Abstract
Previous studies have reported inconsistent findings regarding the efficacy of omega-3 fatty acids on pregnant women with major depressive disorder (MDD). This meta-analysis was conducted to systematically evaluate the clinical applicability of omega-3 fatty acids in treating depression in pregnant women.
Randomized controlled trials (RCTs) that compared omega-3 fatty acids to placebo for short-course treatment of depression in pregnant women were systematically reviewed between March 1999 and April 2015. The search terms used were ‘depression’, ‘omega-3 fatty acids’, ‘fish oil’, ‘eicosapentaenoic acid’ and ‘docosahexaenoic acid’. Standardized difference in means of depression scale was used as the main outcome. Random effect model was used. The effects of baseline depression scores were studying by meta-regression analysis.
patients received omega-3 fatty acids. The pooled standardized difference in means was 0.75 with 95% CI= (0.47, 1.04). The baseline depression scores had no effect on the efficacy. None of the recruited patients was withdrawn.
Key Words: Major depressive disorder, MDD, Omega-3 fatty acids, Meta-analysis, Women
Introduction
Major depressive disorder (MDD) is one of the most prevalent debilitating mental disorders, which puts heavy disease burden on individual, family, and society (1). MDD affects about 10% of the population (2), and women have higher risk than man. Due to the body changes caused by pregnancy, many women suffer from MDD during this time (3). Previous studies showed that MDD could affect 10~ 20% of the perinatal women (4, 5). If untreated, MDD can increase the risk of negative pregnancy outcomes (6). But, up to now, although many researchers have used metabonomics to successfully identify some potential biomarkers for diagnosing unipolar depression (7, 8) and bipolar disorder (9-11). there is still no objective laboratory test method to diagnose MDD. Moreover, as far as we know, none of the currently available antidepressant could treat MDD with 100% response rates. Repetitive transcranial magnetic stimulation could obtain about 40%~48% response rates (12, 13), and the response rates of electroconvulsive therapy varied from below 20 to 70% or higher (14, 15). Generally speaking, about 30% of the patients receiving treatment fail to respond to the first-line treatments (16). Additionally, some researchers have concerns that selective serotonin reuptake inhibitor antidepressants could increase the risk of perinatal complications (17) and have side-effects on the fetus (18). Therefore, there is an urgent need to find a novel and safely treatment for depression in pregnant women.
Omega-3 fatty acids are nutritional compounds that could not be synthesized by the human body (19). Some clinical studies showed that the deficit of omega-3 fatty acids might increase the incidence of MDD (20-22). Researchers also found the decreased omega-3 fatty acids levels in patients with MDD (23). Therefore, researchers assumed that omega-3 fatty acids might have antidepressant efficacy, and completed many meaningful works. Michael et al. found a small, non-significant benefit of omega-3 fatty acids for MDD (24). Freeman et al. found the significant benefit of omega-3 fatty acids over placebo in treating affective disorders (25). Linda et al. suggested that whether omega-3 fatty acids administration was effective in the treatment of perinatal depression or not needed future studies to answer (26). Meanwhile, pregnancy period provides an excellent opportunity to examine the relationship between omega-3 fatty acids and MDD. Therefore, we did this meta-analysis to assess the efficacy of omega-3 fatty acids as monotherapy in treating depression in pregnant women. The results of this work will help clinicians to make an optimal treatment method for pregnant women with MDD.
Experimental
Literature reviewing
Electronic searches were conducted in several international databases, such as PubMed, CCTR, Web of Science, and Embase, and two Chinese databases (CBM-disc, CNKI) between March 1999 and April 2015. The search terms used were ‘depress*’ combined with ‘omega-3 fatty acids’, ‘fish oil’, ‘eicosapentaenoic acid’ and ‘docosahexaenoic acid’. No language restriction was imposed. Studies met the following inclusion criteria were used for the subsequent analysis: (i) randomized controlled trials (RCTs) (ii) omega-3 fatty acids versus placebo (active or not); (iii) depression in pregnant women over 18 y of age; (iv) provided informed consent and (v) outcome assessed by depression scales, such as Hamilton Depression Rating Scale (HDRS), Clinical Global Impression (CGI), Montgomery–Åsberg Depression Rating Scale (MADRS), Geriatric Depression Scale (GDS) or Beck Depression Inventory (BDI). Meanwhile, studies met any following criteria were excluded: (i) duplicate studies; (ii) nonrandom allocation; (iii) depression in men and (iv) case reports and reviews.
Bias Risk Assessment
The quality of the suitable RCTs was independently assessed by two authors according to the Cochrane Collaboration criteria. The items, such as the randomization, allocation concealment, blind assessment and incomplete data were used to assess the bias risk.
Data Extraction
After literature reviewing, two authors independently checked the obtained studies according to the inclusion/exclusion criteria. The two authors extracted the data from the suitable studies. Any disagreement was solved by consulting with a third reviewer. We extracted the following data from the suitable studies: (i) patients’ characteristics (i.e., number of patient, mean age, patient status, country, and depression scale); (ii) parameters of the treatment method (i.e., treatment time, strategy, placebo and daily dose); and (iii) bias risk of the included studies. When the RCTs reported results from kinds of depression scales, HDRS was preferentially selected. Good faith efforts were applied to obtain the data that could not be directly extracted from the RCTs.
Statistical Analysis
Statistical analysis was conducted using Revman 5.1 and SPSS 19.0. The means and standard deviation (SDs) of depression scale before and after treatment in two groups were extracted. We used these data to do pooled analysis (27), and calculated the standardized mean effect by using Hedges adjusted g to correct the small sample bias (28). Mantel-Haenszel random-effects model was used.
Heterogeneity was assessed using I2 and Q statistic test (29). The Egger›s test and funnel plots were used to assess the publication bias. Meta-regression analysis was used to assess the effects of the baseline depression scores. The protocol of this work followed the recommendations for conducting a meta-analysis (30).
Results
Workflow of literature search
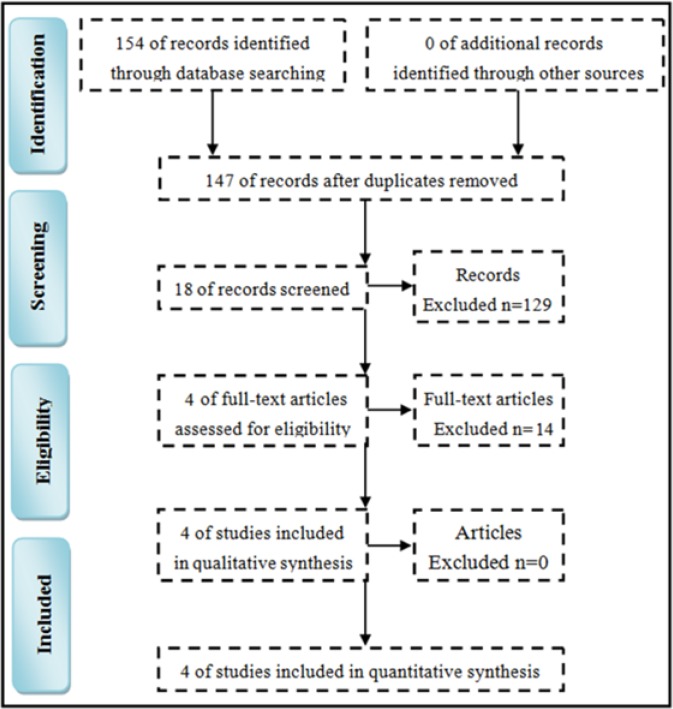
The search was according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (31). Totally, the initial internet search yielded 154 potentially relevant studies. After removing the 7 duplicate studies, 147 studies remained. Among these, 89 studies were excluded by reviewing the titles, 40 studies were excluded by reviewing the abstract and 14 studies were excluded by two authors independently reviewing the full texts. Finally, four RCTs met all the aforementioned criteria and were used for this meta-analysis (Figure 1).
Figure 1.
Literature search
Description of included studies
Totally, these four studies recruited 201 pregnant women with MDD, composed of 102 patients receiving placebo and 99 patients receiving omega-3 fatty acids (32-35). The average age of these patients was about 30 y old. These four studies were from different countries. Two studies used olive oil as placebo; one study used corn oil plus 1% fish oil as placebo; another study used sunola oil as placebo. All the included studies used omega-3 fatty acids as monotherapy to treat patients with six or 8 weeks. The detailed information was described in Table 1 and Table 2.
Table 1.
Clinical characteristics of the patients
| RCT | Subjects, n | Age, (S.D . ) (I/C) | Depression scale | Patient status | E thnic group |
|---|---|---|---|---|---|
| Kaviani et al. 2014 | 80 (40/40) (I/C) | 26.3(4.2)/25.2(4.2) | BDI-21 | pregnancy | Iran |
| Freeman et al. 2008 | 59 (31/28) (I/C) | 31.0(5.8)/29.7(6.2) | HDRS,CGI, EPDS | pregnancy | United States |
| Rees et al. 2008 | 26 (13/13) (I/C) | 31.2(4.4)/34.5(3.8) | HDRS,MADRS, EPDS | pregnancy | Australia |
| Su et al. 2008 | 36 (18/18) (I/C) | 30.9(3.9)/31.3(5.7) | HDRS, EPDS,BDI-21 | pregnancy | China |
RCT: randomized controlled trial; I/C: intervention/control; BDI: Beck Depression Inventory; HDRS: Hamilton Depression Rating Scale; CGI: Clinical Global Impression; EPDS: Edinburgh Postnatal Depression Scale; MADRS: Montgomery- Åsberg Depression Rating Scale; MDD: major depression disorder.
Table 2.
Parameters of the treatment method.
| RCT | Intervention | S trategy | Control | Daily dose | Time |
|---|---|---|---|---|---|
| Kaviani et al. 2014 | omega-3 fatty acids | Monotherapy | olive oil | 1g | 6 weeks |
| Freeman et al. 2008 | omega-3 fatty acids | Monotherapy | corn oil+1% fish oil | 1.1 g EPA+0.8 g DHA | 8 weeks |
| Rees et al. 2008 | omega-3 fatty acids | Monotherapy | sunola oil | 0.42 g EPA. 1.64 g DHA | 6 weeks |
| Su et al. 2008 | omega-3 fatty acids | Monotherapy | olive oil | 2.2 g EPA 1.2 g DHA | 8 weeks |
RCT: randomized controlled trial; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Bias risk of included studies
All the included studies conducted adequate randomization and allocation concealment. The raters and patients were blinded to the treatment methods. The baseline characteristics of patients in two groups were similar. Incomplete data, if existed, was reported by all studies. Generally speaking, the double-blind was difficult to carry out in clinical study. But all the four studies successfully blinded the raters and patients. Thus, these four studies in this meta-analysis were consistently high-quality and displayed no bias risk (Table 3).
Table 3.
Bias risk of the included randomized controlled trials
| Study | Randomization | Blinding | Allocation | Baseline | Incomplete data |
|---|---|---|---|---|---|
| Kaviani et al. 2014 | None | None | None | None | None |
| Freeman et al. 2008 | None | None | None | None | None |
| Rees et al. 2008 | None | None | None | None | None |
| Su et al. 2008 | None | None | None | None | None |
Meta-analysis
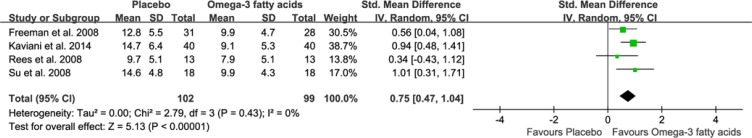
Four studies were used to perform meta-analysis. The pooled standardized difference in means was 0.75 (95% CI=0.47, 1.04) for the random-effects model (Figure 2), which indicated a beneficial effect of the omega-3 fatty acids for pregnant women with MDD compared with placebo. Moreover, heterogeneity in effect size was very low (P=0.43, I2=0%). Meanwhile, meta-regression analysis was conducted to assess the effect of baseline depression scores on the efficacy of omega-3 fatty acids. The results showed the negligible relation between the baseline depression scores and the efficacy (regression coefficient = 0.021, 95% CI=-0.009, 0.033; P=0.37). The inverted funnel plots of these RCTs showed no significant asymmetry. As the total number of studies was too limited to show clear asymmetry, we also performed the Egger’s test. The results (t=-1.54, P=0.27) showed the outcome was not influenced by publication bias.
Figure 2.
Meta-analysis of omega-3 fatty acid versus placebo
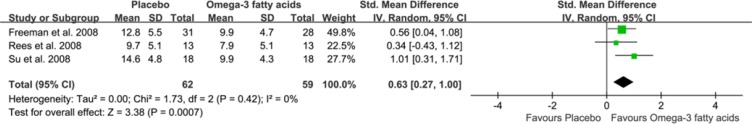
Three studies mainly used HDRS to assess the depressive symptoms, and one study used BDI-21 to assess the depressive symptoms. Therefore, we did sensitivity analysis by excluding this study. This exclusion did not significantly affect the initial effect-size estimates for the whole sample. The pooled standardized difference in means was 0.63 (95% CI=0.27, 1.00) for the random-effects model (Figure 3). Still, no significant heterogeneity was existed (P=0.42, I2=0%).
Figure 3.
Sensitivity analysis of omega-3 fatty acid versus placebo
Discussion
As far as we know, this is the first meta-analysis to study the efficacy of omega-3 fatty acids as monotherapy in the acute treatment of MDD in pregnant women. The results showed that the omega-3 fatty acids as monotherapy produced better efficacy than placebo with standardized difference in means of 0.75 (95% CI=0.47, 1.04). No significantly heterogeneity in effect size was existed. Concerning the acceptability, none of the included patients withdrawn and experienced serious side-effects. Meanwhile, the sensitivity analysis yielded the similar results. Based on these results, omega-3 fatty acids as monotherapy might have a beneficial effect in depression during pregnancy. Considering the safety issue and psychotherapeutic effect for newborns and mothers, the clinical applicability of omega-3 fatty acids showed greater promise and should be further explored. Limited by the small number of the included studies and patients, this conclusion should be interpreted with caution and needed future studies to confirm.
Some researchers proposed omega-3 fatty acids as a novel and potential treatment method for MDD (36). However, previous studies have demonstrated inconsistent findings regarding the efficacy of omega-3 fatty acids vs. placebo in treating depression in pregnant women. Kaviani et al. reported that using omega-3 fatty acids was a suitable method for treating mild depression during pregnancy (35). Su et al. found that omega-3 fatty acids might have antidepressant efficacy for pregnant women with MDD (33). But other studies reported that there was no benefit for omega-3 fatty acids over placebo in treating depression in pregnant women (32, 34). These discrepant conclusions might be caused by the relatively low statistical power among some of the individual studies (37). Therefore, we applied meta-analytical approaches to examine the efficacy of omega-3 fatty acids in pregnant women with MDD. By integrating the findings from multiple studies, the results in this work obtained by this approach should be more accurate and robust (38).
Several limitations should be mentioned here: i) the relatively small number of included studies and depression in pregnant women; ii) the recruited studies had the different population, placebo and dose, which were also the general problems for meta-studies to solve; iii) publication bias, which was difficult to detect and somewhat was controversial when the number of included studies was small (39). However, this pooled analysis of four high-quality double-blinded RCTs found that omega-3 fatty acids was a potential treatment method for pregnant women with MDD.
Conclusion
The abovementioned results showed that omega-3 fatty acids could produce a beneficial effect on depression in pregnant women compared with placebo. The clinical applicability of omega-3 fatty acids showed greater promise and should be further explored.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81102806, No. 81260676, No. 81360679), the opening project of the State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences (No. SKLMR-20100602), Yunnan Provincial Natural Science Foundation (2011FZ166).
References
- 1.Lane R, Mc Donald G. Reducing the economic burden of depression. Int. Clin. Psychopharmacol. . 1994;9:229–43. doi: 10.1097/00004850-199400940-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. National Comorbidity Survey Replication The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. . 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Yonkers KA, Ramin SM, Rush AJ, Navarrete CA, Carmody T, March D, Heartwell SF, Leveno KJ. Onset and persistence of postpartum depression in an inner city maternalhealth clinic system. Am. J. Psychiatry . 2001;158:1856–63. doi: 10.1176/appi.ajp.158.11.1856. [DOI] [PubMed] [Google Scholar]
- 4.Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J. Womens. Health (Larchmt) 2003;12:373–80. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- 5.Evans J, Heron J, Francomb H, Oke S, Golding J. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. . 2001;323:257–60. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wisner KL, Zarin DA, Holmboe ES, Appelbaum PS, Gelenberg AJ, Leonard HL, Frank E. Risk-benefit decision making for treatment of depression during pregnancy. Am. J. Psychiatry. . 2000;157:1933–40. doi: 10.1176/appi.ajp.157.12.1933. [DOI] [PubMed] [Google Scholar]
- 7.Zheng P, Wang Y, Chen L, Yang D, Meng H, Zhou D, Zhong J, Lei Y, Melgiri ND, Xie P. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol. Cell Proteomics. . 2013;12:207–14. doi: 10.1074/mcp.M112.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng P, Chen JJ, Huang T, Wang MJ, Wang Y, Dong MX, Huang YJ, Zhou LK, Xie P. A novel urinary metabolite signature for diagnosing major depressive disorder. J. Proteome. Res. . 2013;12:5904–11. doi: 10.1021/pr400939q. [DOI] [PubMed] [Google Scholar]
- 9.Chen JJ, Liu Z, Fan SH, Yang DY, Zheng P, Shao WH, Qi ZG, Xu XJ, Li Q, Mu J, Yang YT, Xie P. Combined application of NMR- and GC-MS-based metabonomics yields a superior urinary biomarker panel for bipolar disorder. Sci. Rep. . 2014;4:5855. doi: 10.1038/srep05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JJ, Huang H, Zhao LB, Zhou DZ, Yang YT, Zheng P, Yang DY, He P, Zhou JJ, Fang L, Xie P. Sex-specific urinary biomarkers for diagnosing bipolar disorder. PLOS. One. . 2014;9:e115221. doi: 10.1371/journal.pone.0115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JJ, Zhou CJ, Liu Z, Fu YY, Zheng P, Yang DY, Li Q, Mu J, Wei YD, Zhou JJ, Huang H, Xie P. Divergent Urinary Metabolic Phenotypes between Major Depressive Disorder and Bipolar Disorder Identified by a Combined GC-MS and NMR Spectroscopic Metabonomic Approach. J. Proteome. Res. . 2015;14:3382–9. doi: 10.1021/acs.jproteome.5b00434. [DOI] [PubMed] [Google Scholar]
- 12.Jian-jun Chen, Chuanjuan Zhou, Bo Wu, Ying Wang, Qi Li, Youdong Wei, Deyu Yang, Jun Mu, Dan Zhu, Dezhi Zou, Peng Xie. Left versus Right Repetitive Transcranial Magnetic Stimulation in Treating Major Depression: a Meta-Analysis of Randomized Controlled Trials. Psychiatry Resh. . 2013;210:1260–4. doi: 10.1016/j.psychres.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Jian-jun Chen, Zhao Liu, Dan Zhu, Qi Li, Hongzhi Zhang, Hua Huang, Youdong Wei, Jun Mu, Deyu Yang, Peng Xie. Bilateral vs unilateral repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomized controlled trials. Psychiatry Res. . 2014;219:51–7. doi: 10.1016/j.psychres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 14.McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Arch. Gen Psychiatry . 2000;57:438–44. doi: 10.1001/archpsyc.57.5.438. [DOI] [PubMed] [Google Scholar]
- 15.Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, Fitzsimons L, Moody BJ, Clark J. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch. Gen Psychiatry . 2000;57:425–34. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 16.Fava M. Diagnosis and de fi nition of treatment-resistant depression. Biol. Psychiatry . 2003;53:649–59. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 17.Moses-Kolko EL, Bogen D, Perel J, Bregar A, Uhl K, Levin B, Wisner KL. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. JAMA. . 2005;293:2372–83. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 18.Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. Psychological distress in pregnancy and preterm delivery. BMJ. . 1993;307:234–9. doi: 10.1136/bmj.307.6898.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. . 2006;296:1885–99. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 20.Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G, Negroni M, Berra B, Politi P, Rizzo AM. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. J. Am. Coll. Nutr. . 2010;29:55–64. doi: 10.1080/07315724.2010.10719817. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo AM, Corsetto PA, Montorfano G, Opizzi A, Faliva M, Giacosa A, Ricevuti G, Pelucchi C, Berra B, Rondanelli M. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutr. J. . 2012;11:82. doi: 10.1186/1475-2891-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol. Psychiatry. . 2004;55:891–96. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Rondanelli M, Giacosa A, Opizzi A, Pelucchi C, La Vecchia C, Montorfano G, Negroni M, Berra B, Politi P, Rizzo AM. Long chain omega 3 polyunsaturated fatty acids supplementation in the treatment of elderly depression: effects on depressive symptoms, on phospholipids fatty acids profile and on health-related quality of life. J. Nutr. Health. Aging . 2011;15:37–44. doi: 10.1007/s12603-011-0011-y. [DOI] [PubMed] [Google Scholar]
- 24.Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol. Psychiatry . 2012;17:1272–82. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jans LA, Giltay EJ, Van der Does AJ. The efficacy of n-3 fatty acids DHA and EPA (fish oil) for perinatal depression. Br. J. Nutr. . 2010;104:1577–85. doi: 10.1017/S0007114510004125. [DOI] [PubMed] [Google Scholar]
- 26.Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatr. Scand. . 2006;113:31–5. doi: 10.1111/j.1600-0447.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 27.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. . 2000;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jans LA, Giltay EJ, Van der Does AJ. The efficacy of n-3 fatty acids DHA and EPA (fish oil) for perinatal depression. Br. J. Nutr. . 2010;104:1577–85. doi: 10.1017/S0007114510004125. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. . 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacks HS, Berrier J, Reitman D, Ancona-Berk V, Chalmers TC. Meta-analyses of randomized controlled trials. N. Engl. J. Med. . 1987;316:450. doi: 10.1056/NEJM198702193160806. [DOI] [PubMed] [Google Scholar]
- 31.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLOS One . 2013;8:e83138. doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J. Affect Disord. . 2008;110:142–148. doi: 10.1016/j.jad.2007.12.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su KP, Huang SY, Chiu TH, Huang KC, Huang CL, Chang HC, Pariante CM. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiatry. . 2008;69:644–51. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- 34.Rees AM, Austin MP, Parker GB. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust. N. Z. J. Psychiatry . 2008;42:199–205. doi: 10.1080/00048670701827267. [DOI] [PubMed] [Google Scholar]
- 35.Kaviani M, Saniee L, Azima S, Sharif F, Sayadi M. The Effect of Omega-3 Fatty Acid Supplementation on Maternal Depression during Pregnancy: A Double Blind Randomized Controlled Clinical Trial. Int. J. Community Based Nurs. Midwifery . 2014;2:142–7. [PMC free article] [PubMed] [Google Scholar]
- 36.Kraguljac NV, Montori VM, Pavuluri M, Chai HS, Wilson BS, Unal SS. Efficacy of omega-3 Fatty acids in mood disorders-a systematic review and meta-analysis. Psychopharmacol. Bull. . 2009;42:39–54. [PubMed] [Google Scholar]
- 37.Maxwell SE, Kelley K, Rausch JR. Sample size planning for statistical power and accuracy in parameter estimation. Annu. Rev. Psychol. . 2008;59:537–63. doi: 10.1146/annurev.psych.59.103006.093735. [DOI] [PubMed] [Google Scholar]
- 38.Huf W, Kalcher K, Pail G, Friedrich ME, Filzmoser P, Kasper S. Meta-analysis: fact or fiction? How to interpret meta-analyses. World. J. Biol. Psychiatry . 2011;12:188–200. doi: 10.3109/15622975.2010.551544. [DOI] [PubMed] [Google Scholar]
- 39.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. . 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]