as mobile phone technology and provision of incentives need to be evaluated in a variety of settings across the HIV cascade, to determine its implementation effectiveness in linkage to care, time to ART initiation and retention in HIV care.
Acknowledgements
We acknowledge the input of the Thembi Ngubane and Thabisile Maluleka, the phlebotomists on the study, and are indebted to the participants themselves for being part of this project. We thank especially the Lancers Road clinic staff for their co-operation and the eThekwini Municipality Health Unit for permission to conduct the study in their facility. The Medical Research Council HIV Prevention Research Unit for infrastructural support for the study.
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Authors’ contributions
P.K. and A.C. designed the study and provided overall supervision for the study. S.N. recruited patients and obtained informed consents. E.S. collected data from study subjects and N.N. provided clinical oversight. M.S. and S.R. conducted laboratory analyses. T.R. conducted the statistical analyses and M.S., P.K. and A.C. undertook data interpretation. All authors contributed to the writing of the manuscript and approved the final version.
Footnotes
Research Project no.: BE212/11 (BREC UKZN)
How to cite this article: Skhosana M, Reddy S, Reddy T. et al. PIMA™ point-of-care testing for CD4 counts in predicting antiretroviral initiation in HIV-infected individuals in KwaZulu-Natal, Durban, South Africa. S Afr J HIV Med. 2016; 17(1), a444. http://dx.doi.org/10.4102/sajhivmed.v17i1.444
References
- 1.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: Systematic review. Trop Med Int Health. 2010;15(s1):1–15. http://dx.doi.org/10.1111/j.1365-3156.2010.02508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson B, Schnippel K, Ndibongo B, Xulu T, Brennan A, Long L. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: An evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr. 2012;61(2):e13 http://dx.doi.org/10.1097/QAI.0b013e31825eec60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub- Saharan Africa: A systematic review. PLoS Med. 2011;8(7):e1001056 http://dx.doi.org/10.1371/journal.pmed.1001056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: An observational cohort study. Lancet. 2011;378(9802):1572–1579. http://dx.doi.org/10.1016/S0140-6736(11)61052-0 [DOI] [PubMed] [Google Scholar]
- 5.Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: Systematic review and meta-analysis. Trop Med Int Health. 2012;17(12):1509–1520. http://dx.doi.org/10.1111/j.1365-3156.2012.03089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patten GE, Wilkinson L, Conradie K, Isaakidis P, Harries AD, Edginton ME. Impact on ART initiation of point-of-care CD4 testing at HIV diagnosis among HIV-positive youth in Khayelitsha, South Africa. J Int AIDS Soc. 2013;16(1):18518. http://dx.doi.org/10.7448/IAS.16.1.18518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: A systematic review. J Int AIDS Soc. 2014;17(1):18809. http://dx.doi.org/10.7448/IAS.17.1.18809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP. The “ART” of linkage: Pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5(3):e9538 http://dx.doi.org/10.1371/journal.pone.0009538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson BA, Schnippel K, Brennan A, Long L, Xulu T, Maotoe T. Same-Day CD4 testing to improve uptake of HIV care and treatment in South Africa: Point-of-care is not enough. AIDS Res Ther. 2013;2013:941493 http://dx.doi.org/10.1155/2013/941493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachariah R, Reid S, Chaillet P, Massaquoi M, Schouten E, Harries A. Viewpoint: Why do we need a point-of-care CD4 test for low-income countries? Trop Med Int Health. 2011;16(1):37–41. http://dx.doi.org/10.1111/j.1365-3156.2010.02669.x [DOI] [PubMed] [Google Scholar]
- 11.Faal M, Naidoo N, Glencross DK, Venter WD, Osih R. Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic Syndr. 2011;58(3):e54–e9. https://dx.doi.org/10.1097/QAI.0b013e3182303921 [DOI] [PubMed] [Google Scholar]
- 12.Jani IV, Sitoe NE, Chongo PL, Alfai ER, Quevedo JI, Tobaiwa O. Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS. 2011;25(6):807–812. http://dx.doi.org/10.1097/QAD.0b013e328344f424 [DOI] [PubMed] [Google Scholar]
- 13.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: A systematic review. J Int AIDS Soc. 2012;15(2):17383 http://dx.doi.org/10.7448/IAS.15.2.17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassett IV, Wang B, Chetty S. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir immune Defic Syndr. 2009;51(2):135–139. http://dx.doi.org/10.1097/QAI.0b013e3181a44ef2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassett IV, Regan S, Chetty S, Giddy J, Uhler LM, Holst H. Who starts antiretroviral therapy in Durban, South Africa? … not everyone who should. AIDS. 2010;24(Suppl 1):S37.s http://dx.doi.org/10.1097/01.aids.0000366081.91192.1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Micek MA, Gimbel-Sherr K, Baptista AJ, Matediana E, Montoya P, Pfeiffer J. Loss to follow-up of adults in public HIV care systems in Mozambique: Identifying obstacles to treatment. J Acquir immune Defic Syndr. 2009;52(3):397–405. http://dx.doi.org/10.1097/QAI.0b013e3181ab73e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Layer EH, Kennedy CE, Beckham SW. Multi-level factors affecting entry into and engagement in the HIV continuum of care in Iringa, Tanzania. PLoS One. 2014;9(8):e104961 http://dx.doi.org/10.1371/journal.pone.0104961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glencross DK, Coetzee LM, Faal M, Masango M, Stevens WS, Venter WF. Performance evaluation of the Pima™ point-of-care CD4 analyser using capillary blood sampling in field tests in South Africa. J Int AIDS Soc. 2012;15(1):3 http://dx.doi.org/10.1186/1758-2652-15-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wade D, Diaw PA, Daneau G, Camara M, Dieye TN, Mboup S. CD4 T-Cell enumeration in a field setting: Evaluation of CyFlow counter using the CD4 easy count kit-Dry and Pima CD4 systems. PLoS One. 2013;8(9):e75484 http://dx.doi.org/10.1371/journal.pone.0075484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakar M, Mahajan B, Shaikh N, Bagwan S, Sane S, Kabra S. Utility of the point of care CD4 analyzer, PIMA, to enumerate CD4 counts in the field settings in India. AIDS Res Ther. 2012;9(1):26 http://dx.doi.org/10.1186/1742-6405-9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wade D, Daneau G, Aboud S, Vercauteren GH, Urassa WS, Kestens L. WHO multicenter evaluation of FACSCount CD4 and Pima CD4 T-cell count systems: Instrument performance and misclassification of HIV-infected patients. J Acquir immune Defic Syndr. 2014;66(5):e98 http://dx.doi.org/10.1097/QAI.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myer L, Daskilewicz K, McIntyre J, Bekker L-G. Comparison of point-of-care versus laboratory-based CD4 cell enumeration in HIV-positive pregnant women. J Int AIDS Soc. 2013;16(1):18649 http://dx.doi.org/10.7448/IAS.16.1.18649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mwau M, Adungo F, Kadima S, Njagi E, Kirwaye C, Abubakr NS. Evaluation of PIMA™® point of care technology for CD4 T cell enumeration in Kenya. PLoS One. 2013;8(6):e67612 http://dx.doi.org/10.1371/journal.pone.0067612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manabe YC, Wang Y, Elbireer A, Auerbach B, Castelnuovo B. Evaluation of portable point-of-care CD4 counter with high sensitivity for detecting patients eligible for antiretroviral therapy. PLoS One. 2012;7(4):e34319 http://dx.doi.org/10.1371/journal.pone.0034319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The South African antiretroviral treatment guidelines 2013 Version 14 March 2013 Department of Health, South Africa: (accessed 24 May 2016) [Google Scholar]
- 26.Glencross DK, Janossy G, Coetzee LM, Lawrie D, Aggett HM, Scott LE. Large-scale affordable Panleucogated CD4+ testing with proactive internal and external quality assessment: In support of the South African national comprehensive care, treatment and management programme for HIV and AIDS. Cytom Part B Clin Cy. 2008;74(S1):S40–S51. http://dx.doi.org/s10.1002/cyto.b.20384 [DOI] [PubMed] [Google Scholar]
- 27.Tholen DW, Impact of international standards and initiatives on proficiency testing for medical laboratories. Accredit Qual Assur. 2004;9(11–12):653–656. http://dx.doi.org/10.1007/s00769-004-0801-6 [Google Scholar]
- 28.Schnizlein-Bick CT, Spritzler J, Wilkening CL, Nicholson JK, O’Gorman MR. Evaluation of TruCount absolute-count tubes for determining CD4 and CD8 cell numbers in human immunodeficiency virus-positive adults. Clin Diagn Lab Immunol. 2000;7(3):336–343. http://dx.doi.org/10.1128/cdli.7.3.336-343.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bland JM, Altman D, Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. http://dx.doi.org/10.1016/S0140-6736(86)90837-8 [PubMed] [Google Scholar]
- 30.Sukapirom K, Onlamoon N, Thepthai C, Polsrila K, Tassaneetrithep B, Pattanapanyasat K. Performance evaluation of the Alere PIMA CD4 test for monitoring HIV-infected individuals in resource-constrained settings. J Acquir Immune Defic Syndr. 2011;58(2):141–147. http://dx.doi.org/10.1097/QAI.0b013e31822866a2 [DOI] [PubMed] [Google Scholar]
- 31.Mtapuri-Zinyowera S, Chideme M, Mangwanya D, Mugurungi O, Gudukeya S, Hatzold K. Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. J Acquir Immune Defic Syndr. 2010;55(1):1–7. http://dx.doi.org/10.1097/QAI.0b013e3181e93071 [DOI] [PubMed] [Google Scholar]
- 32.Van Schaik N, Kranzer K, Myer L, Raditlhalo E, Thebus E, Davies N, editors. Field validation of the PIMA analyzer in a mobile clinic setting in South Africa. 18th Conference on Retroviruses and Opportunistic Infections; 2011 27 Feb–02 March, Boston, MA. [Google Scholar]
- 33.Diaw PA, Daneau G, Coly AA, Ndiaye BP, Wade D, Camara M. Multisite evaluation of a point-of-care instrument for CD4+ T-cell enumeration using venous and finger-prick blood: The PIMA CD4. J Acquir Immune Defic Syndr. 2011;58(4):e103–e11. http://dx.doi.org/10.1097/QAI.0b013e318235b378 [DOI] [PubMed] [Google Scholar]
- 34.Galiwango RM, Lubyayi L, Musoke R, Kalibbala S, Buwembo M, Kasule J. Field evaluation of PIMA point-of-care CD4 testing in Rakai, Uganda. PLoS One. 2014;9(3):e88928 http://dx.doi.org/10.1371/journal.pone.0088928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Department of Health National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria, South Africa: Department of Health, Republic of South Africa; 2014. [Google Scholar]
- 36.Holstad MM, DiIorio C, McCarty F. Adherence, sexual risk, and viral load in HIV-infected women prescribed antiretroviral therapy. AIDS Patient Care STDS. 2011;25(7):431–438 http://dx.doi.org/10.1089/apc.2010.0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds SJ, Makumbi F, Nakigozi G, Kagaayi J, Gray RH, Wawer M. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25(4):473 http://dx.doi.org/10.1097/QAD.0b013e3283437c2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandormael A, Newell M, Barnighausen T, Tanser F. Use of antiretroviral therapy in households and risk of HIV acquisition in rural KwaZulu-Natal, South Africa, 2004–12: A prospective cohort study. Lancet Glob Health. 2014;2:209–215. http://dx.doi.org/10.1016/S2214-109X(14)70018-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbert S, Edwards S, Carrick G, Copas A, Sandford C, Amphlett M. Evaluation of PIMA point-of-care CD4 testing in a large UK HIV service. Sex Transm Infect. 2012;88(6):413–417. http://dx.doi.org/10.1136/sextrans-2012-050507 [DOI] [PubMed] [Google Scholar]
- 40.Glencross DK, Coetzee LM, Cassim N. An integrated tiered service delivery model (ITSDM) based on local CD4 testing demands can improve turn-around times and save costs whilst ensuring accessible and scalable CD4 services across a national programme. PLoS One. 2014;9(12):e114727 http://dx.doi.org/10.1371/journal.pone.0114727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cassim N, Coetzee LM, Schnippel K, Glencross DK. Estimating implementation and operational costs of an integrated tiered CD4 service including laboratory and point of care testing in a remote health district in South Africa. PLoS One. 2014;9(12):e115420 http://dx.doi.org/10.1371/journal.pone.0115420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gous N, Scott L, Potgieter J, Ntabeni L, Enslin S, Newman R. Feasibility of performing multiple point of care testing for HIV anti-retroviral treatment initiation and monitoring from multiple or single fingersticks. PLoS One. 2013;8(12):e85265 http://dx.doi.org/10.1371/journal.pone.0085265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehe JD, Sitoe NE, Tobaiwa O, Loquiha O, Quevedo JI, Peter TF. Evaluating operational specifications of point-of-care diagnostic tests: A standardized scorecard. PLoS One. 2012;7(10):e47459 http://dx.doi.org/10.1371/journal.pone.0047459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Govindasamy D, Meghij J, Negussi EK, Baggaley RC, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low-and middle-income settings – A systematic review. J Int AIDS Soc. 2014;17(1):19032 http://dx.doi.org/10.7448/IAS.17.1.19032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: A systematic review. AIDS. 2012;26(16):2059–2067. http://dx.doi.org/10.1097/QAD.0b013e3283578b9b [DOI] [PubMed] [Google Scholar]
- 46.Muhamadi L, Tumwesigye NM, Kadobera D, et al. A single-blind randomized controlled trial to evaluate the effect of extended counseling on uptake of pre- antiretroviral care in Eastern Uganda. Trials. 2011;12(1):184 http://dx.doi.org/10.1186/1745-6215-12-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwaan L, Kindra G, Mdutyana L, Coutsoudis A. Prevention is better than cure–the art of avoiding non-adherence to antiretroviral treatment. S Afr J HIV Med. 2010;11(2):8–10. [Google Scholar]
- 48.Coutsoudis A, Spooner E, Kindra G, Ndlovu NI. Evaluation of a Primary Health Care Level, HIV Care and Treatment Programme which included integrated, family-centered, respectful care: Durban 2003–2009. Department of Paediatrics and Child Health, University of KwaZulu-Natal: 2013 [Google Scholar]
- 49.Hyle EP, Jani IV, Lehe J, Su AE, Wood R, Quevedo J. The clinical and economic impact of point-of-care CD4 testing in Mozambique and other resource-limited settings: A cost-effectiveness analysis. PLoS Med. 2014;11(9):e1001725 http://dx.doi.org/10.1371/journal.pmed.1001725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciaranello AL, Myer L, Kelly K, Christensen S, Daskilewicz K, Doherty K. Point- of- care CD4 testing to inform selection of antiretroviral medications in South African antenatal clinics: A cost-effectiveness analysis. PLoS One. 2015;10(3):e0117751 http://dx.doi.org/10.1371/journal.pone.0117751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organisation Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV [homepage on the Internet]. 2015. [cited 2015 Oct 20]. Available from: http://www.who.int/en/ [PubMed]