Abstract
Specific delivery of therapeutic agents to solid tumors and their bioavailability at the target site are the most clinically important and challenging goals in cancer therapy. Liposomes are promising nanocarriers and have been well investigated for cancer therapy. In spite of preferred accumulation in tumors via the enhanced permeability and retention (EPR) effect, inefficient drug release at the target site and endosomal entrapment of long circulating liposomes are very important obstacles for achieving maximum anticancer efficacy. Thus, additional strategies such as stimulus-sensitive drug release are necessary to improve efficacy. Stimuli-sensitive liposomes are stable in blood circulation, however, activated by responding to external or internal stimuli and control the cargo release at the target site. This review focuses on state of the art of stimuli-responsive liposomes. Both external stimuli-responsive liposomes, including hyperthermia (HT), magnetic, light, and ultrasound-sensitive liposomes and internal stimuli (pH, reduction, and enzyme) responsive liposomes are covered.
Key Words: Liposomes, Cancer, Stimuli-responsive, Thermosensitive, Magnetoliposomes, Light, Ultrasound, pH, Redox, Enzyme
Introduction
Cancer is one of the most common causes of death in the world that was responsible for millions of deaths in the 20th century, and still remains one of most challenging diseases to treat. Cancer as a significant cause of morbidity and mortality with more than ten million new cases every year is the biggest public health concern (1, 2). Although chemotherapeutics are widely used for cancer treatment, and effective to some extent, their nonspecific biodistribution to normal tissues and affecting healthy rapidly dividing cells (enterocytes, white blood cells, etc.) cause drug-induced toxicity and numerous serious side effects. In addition, free cancer therapeutics suffer from poor solubility, low stability, rapid in-vivo degradation, and short plasma residence time. To overcome the aforementioned limitations of chemotherapeutics, various nanoparticulate systems including liposomes, polymeric nanoparticles, polymeric micelles, dendrimers, and inorganic nanoparticles have been increasingly investigated for cancer therapy due to several advantages (3-7).
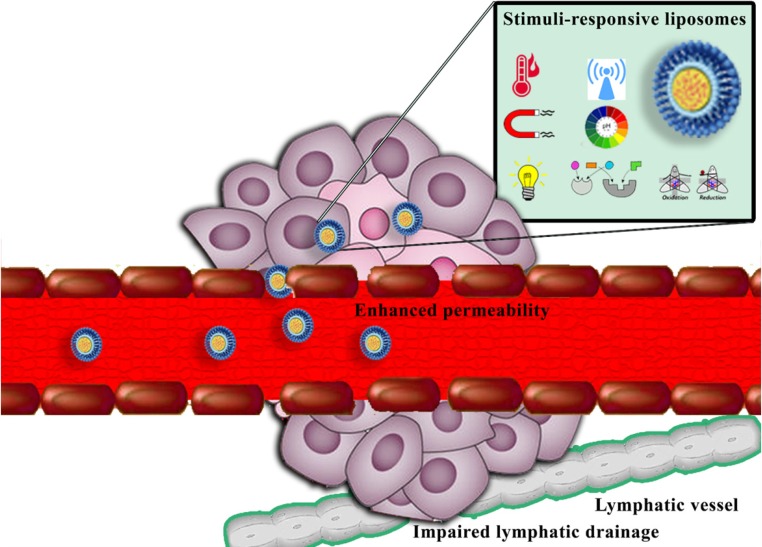
The enhanced permeability and retention (EPR) effect, which is due to unique structural features of many solid tumors, including hypervasculature, poorly aligned defective endothelial cells lacking smooth muscle layer, and impaired lymphatic drainage (Figure 1), has the critical role in nanoparticles accumulation at the tumor site (8, 9). However, the effective nanocarriers of chemotherapeutics cannot only rely on the EPR effect. In order to use beneficial features of the EPR effect for anticancer delivery, nanomedicines should exhibit prolonged circulation time. Size, surface charge, hydrophobicity, composition, PEGylation, and shape are critical parameters in pharmacokinetic properties of nanostructures (10, 11).
Figure 1.
Schematic illustration of enhanced permeability and retention (EPR) effect and passive targeting of nanocarriers to solid tumors
Although nanoparticle accumulation in tumor site is very critical for antitumor efficacy enhancement and reduced adverse effects, inefficient drug release at the target site and endosomal entrapment of nanoparticles are very important obstacles for achieving maximum efficacy. To overcome these problems, stimuli-responsive nanocarriers are designed to trigger drug release by either externally applied stimuli (such as hyperthermia (HT), magnetic, light, and ultrasound) or pathophysiological characteristic of tumors (internal stimuli). Usually in tumors, the pH value is lower and reduction potential and enzyme activity are different. These features provide the opportunity to use these endogenous factors as triggers to control cargo release at the target sites (12-16).
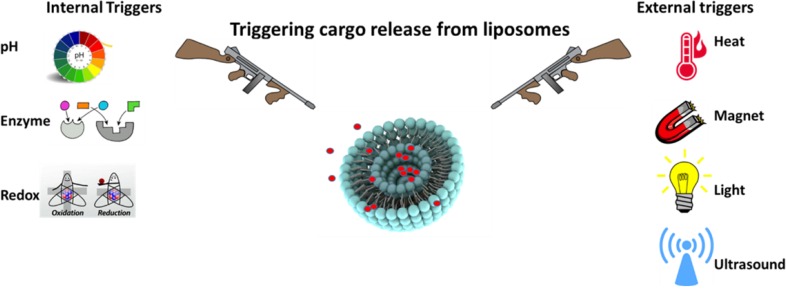
Among various nanoparticles, liposomes are widely-studied colloidal particles for cancer therapeutics delivery. Liposomes are bilayer vesicles composed of phospholipids and cholesterol and formed spontaneously when lipids are dispersed into an aqueous phase. Liposomes have numerous advantages such as biodegradability, excellent biocompatibility, non-immunogenicity, lack of toxicity, ability to incorporate hydrophilic and hydrophobic cargoes, enhanced bioavailability, and high stability that make them a unique carrier for drug and gene delivery (17-20). Beyond cancer, liposomes are used to enhance drug efficacy in various diseases including cardiovascular diseases (21-23), infections (24), autoimmune disorders (25), and skin diseases (26). In this article, we focus on recent studies of stimuli-sensitive liposomes for cancer therapy. Both external stimuli-responsive liposomes, including HT, magnetic, light, and ultrasound-sensitive liposomes and internal stimuli (pH, reduction, and enzyme) responsive liposomes will be covered (Figure 2).
Figure 2.
Schematic illustration of stimuli-responsive liposomes triggered upon external as well as internal stimulation
External stimuli-responsive liposomes
Thermosensitive liposomes
Hyperthermia (HT) was mentioned as a cancer treatment throughout the Middle ages (27). However, unsatisfactory heating techniques and equipment, the lack of precise non-invasive thermometry, and ineffective targeting of deeply-seated tumors hampered clinical applications of HT (28). During the past two decades, testing HT as a component of cancer treatment strategies in a total of 109 trials has improved confidence in its clinical potential (29). The combination of mild HT with chemotherapy and radiation has been shown to improve cancer outcomes (30).
When combined with thermosensitive liposomes (TSL), HT can improve treatment efficacy by various mechanisms: (i) increasing liposomes accumulation in the tumor site by increasing tumor vascular permeability and local blood flow, (ii) triggering cargo release from TSL within tumor vasculature and interstitium, (iii) increasing cancer cell membrane permeability to the released drugs, and (iv) being directly cytotoxic to tumor cells (31, 32). Therefore, combination of HT and TSL administration holds great potential in cancer therapy (Table 1).
Table 1.
Examples of recent studies on thermosensitive liposomes
| Stage of study | Cargo | In-vivo tumor model | Components | Targeting ligand | Reference |
|---|---|---|---|---|---|
| In-vitro | Marker | _ | DPPC, DSPC, DPPG2 | _ | (61) |
| In-vitro | Arsenic trioxide | _ | DPPC, MPPC | _ | (62) |
| In-vitro | DOX | _ | DPPC, HSPC, Chol, DSPE-PEG, p(NIPAAm-co-PAA) | _ | (63) |
| In-vitro | _ | _ | DPPC, C12H25-PNIPAM-COOH, PnBA-PNIPAM | _ | (64) |
| In-vitro | DOX, Marker | _ | DPPC, Chol, Dimyristoylphosphatidic acid, 2C12-p(NIPMAM-co-NIPAM) | _ | (65) |
| In-vitro | DOX | _ | DPPC, DSPC, DSPE-PEG, short-chain glucosylceramide, | _ | (66) |
| In-vitro / In-vivo | DOX, Marker | Murine sarcoma (BFS-1 cells) | DPPC, DSPC, DSPE-PEG | _ | (67) |
| In-vitro / In-vivo | Cisplatin, Marker | Cervical carcinoma (ME-180 cells) | DPPC, DPPG, MSPC, DSPE-PEG | _ | (68) |
| In-vitro / In-vivo | DOX, Marker | Breast cancer (MDA-MB-435 cells) | DPPC, Chol, DSPE-PEG, ammonium bicarbonate, gold nanorods | _ | (69) |
| In-vitro / In-vivo | 5-FU, Marker | Colorectal adenocarcinoma (HT-29 cells) | DPPC, Chol, DSPE-PEG | _ | (70) |
| In-vitro / In-vivo | Cisplatin, Marker | Cervical carcinoma (ME-180 cells) | DPPC, DPPG, MSPC, DSPE-PEG, | _ | (71) |
| In-vitro / In-vivo | Marker | Murine melanoma (B16B16 cells) | DPPC, DSPC, DSPE-PEG, DPTAP | _ | (72) |
| In-vitro / In-vivo | DOX | Melanoma (BLM cells) | DPPC, DSPC, DSPE-PEG | _ | (17) |
| In-vitro / In-vivo | DOX | Murine squamous cell carcinoma (SSC-7 cells) | DPPC, Chol, DSPE-PEG, Elastin-like polypeptide | _ | (73) |
| In-vitro / In-vivo | DOX, Marker | Murine mammary tumor (EMT-6 cells) | DPPC, Chol, DSPE-PEG, Elastin-like polypeptide | _ | (50) |
| In-vitro / In-vivo | Oxaliplatin | Lewis Lung Cancer Cell (LLCC) | DPPC, MSPC, DSPE-PEG, Poloxamer 188 | _ | (52) |
| In-vitro / In-vivo | DOX, Marker | _ | DPPC, Polxamer 188 | _ | (51) |
| In-vitro / In-vivo | DOX, Marker | Murine colon cancer (C26 carcinoma cells) | EPC, DOPE, Chol, DSPE-PEG, EOEOVE-ODVE | _ | (74) |
| In-vitro | DOX | _ | DPPC, Chol, Brij78 | Pamidronate | (75) |
| In-vitro | DOX, Marker | _ | DPPC, DSPC, DSPE-PEG | Cetuximab (Fab' fragments), GE11 peptide | (57) |
| In-vitro / In-vivo | DOX, Marker | Breast cancer (MCF-7 cells) | DPPC, DSPC, Chol, DSPE-PEG, Elastin-like polypeptide | cRGD peptide | (50) |
| In-vitro / In-vivo | DOX, Marker | Murine melanoma (B16B16 cells) | DPPC, DSPC, DSPE-PEG | cRGD peptide | (60) |
| In-vitro / In-vivo | DOX, Marker | Multi resistant breast cancer (MCF-7/ADR cells) | DPPC, MSPC, DSPE-PEG | CREKA peptide | (76) |
| In-vitro / In-vivo | DOX, Marker | Epidermoid carcinoma (KB cells) | DPPC, Chol, DSPE-PEG | Folate | (53) |
| In-vitro / In-vivo | DOX, Marker | Ovarian carcinoma (SK-OV3 cells) | EPC, Chol, DSPE-PEG5000, EOEOVE-ODVE | Trastuzumab | (49) |
| In-vitro / In-vivo | siRNA-CPP, Marker | Fibrosarcoma (HT1080 cells) | DPPC, MSPC, DSPE-PEG | NGR peptide | (77) |
Abbreviations: Brij78: Polyoxyethylene stearyl ether; C12H25-PNIPAM-COOH: C12H25-poly(N-isopropylacrylamide)-COOH; 2C12 (PNIPMAM co NIPAM): 2C12 (poly N-isopropylmethacrylamide co poly N-isopropylacrylamide); Chol: Cholesterol; CREKA peptide: Cys-Arg-Glu-Lys-Ala; cRGD peptide: Cyclic Arg-Gly-Asp peptide; DOPE: 1,2-Dioleoylsn-glycero-3-phosphoethanolamine; DOX: Doxorubicin; DPPC: 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine; DPPG: 1,2-Dipalmitoyl-snglycero-3-phosphoglycerol; DPTAP: 1,2-Dipalmitoyl-3-trimethylammonium-propane; DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine; DSPE-PEG: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-(polyethylene glycol)-2000; EOEOVE-ODVE: Block copolymer compounds of octadecyl vinyl ether and 2-(2-ethoxy)ethoxyethyl vinyl ether; EPC: Egg phosphatidylcholine; 5-FU: 5-Fluorouracil; HSPC: Hydrogenated soy phosphatidylcholine; MPPC: Monopalmitoylphosphatidylcholine; MSPC: Mono-stearoyl-sn-glycero-3-phosphatidylcholine; NGR peptide: Asn-Gly-Arg peptide; PnBA-PNIPAM: Poly(n-butylacrylate-b-N-isoropylacrylamide)-poly(N-isopropylacrylamide); p(NIPAAm-co-PAA): Copolymer of N-isopropylacrylamide conjugated with propylacrilic acid; siRNA-CPPs: Small (or short) interfering RNA via disulfide-bonds conjugated with cell penetrating peptides
The design of TSL is based on pioneering work of Yatvin et al. (33) in 1978 on neomycin liposomal formulation which was the first formulation of traditional TSL. Over the next few decades, traditional TSL were further developed from lipid membranes that undergo phase transition from a gel to a liquid phase upon heating and the encapsulated cargoes leak out of the liposome during the phase transition. Early formulations of traditional TSL were generally composed of dipalmitoylphosphatidylcholine (DPPC), a saturated 16-carbon chain fatty acid with transition temperature (Tc) around 41 °C (32, 34 and 35). Increased drug release in response to heat was observed with pure DPPC TSL, however the amount and rate of release were relatively limited (33).
Adding either distearoyl phosphocholine (DSPC) or hydrogenated soy phosphocholine (HSPC) to the DPPC TSL increased packing incompatibility, bilayer permeability, and the amount and rate of cargo released (32, 36).
Following the development of stealth PEGylated liposomes in 1990, PEGylated TSL were studied in 1994 (37). Koning and his coworkers investigated the optimum 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-PEG(2000) (DSPE-PEG) percentage in TSL to achieve stealth liposomes with enhanced content release under mild HT. Different percentages of DSPE-PEG (1 to 10 mol %) were incorporated in TSL and cargo release as well as vesicle stability were monitored. Cargo leakage at physiological condition was reported with 6 mol% and higher DSPE-PEG. TSL with 5 mol% DSPE-PEG were stable at 37 °C, released 60% carboxyfluorescein in 1 min and almost 100% of cargo in 1 h at 42 °C and reported as the optimum TSL (38).
Dewhirst and Needham proposed the idea of formulating lysolipid based TSL (LTSL) that promoted rapid drug release in mild HT condition (39-42 °C). This formulation, composed of DPPC, 1-stearoyl-2-hydroxy-sn-glycero-3-phosphatidylcholine (MSPC), and DSPE-PEG (at 86:10:4 molar ratio), has been commercialized by Celsion under the trade name of ThermoDox®, the first and only TSL reaching clinical development. To ensure a sharp transition temperature, cholesterol was not added to the formulation; however, ThermoDox® has a relatively short plasma residence time (39, 40).
Banno et al. found out the lysolipid (MSPC, 0-10 mol%) had a concentration-dependent effect on In-vitro drug release at 42 °C. However, within 1 h postinjection of LTSL, approximately 70% of lysolipid was lost (41).
There are two proposed mechanisms for cargo release upon HT with LTSL: i) formation of nanopores by lysolipids and DSPE-PEG in the bilayer during the phase transition (39) and ii) desorption of lysolipids from the bilayer membrane at Tc and formation of molecular scale defects for drugs escape (41). Lysolipids transfer from LTSL into biological membranes and their dissociation upon LTSL dilution in the blood stream may result in a negative impact on the temperature-sensitivity, premature drug release at physiological conditions, and its associated adverse effects (32).
The HEAT trial evaluating the combination of ThermoDox® and radiofrequency ablation in comparison to radiofrequency ablation alone for inoperable hepatocellular carcinoma treatment failed to reach its primary endpoint in progression-free survival. Allen and her coworkers extensively reviewed the possible reasons and factors underlying this failure (42).
Lindner‘s group described a TSL based formulation based on 1,2-dipalmitoyl-sn-glycero-3-phosphodiglycerol (DPPG2) for doxorubicin (DOX) (43) and pyrimidine analogue gemcitabine (44). While similar release profile with ThermoDox® was obtained (43), significantly prolonged plasma residence time was achieved (i.e. half-life of 5 h in rats and 9.6 h in hamsters) (45). Gemcitabine prodrug loaded DPPG2 TSL were also stable at 37 °C in serum and exhibited a temperature dependent cargo release > 40 °C. Plasma half-life of gemcitabine was significantly increased from 0.07 h (free drug) to 0.53-2.59 h (liposomal formulations). Therapy of soft tissue sarcoma BN175 with combination of gemcitabine loaded DPPG2 TSL and HT was the most effective treatment strategy (44).
Another approach for heat sensitizing of liposomal nanocarriers is to add synthetic polymers that are bilayer membrane disruptive in response to HT into lipid composition. Such polymers can either add to TSL to improve their heat-responsive functionality or incorporate to non-thermosensitive liposomes to make them thermosensitive. Temperature-sensitive polymers such as poly(N-isopropylacrylamide) (p(NIPAAm)) (46, 47), poly(N-vinyl ethers) (48, 49), elastin-like polypeptide (50), and poloxamers (Pluronic®) (51, 52), are among the most extensively studied temperature-sensitive polymer incorporated TSL.
Most of heat-responsive nanoparticles were not decorated with ligands for receptor-mediated targeting. However, a few recent studies have focused on design of next generation of TSL that combine targeting and triggered drug release. Various ligands such as folate (53, 54), trastuzumab antibody (herceptin) (49, 55 and 56), epidermal growth factor receptor (EGFR) specific Fab̕ fragment (57), affibodies (58), and peptides (57, 59 and 60) were conjugated to TSL.
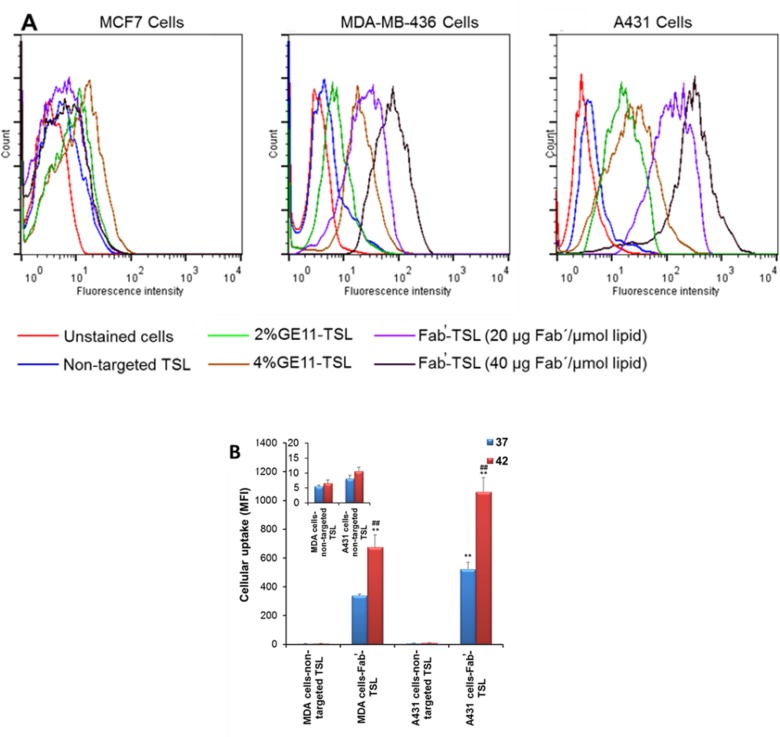
Multifunctional TSL conjugated with anti-EGFR ligands (GE11 peptide and Fab̕ fragments of cetuximab) for targeted delivery and localized HT triggered release of chemotherapy were designed by our team (57) (Figure 3). Ligand decoration did not significantly alter the physicochemical characteristics of TSL. Compared to GE11 conjugated TSL, Fab̕-coated TSL (Fab̕-TSL) bound to the EGFR overexpressed cancer cells more specifically and efficiently as shown by flow cytometry and live cell imaging analyses. Calcein loaded Fab̕-TSL exhibited adequate stability at the physiological condition (<4% calcein released after 1 h at 37 °C in serum) and a temperature dependent release at > 40 °C. Combination of HT and Fab̕ modification enhanced cytotoxicity of DOX encapsulated TSL. The drug loaded Fab̕-TSL cytotoxicity was also correlated to EGFR density on the cancer cells (Figure 3) (57).
Figure 3.
EGFR targeted thermosensitive liposomes (TSL) were successfully prepared for simultaneous tumor targeted and stimulus-responsive drug delivery. (A) Cellular uptake of labeled non-targeted and targeted TSL by cancer cells with different expression of EGFR receptors. Fab̕ modified TSL can more efficiently bind to the EGFR overexpressed cells as compared to GE11 decorated TSL. (B) Upon internalization dramatic intracellular cargo release was observed upon hyperthermia as confirmed by flow cytometry analysis. Reprinted from reference (57), Copyright 2016, with permission from Elsevier
Magnetic field-sensitive liposomes
Magnetic nanoparticles, especially iron oxide nanoparticles, show great promise in biomedical applications due to their biocompatibility and unique features. They are used for magnetic resonance imaging (MRI), gene transfection, heat generation (or HT) under an alternating current (AC) magnetic field, targeted drug delivery, cell sorting, and cancer treatments (78-80).
The combination of magnetic nanoparticles and liposomes, commonly called “magnetoliposomes”, was first introduced in 1988 by De Cuyper and Joniau (81). Since then, magnetoliposomes have been used in MRI imaging, targeted drug delivery, and HT-mediated controlled drug release (82, 83).
Both bottom up and top down methods have been used for preparation of iron oxide nanoparticles. However, top down methods have some limitations on particle size and scale of production, thus bottom up techniques such as coprecipitation and thermal decomposition are more frequently used to prepare nanoparticles with hydrophilic and hydrophobic surfaces (84, 85).
Magnetoliposomes can be formed by three different approaches: i) encapsulation of hydrophilic nanoparticle in the aqueous core of liposomes; ii) incorporation of hydrophobic nanoparticle within lipidic bilayer of liposomes; iii) binding magnetic nanoparticles on the surface of liposomes (80, 86). The first two methods are more commonly used.
Magnetic nanoparticles are one of the promising carriers for targeted delivery. By applying external field gradient from magnet, magnetic nanoparticles are attracted to the magnetic force. One of the main applications of magnetoliposomes is heat triggered cargo release mediated by an externally applied AC magnetic field at specific region. The heating of the magnetic nanoparticles depends on magnetic property of nanoparticles, frequency and amplitude of the magnetic field, and surrounding environment. Therefore, in order to control the temperature rise of magnetic nanoparticles, in depth optimization of magnetic field condition and surrounding environment is crucial for each magnetic nanocarrier (80). HT generated by magnetic nanoparticle under AC magnetic field can result in phase transition of the lipid bilayer from gel to liquid and trigger and control release of drugs encapsulated in magnetoliposomes. By tuning the bilayer composition of liposomes, the membrane phase transition can be adjusted to be around HT temperature (34, 82). Table 2 summarizes some recent researches of HT-mediated triggered release from magnetoliposomes. As an example of these researches, docetaxel loaded magnetoliposomes were prepared from purified magnetite and liposomes for gastric cancers. Tumor volume on 7th day after treatment was at least 6 times lower in the animal group received docetaxel loaded magnetoliposomes in addition to applying magnetic field compared to free drug group. However, drug loaded liposomes treatment group without exposing to AC magnetic field showed comparable efficacy to drug solution group (87).
Table 2.
Examples of recent studies on magnetic field-sensitive liposomes
| Stage of study | Cargo | In-vivo tumor model | Components | Magnetic field specifications1 | Reference |
|---|---|---|---|---|---|
| In-vitro | 5-FU | _ | PC, Fe3O4 nanoparticles | f = 250 kHz, H = 4 kA/m | (99) |
| In-vitro | Gemcitabine | _ | DPPC, Chol, Fe3O4 nanoparticles | f = 356 kHz, H = 30 kA/m, B = 2T | (100) |
| In-vitro | Paclitaxel | _ | DPPC, PG, Fe3O4 nanoparticles | f = 423kHz, H = 10 kA/m | (101) |
| In-vitro | Marker | _ | DSPC, POPC, SOPC, DSPE-PEG, Palmityl-nitroDOPA, Iron oxide nanoparticles | f = 230 kHz | (102) |
| In-vitro | Curcumin | _ | DPPC, Chol, DSPE-PEG, Fe3O4 nanoparticles | (103) | |
| In-vitro / In-vivo | Arsenic trioxide | Hepatocarcinoma (SMMC-7721 cells) | DPPC, Chol, Mn0.5Zn0.5Fe2O4 nanoparticles | f = 230 kHz | (104) |
| In-vitro / In-vivo | Docetaxel | Gastric cancer (MKN45 cells) | DLPC, DOPE, FeFe2O4 nanoparticles | f = 478 kHz, H = 6.36 kA/m, P = 1kW | (87) |
| In-vitro / In-vivo | DOX-CPP conjugate, Marker | Breast adenocarcinoma (MCF-7 cells) | DPPC, MSPC, Fe3O4 nanoparticles | f = 423kHz, H = 10 k A/m | (95) |
| In-vitro | Marker | _ | SPC, Fe3O4 nanoparticles | f = 20 kHz, H = 100 A/m | (105) |
| In-vitro | Marker | _ | HSPC, Chol, Fe3O4 nanoparticles | f = 20 kHz, H = 60 A/m | (106) |
| In-vitro | Marker | _ | Cetyltrimethylammonium chloride, Myristic acid, Fe3O4 nanoparticles | f = 520 kHz, H = 28 kA/m, B = 145 mT | (107) |
| In-vitro | DOX | _ | SPC, Fe3O4 nanoparticles | f = 50 Hz, B = 30 mT | (89) |
| In-vitro | Marker | _ | DPPC, DSPC, Chol, Fe3O4 nanoparticles | f = 214.8 Hz, B = 3 T | (88) |
| In-vitro / In-vivo | Oxaliplatin, Gemcitabine | Breast cancer (MCF-7 cells) | PC, DMPG, Chol, Fe3O4 nanoparticles | B = 0.5 T | (108) |
| In-vitro | DOX, Marker | _ | DPPC, Chol, DSPE-PEG, Fe3O4 nanoparticles | f = 290 kHz, H = 12 kA/m | (109)2 |
| In-vitro | DOX | _ | DOPC, DSPE-PEG, DPTAP, Fe2O3 nanoparticles | f = 287 kHz, H = 5.9 ×105 kA/m, P = 1 kW | (90)2 |
| In-vitro / In-vivo | Marker | Hepatocarcinoma (CD90+ stem cells) | DPPC, DSPE-PEG, Fe3O4 nanoparticles | f = 200 kHz | (110)3 |
Abbreviations: Chol: Cholesterol; CPP: Cell penetrating peptide; DLPC: Dilauroylphosphatidylcholine; DMPG: 1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol; DOPC: 1,2-Dioleoyl-sn-glycero-3-phosphocholine; DOPE: 1,2-Dioleoylsn-glycero-3-phosphoethanolamine; DOX: Doxorubicin; DPPC: 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine ; DPTAP: 1,2-Dipalmitoyl-3-trimethylammonium-propane; DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine; DSPE-PEG: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-(polyethylene glycol)-2000; 5-FU: 5-Fluorouracil; HSPC: Hydrogenated soy phosphatidylcholine; MSPC: Mono-stearoyl-sn-glycero-3-phosphatidylcholine; Palmityl-nitroDOPA: Palmityl-nitro 3,4-dihydroxyphenylalanine; PC: Phosphatidylcholine; PG: 1-Palmitoyl-2-oleoyl-sn-glycero-3-phospho-rac-glycerol; POPC: 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; SOPC: 1-Stearoyl-2-oleoyl-sn-glycero-3-phosphocholine; SPC: Soybean phosphatidylcholine
B, Magnetic induction; f, Frequency; H, Magnetic intensity; M, Magnetization; P, Magnetic power.
Folate decorated nanocarrier for targeted delivery.
CD90 antibody decorated nanocarrier for targeted delivery.
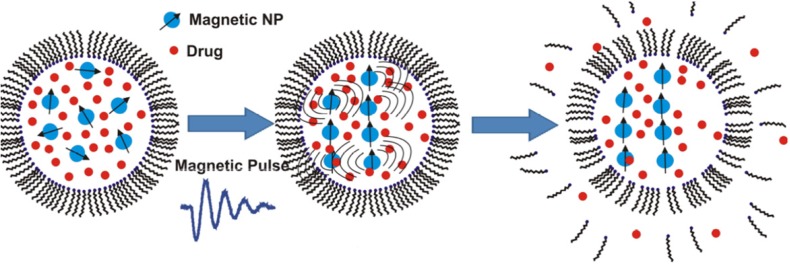
Stimulating drug release from magnetoliposomes by pulsed or low frequency magnetic field is a recent area of research that draws a lot of attention for triggering cargo release under controlled temperature (Table 2). For achieving fast drug release, short magnetic pulses were applied to disrupt the membrane of magnetoliposomes (Figure 4). Ultrasound generation under this magnetic field may also play a role in the drug release from the magnetic liposomes (88). Carboxymethyl dextran-coated magnetoliposomes with high loading ability for DOX were prepared and showed on-demand drug release under low-frequency alternating magnetic field. The hybrid nanostructures were demonstrated as a potential T2-weighted contrast agent for In-vitro MRI measurements (89).
Figure 4.
Fast release of the liposomes’ payload by using short magnetic pulses to disrupt the lipid bilayer of liposomes loaded with magnetic nanoparticles. Reprinted from reference (88) Copyright 2014, with permission from American Chemical Society
Ligand conjugated magnetoliposomes have been also studied to achieve more specific drug delivery. Magnetoliposomes have been decorated with folate (90), hyaluronic acid (91, 92), anti-αvβ3 antibody (93), sugar moieties (94), and cell-penetrating peptides (CPPs) (95) to achieve ligand targeted magnetic liposomes. Hyaluronic acid, specifically bind to the CD44, is a promising ligand for tumor targeting due to overexpression of CD44 on various tumors including the colon, pancreas, breast, and ovarian (96). Hyaluronic acid decorated magnetoliposomes have been investigated for triggered release and targeted delivery of an anticancer drug (docetaxel). Docetaxel was incorporated in the vesicle bilayers while citric acid-coated magnetic nanoparticles were encapsulated in the aqueous cores. Targeted nanoparticles were about 190 nm and spherical in shape. Drug loaded targeted magnetoliposomes showed accelerated drug release under near-infrared laser irradiation and superior cellular uptake in comparison with the conventional non-targeted liposomes (91).
CPPs are attractive ligands in targeted drug delivery with the ability to transport various small and bulk cargoes intracellularly (97). However, their lack of specificity is a major limitation for CPPs’ systemic application (98). In order to control their biodistribution and present CPPs at target site, in a recent study, Lin et al. reported multifunctional targeted magnetoliposomes that encapsulated these ligands (95). CPP derived from penetratin was conjugated to DOX. CPP-DOX conjugate and Fe3O4 were co-encapsulated into lysolipid based TSL composed of DPPC:MSPC:DSPE-PEG2000 (87:3:10 mass ratio). The results demonstrated that the vesicles possessed appropriate size (98 nm) and encapsulation efficiency (87%). When AC magnetic field was applied for 30 min, about 86% CPP-DOX was released from the liposomes (12-fold compared to control condition). In-vitro cytotoxicity studies showed both CPP conjugation as well as HT-mediated by AC magnetic field improved anticancer efficacy. Moreover, in-vivo study in a breast xenograft model showed superior antitumor efficacy of multifunctional magnetoliposomes activated by AC magnetic field (95).
Light-sensitive liposomes
The success of light-triggered delivery system is dependent on adequate light source selection that can penetrate the tissues, photosensitizing properties of the therapeutic agents, and instrumentation. The preferred wavelengths are in the near-infrared (NIR) regions (~700 nm to 1100 nm) as the light penetration is more than 1 cm depth in the body (111, 112). Light absorbing pharmaceutical agents typically called as photosensitizers are promising candidates for photodynamic therapy (PDT). PDT is a minimally invasive cancer treatment generally based on light-mediated excitation of a photosensitizer resulting in localized production of reactive oxygen species (ROS) and destruction of nearby unwanted biological agents (113, 114). Due to hydrophobic properties and non-specific biodistribution of photosensitizers, their application in cancer therapy meets technical challenges. Nanoparticles especially liposomal formulations of photosensitizers are attractive systems for improved and targeted delivery of photosensitizers (114, 115) (Table 3). In formulation of successful photo-triggerable liposomes, retention of vesicle stability and entrapped cargo before accumulation at the target site as well as efficient activation/destabilization of liposomes in the tissue by the source light are very important parameters (111, 112). Visudyne is a successful example of photosensitizer (verteporfin) liposomal formulations that is currently clinically used.
Table 3.
Examples of recent studies on light-sensitive liposomes
| Stage of study | Cargo | In-vivo tumor model | Components | Light wavelength | Photosensitizing agent or group | Reference |
|---|---|---|---|---|---|---|
| In-vitro | Marker | _ | DSPC, DMPC, Chol | UV (365 nm) | ZnPC | (128) |
| In-vitro | Marker | _ | DPPC, DSPC, Lyso PC, DSPE-PEG | NIR (808 nm) | ICG | (129) |
| In-vitro | Marker | _ | SOPC, DOPC, SLPC | Visible (590 nm) | m-THPP, Pheophorbide a, Verteporfin | (130) |
| In-vitro | DOX, Marker | _ | DPPC, DSPE-PEG, DC8,9PC | Visible (514 nm) | _ | (125) |
| In-vitro | Marker | _ | DPPC, MPPC, DPPE-PEG | NIR (760 nm) | Gold nanoparticles | (131) |
| In-vitro | Marker | _ | DSPC, DPPC | UV (365 nm) | Gold nanoparticles (Au NPs) | (132) |
| In-vitro | Marker | _ | EPC, DPPC, DOPC, DLiPC, DPhPC | Visible (532, 633 nm) | AlPcS3, ZnPcGlyc4, Chlorin e6 | (133) |
| In-vitro | Marker | _ | EPC, PVA carrying a malachite green moiety | UV | _ | (134) |
| In-vitro | Marker | _ | DPPC, DSPE-PEG, DC8,9PC | Visible (514 nm) | _ | (126) |
| In-vitro | Marker | _ | DSPC, photocleavable lipid | UV (365 nm) | Amphiphilic lipids containing amino acids and o-nitrobenzyl groups | (123) |
| In-vitro | DOX | _ | HSPC, DMPG, Chol | UV (365 nm) | Azobenzene moiety | (120) |
| In-vitro | Marker | _ | DMPC, DMPG, DMPE, DMPS, | UV (365 nm), Visible (532 nm) | Azobenzene moiety | (135) |
| In-vitro / In-vivo | DOX | Breast cancer (MCF 7 cells) | DSPC, DSPE-PEG | NIR (980 nm) | Upconversion nanoparticles; Azobenzene moiety | (121)1 |
| In-vitro / In-vivo | DOX, Marker | Ovarian carcinoma (SKOV3 cells), Lung adenocarcinoma (A549 cells) | DPPC, HSPC, Chol, DSPE-PEG | NIR | Gold nanoparticles | (136)2 |
Abbreviations: AlPcs3: Aluminum trisulfophthalocyanine; Chol: Cholesterol; DC8,9PC: 1,2-Bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine; DLiPC: Dilinoleoylphosphatidylcholine; DMPC: 1,2-Dimyristoyl-sn-glycero-3-phosphocholine; DMPE: Dimyristoyl phosphatidylethanolamine; DMPG: 1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol; DMPS: Dimyristoyl phosphatidylserine; DOPC: 1,2-Dioleoyl-sn-glycero-3-phosphocholine; DOX: Doxorubicin; DPhPC: Diphytanoylphosphatidylcholine; DPPC: 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine; DPPE-PEG: Dipalmitoylphosphatidylethanolamine-[N-methoxy(polyethylene glycol)-2000]; DSPE-PEG: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-(polyethylene glycol)-2000; DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine; EPC: Egg phosphatidylcholine; HSPC: Hydrogenated soy phosphatidylcholine; ICG: Indocyanine green; Lyso PC: 1-Stearoyl-2-hydroxy-sn-glycero-3-phosphocholine; MPPC: Monopalmitoylphosphatidylcholine; m-THPP: [5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin]; NIR: Near infrared; PC: Phosphatidylcholine; PVA: Poly(vinyl alcohol); SLPC: 1-Stearoyl-2-linoleoyl-sn-glycero-3-phosphocholine; SOPC: 1-Stearoyl-2-oleoyl-sn-glycero-3-phosphocholine; UV: Ultraviolet; ZnPC: Zinc phthalocyanine; ZnPcGlyc4: Neutral zinc phthalocyanine with four glycerols attached to the peripheral (4,5) positions of the isoindoline subunits
Folate decorated nanocarrier for targeted delivery.
HER2 antibody decorated nanocarrier for targeted delivery.
A number of photo-triggerable synthetic phospholipids have been investigated to undergo various chemical processes including photosensitization, photopolymerization, photooxidation, photoisomerization, or the degradation of photocleavable lipids that have been explained in recent review articles (116, 117). The majority of these nanosystems were based on light-triggered modifications in conjunction with a photosensitizing molecule either incorporated in the bilayer membrane or encapsulated in the aqueous core. The photo-induced modifications were mostly irreversible changes with the exception of phospholipid molecules with photo-triggering capability via the cis–trans isomerization (111, 112 and 117).
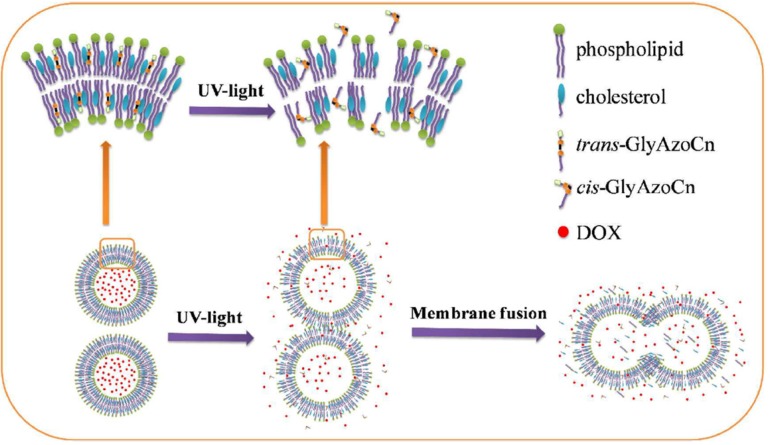
Azobenzenes are a class of chemical compounds that undergo photoisomerization of their cis and trans isomers. Bis-Azo PC (a photochromic lipid) and azobenzene cholesterol derivatives were studied (118, 119). Recently, newly structured azobenzene derivatives, azobenzene-contained glycolipids, have been synthesized. The photo-induced control of DOX release from liposomes was investigated (Figure 5). The isomerization process in ethanol solution was much faster than that in the liposome bilayer, indicating the hindering effect of surrounding lipids in the liposomal bilayer. Among the synthesized azobenzene-contained glycolipids, GlyAzoC7 was shown to be the most favorable photosensitive actuator for controlling cargo release. In the dark, less than 10% drug leakage was observed in 10 h but nearly 100% of cargoes instantaneously released with ultraviolet (UV) irradiation (120). However, due to limited tissue penetration of UV/visible wavelengths, achieving a suitable photo-triggering at in-vivo conditions was a challenging issue. A new hybrid vesicle based on azobenzene liposome and phosphatidylcholine modified upconversion nanoparticle (UCNP) was designed for precise remote control of drug release using NIR light. The encapsulated UCNPs converted NIR light into the UV/visible region emissions which can be immediately absorbed by the photoresponsive azobenzene amphiphilic molecules in the liposomal bilayer (121).
Figure 5.
The diagram of photoisomerization induced burst release of doxorubicin from liposomes embedded by azobenzene-contained glycolipid. Reprinted from reference (120) Copyright 2017, with permission from American Chemical Society
Photocleavable liposome is another approach to trigger drug release in response to light and designed on the basis of destabilization and disruption of liposome membrane by breakdown products of irradiation. Chandra and co-workers synthesized several amphiphiles containing a nitrobenzyl moiety separating a polar amino acid headgroup from a long hydrophobic tail (122, 123). To develop analogues with closer structure to lipids, a photocleavable 2-nitrobenzyl group embedded within the acyl chain was synthesized by Bayer et al. (124).
Another research groups have focused on photopolymerization rendering the destabilization of liposomal bilayer by intermolecular photo-crosslinking of phospholipids. bis-sorbyl phosphatidylcholine and 1,2-bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine (DC8,9PC) are some examples of photopolymerizable lipids that have been studied. Puri and her coworkers developed DPPC:DC8,9PC formulations and the cargo release (DOX or calcein) occurred upon treatment with a 514 nm laser. Photo-triggering occurred primarily via a type-I photoreaction process (125, 126).
In addition to light-sensitive liposomes, photo-stabilized liposomes are attractive candidates for sustained drug delivery. In this approach, a photopolymerizable group was introduced into lipid bilayer to prepare plasma stable liposomes. An example of photo-stabilized liposomes was vesicles prepared from the polymerizable lipid, 1,2-dipalmitoyl-sn-glycero-3-phospho-N-(2-hydroxymethyl)-3,5-divinylbenzamide (DPPE-DVBA), that have been demonstrated to photo-crosslink in the presence of UV light (127).
Ultrasound-sensitive liposomes
Ultrasound offers an easy and non-invasive method for precise drug delivery because its energy can disrupt nanostructures that stably encapsulated cargoes before triggering. Furthermore, enhancement of drug transport across cell membranes and a synergistic effect between the pharmacological activity of some drugs and ultrasound effects were observed. In ultrasound-mediated triggered drug carriers, acoustic parameters should be carefully tuned to be energetic enough to actuate drug release while avoiding harmful damage to cells and tissues (137, 138). Compared to high-frequency ultrasound (1–3 MHz), low frequency ultrasound (20–100 kHz) can be a more effective trigger for drug release and penetrate deeper into tissues. However, it does not allow for sharp focusing (139). In this part, non-thermal effects of ultrasound to enhance drug release from liposomal carrier are discussed and examples of recent studies are presented in Table 4.
Table 4.
Examples of recent studies on ultrasound-sensitive liposomes
| Stage of study | Cargo | In-vivo tumor model | Components | Gas type | Ultrasound frequency | Reference |
|---|---|---|---|---|---|---|
| In-vitro | DOX, Marker | _ | DSPC, Chol, DSPE-PEG, DOPE | _ | 40 kHz | (153, 154) |
| In-vitro | Marker | _ | DSPC, Chol, DSPE-PEG, DOPE | _ | 1.13 MHz | (155) |
| In-vitro | DOX | _ | DPPC, Chol, DSPE-PEG-SPDP | Decafluorobutane | 1 MHz | (156) |
| In-vitro | Marker | _ | POPC, Lipopeptide | _ | 3 MHz | (157) |
| In-vitro | Thrombin, Marker | _ | PC, Chol, PEG150 stearate, Biotin-PEG3400-PC | Decafluorobutane | 1 MHz | (149) |
| In-vitro | _ | _ | DPPC | Perfluorohexane | 20 kHz | (146) |
| In-vitro / In-vivo | Marker | _ | DPPC, Chol, DSPE-PEG, PDP | Decafluorobutane | 1 MHz | (148) |
| In-vitro / In-vivo | Marker | Prostate tumor (22Rv1 cells) | DSPC, Chol, DSPE-PEG, DOPE | _ | 1.1 MHz | (158) |
| In-vitro / In-vivo | DOX, Marker | Metastatic murine melanoma (B16F10 luciferase cells) | HSPC, Chol, DSPE, DSPE-PEG | Sulphur hexafluoride | 0.5 MHz | (159) |
| In-vitro / In-vivo | IL-12 corded pDNA | Murine ovarian carcinoma (OV-HM cells) | DSPC, DSPE-PEG | Perfluoropropane | 1 MHz | (143) |
| In-vitro | Marker | _ | DPPC, DSPC, DMPC, DSPE-PEG, DLPA, DPPA |
Perfluorohexane, Perfluoropentane | 20 kHz | (145)1 |
Abbreviations: Chol: Cholesterol; DLPA: 1,2-Didodecanoyl-snglycero-3-phosphate (sodium salt); DMPC: 1,2-Dimyristoyl-sn-glycero-3-phosphocholine; DOPE: 1,2-Dioleoylsn-glycero-3-phosphoethanolamine; DOX: Doxorubicin; DPPA: 1,2-Dipalmitoyl-sn-glycero-3-phosphate; DPPC: 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine; DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine; DSPE: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine; DSPE-PEG: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-(polyethylene glycol)-2000; DSPE-PEG-SPDP: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-[N-PDP(polyethylene glycol)-2000]; HSPC: Hydrogenated soy phosphocholine; PC: Phosphatidylcholine; pDNA: Plasmid DNA; PDP: 3-(2-Pyridyldithio)-Propionate; POPC: 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
Folate decorated nanocarrier for targeted delivery.
Liposomes are rather transparent to ultrasound; however, need to contain a gas phase for being sensitive to ultrasound. Therefore, acoustically active liposomes (echogenic liposomes and bubble liposomes) that contain a gas phase are designed in order to respond to ultrasound. To prepare acoustically triggered liposomes, different strategies including an internal gas bubble, a liquid phase changeable to a gas bubble upon insonation, bubbles attached to the vesicle exterior parts, and bubbles reside in the close proximity of liposomes have been used. Two main mechanisms have been proposed for shear stress and cavitation of ultrasound to trigger vesicle content release: i) producing small pores for enhanced permeability or ii) destabilization and disruption of the entire liposome.
In the first strategy to prepare ultrasound-responsive liposomes, gas bubbles (generally micron-sized) are nested inside liposomal vesicles. These delivery systems can be prepared by either mixing and sonication of gas bubbles with phospholipid mixture (140), or sonication of liposomes in the presence of perfluorocarbon gas (141), or gas generation inside the liposomes by a chemical reaction (e.g. bicarbonate solution) (142).
Suzuki et al. reported effective gene delivery system by using the bubble liposome and sonoporation for IL-12 corded plasmid DNA delivery (143). This approach dramatically suppressed tumor growth and the therapeutic effect was T-cell dependent (143). One advantage of bubble liposomes over echogenic liposomes is that the bubble liposomes are smaller in size with average diameter generally less than 500 nm, compared to micron sizes for echogenic liposomes (143, 144).
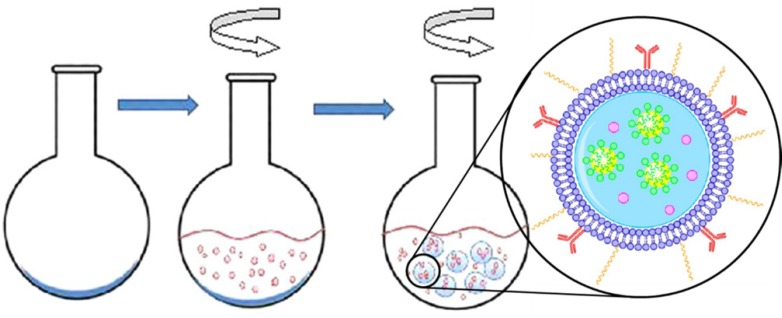
To achieve nanosized vesicles for extravasation or endocytosis, another strategy has been invented that the gas bubbles are not pre-existing, but rather form upon the ultrasound triggering event. This approach allows vesicles with diameter less than 500 nm. Pitt and his coworkers developed an emulsion-containing liposome (eLiposome). The ultrasound application can cause changing the emulsion droplet to gas, thus increasing the volume inside the liposomes and leading to vesicle rupture and the cargo release (145-147). Two methods were proposed for eLiposome preparation. In the first method, emulsion droplets were made of perfluorohexane or perfluoropentane and stabilized with phospholipids. A thin layer of phospholipids was dried in a round-bottomed flask. The emulsion was added to the flask and hydrated the phospholipids forming liposomes around the emulsions (one-step preparation, Figure 6). In the second method, liposomes and emulsions were made separately, and then mixed using ultrasound (145).
Figure 6.
One-step process of eLiposomes production. Phospholipid is deposited on the flask and an emulsion is added. eLiposomes form while the flask is rotated. Reprinted with minor modification from reference (145), with permission from American Chemical Society, Copyright 2012
The third category of ultrasound-sensitive liposomes is designed by small liposomes that are attached to larger gas bubbles. The liposomes are usually around 100 nm and loaded with the therapeutic agents while the bubbles are ~1–3 μm and contain a perfluorocarbon gas (148, 149). When exposed to ultrasound the bubble cavitates violently, and the resulting shock waves and shear forces disrupt the nearby vesicle bilayer and release its content. As an example of these studies, Cool et al. prepared microbubbles with drug containing liposomes at their surface in one single step. Liposomes prepared from DPPC, cholesterol, and DSPE-PEG and loaded with indocyanine green (ICG) as a model drug. Mirobubbles were composed of the perfluorobutane gas. This strategy led to enhanced liposome extravasation (148).
Some studies have described ligand targeted ultrasound-responsive liposomes by using antibodies (150), folate (151), and CPPs (152).
Internal stimuli-responsive liposomes
pH-sensitive liposomes
pH-sensitive liposomes have been designed to trigger and promote efficient release of entrapped cargoes in response to an acidic environment. The pH of blood and extracellular fluid of normal tissues is approximately 7.4 whereas in extracellular microenvironment of tumor, pH is between 6.0 and 7.0 (160, 161). The acidosis in tumor tissue can be explained by the poor organization and dysfunctional vasculature, heterogeneous blood flow, and insufficient nutrient delivery. This condition ultimately forces cells to generate energy from anaerobic glycolytic metabolism of glucose to lactic acid. Limited clearance and increased accumulation of lactic acid due to reduced blood flow lead to a pH reduction in the tumor microenvironment, as mentioned above (162). However, the pH value of the tumor interstitial fluid rarely declines below 6.5, thus designing liposomal carrier to disrupt in response to such a narrow pH change is technically challenging (161). On the other hand, following binding to cancer cells, the vesicles can be up-taken and internalized through endocytosis and retained in endosomal and lysosomal compartments. The promising potential of pH-sensitive liposomes lies in their ability in fusion or destabilization after cell internalization at the endosomal stage with pH values in range of 4.5 – 5.5 (mainly due to the activity of vacuolar-type proton ATPase) (163), thereby preventing their contents degradation at the lysosomal level and promoting cargo release into the cytoplasm. This process known as ‘endosomal escape’ results in releasing drug payload into the cytosol and also far from the transmembrane efflux pumps, thereby at least partly preventing lysosomal degradation and circumventing drug resistance development in tumor cells (164, 165). To date, various liposomal carriers have been designed to respond to either low extracellular pH in tumors or endosomal pH compartments (Table 5).
Table 5.
Examples of recent studies on pH-sensitive liposomes
| Stage of study | Cargo | In-vivo tumor model | Components | Targeting ligand | Reference |
|---|---|---|---|---|---|
| In-vitro | Docetaxel | _ | PE, Chol, Oleic acid, Linoleic acid, CHEMS | _ | (191) |
| In-vitro | DOX, Marker | _ | SPC, Chol, DSPE-PEG, PEtOz-CHEMS | _ | (192) |
| In-vitro | DOX, Marker | _ | HSPC, DOPC, Chol, PEGm-PDPAn-PEGm | _ | (193) |
| In-vitro | Paclitaxel | _ | DOPE, DSPE-PEG, CHEMS | _ | (194) |
| In-vitro / In-vivo | DOX, Marker | Colorectal cancer (HCT116 cells) | DPPC, mPEG-P(HPMA-g-His)-Col | _ | (195) |
| In-vitro / In-vivo | Temsirolimus | Murine renal carcinoma (A498 cells) | SPC, Chol, a synthetic smart lipid (HHG2C18) | _ | (196) |
| In-vitro / In-vivo | Paclitaxel | _ | SPC, DSPE-PEG, CHEMS-PEG, CHEMS-Hz-PEG | _ | (179) |
| In-vitro / In-vivo | miRNA, Marker | _ | Chol, DMG-PEG, a pH-sensitive lipid | _ | (197) |
| In-vitro / In-vivo | Ovalbumin, Marker | Mouse lymphoma (E.G7-OVA cells) | EPC, 3,5-Didodecyloxybenzamidine hydrochloride, MGlu-HPG |
_ | (174) |
| In-vitro | siRNA, Marker | _ | DOPC, DODAP, N-dod-DOPE | Anti-CXCR4 antibodies | (182) |
| In-vitro | DOX, Marker | _ | 2IPC, DSPA, DSPE-PEG | Folate | (198) |
| In-vitro / In-vivo | Paclitaxel, Marker | Murine mammary carcinoma (4T1 cells) | SPC, Chol, DSPE-PEG, PEG5000-Hz-PE | R8 peptide | (178) |
| In-vitro / In-vivo | Paclitaxel, Marker | Murine melanoma tumor (B16F1 cells) | SPC, Chol, DSPE-PEG, DSPE-SS-PEG5000 | TAT peptide | (199) |
| In-vitro / In-vivo | DOX | Breast adenocarcinoma (MDA-MB-231 cells) | DOPE, DSPE-PEG3400, CHEMS | Alendronate | (200) |
| In-vitro / In-vivo | Paclitaxel, Marker | Murine hepatocellular carcinoma (HepG2 cells) | SPC, Chol | CPP, Hyaluronic acid | (201) |
| In-vitro / In-vivo | DOX, Marker | Colon adenocarcinoma (HT29 cells) | SPC, Chol, DSPE-PEG | STP peptide | (202) |
| In-vitro / In-vivo | Paclitaxel, Marker | Murine melanoma (B16F10 cells) | SPC, Chol, DSPE-PEG | pH-responsive CPP and cRGD peptide | (203) |
| In-vitro / In-vivo | Paclitaxel, Marker | Colon adenocarcinoma (C26 cells) | SPC, Chol, DSPE-PEG, [D]-H6L9 peptide | cRGD peptide | (204) |
| In-vitro/In-vivo | DOX, Marker | Rat glioma (C6 cells) | Chol, DSPE-PEG, DOPE | pH-responsive CPP | (185) |
| In-vitro/In-vivo | DOX, Marker | Epidermoid carcinoma (KB cells) | PC, Chol, DSPE-PEG, DOTAP, Malachite green carbinol base | Folate | (205) |
| In-vitro / In-vivo | DOX | Breast carcinoma (MCF7 cells) | HSPC, DOPE, CHEMS, DSPE | Estrone | (189) |
Abbreviations: CHEMS: Cholesteryl hemisuccinate; CHEMS-Hz-PEG: Cholesteryl hemisuccinate-Hidrazone activated-(polyethylene glycol)-2000 (An acid cleavable PEG-lipid derivative); CHEMS-PEG: Cholesteryl hemisuccinste-(polyethylene glycol)-2000; Chol: Cholesterol; CPP: Cell-penetrating peptide; cRGD peptide: Cyclic Arg-Gly-Asp peptide; CXCR4: C-X-C chemokine receptor type 4; [D]-H6L9 peptide: A pH-responsive anti-microbial peptide; DMG-PEG: 1,2-Dimyristoyl-sn-glycerol-methoxy(polyethylene glycol)-2000; DODAP: 1,2-Dioleoyl-3-dimethylammonium-propane; DOPC: 1,2-Dioleoyl-sn-glycero-3-phosphocholine; DOPE: 1,2-Dioleoylsn-glycero-3-phosphoethanolamine; DOTAP: 1,2-Dioleoyl-3-trimethylammoniumpropane; DOX: Doxorubicin; DPPC: 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine; DSPE: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine; DSPE-PEG: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-(polyethylene glycol)-2000; DSPE-SS-PEG: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine thiolytic cleavable-(polyethylene glycol)-2000; DSPA: 1,2-Distearoyl-sn-glycero-3-phosphate; EPC: Egg phosphatidylcholine; HHG2C18-L: (1,5-Dioctadecyl-l-glutamyl2-histidyl-hexahydrobenzoic acid-liposome) (A zwitterionic oligopeptide liposomes; HSPC: Hydrogenated soy phosphocholine; 2IPC: 1,2-diheneicosanoyl-sn-glycero-3-phosphocholine; MGlu-HPG: 3-Methylglutarylated hyperbranched poly(glycidol); miRNA: MicroRNA; mPEG-P(HPMA-g-His)-Chol: Methoxy-(polyethylene glycol)-b-poly(N-2-hydroxypropyl methacrylamide-co-histidine)-cholesterol; N-dod-PE: 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine-N-dodecanoyl; PEG5000-Hz-PE: (Polyethylene glycol)-5000-Hidrazone activated-Polyethylene; PC: Phosphatidylcholine; PE: Polyethylene; PEGm-PDPAn-PEGm: Di-block copolymer PEG8-PDPA15; PEtOz-CHEMS: Poly(2-ethyl-2-oxazoline)-cholesteryl hemisuccinate; R8 peptide: Octaarginine peptide; siRNA: Small (or short) interfering RNA; SPC: Soybean phosphatidylcholine; STP peptide: Dual-recognition (SKDEEWHKNNFPLSP) peptide; TAT peptide: (GRKKRRQRRRPQ) peptide.
Dioleoylphosphatidylethanolamine (DOPE) is the most commonly used pH-sensitive lipid. DOPE has a cone shape due to small and minimally hydrated polar head group that occupies a lower volume compared with its acyl chains. The inverted cone shape of DOPE lipid has a tendency to form an inverted hexagonal HII shape at physiological pH because of strong interactions between the phosphate and amine groups of the polar head groups. DOPE by itself with these structural aspects cannot form lipid bilayers at neutral pH. DOPE combined with amphiphilic molecules containing a protonatable acidic group, such as cholesteryl hemisuccinate (CHEMS) and oleic acid have been used to prepare pH-sensitive liposomes. The electrostatic repulsion between deprotonated carboxylate and phosphate groups allows the formation of bilayer structures at neutral pH. At acidic pH, destabilization of liposomes is mediated by the protonation of carboxylate groups, suppressing charge repulsion in the bilayer, and consequently resulting in the reversion of DOPE molecules into their inverted hexagonal phase (161, 166 and 167).
Besides a considerable number of researches on pH-sensitive liposomes prepared from DOPE derivatives, a few studies have recently described incorporation of novel pH-sensitive lipids. Szoka and his coworkers synthetized a novel acid-labile lipid containing a linear ortho ester linker between cholesterol-derived lipid tail and its dimethylethanolamine-type cationic head group. Liposomes, composed of this acid-labile lipid and DOPE, were used for gene delivery. Compared to the acid-stable control, pH-sensitive lipoplexes increased the luciferase gene expression by 5- to 10-fold both in CV-1 cells (a monkey fibroblast cell line) and following intratracheal administration in CD-1 mice (168). Harashima group introduced another cationic pH-sensitive lipid, YSK05 (a tertiary amine containing lipid with structure similar to DOTAP) for improving the delivery of liposomal siRNA and gene silencing (169, 170).
The other approach to prepare pH-sensitive liposomes is by incorporation of pH-sensitive fusogenic peptides either derived from viruses (like haemagglutinin, gp41, and diINF-7), bacteria (such as listeriolysin O and diphtheria toxin), and plants (e.g. ricin, saporin, and gelonin) or synthetic materials (e.g. GALA, KALA, and surfactants) (for review see (171)).
Liposomes enriching with pH-sensitive polymers such as N-isopropylacrylamide (NIPAM) (172, 173), poly(glycidol)s (174, 175), and poly(alkyl acrylic acid)s (176, 177) have also proposed for acid-responsive delivery. Simplicity of preparation and lower immunogenicity are probably two main advantages of these carriers to peptidic pH sensitizers. As an example of these studies, Yuba et al. (175) investigated the relationship between backbone structure of pH-sensitive poly(glycidol) derivatives and their interaction with the membrane. A stronger interaction with the membrane was observed with hyperbranched poly(glycidol) derivatives than the linear polymers. Increasing degree of polymerization of hyperbranched poly(glycidol) derivatives enhanced their bilayer interaction as well. Liposomes modified with these polymers effectively delivered their contents into the cytosol of dendritic cells (175).
In spite of the success of ‘stealth’ nanosystems (PEGylated pH-sensitive liposomes) in achieving stability and long-circulation, DSPE-PEG containing pH-sensitive liposomes showed reduced pH-sensitivity and cellular uptake. The reduced ability of PEGylated shell to come into close proximity of cancer cells and endosomal membranes is one of main explanations for reduced internalization and pH-responsiveness. Aiming to overcome the instability of non-PEGylated liposomes in addition to maintain adequate pH sensitivity, some studies have been focused on designing pH-sensitive liposomes with a cleavable PEG chain (178-181).
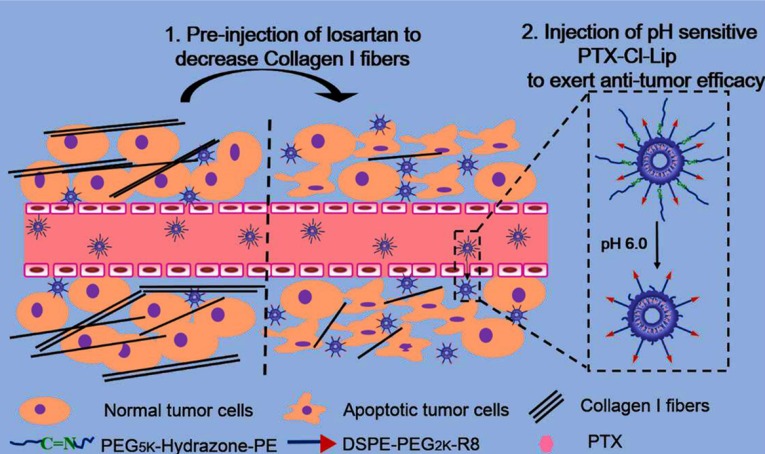
In a recent study, CPP decorated pH-sensitive cleavable liposomes were designed and loaded with paclitaxel. The CPP (R8 peptide) was conjugated to a short PEG group and long PEG chains were linked to liposomal surface by hydrazone bond. Before liposomes injection, free losartan was administered to deplete the collagen I and facilitated liposomes deep penetration into tumors. After taking the advantage of increasing plasma residence time and passive targeting by the long PEG, low extracellular pH in the cancer cell proximity caused long PEG detachment and exposed CPP to the tumor cell. This approach can overcome limited uptake of PEGylated liposomes as well as non-specificity of R8 peptide (Figure 7) (178).
Figure 7.
CPP decorated pH-sensitive cleavable liposomes are designed and loaded with paclitaxel. The CPP (R8 peptide) is conjugated to a short PEG and long PEG chains are linked to liposomal surface by acid-sensitive hydrazone bond. Before liposomes injection, free losartan is administered to deplete the collagen I and facilitate liposomes deep penetration into tumors. Low extracellular pH in the cancer cell proximity causes long PEG detachment and exposes CPP to the tumor cell. Reprinted from reference (178) Copyright 2015, with permission from American Chemical Society
To overcome limited uptake and specificity of stealth liposomes, some studies have been designed targeted pH-sensitive liposomes by conjugating various ligands such as antibodies (182, 183), peptides (178, 184 and 185), hyaluronic acid (186), transferrin (187), and folate (188) to the vesicle surfaces. Estrone decorated pH-sensitive liposomes were also designed for intracellular delivery of DOX to estrogen receptor on breast cancer cells (189). The estrogen receptor expression amplifies in breast carcinomas (190). Liposomes were prepared from DOPE, HSPC, CHEMS, and cholesterol. The targeted pH triggered formulation showed enhanced nuclear drug delivery, improved therapeutic efficacy, and reduced cardiotoxicity compared with non-triggered formulation and free drug (189).
Enzyme-sensitive liposomes
In some pathological conditions, such as cancer, inflammation, and infection, the concentrations of different extracellular and intracellular enzymes are elevated. Enzyme-responsive nanocarriers can be designed to undergo structural transformation and release the encapsulated cargoes by this biochemical abnormality (206, 207).
Enzyme-responsive liposomes have a number of advantages. The payload release is controlled by an enzyme at the target site without any external equipment for triggering. The amount of drug release in the targeted tissue is usually proportional to the concentration of the active enzyme and the severity of the pathological condition. Furthermore, some bioactive molecules are produced following enzyme digestion that may have synergistic therapeutic effects or facilitate the uptake of the drug. Secreted phospholipase A2, matrix metalloproteinases, urokinase plasminogen activator, elastase, and prostate-specific antigen are extracellular enzymes and cathepsin B is an intracellular enzyme used as triggers for drug release from liposomal carriers (206-208). Table 6 represents recent studies on enzyme-responsive liposomes.
Table 6.
Examples of recent studies on enzyme-sensitive liposomes
| Stage of study | Cargo | In-vivo tumor model | Components | Type of enzyme | Targeting ligand | Reference |
|---|---|---|---|---|---|---|
| In-vitro | Marker | _ | DPPC, DMPC, DPPG, DMPG | Secretory phospholipase A2 | _ | (213) |
| In-vitro | _ | _ | DSPG, thio-ester pro anticancer ether lipid | Secretory phospholipase A2 | _ | (223) |
| In-vitro | DOX, Marker | _ | DPPC, Poloxamer 188 | Secretory phospholipase A2 | _ | (224) |
| In-vitro/In-vivo | Oxaliplatin | Breast cancer (MT3-cells) | POPC, POPG, Chol, DSPE-PEG | Secretory phospholipase A2 | _ | (212) |
| In-vitro/In-vivo | DOX, Marker | Prostate cancer (PC-3 cells) | DSPC, DSPG, Chol, DSPE, DSPE-PEG | Secretory phospholipase A2 | _ | (225) |
| In-vitro | Marker | _ | POPC, Stearic acid conjugated collagen-mimetic peptides | MMP-9 | _ | (217) |
| In-vitro | Marker | _ | DOPC, DSPC, POPC, MMP-9-sensitive lipopeptide | MMP-9 | _ | (216) |
| In-vitro | Marker | EPC, Chol, DSPE-PEG, DSPE-peptide-PEG3400 | MMP-2 | 2C5 monoclonal antibody, TAT peptide | (215) | |
| In-vitro | Marker | _ | DOPE, DODAP, DOPE conjugated to elastase-sensitive peptide | Protease (Elastase) | _ | (226) |
| In-vitro | pDNA | _ | DOPE, DOTAP, PEG lipid with an enzymatically-cleavable linker | Cathepsin B | _ | (221) |
| In-vitro/In-vivo | Paclitaxel conjugated to dendrimers by enzyme-sensitive linker, Marker | Breast cancer (MDA-MB-231 cells) | EPC, Chol, DSPE-PEG | Cathepsin B | Folate | (222) |
| In-vitro/In-vivo | siRNA, Marker | Prostate cancer (22Rv1 cells) | SPC, 3b[N-(N0 ,N0-dimethylaminoethane)-carbamoyl] chol, DSPE-PEG | PSA enzyme | Folate, CPPs | (219) |
Abbreviations: Chol: Cholesterol; CPPs: Cell penetrating peptides; DMPC: 1,2-Dimyristoyl-sn-glycero-3-phosphocholine; DMPG: 1,2-Dimyristoyl-sn-glycero-3-phosphoglycerol; DODAP: 1,2-Dioleoyl-3-dimethylammonium-propane; DOPC: 1,2-Dioleoyl-sn-glycero-3-phosphocholine; DOPE: 1,2-Dioleoylsn-glycero-3-phosphoethanolamine; DOTAP: 1,2-Dioleoyl-3-trimethylammoniumpropane; DOX: Doxorubicin; DPPC: 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine; DPPG: 1,2-Dipalmi-toyl-sn-glycero-3-phosphoglycerol; DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine; DSPE: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine; DSPE-PEG: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-(polyethylene glycol)-2000; DSPG: 1,2-Distearoyl-snglycero-3-phospho-(1'-rac-glycerol); EPC: Egg phosphatidylcholine; MMP-2: Matrix mettaloprotease-2; MMP-9: Matrix mettaloprotease-9; pDNA: Plasmid DNA; POPC: 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; POPG: 1-Palmitoyl-2-oleoyl-sn- glycero-3-phospho-(1’-rac-glycerol); PSA: Prostate-specific antigen; siRNA: Small (or short) interfering RNA; SPC: Soybean phosphatidylcholine; TAT peptide: (GRKKRRQRRRPQ) peptide
Secreted phospholipase A2 level increases in cancers (especially in the prostate, pancreatic, colon, and breast tumors), inflammatory diseases, cardiovascular diseases, and immune disorders (209). Therefore, phospholipase A2-responsive liposomes are attractive nanocarriers for the targeted release of anticancer agents at the tumor tissues.
Enzyme-mediated phospholipids hydrolysis disrupts the integrity of the lipid bilayer and triggers drug release. Involvement of phospholipase A2 receptor in the uptake of this responsive liposome has also been proposed. Another possible mechanism is cleavage of a lipophilic drug attached to the carrier by a phospholipase A2-sensitive bond (206, 210 and 211). A number of factors influence phospholipase A2 hydrolytic activity including the enzyme isoforms, lipid assembly, lipid physical properties, liposomal composition, and presence of lipopolymer. Incorporation of short acyl chain lipids, anionic polar head groups, and PEG grafted lipids have shown to increase the hydrolytic activity of phospholipase A2 (212, 213).
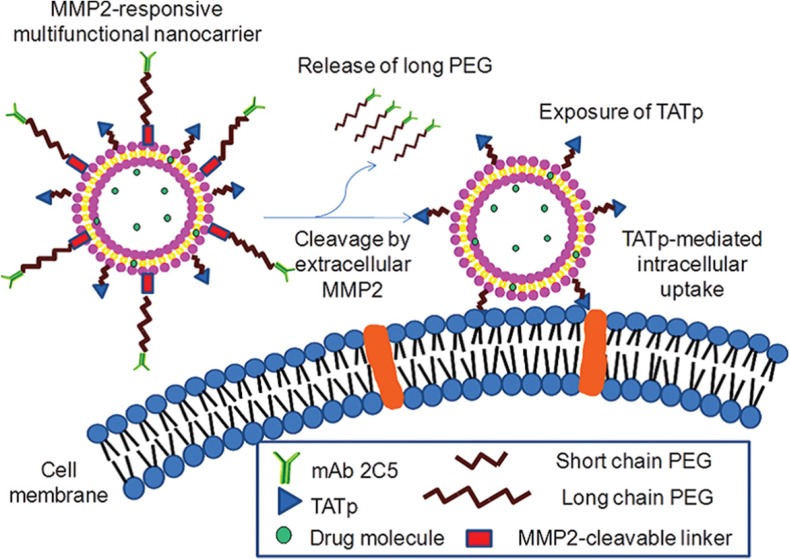
Matrix metalloproteinases (MMPs) are responsible for the proteolytic degradation of extracellular matrix. These enzymes (particularly, MMP-2 and MMP-9) are overexpressed in pancreatic, colorectal, breast, and lung tumors and play important roles in tumor growth, invasion, and metastasis (214). There are two main strategies to prepare MMP-responsive liposomes (206). First, MMP-sensitive peptides are synthetized and linked the shielding PEG groups to liposomal surface. At target site, the peptide is cleaved leading to the release of PEG and subsequently ligands promoting cellular uptake of nanocarriers are exposed to the target cancer cells. Torchilin and his coworkers designed a dual antinucleosome monoclonal antibody and TAT peptide targeted MMP-2-responsive multifunctional liposomal delivery system. Upon nanocarrier accumulation in tumors and specific targeting of cancer cells, in response to up-regulated extracellular MMP-2 in tumors, the hidden surface-attached TAT peptides exposed and enhanced cellular internalization of liposomes (Figure 8) (215). Another strategy is the incorporation of MMP-cleavable lipopeptides into the liposomal membrane. The lipopeptide cleavage in response to elevated enzyme concentration at the tumor tissues leads to liposomes destabilization and content release (216, 217).
Figure 8.
A dual antinucleosome monoclonal antibody and TAT peptide targeted MMP-2-responsive multifunctional liposomal delivery system is designed. Upon nanocarrier accumulation in tumors and specific targeting of cancer cells, in response to up-regulated extracellular MMP-2 in tumors, the hidden surface-attached TAT peptides expose and enhance cellular internalization of liposomes. Reprinted from reference (215) Copyright 2012, with permission from American Chemical Society
The urokinase plasminogen activator and prostate-specific antigen are serine proteases. Urokinase plasminogen activator elevated levels have been reported in a variety of cancers including colon, bladder, breast, and ovarian tumors (218). Therefore, liposomes containing urokinase plasminogen activator cleavable peptides can release the encapsulated payloads at target sites. Prostate-specific antigen-activated nanoparticles developed from the enzyme-cleavable peptides can exhibit very selective antitumor activity against prostate cancer (219).
Cathepsin B is a lysosomal cysteine proteinase of the papain family enhanced extracellular matrix degradation and overexpressed in several malignancies, such as colon, prostate, brain, breast, and lung tumors (220). Cathepsin B has been reported to increase fusogenicity of liposomes at the target sites. Since cathepsin B is mainly found in lysosomes, dual pH and cathepsin B-responsive liposomes have been developed for targeted intracellular cargo delivery. In a recent article, PEG was attached to a lipid by an enzymatically-cleavable linker (glycine-phenylalanine-leucine-glycine). In the endosome, the detachment of PEG shell following the degradation of the peptide linker by cathepsin B caused vesicle destabilization, endosomal disruption, and triggering the controlled plasmid DNA release into cytoplasm (221). In another study, Satsangi et al. designed paclitaxel conjugated poly(amidoamine) dendrimers by cathepsin B-cleavable tetrapeptide and encapsulated this conjugate within folate receptor targeting liposomes (222).
Redox-sensitive liposomes
Redox-responsive delivery systems are one of the most efficient stimulus-responsive carriers for cancer drug and gene therapy. Glutathione (GSH) is a cysteine-containing tripeptide and the key intracellular reducing agent which plays an important role in cell growth and function as well as maintaining cellular redox homeostasis (227, 228). Much higher concentration of GSH (~100-1000 fold) in the intracellular compartments, especially in cytosol, mitochondria, and cell nucleus, compared to its levels in blood and extracellular matrix along with high redox potential difference existing between normal and tumor tissue provide a good rationale for redox-responsive nanocarriers as an intracellular drug delivery and tumor specific strategy. Typically, redox-sensitive nanostructures contain the cleavable/reversible disulfide bonds in their structures to render redox-responsive character (229-231). Redox-responsive liposomes have been often destabilized either by changes in hydrophilicity and/or charge of the amphiphile with reducing agents, or by cross-linker removal to cause lipid phase transitions (232). Table 7 summarizes recent studies on redox-responsive liposomes.
Table 7.
Examples of recent studies on redox-sensitive liposomes
| Stage of study | Cargo | In-vivo tumor model | Components | Targeting ligand | Reference |
|---|---|---|---|---|---|
| In-vitro | Marker | _ | DOPE, Q-DOPE, DOPE-PEG | _ | (240) |
| In-vitro | DOX, Marker | _ | DSPC, DSPG, Ferrocene modified phospholipid prepared from DSPE and ferroceneacetic acid | _ | (241) |
| In-vitro | Redox-responsive docetaxel prodrug, Marker | _ | SPC, Chol, DSPE-PEG | _ | (242) |
| In-vitro | Marker | _ | POPC, POPE, Gallate derivative with three propargyl groups | _ | (243) |
| In-vitro | DOX | _ | Cerasome forming lipid with disulfide bond | _ | (244) |
| In-vitro | pDNA, Marker | _ | DOPE, Redox-sensitive gemini cationic Chol lipids | _ | (245) |
| In-vitro/In-vivo | Paclitaxel, siRNA, Marker | Breast cancer (4T1 cells) | SPC, Chol, redox-sensitive cationic lipid | _ | (234) |
| In-vitro/In-vivo | DOX | Murine osteosarcoma (MG63 cells) | SPC, Chol-SS-COOH, Chitooligosaccharides | _ | (233) |
| In-vitro/In-vivo | Redox-responsive docetaxel prodrug | Lung carcinoma (A549 cells) | SPC, Chol, DSPE-PEG | _ | (236) |
| In-vitro | DOX, Marker | _ | EPC, DOPE, lipid like conjugate with disulfide bond and a biotin moiety | anti-HER2 antibody | (237) |
| In-vitro | Marker | _ | DPPC, DOPE, CHEMS, DOPE-S-S-PEG | R8 peptide | (246) |
| In-vitro/In-vivo | DOX | Murine osteosarcoma (MG63 cells) | SPC, DOPE, DOTAP, Chol-SS-mPEG | Hyaluronic acid | (238) |
| In-vitro/In-vivo | Paclitaxel, Marker | Murine melanoma (B16F1) | SPC, Chol, DSPE-PEG, DSPE-SS- PEG5000 |
TAT peptide | (199) |
| In-vitro/In-vivo | Marker | Colon carcinoma (C26 cells) | EPC, Chol, Chol-S-S-PEG5000 | R8 peptide | (239) |
| In-vitro/In-vivo | DOX, Verapamil, Marker | Breast cancer (MCF7 cells) | EPC, Chol, Chol-PEG2000, Chol-S-S-PEG5000 | R8 peptide | (235) |
Abbreviations: CHEMS: Cholesteryl hemisuccinate; Chol: Cholesterol; Chol-PEG: Cholesterol anchored modified-(polyethylene glycol)-2000; Chol-SS-COOH: Cholesterol anchored reduction-sensitive COOH; Chol-SS-PEG: Cholesterol anchored reduction-sensitive-(polyethylene glycol)-2000; DOPE: 1,2-Dioleoylsn-glycero-3-phosphoethanolamine; DOPE-PEG: 1,2-Dioleoylsn-glycero-3-phosphoethanolamine (polyethylene glycol)-2000; DOPE-SS-PEG: 1,2-Dioleoylsn-glycero-3-phosphoethanolamine anchored modified (polyethylene glycol)-2000; DOTAP: 1,2-Dioleoyl-3-trimethylammonium-propan; DOX: Doxorubicin; DPPC: 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine; DSPC: 1,2-Distearoyl-sn-glycero-3-phosphocholine; DSPE: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine; DSPE-PEG: 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-(polyethylene glycol)-2000; DSPG: 1,2-Distearoyl-snglycero-3-phospho-(1'-rac-glycerol); EPC: Egg phosphatidylcholine; HER2: Human epidermal growth factor receptor 2; pDNA: Plasmid DNA; POPC: 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; POPE: Palmitoyl-oleoyl-phosphoethanolamine; Q-DOPE: Quinone-dioleoyl phosphatidylethanolamine; R8 peptide: Octaarginine peptide; siRNA: Small (or short) interfering RNA; SPC: Soybean phosphatidylcholine; TAT peptide: (GRKKRRQRRRPQ) peptide
A reduction-sensitive fusogenic liposome was prepared by vesicle surface-coating with chotooligosaccharides, hydrolytic products of chitosan, via a disulfide linker (233). The hydrophilic backbone, low degree of polymerization, high water-solubility, cationic nature, cell adhesion properties, and wider biological activities such as anti-angiogenesis and radical scavenging efficacy make them as candidates for modification of tumor-targeted liposomes (233). Modified liposomes were stable under physiological conditions but destabilized in the presence of the cytosolic level of reducing agents most likely due to disulfide bond breakage. Chotooligosaccharide coated liposomes exhibited a prolonged half-life of DOX by 4-5.5 fold and strong inhibitory effect on tumor growth in osteosarcomas animal model compared to free drug (233).
The survivin overexpression is an important factor involved in paclitaxel resistance of breast cancer cells. In a recent study, Chen et al. proposed redox-sensitive oligopeptide liposomes for co-delivery of paclitaxel and anti-survivin siRNA for the synergistic treatment and efficient anti-metastasis strategy against breast cancer (234). The nanosystem was composed of soybean phosphatidylcholine, cholesterol, and a redox-sensitive cationic oligopeptide lipid with a proton sponge effect. The vesicles disassembled in the presence of 10 mM GSH as confirmed by monitoring size, zeta potential, and morphology changes. The system offered several advantages including improved cellular uptake, reduced survivin expression, efficient endolysosomal escape, higher cell cytotoxicity, synergistic in-vivo inhibitory effect on tumor growth, and reduced pulmonary metastasis of breast cancer (234). Another co-delivery approach has been reported for combination of DOX and P-glycoprotein, verapamil, by a redox-responsive liposome to overcome multidrug resistance (235).
In contrast to studies utilized disulfide bonds in lipid components of vesicles, Ren et al. prepared a redox-responsive prodrug of docetaxel prodrug by conjugation of the drug molecule to vitamin E via a disulfide linker and incorporated it in liposomes (236).
A few studies have focused on designing targeted redox-responsive liposomal carriers by employing antibody (237), hyaluronic acid (238), and CPPs (199, 239). Cationic redox-sensitive liposomes were prepared with a novel detachable PEG conjugated with cholesterol through a disulfide linker and hyaluronic acid, a ligand for CD44, was non-covalently coated on the cationic vesicles. This nanosystem destabilized in reducing conditions and released higher cargo levels compared to redox insensitive liposomes. The proposed nanostructure was an excellent CD44-mediated intracellular delivery system for osteosarcoma treatment in animal models (238).
Conclusion
Liposomes are a viable carrier to improve both the safety and efficacy of antineoplastic therapeutics which have already resulted in marketed anticancer products (i.e., Doxil®, DaunoXome®, and Depocyt®) (247). To improve their efficacy and overcome the limitations of conventional liposomes, modified formulations have been investigated including stimuli-sensitive liposomes. This review evidences that numerous research efforts have been recently devoted to the optimization of liposomal carriers that allow delivering chemotherapeutics locally upon external as well as internal stimulation. Herein we have summarized the latest researches on stimuli-responsive liposomes. We also try to mention researches on combination of active targeting and active triggering for cancer therapy. Further attempts on industrialization are in great demand to bring these developments closer to oncology clinics.
References
- 1.Moore MA. Cancer control programs in East Asia: Evidence from the international literature. J. Prev. Med. Public Health . 2014;47:183–200. doi: 10.3961/jpmph.2014.47.4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore MA, Yoo KY, Tuncer M, Sobue T. Overview of players and information in the cancer epidemiology and control world in Asia. Asian Pac. J. Cancer Prev. . 2010;11 (Suppl 2):1–10. [PubMed] [Google Scholar]
- 3.Guo S, Huang L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv. . 2014;32:778–88. doi: 10.1016/j.biotechadv.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain V, Jain S, Mahajan SC. Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr. Drug Deliv. . 2015;12:177–91. doi: 10.2174/1567201811666140822112516. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. . 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Alavi S, Haeri A, Dadashzadeh S. Utilization of chitosan-caged liposomes to push the boundaries of therapeutic delivery. Carbohydr. Polym. . 2017;157:991–1012. doi: 10.1016/j.carbpol.2016.10.063. [DOI] [PubMed] [Google Scholar]
- 7.Daeihamed M, Haeri A, Ostad SN, Akhlaghi MF, Dadashzadeh S. Doxorubicin-loaded liposomes: Enhancing the oral bioavailability by modulation of physicochemical characteristics. Nanomedicine (Lond) 2017;12:1187–202. doi: 10.2217/nnm-2017-0007. [DOI] [PubMed] [Google Scholar]
- 8.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in-vivo. Adv. Drug Deliv. Rev. . 2013;65:71–9. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Stylianopoulos T, Economides EA, Baish JW, Fukumura D, Jain RK. Towards optimal design of cancer nanomedicines: Multi-stage nanoparticles for the treatment of solid tumors. Ann. Biomed. Eng. . 2015;43:2291–300. doi: 10.1007/s10439-015-1276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernsting MJ, Murakami M, Roy A, Li SD. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release . 2013;172:782–94. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang H, Mintri S, Menon AV, Lee HY, Choi HS, Kim J. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale . 2015;7:18848–62. doi: 10.1039/c5nr05264e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crucho CI. Stimuli-responsive polymeric nanoparticles for nanomedicine. ChemMedChem. . 2015;10:24–38. doi: 10.1002/cmdc.201402290. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Lane LA, Nie S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release . 2015;219:205–14. doi: 10.1016/j.jconrel.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fumoto S, Kawakami S. Combination of nanoparticles with physical stimuli toward cancer therapy. Biol. Pharm. Bull. . 2014;37:212–6. doi: 10.1248/bpb.b13-00703. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Gao GH, Lee DS. Stimulus-sensitive polymeric nanoparticles and their applications as drug and gene carriers. Adv. Healthcare Mater. . 2013;2:388–417. doi: 10.1002/adhm.201200313. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Chu X, Hou Y. Stimuli-responsive cancer therapy based on nanoparticles. Chem. Commun. (Camb) 2014;50:11614–30. doi: 10.1039/c4cc03984j. [DOI] [PubMed] [Google Scholar]
- 17.Haeri A, Alinaghian B, Daeihamed M, Dadashzadeh S. Preparation and characterization of stable nanoliposomal formulation of fluoxetine as a potential adjuvant therapy for drug-resistant tumors. Iran. J. Pharm. Res. . 2014;13:3–14. [PMC free article] [PubMed] [Google Scholar]
- 18.Haeri A, Sadeghian S, Rabbani S, Anvari MS, Boroumand MA, Dadashzadeh S. Use of remote film loading methodology to entrap sirolimus into liposomes: Preparation, characterization and in-vivo efficacy for treatment of restenosis. Int. J. Pharm. . 2011;414:16–27. doi: 10.1016/j.ijpharm.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 19.Daeihamed M, Dadashzadeh S, Haeri A, Akhlaghi MF. Potential of liposomes for enhancement of oral drug absorption. Curr. Drug Deliv. . 2017;14:289–303. doi: 10.2174/1567201813666160115125756. [DOI] [PubMed] [Google Scholar]
- 20.Mallick S, Choi JS. Liposomes: Versatile and biocompatible nanovesicles for efficient biomolecules delivery. J. Nanosci. Nanotechnol. . 2014;14:755–65. doi: 10.1166/jnn.2014.9080. [DOI] [PubMed] [Google Scholar]
- 21.Haeri A, Sadeghian S, Rabbani S, Anvari MS, Ghassemi S, Radfar F, Dadashzadeh S. Effective attenuation of vascular restenosis following local delivery of chitosan decorated sirolimus liposomes. Carbohydr. Polym. . 2017;157:1461–9. doi: 10.1016/j.carbpol.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Haeri A, Sadeghian S, Rabbani S, Anvari MS, Lavasanifar A, Amini M, Dadashzadeh S. Sirolimus-loaded stealth colloidal systems attenuate neointimal hyperplasia after balloon injury: A comparison of phospholipid micelles and liposomes. Int. J. Pharm. . 2013;455:320–30. doi: 10.1016/j.ijpharm.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Haeri A, Sadeghian S, Rabbani S, Shirani S, Anvari MS, Dadashzadeh S. Physicochemical characteristics of liposomes are decisive for their antirestenosis efficacy following local delivery. Nanomedicine (Lond) 2017;12:131–45. doi: 10.2217/nnm-2016-0294. [DOI] [PubMed] [Google Scholar]
- 24.Alhariri M, Azghani A, Omri A. Liposomal antibiotics for the treatment of infectious diseases. Expert Opin. Drug Deliv. . 2013;10:1515–32. doi: 10.1517/17425247.2013.822860. [DOI] [PubMed] [Google Scholar]
- 25.Tabansky I, Messina MD, Bangeranye C, Goldstein J, Blitz-Shabbir KM, Machado S, Jeganathan V, Wright P, Najjar S, Cao Y, Sands W, Keskin DB, Stern JN. Advancing drug delivery systems for the treatment of multiple sclerosis. Immunol. Res. . 2015;63:58–69. doi: 10.1007/s12026-015-8719-0. [DOI] [PubMed] [Google Scholar]
- 26.Garg T. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif. Cells Nanomed. Biotechnol. . 2016;44:98–105. doi: 10.3109/21691401.2014.916715. [DOI] [PubMed] [Google Scholar]
- 27.Baronzio GF, Hager ED. Hyperthermia in cancer treatment: A primer. Springer Science & Business Media; 2008. [Google Scholar]
- 28.Stewart JR, Gibbs FA Jr. Hyperthermia in the treatment of cancer. Perspectives on its promise and its problems.Cancer . 1984;54:2823–30. doi: 10.1002/1097-0142(19841201)54:2+<2823::aid-cncr2820541430>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, Beck M, Nadobny J, Budach V, Wust P, Ghadjar P. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials. gov registry.Int. J. Hyperthermia . 2015;31:609–14. doi: 10.3109/02656736.2015.1040471. [DOI] [PubMed] [Google Scholar]
- 30.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol. . 2002;3:487–97. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 31.Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Koning GA, ten Hagen TL, Needham D, Dewhirst MW. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. . 2012;72:5566–75. doi: 10.1158/0008-5472.CAN-12-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ta T, Porter TM. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release . 2013;169:112–25. doi: 10.1016/j.jconrel.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science . 1978;202:1290–3. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 34.Kneidl B, Peller M, Winter G, Lindner LH, Hossann M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomedicine . 2014;9:4387–98. doi: 10.2147/IJN.S49297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, ten Hagen TL, Haeri A, Soullie T, Scholten C, Seynhaeve AL, Eggermont AM, Koning GA. A novel two-step mild hyperthermia for advanced liposomal chemotherapy. J. Control. Release . 2014;174:202–8. doi: 10.1016/j.jconrel.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Maruyama K, Unezaki S, Takahashi N, Iwatsuru M. Enhanced delivery of doxorubicin to tumor by long-circulating thermosensitive liposomes and local hyperthermia. Biochim. Biophys. Acta . 1993;1149:209–16. doi: 10.1016/0005-2736(93)90203-c. [DOI] [PubMed] [Google Scholar]
- 37.Unezaki S, Maruyama K, Takahashi N, Koyama M, Yuda T, Suginaka A, Iwatsuru M. Enhanced delivery and antitumor activity of doxorubicin using long-circulating thermosensitive liposomes containing amphipathic polyethylene glycol in combination with local hyperthermia. Pharm. Res. . 1994;11:1180–5. doi: 10.1023/a:1018949218380. [DOI] [PubMed] [Google Scholar]
- 38.Li L, ten Hagen TL, Schipper D, Wijnberg TM, van Rhoon GC, Eggermont AM, Lindner LH, Koning GA. Triggered content release from optimized stealth thermosensitive liposomes using mild hyperthermia. J. Control. Release . 2010;143:274–9. doi: 10.1016/j.jconrel.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Landon CD, Park JY, Needham D, Dewhirst MW. Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low Temperature-Sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed. J. . 2011;3:38–64. doi: 10.2174/1875933501103010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Needham D, Anyarambhatla G, Kong G, Dewhirst MW. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. . 2000;60:1197–201. [PubMed] [Google Scholar]
- 41.Banno B, Ickenstein LM, Chiu GN, Bally MB, Thewalt J, Brief E, Wasan EK. The functional roles of poly(ethylene glycol)-lipid and lysolipid in the drug retention and release from lysolipid-containing thermosensitive liposomes in-vitro and in-vivo. J. Pharm. Sci. . 2010;99:2295–308. doi: 10.1002/jps.21988. [DOI] [PubMed] [Google Scholar]
- 42.Dou Y, Hynynen K, Allen C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Control. Release . 2017;249:63–73. doi: 10.1016/j.jconrel.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 43.Hossann M, Wiggenhorn M, Schwerdt A, Wachholz K, Teichert N, Eibl H, Issels RD, Lindner LH. In-vitro stability and content release properties of phosphatidylglyceroglycerol containing thermosensitive liposomes. Biochim. Biophys. Acta . 2007;1768:2491–9. doi: 10.1016/j.bbamem.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Limmer S, Hahn J, Schmidt R, Wachholz K, Zengerle A, Lechner K, Eibl H, Issels RD, Hossann M, Lindner LH. Gemcitabine treatment of rat soft tissue sarcoma with phosphatidyldiglycerol-based thermosensitive liposomes. Pharm. Res. . 2014;31:2276–86. doi: 10.1007/s11095-014-1322-6. [DOI] [PubMed] [Google Scholar]
- 45.Lindner LH, Eichhorn ME, Eibl H, Teichert N, Schmitt-Sody M, Issels RD, Dellian M. Novel temperature-sensitive liposomes with prolonged circulation time. Clin. Cancer Res. . 2004;10:2168–78. doi: 10.1158/1078-0432.ccr-03-0035. [DOI] [PubMed] [Google Scholar]
- 46.Ta T, Bartolak-Suki E, Park EJ, Karrobi K, McDannold NJ, Porter TM. Localized delivery of doxorubicin in-vivo from polymer-modified thermosensitive liposomes with MR-guided focused ultrasound-mediated heating. J. Control. Release . 2014;194:71–81. doi: 10.1016/j.jconrel.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pippa N, Meristoudi A, Pispas S, Demetzos C. Temperature-dependent drug release from DPPC:C12H25-PNIPAM-COOH liposomes: Control of the drug loading/release by modulation of the nanocarriers› components. Int. J. Pharm. . 2015;485:374–82. doi: 10.1016/j.ijpharm.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 48.Kono K, Nakashima S, Kokuryo D, Aoki I, Shimomoto H, Aoshima S, Maruyama K, Yuba E, Kojima C, Harada A, Ishizaka Y. Multi-functional liposomes having temperature-triggered release and magnetic resonance imaging for tumor-specific chemotherapy. Biomaterials . 2011;32:1387–95. doi: 10.1016/j.biomaterials.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 49.Kono K, Takashima M, Yuba E, Harada A, Hiramatsu Y, Kitagawa H, Otani T, Maruyama K, Aoshima S. Multifunctional liposomes having target specificity, temperature-triggered release, and near-infrared fluorescence imaging for tumor-specific chemotherapy. J. Control. Release . 2015;216:69–77. doi: 10.1016/j.jconrel.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Park SM, Cha JM, Nam J, Kim MS, Park SJ, Park ES, Lee H, Kim HR. Formulation optimization and in-vivo proof-of-concept study of thermosensitive liposomes balanced by phospholipid, elastin-like polypeptide, and cholesterol. PLoS One . 2014;9:e103116. doi: 10.1371/journal.pone.0103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tagami T, Kubota M, Ozeki T. Effective remote loading of doxorubicin into DPPC/Poloxamer 188 hybrid liposome to retain thermosensitive property and the assessment of carrier-based acute cytotoxicity for pulmonary administration. J. Pharm. Sci. . 2015;104:3824–32. doi: 10.1002/jps.24593. [DOI] [PubMed] [Google Scholar]
- 52.Zeng C, Yu F, Yang Y, Cheng X, Liu Y, Zhang H, Zhao S, Yang Z, Li M, Li Z, Mei X. Preparation and evaluation of oxaliplatin thermosensitive liposomes with rapid release and high stability. PLoS One . 2016;11:e0158517. doi: 10.1371/journal.pone.0158517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo F, Yu M, Wang J, Tan F, Li N. Smart IR780 theranostic nanocarrier for tumor-specific therapy: Hyperthermia-mediated bubble-generating and folate-targeted liposomes. ACS Appl. Mater. Interfaces . 2015;7:20556–67. doi: 10.1021/acsami.5b06552. [DOI] [PubMed] [Google Scholar]
- 54.Jain A, Jain SK. Multipronged, strategic delivery of paclitaxel-topotecan using engineered liposomes to ovarian cancer. Drug Dev. Ind. Pharm. . 2016;42:136–49. doi: 10.3109/03639045.2015.1036066. [DOI] [PubMed] [Google Scholar]
- 55.Kullberg M, Mann K, Owens JL. A two-component drug delivery system using Her-2-targeting thermosensitive liposomes. J. Drug Target. . 2009;17:98–107. doi: 10.1080/10611860802471562. [DOI] [PubMed] [Google Scholar]
- 56.Kullberg M, Owens JL, Mann K. Listeriolysin O enhances cytoplasmic delivery by Her-2 targeting liposomes. J. Drug Target. . 2010;18:313–20. doi: 10.3109/10611861003663549. [DOI] [PubMed] [Google Scholar]
- 57.Haeri A, Zalba S, Ten Hagen TL, Dadashzadeh S, Koning GA. EGFR targeted thermosensitive liposomes: A novel multifunctional platform for simultaneous tumor targeted and stimulus responsive drug delivery. Colloids Surf. B Biointerfaces . 2016;146:657–69. doi: 10.1016/j.colsurfb.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Smith B, Lyakhov I, Loomis K, Needle D, Baxa U, Yavlovich A, Capala J, Blumenthal R, Puri A. Hyperthermia-triggered intracellular delivery of anticancer agent to HER2(+) cells by HER2-specific affibody (ZHER2-GS-Cys)-conjugated thermosensitive liposomes (HER2(+) affisomes) J. Control. Release . 2011;153:187–94. doi: 10.1016/j.jconrel.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Yang Y, Xie X, Cai X, Zhang H, Gong W, Wang Z, Mei X. PEGylated liposomes with NGR ligand and heat-activable cell-penetrating peptide-doxorubicin conjugate for tumor-specific therapy. Biomaterials . 2014;35:4368–81. doi: 10.1016/j.biomaterials.2014.01.076. [DOI] [PubMed] [Google Scholar]
- 60.Dicheva BM, ten Hagen TL, Seynhaeve AL, Amin M, Eggermont AM, Koning GA. Enhanced specificity and drug delivery in tumors by crgd-anchoring thermosensitive liposomes. Pharm. Res. . 2015;32:3862–76. doi: 10.1007/s11095-015-1746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hossann M, Wang T, Syunyaeva Z, Wiggenhorn M, Zengerle A, Issels RD, Reiser M, Lindner LH, Peller M. Non-ionic Gd-based MRI contrast agents are optimal for encapsulation into phosphatidyldiglycerol-based thermosensitive liposomes. J. Control. Release . 2013;166:22–9. doi: 10.1016/j.jconrel.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Winter ND, Murphy RK, O›Halloran TV, Schatz GC. Development and modeling of arsenic-trioxide-loaded thermosensitive liposomes for anticancer drug delivery. J. Liposome Res. . 2011;21:106–15. doi: 10.3109/08982104.2010.483597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ta T, Convertine AJ, Reyes CR, Stayton PS, Porter TM. Thermosensitive liposomes modified with poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin. Biomacromolecules . 2010;11:1915–20. doi: 10.1021/bm1004993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chountoulesi M, Kyrili A, Pippa N, Meristoudi A, Pispas S, Demetzos C. The modulation of physicochemical characterization of innovative liposomal platforms: the role of the grafted thermoresponsive polymers. Pharm. Dev. Technol. . 2017;22:330–5. doi: 10.3109/10837450.2015.1121497. [DOI] [PubMed] [Google Scholar]
- 65.Ninomiya K, Kawabata S, Tashita H, Shimizu N. Ultrasound-mediated drug delivery using liposomes modified with a thermosensitive polymer. Ultrason. Sonochem. . 2014;21:310–6. doi: 10.1016/j.ultsonch.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Haeri A, Pedrosa LR, Ten Hagen TL, Dadashzadeh S, Koning GA. A novel combined approach of short-chain sphingolipids and thermosensitive liposomes for improved drug delivery to tumor cells. J. Biomed. Nanotechnol. . 2016;12:630–44. doi: 10.1166/jbn.2016.2199. [DOI] [PubMed] [Google Scholar]
- 67.Lokerse WJ, Kneepkens EC, ten Hagen TL, Eggermont AM, Grull H, Koning GA. In depth study on thermosensitive liposomes: Optimizing formulations for tumor specific therapy and in-vitro to in-vivo relations. Biomaterials . 2016;82:138–50. doi: 10.1016/j.biomaterials.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 68.Dou YN, Zheng J, Foltz WD, Weersink R, Chaudary N, Jaffray DA, Allen C. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release . 2014;178:69–78. doi: 10.1016/j.jconrel.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 69.Yu M, Guo F, Tan F, Li N. Dual-targeting nanocarrier system based on thermosensitive liposomes and gold nanorods for cancer thermo-chemotherapy. J. Control. Release . 2015;215:91–100. doi: 10.1016/j.jconrel.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Al Sabbagh C, Tsapis N, Novell A, Calleja-Gonzalez P, Escoffre JM, Bouakaz A, Chacun H, Denis S, Vergnaud J, Gueutin C, Fattal E. Formulation and pharmacokinetics of thermosensitive stealth(R) liposomes encapsulating 5-Fluorouracil. Pharm. Res. . 2015;32:1585–603. doi: 10.1007/s11095-014-1559-0. [DOI] [PubMed] [Google Scholar]
- 71.Dou YN, Weersink RA, Foltz WD, Zheng J, Chaudary N, Jaffray DA, Allen C. Custom-designed Laser-based heating apparatus for triggered release of cisplatin from thermosensitive liposomes with magnetic resonance image guidance. J. Vis. Exp. . 2015:e53055. doi: 10.3791/53055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dicheva BM, ten Hagen TL, Li L, Schipper D, Seynhaeve AL, van Rhoon GC, Eggermont AM, Lindner LH, Koning GA. Cationic thermosensitive liposomes: A novel dual targeted heat-triggered drug delivery approach for endothelial and tumor cells. Nano Lett. . 2013;13:2324–31. doi: 10.1021/nl3014154. [DOI] [PubMed] [Google Scholar]
- 73.Park SM, Kim MS, Park SJ, Park ES, Choi KS, Kim YS, Kim HR. Novel temperature-triggered liposome with high stability: Formulation, in-vitro evaluation and in-vivo study combined with high-intensity focused ultrasound (HIFU) J. Control. Release . 2013;170:373–9. doi: 10.1016/j.jconrel.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Kokuryo D, Nakashima S, Ozaki F, Yuba E, Chuang KH, Aoshima S, Ishizaka Y, Saga T, Kono K, Aoki I. Evaluation of thermo-triggered drug release in intramuscular-transplanted tumors using thermosensitive polymer-modified liposomes and MRI. Nanomedicine . 2015;11:229–38. doi: 10.1016/j.nano.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Song H, Zhang J, Liu X, Deng T, Yao P, Zhou S, Yan W. Development of a bone targeted thermosensitive liposomal doxorubicin formulation based on a bisphosphonate modified non-ionic surfactant. Pharm. Dev. Technol. . 2016;21:680–7. doi: 10.3109/10837450.2015.1045617. [DOI] [PubMed] [Google Scholar]
- 76.Wang C, Wang X, Zhong T, Zhao Y, Zhang WQ, Ren W, Huang D, Zhang S, Guo Y, Yao X, Tang YQ, Zhang X, Zhang Q. The antitumor activity of tumor-homing peptide-modified thermosensitive liposomes containing doxorubicin on MCF-7/ADR: In-vitro and in-vivo. Int. J. Nanomedicine. . 2015;10:2229–48. doi: 10.2147/IJN.S79840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang Y, Yang Y, Xie X, Xu X, Xia X, Wang H, Li L, Dong W, Ma P, Liu Y. Dual stimulus of hyperthermia and intracellular redox environment triggered release of siRNA for tumor-specific therapy. Int. J. Pharm. . 2016;506:158–73. doi: 10.1016/j.ijpharm.2016.04.035. [DOI] [PubMed] [Google Scholar]
- 78.Lee JH, Kim JW, Cheon J. Magnetic nanoparticles for multi-imaging and drug delivery. Mol. Cells. . 2013;35:274–84. doi: 10.1007/s10059-013-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh D, McMillan JM, Kabanov AV, Sokolsky-Papkov M, Gendelman HE. Bench-to-bedside translation of magnetic nanoparticles. Nanomedicine (Lond) 2014;9:501–16. doi: 10.2217/nnm.14.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hervault A, Thanh NT. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale . 2014;6:11553–73. doi: 10.1039/c4nr03482a. [DOI] [PubMed] [Google Scholar]
- 81.De Cuyper M, Joniau M. Magnetoliposomes Formation and structural characterization. Eur. Biophys. J. . 1988;15:311–9. doi: 10.1007/BF00256482. [DOI] [PubMed] [Google Scholar]
- 82.Bakandritsos A, Fatourou AG, Fatouros DG. Magnetoliposomes and their potential in the intelligent drug-delivery field. Ther. Deliv. . 2012;3:1469–82. doi: 10.4155/tde.12.129. [DOI] [PubMed] [Google Scholar]
- 83.Fattahi H, Laurent S, Liu F, Arsalani N, Vander Elst L, Muller RN. Magnetoliposomes as multimodal contrast agents for molecular imaging and cancer nanotheragnostics. Nanomedicine (Lond) 2011;6:529–44. doi: 10.2217/nnm.11.14. [DOI] [PubMed] [Google Scholar]
- 84.Lim EK, Haam S, Lee K, Huh YM. Design and synthesis of biofunctionalized metallic/magnetic nanomaterials. Methods Mol. Biol. . 2011;751:583–95. doi: 10.1007/978-1-61779-151-2_36. [DOI] [PubMed] [Google Scholar]
- 85.Tombacz E, Turcu R, Socoliuc V, Vekas L. Magnetic iron oxide nanoparticles: Recent trends in design and synthesis of magnetoresponsive nanosystems. Biochem. Biophys. Res. Commun. . 2015;468:442–53. doi: 10.1016/j.bbrc.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 86.Langereis S, Geelen T, Grull H, Strijkers GJ, Nicolay K. Paramagnetic liposomes for molecular MRI and MRI-guided drug delivery. NMR Biomed. . 2013;26:728–44. doi: 10.1002/nbm.2971. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida M, Sato M, Yamamoto Y, Maehara T, Naohara T, Aono H, Sugishita H, Sato K, Watanabe Y. Tumor local chemohyperthermia using docetaxel-embedded magnetoliposomes: Interaction of chemotherapy and hyperthermia. J. Gastroenterol. Hepatol. . 2012;27:406–11. doi: 10.1111/j.1440-1746.2011.06972.x. [DOI] [PubMed] [Google Scholar]
- 88.Podaru G, Ogden S, Baxter A, Shrestha T, Ren S, Thapa P, Dani RK, Wang H, Basel MT, Prakash P, Bossmann SH, Chikan V. Pulsed magnetic field induced fast drug release from magneto liposomes via ultrasound generation. J. Phys. Chem. B . 2014;118:11715–22. doi: 10.1021/jp5022278. [DOI] [PubMed] [Google Scholar]
- 89.Guo H, Chen W, Sun X, Liu YN, Li J, Wang J. Theranostic magnetoliposomes coated by carboxymethyl dextran with controlled release by low-frequency alternating magnetic field. Carbohydr. Polym. . 2015;118:209–17. doi: 10.1016/j.carbpol.2014.10.076. [DOI] [PubMed] [Google Scholar]
- 90.Bothun GD, Lelis A, Chen Y, Scully K, Anderson LE, Stoner MA. Multicomponent folate-targeted magnetoliposomes: Design, characterization and cellular uptake. Nanomedicine . 2011;7:797–805. doi: 10.1016/j.nano.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen VD, Zheng S, Han J, Le VH, Park JO, Park S. Nanohybrid magnetic liposome functionalized with hyaluronic acid for enhanced cellular uptake and near-infrared-triggered drug release. Colloids Surf. B Biointerfaces . 2017;154:104–14. doi: 10.1016/j.colsurfb.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Park JH, Cho HJ, Yoon HY, Yoon IS, Ko SH, Shim JS, Cho JH, Park JH, Kim K, Kwon IC, Kim DD. Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control. Release . 2014;174:98–108. doi: 10.1016/j.jconrel.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 93.Yan C, Wu Y, Feng J, Chen W, Liu X, Hao P, Yang R, Zhang J, Lin B, Xu Y, Liu R. Anti-alphavbeta3 antibody guided three-step pretargeting approach using magnetoliposomes for molecular magnetic resonance imaging of breast cancer angiogenesis. Int. J. Nanomedicine. . 2013;8:245–55. doi: 10.2147/IJN.S38678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soenen SJ, Brisson AR, Jonckheere E, Nuytten N, Tan S, Himmelreich U, De Cuyper M. The labeling of cationic iron oxide nanoparticle-resistant hepatocellular carcinoma cells using targeted magnetoliposomes. Biomaterials . 2011;32:1748–58. doi: 10.1016/j.biomaterials.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 95.Lin W, Xie X, Yang Y, Fu X, Liu H, Yang Y, Deng J. Thermosensitive magnetic liposomes with doxorubicin cell-penetrating peptides conjugate for enhanced and targeted cancer therapy. Drug Deliv. . 2016;23:3436–43. doi: 10.1080/10717544.2016.1189983. [DOI] [PubMed] [Google Scholar]
- 96.Mattheolabakis G, Milane L, Singh A, Amiji MM. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. . 2015;23:605–18. doi: 10.3109/1061186X.2015.1052072. [DOI] [PubMed] [Google Scholar]
- 97.Wang F, Wang Y, Zhang X, Zhang W, Guo S, Jin F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release . 2014;174:126–36. doi: 10.1016/j.jconrel.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 98.Jhaveri A, Torchilin V. Intracellular delivery of nanocarriers and targeting to subcellular organelles. Expert Opin. Drug Deliv. . 2016;13:49–70. doi: 10.1517/17425247.2015.1086745. [DOI] [PubMed] [Google Scholar]
- 99.Clares B, Biedma-Ortiz RA, Saez-Fernandez E, Prados JC, Melguizo C, Cabeza L, Ortiz R, Arias JL. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. . 2013;85:329–38. doi: 10.1016/j.ejpb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 100.Ferreira RV, Martins TM, Goes AM, Fabris JD, Cavalcante LC, Outon LE, Domingues RZ. Thermosensitive gemcitabine-magnetoliposomes for combined hyperthermia and chemotherapy. Nanotechnology . 2016;27:085105. doi: 10.1088/0957-4484/27/8/085105. [DOI] [PubMed] [Google Scholar]
- 101.Kulshrestha P, Gogoi M, Bahadur D, Banerjee R. In-vitro application of paclitaxel loaded magnetoliposomes for combined chemotherapy and hyperthermia. Colloids Surf. B Biointerfaces . 2012;96:1–7. doi: 10.1016/j.colsurfb.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 102.Amstad E, Kohlbrecher J, Muller E, Schweizer T, Textor M, Reimhult E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. . 2011;11:1664–70. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 103.Hardiansyah A, Yang MC, Liu TY, Kuo CY, Huang LY, Chan TY. Hydrophobic drug-loaded PEGylated magnetic liposomes for drug-controlled release. Nanoscale Res. Lett. . 2017;12:355. doi: 10.1186/s11671-017-2119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L, Zhang J, An Y, Wang Z, Liu J, Li Y, Zhang D. A study on the thermochemotherapy effect of nanosized As2O3/MZF thermosensitive magnetoliposomes on experimental hepatoma in-vitro and in-vivo. Nanotechnology . 2011;22:315102. doi: 10.1088/0957-4484/22/31/315102. [DOI] [PubMed] [Google Scholar]
- 105.Spera R, Petralito S, Liberti M, Merla C, d›Inzeo G, Pinto R, Apollonio F. Controlled release from magnetoliposomes aqueous suspensions exposed to a low intensity magnetic field. Bioelectromagnetics . 2014;35:309–12. doi: 10.1002/bem.21841. [DOI] [PubMed] [Google Scholar]
- 106.Spera R, Apollonio F, Liberti M, Paffi A, Merla C, Pinto R, Petralito S. Controllable release from high-transition temperature magnetoliposomes by low-level magnetic stimulation. Colloids Surf. B Biointerfaces . 2015;131:136–40. doi: 10.1016/j.colsurfb.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 107.Bealle G, Lartigue L, Wilhelm C, Ravaux J, Gazeau F, Podor R, Carriere D, Menager C. Surface decoration of catanionic vesicles with superparamagnetic iron oxide nanoparticles: A model system for triggered release under moderate temperature conditions. Phys. Chem. Chem. Phys. . 2014;16:4077–81. doi: 10.1039/c3cp54484b. [DOI] [PubMed] [Google Scholar]
- 108.Ye H, Tong J, Liu J, Lin W, Zhang C, Chen K, Zhao J, Zhu W. Combination of gemcitabine-containing magnetoliposome and oxaliplatin-containing magnetoliposome in breast cancer treatment: A possible mechanism with potential for clinical application. Oncotarget . 2016;7:43762–78. doi: 10.18632/oncotarget.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doblinger M, Banerjee R, Bahadur D, Plank C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release . 2010;142:108–21. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 110.Yang R, An LY, Miao QF, Li FM, Han Y, Wang HX, Liu DP, Chen R, Tang SQ. Effective elimination of liver cancer stem-like cells by CD90 antibody targeted thermosensitive magnetoliposomes. Oncotarget . 2016;7:35894–916. doi: 10.18632/oncotarget.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yavlovich A, Smith B, Gupta K, Blumenthal R, Puri A. Light-sensitive lipid-based nanoparticles for drug delivery: Design principles and future considerations for biological applications. Mol. Membr. Biol. . 2010;27:364–81. doi: 10.3109/09687688.2010.507788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leung SJ, Romanowski M. Light-activated content release from liposomes. Theranostics . 2012;2:1020–36. doi: 10.7150/thno.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Allison RR. Photodynamic therapy: Oncologic horizons. Future Oncol. . 2014;10:123–4. doi: 10.2217/fon.13.176. [DOI] [PubMed] [Google Scholar]
- 114.Voon SH, Kiew LV, Lee HB, Lim SH, Noordin MI, Kamkaew A, Burgess K, Chung LY. In-vivo studies of nanostructure-based photosensitizers for photodynamic cancer therapy. Small . 2014;10:4993–5013. doi: 10.1002/smll.201401416. [DOI] [PubMed] [Google Scholar]
- 115.Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem. Rev. . 2015;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 116.Bansal A, Zhang Y. Photocontrolled nanoparticle delivery systems for biomedical applications. Acc. Chem. Res. . 2014;47:3052–60. doi: 10.1021/ar500217w. [DOI] [PubMed] [Google Scholar]
- 117.Fomina N, Sankaranarayanan J, Almutairi A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. . 2012;64:1005–20. doi: 10.1016/j.addr.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu XM, Yang B, Wang YL, Wang JY. Photoisomerisable cholesterol derivatives as photo-trigger of liposomes: Effect of lipid polarity, temperature, incorporation ratio and cholesterol. Biochim. Biophys. Acta . 2005;1720:28–34. doi: 10.1016/j.bbamem.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 119.Bisby RH, Mead C, Morgan CG. Wavelength-programmed solute release from photosensitive liposomes. Biochem. Biophys. Res. Commun. . 2000;276:169–73. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- 120.Liu D, Wang S, Xu S, Liu H. Photocontrollable intermittent release of doxorubicin hydrochloride from liposomes embedded by azobenzene-contained glycolipid. Langmuir . 2017;33:1004–12. doi: 10.1021/acs.langmuir.6b03051. [DOI] [PubMed] [Google Scholar]
- 121.Yao C, Wang P, Li X, Hu X, Hou J, Wang L, Zhang F. Near-infrared-triggered azobenzene-liposome/upconversion nanoparticle hybrid vesicles for remotely controlled drug delivery to overcome cancer multidrug resistance. Adv. Mater. . 2016;28:9341–8. doi: 10.1002/adma.201503799. [DOI] [PubMed] [Google Scholar]
- 122.Chandra B, Mallik S, Srivastava DK. Design of photocleavable lipids and their application in liposomal "uncorking". Chem. Commun. (Camb) 2005:3021–3. doi: 10.1039/b503423j. [DOI] [PubMed] [Google Scholar]
- 123.Chandra B, Subramaniam R, Mallik S, Srivastava DK. Formulation of photocleavable liposomes and the mechanism of their content release. Org. Biomol. Chem. . 2006;4:1730–40. doi: 10.1039/b518359f. [DOI] [PubMed] [Google Scholar]
- 124.Bayer AM, Alam S, Mattern-Schain SI, Best MD. Triggered liposomal release through a synthetic phosphatidylcholine analogue bearing a photocleavable moiety embedded within the sn-2 acyl chain. Chemistry . 2014;20:3350–7. doi: 10.1002/chem.201304094. [DOI] [PubMed] [Google Scholar]
- 125.Yavlovich A, Singh A, Blumenthal R, Puri A. A novel class of photo-triggerable liposomes containing DPPC:DC(8,9)PC as vehicles for delivery of doxorubcin to cells. Biochim. Biophys. Acta . 2011;1808:117–26. doi: 10.1016/j.bbamem.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yavlovich A, Viard M, Gupta K, Sine J, Vu M, Blumenthal R, Tata DB, Puri A. Low-visibility light-intensity laser-triggered release of entrapped calcein from 1,2-bis (tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine liposomes is mediated through a type I photoactivation pathway. Int. J. Nanomedicine . 2013;8:2575–87. doi: 10.2147/IJN.S44993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Puri A. Phototriggerable liposomes: Current research and future perspectives. Pharmaceutics . 2013;6:1–25. doi: 10.3390/pharmaceutics6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aygun A, Torrey K, Kumar A, Stephenson LD. Investigation of factors affecting controlled release from photosensitive DMPC and DSPC liposomes. Appl. Biochem. Biotechnol. . 2012;167:743–57. doi: 10.1007/s12010-012-9724-6. [DOI] [PubMed] [Google Scholar]
- 129.Lajunen T, Kontturi LS, Viitala L, Manna M, Cramariuc O, Rog T, Bunker A, Laaksonen T, Viitala T, Murtomaki L, Urtti A. Indocyanine green-loaded liposomes for light-triggered drug release. Mol. Pharm. . 2016;13:2095–107. doi: 10.1021/acs.molpharmaceut.6b00207. [DOI] [PubMed] [Google Scholar]
- 130.Massiot J, Makky A, Di Meo F, Chapron D, Trouillas P, Rosilio V. Impact of lipid composition and photosensitizer hydrophobicity on the efficiency of light-triggered liposomal release. Phys. Chem. Chem. Phys. . 2017;19:11460–73. doi: 10.1039/c7cp00983f. [DOI] [PubMed] [Google Scholar]
- 131.Leung SJ, Kachur XM, Bobnick MC, Romanowski M. Wavelength-Selective Light-Induced Release from Plasmon Resonant Liposomes. Adv. Funct. Mater. . 2011;21:1113–21. doi: 10.1002/adfm.201002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paasonen L, Sipila T, Subrizi A, Laurinmaki P, Butcher SJ, Rappolt M, Yaghmur A, Urtti A, Yliperttula M. Gold-embedded photosensitive liposomes for drug delivery: triggering mechanism and intracellular release. J. Control. Release . 2010;147:136–43. doi: 10.1016/j.jconrel.2010.07.095. [DOI] [PubMed] [Google Scholar]
- 133.Pashkovskaya A, Kotova E, Zorlu Y, Dumoulin F, Ahsen V, Agapov I, Antonenko Y. Light-triggered liposomal release: Membrane permeabilization by photodynamic action. Langmuir . 2010;26:5726–33. doi: 10.1021/la903867a. [DOI] [PubMed] [Google Scholar]
- 134.Uda RM, Kato Y, Takei M. Photo-triggered release from liposomes without membrane solubilization, based on binding to poly(vinyl alcohol) carrying a malachite green moiety. Colloids Surf. B Biointerfaces . 2016;146:716–21. doi: 10.1016/j.colsurfb.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 135.Hester TJ, Dennison SR, Baker MJ, Snape TJ. Functionalising the azobenzene motif delivers a light-responsive membrane-interactive compound with the potential for photodynamic therapy applications. Org. Biomol. Chem. . 2015;13:8067–70. doi: 10.1039/c5ob00465a. [DOI] [PubMed] [Google Scholar]
- 136.Li Q, Tang Q, Zhang P, Wang Z, Zhao T, Zhou J, Li H, Ding Q, Li W, Hu F, Du Y, Yuan H, Chen S, Gao J, Zhan J, You J. Human epidermal growth factor receptor-2 antibodies enhance the specificity and anticancer activity of light-sensitive doxorubicin-labeled liposomes. Biomaterials . 2015;57:1–11. doi: 10.1016/j.biomaterials.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 137.Husseini GA, Pitt WG, Martins AM. Ultrasonically triggered drug delivery: Breaking the barrier. Colloids Surf. B Biointerfaces . 2014;123:364–86. doi: 10.1016/j.colsurfb.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 138.Ahmed SE, Martins AM, Husseini GA. The use of ultrasound to release chemotherapeutic drugs from micelles and liposomes. J. Drug. Target. . 2015;23:16–42. doi: 10.3109/1061186X.2014.954119. [DOI] [PubMed] [Google Scholar]
- 139.Jhaveri A, Shvets V, Torchilin V. In: Stimuli-Sensitive Nanopreparations: Overview. Torchilin V, editor. Smart Pharmaceutical Nanocarriers. World Scientific; 2015. pp. 1–48. [Google Scholar]
- 140.Ibsen S, Benchimol M, Simberg D, Schutt C, Steiner J, Esener S. A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J. Control. Release . 2011;155:358–66. doi: 10.1016/j.jconrel.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yin T, Wang P, Li J, Zheng R, Zheng B, Cheng D, Li R, Lai J, Shuai X. Ultrasound-sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials . 2013;34:4532–43. doi: 10.1016/j.biomaterials.2013.02.067. [DOI] [PubMed] [Google Scholar]
- 142.Chung MF, Chen KJ, Liang HF, Liao ZX, Chia WT, Xia Y, Sung HW. A liposomal system capable of generating CO2 bubbles to induce transient cavitation, lysosomal rupturing and cell necrosis. Angew. Chem. Int. Ed. Engl. . 2012;51:10089–93. doi: 10.1002/anie.201205482. [DOI] [PubMed] [Google Scholar]
- 143.Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, Hirata K, Taira Y, Utoguchi N, Negishi Y, Nakagawa S, Maruyama K. Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure. J. Control. Release . 2010;142:245–50. doi: 10.1016/j.jconrel.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 144.Negishi Y, Endo Y, Fukuyama T, Suzuki R, Takizawa T, Omata D, Maruyama K, Aramaki Y. Delivery of siRNA into the cytoplasm by liposomal bubbles and ultrasound. J. Control. Release . 2008;132:124–30. doi: 10.1016/j.jconrel.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 145.Javadi M, Pitt WG, Belnap DM, Tsosie NH, Hartley JM. Encapsulating nanoemulsions inside eLiposomes for ultrasonic drug delivery. Langmuir . 2012;28:14720–9. doi: 10.1021/la303464v. [DOI] [PubMed] [Google Scholar]
- 146.Lattin JR, Belnap DM, Pitt WG. Formation of eLiposomes as a drug delivery vehicle. Colloids Surf. B Biointerfaces . 2012;89:93–100. doi: 10.1016/j.colsurfb.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 147.Lin CY, Javadi M, Belnap DM, Barrow JR, Pitt WG. Ultrasound sensitive eLiposomes containing doxorubicin for drug targeting therapy. Nanomedicine . 2014;10:67–76. doi: 10.1016/j.nano.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 148.Cool SK, Geers B, Roels S, Stremersch S, Vanderperren K, Saunders JH, De Smedt SC, Demeester J, Sanders NN. Coupling of drug containing liposomes to microbubbles improves ultrasound triggered drug delivery in mice. J. Control. Release . 2013;172:885–93. doi: 10.1016/j.jconrel.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 149.Klibanov AL, Shevchenko TI, Raju BI, Seip R, Chin CT. Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: A tool for targeted drug delivery. J. Control. Release . 2010;148:13–7. doi: 10.1016/j.jconrel.2010.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Herbst SM, Klegerman ME, Kim H, Qi J, Shelat H, Wassler M, Moody MR, Yang CM, Ge X, Zou Y, Kopechek JA, Clubb FJ, Kraemer DC, Huang S, Holland CK, McPherson DD, Geng YJ. Delivery of stem cells to porcine arterial wall with echogenic liposomes conjugated to antibodies against CD34 and intercellular adhesion molecule-1. Mol. Pharm. . 2010;7:3–11. doi: 10.1021/mp900116r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Javadi M, Pitt WG, Tracy CM, Barrow JR, Willardson BM, Hartley JM, Tsosie NH. Ultrasonic gene and drug delivery using eLiposomes. J. Control. Release . 2013;167:92–100. doi: 10.1016/j.jconrel.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 152.Omata D, Negishi Y, Hagiwara S, Yamamura S, Endo-Takahashi Y, Suzuki R, Maruyama K, Nomizu M, Aramaki Y. Bubble liposomes and ultrasound promoted endosomal escape of TAT-PEG liposomes as gene delivery carriers. Mol. Pharm. . 2011;8:2416–23. doi: 10.1021/mp200353m. [DOI] [PubMed] [Google Scholar]
- 153.Evjen TJ, Nilssen EA, Barnert S, Schubert R, Brandl M, Fossheim SL. Ultrasound-mediated destabilization and drug release from liposomes comprising dioleoylphosphatidylethanolamine. Eur. J. Pharm. Sci. . 2011;42:380–6. doi: 10.1016/j.ejps.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Evjen TJ, Hupfeld S, Barnert S, Fossheim S, Schubert R, Brandl M. Physicochemical characterization of liposomes after ultrasound exposure - mechanisms of drug release. J Pharm. Biomed. Anal. . 2013;78-79:118–22. doi: 10.1016/j.jpba.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 155.Evjen TJ, Nilssen EA, Fowler RA, Rognvaldsson S, Brandl M, Fossheim SL. Lipid membrane composition influences drug release from dioleoylphosphatidylethanolamine-based liposomes on exposure to ultrasound. Int. J. Pharm. . 2011;406:114–6. doi: 10.1016/j.ijpharm.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 156.Geers B, Lentacker I, Sanders NN, Demeester J, Meairs S, De Smedt SC. Self-assembled liposome-loaded microbubbles: The missing link for safe and efficient ultrasound triggered drug-delivery. J. Control. Release . 2011;152:249–56. doi: 10.1016/j.jconrel.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 157.Nahire R, Paul S, Scott MD, Singh RK, Muhonen WW, Shabb J, Gange KN, Srivastava DK, Sarkar K, Mallik S. Ultrasound enhanced matrix metalloproteinase-9 triggered release of contents from echogenic liposomes. Mol. Pharm. . 2012;9:2554–64. doi: 10.1021/mp300165s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Evjen TJ, Hagtvet E, Moussatov A, Rognvaldsson S, Mestas JL, Fowler RA, Lafon C, Nilssen EA. In-vivo monitoring of liposomal release in tumours following ultrasound stimulation. Eur. J. Pharm. Biopharm. . 2013;84:526–31. doi: 10.1016/j.ejpb.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 159.Graham SM, Carlisle R, Choi JJ, Stevenson M, Shah AR, Myers RS, Fisher K, Peregrino MB, Seymour L, Coussios CC. Inertial cavitation to non-invasively trigger and monitor intratumoral release of drug from intravenously delivered liposomes. J. Control. Release . 2014;178:101–7. doi: 10.1016/j.jconrel.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 160.Simoes S, Moreira JN, Fonseca C, Duzgunes N, de Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. . 2004;56:947–65. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 161.Ferreira Ddos S, Lopes SC, Franco MS, Oliveira MC. pH-sensitive liposomes for drug delivery in cancer treatment. Ther. Deliv. . 2013;4:1099–123. doi: 10.4155/tde.13.80. [DOI] [PubMed] [Google Scholar]
- 162.Romero-Garcia S, Lopez-Gonzalez JS, Baez-Viveros JL, Aguilar-Cazares D, Prado-Garcia H. Tumor cell metabolism: An integral view. Cancer Biol. Ther. . 2011;12:939–48. doi: 10.4161/cbt.12.11.18140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hernandez A, Serrano G, Herrera-Palau R, Perez-Castineira JR, Serrano A. Intraorganellar acidification by V-ATPases: A target in cell proliferation and cancer therapy. Recent Pat. Anticancer Drug Discov. . 2010;5:88–98. doi: 10.2174/157489210790936216. [DOI] [PubMed] [Google Scholar]
- 164.Shete HK, Prabhu RH, Patravale VB. Endosomal escape: A bottleneck in intracellular delivery. J. Nanosci. Nanotechnol. . 2014;14:460–74. doi: 10.1166/jnn.2014.9082. [DOI] [PubMed] [Google Scholar]
- 165.Yang X, Yi C, Luo N, Gong C. Nanomedicine to overcome cancer multidrug resistance. Curr. Drug Metab. . 2014;15:632–49. doi: 10.2174/1389200215666140926154443. [DOI] [PubMed] [Google Scholar]
- 166.Paliwal SR, Paliwal R, Vyas SP. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. . 2015;22:231–42. doi: 10.3109/10717544.2014.882469. [DOI] [PubMed] [Google Scholar]
- 167.Kanamala M, Wilson WR, Yang M, Palmer BD, Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials . 2016;85:152–67. doi: 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 168.Guo X, Gagne L, Chen H, Szoka FC. Novel ortho ester-based, pH-sensitive cationic lipid for gene delivery in-vitro and in-vivo. J. Liposome Res. . 2014;24:90–8. doi: 10.3109/08982104.2013.848886. [DOI] [PubMed] [Google Scholar]
- 169.Sato Y, Hatakeyama H, Sakurai Y, Hyodo M, Akita H, Harashima H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in-vitro and in-vivo. J. Control. Release . 2012;163:267–76. doi: 10.1016/j.jconrel.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 170.Hatakeyama H, Murata M, Sato Y, Takahashi M, Minakawa N, Matsuda A, Harashima H. The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice. J. Control. Release . 2014;173:43–50. [PubMed] [Google Scholar]
- 171.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J. Control. Release . 2011;151:220–8. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 172.Bertrand N, Simard P, Leroux JC. Serum-stable, long-circulating, pH-sensitive PEGylated liposomes. Methods Mol. Biol. . 2010;605:545–58. doi: 10.1007/978-1-60327-360-2_36. [DOI] [PubMed] [Google Scholar]
- 173.Simard P, Leroux JC. In-vivo evaluation of pH-sensitive polymer-based immunoliposomes targeting the CD33 antigen. Mol. Pharm. . 2010;7:1098–107. doi: 10.1021/mp900261m. [DOI] [PubMed] [Google Scholar]
- 174.Yoshizaki Y, Yuba E, Sakaguchi N, Koiwai K, Harada A, Kono K. Potentiation of pH-sensitive polymer-modified liposomes with cationic lipid inclusion as antigen delivery carriers for cancer immunotherapy. Biomaterials . 2014;35:8186–96. doi: 10.1016/j.biomaterials.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 175.Yuba E, Harada A, Sakanishi Y, Kono K. Carboxylated hyperbranched poly(glycidol)s for preparation of pH-sensitive liposomes. J. Control. Release . 2011;149:72–80. doi: 10.1016/j.jconrel.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 176.Naziris N, Pippa N, Meristoudi A, Pispas S, Demetzos C. Design and development of pH-responsive HSPC:C12H25-PAA chimeric liposomes. J. Liposome Res. . 2017;27:108–17. doi: 10.3109/08982104.2016.1166512. [DOI] [PubMed] [Google Scholar]
- 177.Pippa N, Chountoulesi M, Kyrili A, Meristoudi A, Pispas S, Demetzos C. Calorimetric study on pH-responsive block copolymer grafted lipid bilayers: Rational design and development of liposomes. J. Liposome Res. . 2016;26:211–20. doi: 10.3109/08982104.2015.1076464. [DOI] [PubMed] [Google Scholar]
- 178.Zhang L, Wang Y, Yang Y, Liu Y, Ruan S, Zhang Q, Tai X, Chen J, Xia T, Qiu Y, Gao H, He Q. High tumor penetration of paclitaxel loaded pH sensitive cleavable liposomes by depletion of tumor collagen I in breast cancer. ACS Appl. Mater. Interfaces . 2015;7:9691–701. doi: 10.1021/acsami.5b01473. [DOI] [PubMed] [Google Scholar]
- 179.Chen D, Liu W, Shen Y, Mu H, Zhang Y, Liang R, Wang A, Sun K, Fu F. Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int. J. Nanomedicine . 2011;6:2053–61. doi: 10.2147/IJN.S24344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim HK, Thompson DH, Jang HS, Chung YJ, Van den Bossche J. pH-responsive biodegradable assemblies containing tunable phenyl-substituted vinyl ethers for use as efficient gene delivery vehicles. ACS Appl. Mater. Interfaces . 2013;5:5648–58. doi: 10.1021/am400977t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chan CL, Majzoub RN, Shirazi RS, Ewert KK, Chen YJ, Liang KS, Safinya CR. Endosomal escape and transfection efficiency of PEGylated cationic liposome-DNA complexes prepared with an acid-labile PEG-lipid. Biomaterials . 2012;33:4928–35. doi: 10.1016/j.biomaterials.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Guo P, You JO, Yang J, Jia D, Moses MA, Auguste DT. Inhibiting metastatic breast cancer cell migration via the synergy of targeted, pH-triggered siRNA delivery and chemokine axis blockade. Mol. Pharm. . 2014;11:755–65. doi: 10.1021/mp4004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim MJ, Lee HJ, Lee IA, Kim IY, Lim SK, Cho HA, Kim JS. Preparation of pH-sensitive, long-circulating and EGFR-targeted immunoliposomes. Arch. Pharm. Res. . 2008;31:539–46. doi: 10.1007/s12272-001-1190-9. [DOI] [PubMed] [Google Scholar]
- 184.Garg A, Kokkoli E. pH-Sensitive PEGylated liposomes functionalized with a fibronectin-mimetic peptide show enhanced intracellular delivery to colon cancer cell. Curr. Pharm. Biotechnol. . 2011;12:1135–43. doi: 10.2174/138920111796117328. [DOI] [PubMed] [Google Scholar]
- 185.Zhao Y, Ren W, Zhong T, Zhang S, Huang D, Guo Y, Yao X, Wang C, Zhang WQ, Zhang X, Zhang Q. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release . 2016;222:56–66. doi: 10.1016/j.jconrel.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 186.Paliwal SR, Paliwal R, Agrawal GP, Vyas SP. Hyaluronic acid modified pH-sensitive liposomes for targeted intracellular delivery of doxorubicin. J. Liposome Res. . 2016;26:276–87. doi: 10.3109/08982104.2015.1117489. [DOI] [PubMed] [Google Scholar]
- 187.Fonseca C, Moreira JN, Ciudad CJ, Pedroso de Lima MC, Simoes S. Targeting of sterically stabilised pH-sensitive liposomes to human T-leukaemia cells. Eur. J. Pharm. Biopharm. . 2005;59:359–66. doi: 10.1016/j.ejpb.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 188.Shi G, Guo W, Stephenson SM, Lee RJ. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J. Control. Release . 2002;80:309–19. doi: 10.1016/s0168-3659(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 189.Paliwal SR, Paliwal R, Pal HC, Saxena AK, Sharma PR, Gupta PN, Agrawal GP, Vyas SP. Estrogen-anchored pH-sensitive liposomes as nanomodule designed for site-specific delivery of doxorubicin in breast cancer therapy. Mol. Pharm. . 2012;9:176–86. doi: 10.1021/mp200439z. [DOI] [PubMed] [Google Scholar]
- 190.Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. . 2014;10:2293–301. doi: 10.2217/fon.14.110. [DOI] [PubMed] [Google Scholar]
- 191.Fan Y, Chen C, Huang Y, Zhang F, Lin G. Study of the pH-sensitive mechanism of tumor-targeting liposomes. Colloids Surf. B Biointerfaces . 2017;151:19–25. doi: 10.1016/j.colsurfb.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 192.Xu H, Hu M, Yu X, Li Y, Fu Y, Zhou X, Zhang D, Li J. Design and evaluation of pH-sensitive liposomes constructed by poly(2-ethyl-2-oxazoline)-cholesterol hemisuccinate for doxorubicin delivery. Eur. J. Pharm. Biopharm. . 2015;91:66–74. doi: 10.1016/j.ejpb.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 193.Hao W, Han X, Shang Y, Xu S, Liu H. Insertion of pH-sensitive bola-type copolymer into liposome as a "stability anchor" for control of drug release. Colloids Surf. B Biointerfaces . 2015;136:809–16. doi: 10.1016/j.colsurfb.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 194.Monteiro LO, Lopes SC, Barros AL, Magalhaes-Paniago R, Malachias A, Oliveira MC, Leite EA. Phase behavior of dioleyphosphatidylethanolamine molecules in the presence of components of pH-sensitive liposomes and paclitaxel. Colloids Surf. B Biointerfaces . 2016;144:276–83. doi: 10.1016/j.colsurfb.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 195.Chiang YT, Lo CL. pH-responsive polymer-liposomes for intracellular drug delivery and tumor extracellular matrix switched-on targeted cancer therapy. Biomaterials . 2014;35:5414–24. doi: 10.1016/j.biomaterials.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 197.Hatakeyama H, Murata M, Sato Y, Takahashi M, Minakawa N, Matsuda A, Harashima H. The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice. J. Control. Release . 2014;173:43–50. [PubMed] [Google Scholar]
- 198.Mamasheva E, O›Donnell C, Bandekar A, Sofou S. Heterogeneous liposome membranes with pH-triggered permeability enhance the in-vitro antitumor activity of folate-receptor targeted liposomal doxorubicin. Mol. Pharm. . 2011;8:2224–32. doi: 10.1021/mp200079y. [DOI] [PubMed] [Google Scholar]
- 199.Fu H, Shi K, Hu G, Yang Y, Kuang Q, Lu L, Zhang L, Chen W, Dong M, Chen Y, He Q. Tumor-targeted paclitaxel delivery and enhanced penetration using TAT-decorated liposomes comprising redox-responsive poly(ethylene glycol) J. Pharm. Sci. . 2015;104:1160–73. doi: 10.1002/jps.24291. [DOI] [PubMed] [Google Scholar]
- 200.Ferreira Ddos S, Faria SD, Lopes SC, Teixeira CS, Malachias A, Magalhaes-Paniago R, de Souza Filho JD, Oliveira BL, Guimaraes AR, Caravan P, Ferreira LA, Alves RJ, Oliveira MC. Development of a bone-targeted pH-sensitive liposomal formulation containing doxorubicin: Physicochemical characterization, cytotoxicity and biodistribution evaluation in a mouse model of bone metastasis. Int. J. Nanomedicine . 2016;11:3737–51. doi: 10.2147/IJN.S109966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jiang T, Zhang Z, Zhang Y, Lv H, Zhou J, Li C, Hou L, Zhang Q. Dual-functional liposomes based on pH-responsive cell-penetrating peptide and hyaluronic acid for tumor-targeted anticancer drug delivery. Biomaterials . 2012;33:9246–58. doi: 10.1016/j.biomaterials.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 202.Han Q, Wang W, Jia X, Qian Y, Li Q, Wang Z, Zhang W, Yang S, Jia Y, Hu Z. Switchable liposomes: Targeting-peptide-functionalized and pH-triggered cytoplasmic delivery. ACS Appl. Mater. Interfaces . 2016;8:18658–63. doi: 10.1021/acsami.6b05678. [DOI] [PubMed] [Google Scholar]
- 203.Shi K, Li J, Cao Z, Yang P, Qiu Y, Yang B, Wang Y, Long Y, Liu Y, Zhang Q, Qian J, Zhang Z, Gao H, He Q. A pH-responsive cell-penetrating peptide-modified liposomes with active recognizing of integrin αvβ3 for the treatment of melanoma. J. Control. Release . 2015;217:138–50. doi: 10.1016/j.jconrel.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 204.Zhang Q, Lu L, Zhang L, Shi K, Cun X, Yang Y, Liu Y, Gao H, He Q. Dual-functionalized liposomal delivery system for solid tumors based on RGD and a pH-responsive antimicrobial peptide. Sci. Rep. . 2016;6:19800. doi: 10.1038/srep19800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu Y, Gao FP, Zhang D, Fan YS, Chen XG, Wang H. Molecular structural transformation regulated dynamic disordering of supramolecular vesicles as pH-responsive drug release systems. J. Control. Release . 2014;173:140–7. doi: 10.1016/j.jconrel.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 206.Fouladi F, Steffen KJ, Mallik S. Enzyme-responsive liposomes for the delivery of anticancer drugs. Bioconjug. Chem. . 2017;28:857–68. doi: 10.1021/acs.bioconjchem.6b00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hu J, Zhang G, Liu S. Enzyme-responsive polymeric assemblies, nanoparticles and hydrogels. Chem. Soc. Rev. . 2012;41:5933–49. doi: 10.1039/c2cs35103j. [DOI] [PubMed] [Google Scholar]
- 208.Hu Q, Katti PS, Gu Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale . 2014;6:12273–86. doi: 10.1039/c4nr04249b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Quach ND, Arnold RD, Cummings BS. Secretory phospholipase A2 enzymes as pharmacological targets for treatment of disease. Biochem. Pharmacol. . 2014;90:338–48. doi: 10.1016/j.bcp.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zuo J, Tong L, Du L, Yang M, Jin Y. Biomimetic nanoassemblies of 1-O-octodecyl-2-conjugated linoleoyl-sn-glycero-3-phosphatidyl gemcitabine with phospholipase A2-triggered degradation for the treatment of cancer. Colloids Surf. B Biointerfaces . 2017;152:467–74. doi: 10.1016/j.colsurfb.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 211.Quach ND, Mock JN, Scholpa NE, Eggert MW, Payre C, Lambeau G, Arnold RD, Cummings BS. Role of the phospholipase A2 receptor in liposome drug delivery in prostate cancer cells. Mol. Pharm. . 2014;11:3443–51. doi: 10.1021/mp500174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ostrem RG, Parhamifar L, Pourhassan H, Clergeaud G, Nielsen OL, Kjaer A, Hansen AE, Andresen TL. Secretory phospholipase A2 responsive liposomes exhibit a potent anti-neoplastic effect in-vitro, but induce unforeseen severe toxicity in-vivo. J. Control. Release . 2017;262:212–21. doi: 10.1016/j.jconrel.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 213.Hansen AH, Mouritsen OG, Arouri A. Enzymatic action of phospholipase A(2) on liposomal drug delivery systems. Int. J. Pharm. . 2015;491:49–57. doi: 10.1016/j.ijpharm.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 214.Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G. Matrix metalloproteinases and cancer - roles in threat and therapy. Asian Pac. J. Cancer Prev. . 2014;15:1085–91. doi: 10.7314/apjcp.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 215.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. . 2012;6:3491–8. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Elegbede AI, Banerjee J, Hanson AJ, Tobwala S, Ganguli B, Wang R, Lu X, Srivastava DK, Mallik S. Mechanistic studies of the triggered release of liposomal contents by matrix metalloproteinase-9. J. Am. Chem. Soc. . 2008;130:10633–42. doi: 10.1021/ja801548g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sarkar N, Banerjee J, Hanson AJ, Elegbede AI, Rosendahl T, Krueger AB, Banerjee AL, Tobwala S, Wang R, Lu X, Mallik S, Srivastava DK. Matrix metalloproteinase-assisted triggered release of liposomal contents. Bioconjug. Chem. . 2008;19:57–64. doi: 10.1021/bc070081p. [DOI] [PubMed] [Google Scholar]
- 218.Mekkawy AH, Pourgholami MH, Morris DL. Involvement of urokinase-type plasminogen activator system in cancer: An overview. Med. Res. Rev. . 2014;34:918–56. doi: 10.1002/med.21308. [DOI] [PubMed] [Google Scholar]
- 219.Xiang B, Dong DW, Shi NQ, Gao W, Yang ZZ, Cui Y, Cao DY, Qi XR. PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials . 2013;34:6976–91. doi: 10.1016/j.biomaterials.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 220.Aggarwal N, Sloane BF. Cathepsin B: Multiple roles in cancer. Proteomics Clin. Appl. . 2014;8:427–37. doi: 10.1002/prca.201300105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Song SJ, Lee S, Lee Y, Choi JS. Enzyme-responsive destabilization of stabilized plasmid-lipid nanoparticles as an efficient gene delivery. Eur. J. Pharm. Sci. . 2016;91:20–30. doi: 10.1016/j.ejps.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 222.Satsangi A, Roy SS, Satsangi RK, Tolcher AW, Vadlamudi RK, Goins B, Ong JL. Synthesis of a novel, sequentially active-targeted drug delivery nanoplatform for breast cancer therapy. Biomaterials . 2015;59:88–101. doi: 10.1016/j.biomaterials.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 223.Linderoth L, Fristrup P, Hansen M, Melander F, Madsen R, Andresen TL, Peters GH. Mechanistic study of the sPLA2-mediated hydrolysis of a thio-ester pro anticancer ether lipid. J. Am. Chem. Soc. . 2009;131:12193–200. doi: 10.1021/ja901412j. [DOI] [PubMed] [Google Scholar]
- 224.Tagami T, Ando Y, Ozeki T. Fabrication of liposomal doxorubicin exhibiting ultrasensitivity against phospholipase A2 for efficient pulmonary drug delivery to lung cancers. Int. J. Pharm. . 2017;517:35–41. doi: 10.1016/j.ijpharm.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 225.Mock JN, Costyn LJ, Wilding SL, Arnold RD, Cummings BS. Evidence for distinct mechanisms of uptake and antitumor activity of secretory phospholipase A2 responsive liposome in prostate cancer. Integr. Biol. (Camb) 2013;5:172–82. doi: 10.1039/c2ib20108a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pak CC, Erukulla RK, Ahl PL, Janoff AS, Meers P. Elastase activated liposomal delivery to nucleated cells. Biochim. Biophys. Acta . 1999;1419:111–26. doi: 10.1016/s0005-2736(98)00256-9. [DOI] [PubMed] [Google Scholar]
- 227.Aoyama K, Nakaki T. Glutathione in cellular redox homeostasis: Association with the excitatory amino acid carrier 1 (EAAC1) Molecules . 2015;20:8742–58. doi: 10.3390/molecules20058742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marengo B, Nitti M, Furfaro AL, Colla R, Ciucis CD, Marinari UM, Pronzato MA, Traverso N, Domenicotti C. Redox homeostasis and cellular antioxidant systems: Crucial players in cancer growth and therapy. Oxid. Med. Cell. Longev. . 2016;2016:6235641. doi: 10.1155/2016/6235641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sun H, Meng F, Cheng R, Deng C, Zhong Z. Reduction-sensitive degradable micellar nanoparticles as smart and intuitive delivery systems for cancer chemotherapy. Expert Opin. Drug Deliv. . 2013;10:1109–22. doi: 10.1517/17425247.2013.783009. [DOI] [PubMed] [Google Scholar]
- 230.Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. . 2012;64:866–84. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 231.Jhaveri A, Deshpande P, Torchilin V. Stimuli-sensitive nanopreparations for combination cancer therapy. J. Control. Release . 2014;190:352–70. doi: 10.1016/j.jconrel.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 232.Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. . 2017;9 doi: 10.1002/wnan.1450. doi:10.1002/wnan.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yin X, Chi Y, Guo C, Feng S, Liu J, Sun K, Wu Z. Chitooligosaccharides modified reduction-sensitive liposomes: Enhanced cytoplasmic drug delivery and osteosarcomas-tumor inhibition in animal models. Pharm. Res. doi: 10.1007/s11095-017-2225-0. 10.1007/s11095-017-2225-0. [DOI] [PubMed] [Google Scholar]
- 234.Chen X, Zhang Y, Tang C, Tian C, Sun Q, Su Z, Xue L, Yin Y, Ju C, Zhang C. Co-delivery of paclitaxel and anti-survivin siRNA via redox-sensitive oligopeptide liposomes for the synergistic treatment of breast cancer and metastasis. Int. J. Pharm. . 2017;529:102–15. doi: 10.1016/j.ijpharm.2017.06.071. [DOI] [PubMed] [Google Scholar]
- 235.Tang J, Zhang L, Gao H, Liu Y, Zhang Q, Ran R, Zhang Z, He Q. Co-delivery of doxorubicin and P-gp inhibitor by a reduction-sensitive liposome to overcome multidrug resistance, enhance anti-tumor efficiency and reduce toxicity. Drug Deliv. . 2016;23:1130–43. doi: 10.3109/10717544.2014.990651. [DOI] [PubMed] [Google Scholar]
- 236.Ren G, Liu D, Guo W, Wang M, Wu C, Guo M, Ai X, Wang Y, He Z. Docetaxel prodrug liposomes for tumor therapy: Characterization, in-vitro and in-vivo evaluation. Drug Deliv. . 2016;23:1272–81. doi: 10.3109/10717544.2016.1165312. [DOI] [PubMed] [Google Scholar]
- 237.Goldenbogen B, Brodersen N, Gramatica A, Loew M, Liebscher J, Herrmann A, Egger H, Budde B, Arbuzova A. Reduction-sensitive liposomes from a multifunctional lipid conjugate and natural phospholipids: Reduction and release kinetics and cellular uptake. Langmuir . 2011;27:10820–9. doi: 10.1021/la201160y. [DOI] [PubMed] [Google Scholar]
- 238.Chi Y, Yin X, Sun K, Feng S, Liu J, Chen D, Guo C, Wu Z. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J. Control. Release . 2017;261:113–25. doi: 10.1016/j.jconrel.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 239.Tang J, Zhang L, Fu H, Kuang Q, Gao H, Zhang Z, He Q. A detachable coating of cholesterol-anchored PEG improves tumor targeting of cell-penetrating peptide-modified liposomes. Acta Pharm. Sin. B . 2014;4:67–73. doi: 10.1016/j.apsb.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Loew M, Forsythe JC, McCarley RL. Lipid nature and their influence on opening of redox-active liposomes. Langmuir . 2013;29:6615–23. doi: 10.1021/la304340e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Noyhouzer T, L›Homme C, Beaulieu I, Mazurkiewicz S, Kuss S, Kraatz HB, Canesi S, Mauzeroll J. Ferrocene-modified phospholipid: An innovative precursor for redox-triggered drug delivery vesicles selective to cancer cells. Langmuir . 2016;32:4169–78. doi: 10.1021/acs.langmuir.6b00511. [DOI] [PubMed] [Google Scholar]
- 242.Ren G, Jiang M, Guo W, Sun B, Lian H, Wang Y, He Z. Construction and cellular uptake behavior of redox-sensitive docetaxel prodrug-loaded liposomes. Pharm. Dev. Technol. . 2017:1–31. doi: 10.1080/10837450.2017.1287728. [DOI] [PubMed] [Google Scholar]
- 243.Zhang S, Zhao Y. Controlled release from cleavable polymerized liposomes upon redox and pH stimulation. Bioconjug. Chem. . 2011;22:523–8. doi: 10.1021/bc1003197. [DOI] [PubMed] [Google Scholar]
- 244.Zhou G, Li L, Xing J, Jalde S, Li Y, Cai J, Chen J, Liu P, Gu N, Ji M. Redox responsive liposomal nanohybrid cerasomes for intracellular drug delivery. Colloids Surf. B Biointerfaces . 2016;148:518–25. doi: 10.1016/j.colsurfb.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 245.Vulugundam G, Kumar K, Kondaiah P, Bhattacharya S. Efficacious redox-responsive gene delivery in serum by ferrocenylated monomeric and dimeric cationic cholesterols. Org. Biomol. Chem. . 2015;13:4310–20. doi: 10.1039/c4ob02513j. [DOI] [PubMed] [Google Scholar]
- 246.Maeda T, Fujimoto K. A reduction-triggered delivery by a liposomal carrier possessing membrane-permeable ligands and a detachable coating. Colloids Surf. B Biointerfaces . 2006;49:15–21. doi: 10.1016/j.colsurfb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 247.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. . 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]