Abstract
Objectives
Investigate the impact of Right Ventricular (RV) Internal Work (IW), ratio of arterial to ventricular end-systolic elastance (Ea/Emax), and RV Insertion Point (IP) Late Gadolinium Enhancement (LGE) on outcome in Pulmonary Hypertension (PH) patients.
Background
LGE is well known to be present within the RVIPs and Inter Ventricular Septum (IVS) in PH patients, but its prognostic role remains complex and potentially overestimated via 2D qualitative relative to the 3D quantitative measures now available. However, Ea/Emax, a measure of ventricular-arterial coupling and IW, when added to external cardiac work i.e. the P-V loop area as correlates to the heart’s energy demands, might fundamentally improve measures of prognosis as they interrogate physiology beyond just the RV.
Methods
Cardiac Magnetic Resonance Imaging (CMR) of 124 PH patients (age = 60±13, 85F) referred to a large tertiary PH center, was retrospectively examined for RV volumetric and functional indices and RVIP LGE%. Right Heart Catheterizations (RHC) performed within 1±2 months of the CMR were reviewed. Ea/Emax was derived as RV End-Systolic Volume (ESV/RVSV). IW was estimated as RVESV ×(RV end-systolic pressure-RV diastolic pressure). Patients were followed from date of CMR for up to 5 years for MACE (death, hospitalized RV failure, initiation of parenteral prostacyclin, sustained ventricular arrhythmia or referral for lung transplantation).
Results
MACE was high; 48/124 (39%) patients had MACE by 1.6±1.3 years. Neither RVIP nor IVS LGE using visual assessment or even 3D quantization predicted MACE. The strongest predictor of MACE was RVIW (OR=1.00013, p<0.002), vs. mPAP, RV mass, RV EF and IP LGE.
Conclusions
Surprisingly, neither a single time-point RVIP nor whole IVS LGE% can predict outcome in the largest cohort of PH patients studied to date when compared with conventional or contemporary metrics of disease progression. CMR-LGE appears to lose its’ prognostic value in PH patients in stark contradistinction to all other left and right-sided human myocardial pathologies.
Keywords: CMR, Ventriculoarterial Coupling, Internal Mechanical Work, Insertion Point LGE, Prognosis, Pulmonary Hypertension, Cardiac MRI
Background
Since 2007, several Cardiac Magnetic Resonance imaging (CMR) parameters have been shown to hold a prognostic value in Pulmonary Arterial Hypertension (PAH) [1–5]. In many of these studies, the wide prevalence of Right Ventricular Insertion Point (RVIP) Late Gadolinium Enhancement (LGE) in such PAH patients as well as in other clinical subgroups of Pulmonary Hypertension (PH) has been observed and naturally presumed to represent an adverse prognosis paralleling other disease entities. In most of those studies, RVIP was shown to have varying correlations to RV morphology and/or function± hemodynamic parameters [6–9]. In one study [10], RVIP LGE correlated only with duration of disease.
Only two studies [9,11] examined the prognostic value of such finding. In 58 patients, the authors demonstrated that RVIP-LGE is a marker for more advanced disease and poor prognosis, albeit only as a univariate predictor of outcome but not on multivariate analysis. Again, in 162 patients similar results were shown, but only if LGE was not only confined to the IPs but also involved the Inter Ventricular Septum (IVS) [11].
On the other hand, the relationship between the ventricle and its after load is key to the concept of ventricle-arterial coupling [12]. Fustier and Walsh in 2011 reported that ventricular efficiency is maximal i.e. the most favorable coupling occurs when the Ea/ Emax lies in the range 0.5–1.0; where Ea is the arterial elastance and E max is the end-systolic ventricular elastance. It is conceptualized, then, that in the face of a chronically increased after load, the RV has to increase its contractility through remodeling and increasing its muscle mass to alleviate its wall stress and maintain its stroke volume. Beyond a critical point, the myocardial energy metabolism will be inefficient and the RV will start to fail [13].
In addition, the best assessment of ventricular contractility should be derived from an actual plot of the systolic pressure-volume curves for that ventricle [14]. Kuehne, et al. in 2004 validated such a use of CMR-derived pressure-volume loops for assessment of RV myocardial contractility and applied this technique in patients with chronic RV pressure overload [15].
The external physical work (pressure times volume) performed by the heart, is exactly represented by the area within the ejection loop. That area, however, correlates very poorly with the heart’s oxygen requirements until an internal work component, is added to the external work [14]. Thus, the stage is set for a more careful and clears interrogation of CMR-LGE in pulmonary hypertension patients. This study aims to test the following
Hypotheses
RVIP LGE has a prognostic role in PH patients.
Ea/Emax and internal mechanical RV work (2 parameters of patho physiological relevance) predict outcome in PH patients.
Methods
Study population and design
We evaluated 124 patients derived from all WHO groups of RHC-proven PH who had a CMR scan with gadolinium contrast between January 2008 and June 2014 referred to our tertiary care Pulmonary Hypertension Center of Excellence in Pittsburgh, Pennsylvania. RHC spars formed on average within 1±2 months of the CMR scan were included in the analysis. Patient characteristics are summarized in Tables 1 and 2.
Table 1.
Clinical characteristics of patient population.
| All patients (n=124) | |
|---|---|
| Demographics | |
| Age (years) | 60±13 |
| Women, n(%) | 85(68%) |
| BMI (Kg/m2) | 30.22±7 |
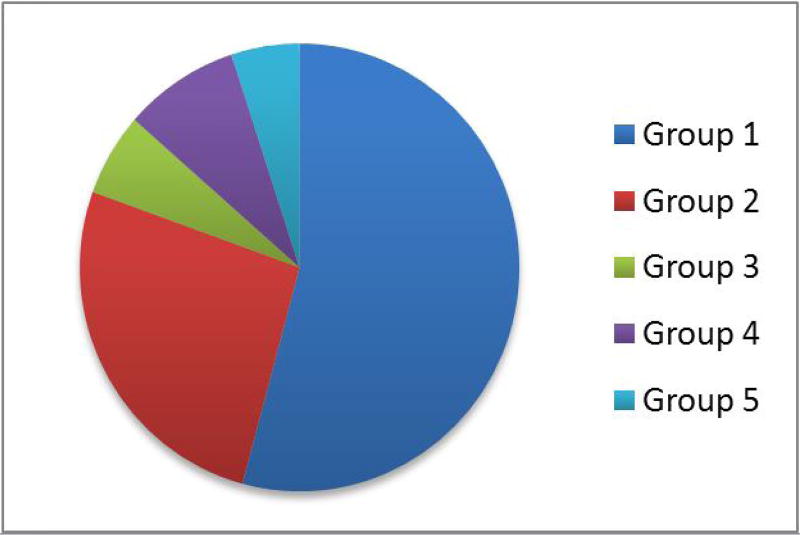
| WHO Categorizations, n(%) (Figure 1) | |
| Group I | 64(51.6%) |
| Idiopathic | 27 |
| Other subgroups | 37 |
| Group II | 31(25%) |
| Group III | 12(9.7%) |
| Group IV | 10(8.1%) |
| Group V | 6(4.8%) |
| Hemodynamic & functional parameters, M±SD(range) | |
| mPAP | 45.5±13.7(22–80) |
| RVEF | 43.6±13.7(16–71) |
Table 2.
WHO sub categorizations of Group 1 study patients.
| WHO sub-categorizations (& actual clinical diagnoses), n(%) |
Group 1 patients (n=64) |
|---|---|
| Group 1.1 (idiopathic) | 28(43.8%) |
| Group 1.2 (BMPR2) | 2(3.1%) |
| Group 1.3 (Anorexigen-induced) | 1(1.6%) |
| Group 1.4.1 (SLE, Scleroderma [including CREST], polymyositis/dermatomyositis, poly mystic with overlap syndrome, ill defined [RA vs Sjögren’s]) | 23(36%) |
| Group 1.4.3 (portal hypertension) | 5(7.8%) |
| Group 1.4.4 (ASD) | 5(7.8%) |
Medical records were reviewed for a composite MACE end-point of 1) initiating IV prostacyclin therapy, 2) hospitalization for heart failure, 3) referral for lung transplantation, 4) life-threatening ventricular arrhythmias and 5) death. All deaths were confirmed by the social security death index.
Follow-up CMR studies for 44/124 patients (35%) were available and were further evaluated. The very latest CMR scan for each individual patient was considered. The time lapse from the index CMR scan to the follow-up scan was on average 21±14 months. The study was approved by the AGH Institutional Review Board.
Right heart catheterization
All patients underwent right heart catheterization. The following parameters were considered for each patient: Mean Pulmonary Arterial Pressure (m PAP), systolic and Diastolic RV Pressures (s RVP and d RVP), and mean Right A Trial Pressure (m RAP), Pulmonary Vascular Resistance Index (PVRI) and Mixed Venous Oxygen Saturation (MVO2).
CMR acquisition and image processing
The following parameters were obtained from the medical records for each patient: RV End-Systolic Volume (RVESV), RV stroke volume derived by Phase Velocity Mapping (RVSV by PVM), 3D RV Ejection Fraction (RVEF) and RV Mass (RVM). The Short-Axis (SA) LGE images were then evaluated for the amount/ extent of LGE in RVIPs and IVS in the following manner.
A semi-quantitative visual scoring method
(Range 0 to 4) as previously described by Sanz, et al. [8] was used while being blinded to the results of formal LGE quantification to assess the accuracy of this relatively feasible approach.
Formal LGE quantification
To provide greater objectivity: Patients had on average 5 SA LGE slices (range 3–7). Images were analyzed with a specialized software (Q MASS® MR 7.5, Medis medical imaging systems, Inc.); the “DSI analysis” tool was used. Endocardial and epicar-dial contours were drawn on each slice to delineate the insertion points excluding the mid-interventricular-septum and then to delineate the whole IVS including that mid-portion (RVIP LGE was observed to extend to varying degrees into the mid-portion of the IVS). A region of reference normal myocardium was defined within the LV free wall. A signal threshold was set to define LGE as a signal intensity that was 4 Standard Deviations (SD) higher than the mean SI of the reference myocardium [17]. Manual editing of the automatically demarcated areas of LGE was performed in the following instances: i) if blood pool or epicardial fat was deemed to have been inadvertently included, ii) to disregard LGE that is confluent with a larger area of LGE representing an inferoseptal-inferior wall myocardial infarct, or Iii) to disregard breathing-related high signal artifacts. The software then automatically calculated the percentage of LGE out of the total contoured myocardial mass.
The following are characteristics of the relevant sequence acquisitions: CMR images were acquired on a 1.5 T GE Signa scanner using dedicated surface coils (GE Healthcare, Milwaukee, Wisconsin). Retrospectively gated cine images were obtained during end-expiratory breath-holds preceded by brief hyperventilation using the Steady-State Free Precession (SSFP) sequence (TR 4.1 ms; TE 2.1 ms; flip angle 45°; voxel size 1.5 × 1.5 × 8mm; and temporal resolution ~40ms; parallel imaging sometimes employed). LGE images of the short axis were obtained 2 mins after infusion of gadobenate dimeglumine (Multi Hance® 529 mg/mL; 0.15 mmol/kg [Bracco Diagnostic Inc., Monroe Township, NJ]) using T1-weighted gradient echo pulse sequence during breath-holds (TR 4.8 ms; TE 1.3 ms; TI 130 ms; flip angle 20°; voxel size 2 × 2 × 8 mm). Pulmonary PVM/flow imaging: phase-contrast images were acquired with segmented fast gradient echo MR sequence, with velocity encoding perpendicular to the imaging plane which was in turn perpendicular to the pulmonary trunk and a predefined upper velocity limit of 250cm/sec. Imaging parameters were TR/ TE 7.5/3.1; flip angle 20°; section thickness 8mm; field of view 30–40cm; matrix 256×192 (typical in-plane resolution 1.9× 1.4mm); number of segments 2; temporal resolution 20msec; number of reconstructed cardiac phases 20–40.
Calculating Ea/Emax and RV Internal Mechanical Work (RV IW)
Ea/Emax was calculated entirely from CMR-based RVESV and RVSV (PVM-derived) as previously proposed by Sanz, et al. 2011:
RV Ea/Emax = RVESV/RVSV [18]
RV pressures obtained from RHC together with RVESV obtained from CMR were used to calculate the RV IW:
RV IW = RVESV × (RVSP-RVDP)
Statistical analysis
Categorical variables were expressed as percentages and continuous variables as mean± standard deviation. Kolmogorov-Smirnov test was used to evaluate normality and non-parametric analysis was used when the assumption of normality was broken.
Univariate logistic regression was performed for the binary composite MACE end-point on the following predictor variables: m RAP, m PAP, RVM, RVEDVI, RVESVI, RVEF, Cardiac Index (CI) by Fick and thermo-dilution method, PVRI, MVO2, RV IW, RV Ea/Emax, RVIP LGE % and whole IVS LGE%. Multivariate regression (forward stepwise variable selection) was then performed for significant predictors on univariate regression.
Overall, a pre-set two-tailed level of significance at 0.05 was employed. All above statistical analyses were performed using IBM SPSS Statistics Software, version 20 (Armonk, NY: IBM Corp.).
Results
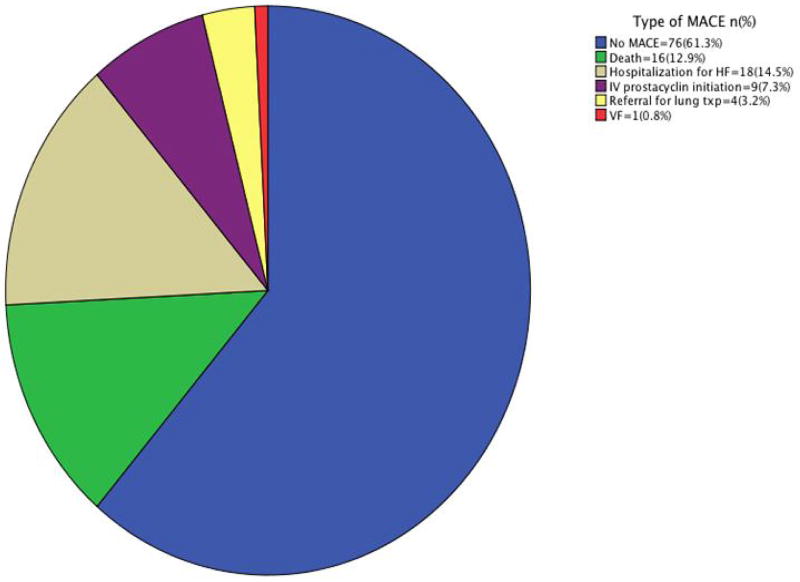
MACE was high; 48/124 patients (39%) had MACE at 1.6±1.3 years. Figure 2 summarizes the type of earliest MACE.
Figure 2.
Type of earliest MACE in the study population.
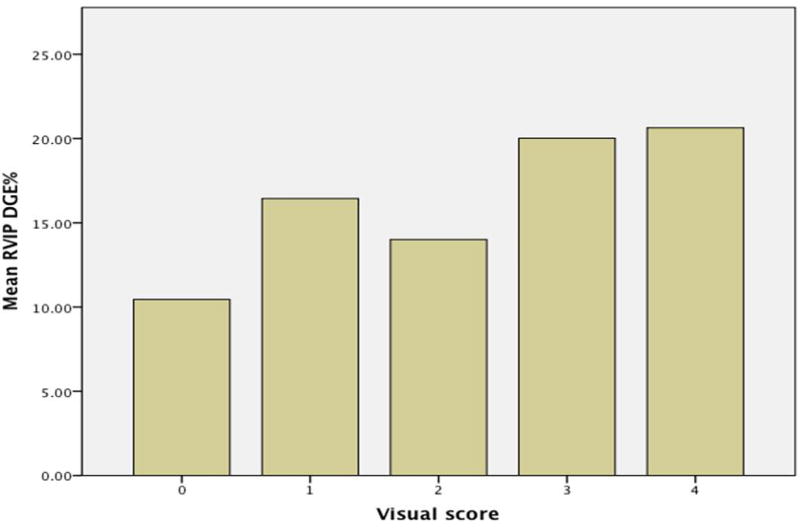
Visual score and LGE quantification
The mere visual assessment showed a reasonable agreement with meticulous 3D LGE quantification as shown in Figure 3.
Figure 3.
Comparison of visual LGE scoring and formal quantification.
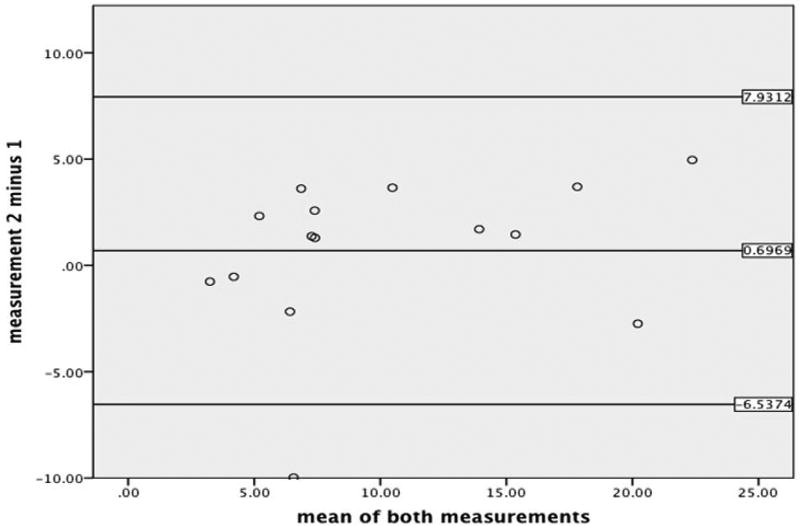
Reader agreement for LGE quantification
In 15/124 patients (12%) randomly selected patients, intra-observer was assessed by means of Bland-Altman analysis. For intra-observer repeatability, the Bias was 0.7 (non-significantly different from zero, P=0.156); limits of agreement=7.2343 (see Figure 4).
Figure 4.
Bland-Altman analysis for intra-observer agreement.
Predictor variables and outcome
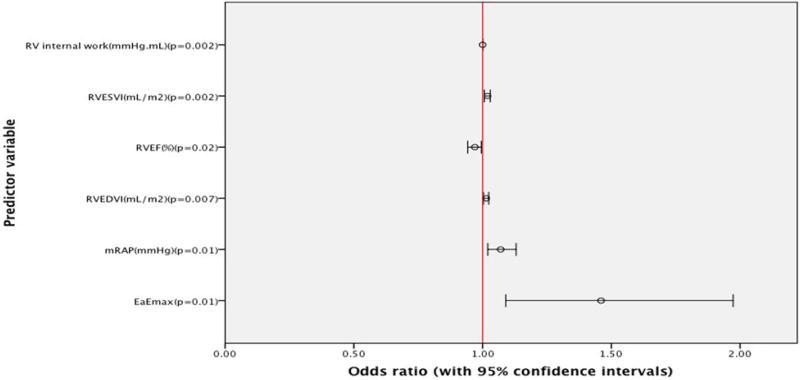
Neither RVIP LGE% nor whole IVS LGE% predicted MACE (p=0.66; 0.69 respectively).
The following were univariate predictors of MACE: m RAP (odds ratio=1.07; CI=1.02 to 1.13, P=0.01), RVEDVI (OR=1.014; CI=1.004 to 1.024, P=0.007), RVESVI (OR=1.019; CI=1.007 to 1.03, P=0.002), RVEF (odds ratio=0.97; CI=0.942 to 0.995, P=0.02), Ea/Emax (odds ratio=1.46; CI=1.09 to 1.973, P=0.01), and RV IW(odds ratio=1.000126; CI=1.000044 to 1.000207, P=0.002).
However, on multivariate regression analysis only RVIW retained its predictive ability (OR=1.00013; CI=1.000047 to 1.000213, P=0.002) (Figure 5).
Figure 5.
Forest plot of Univariate Logistic regression modeling.
Discussion
This study shows a reasonable agreement between mere visual scoring of RVIP LGE and formal quantification which supports using visual scoring as a time-efficient surrogate. However, neither RVIP nor whole IVS LGE% demonstrated any predictive capacity as to patient outcome. A potential explanation would be that normal insertion-region architecture is exaggerated in PH by hypertrophy of the right ventricle and IVS coupled to shear forces. It follows, then, that LGE is related to contrast pooling within areas of myocardial disarray and the “plexiform” fibrosis therein. That said, if the elements behind RVIP LGE are normal but accentuated, rather than pathologic, it would seem likely that this finding in PH would have no incremental prognostic value over existing indices of RV remodeling [19]. This pattern of plexiform rather than replacement fibrosis has been previously shown in 3 cases where a correlation of RVIP LGE and pathologic RVIP microscopy was attempted [19,7]. Of note, LGE CMR, even when T1-mapping is employed, cannot differentiate between interstitial (reactive) fibrosis, and replacement (scar) fibrosis. This has been confirmed using ex-vivo myocardial tissue samples [20]. Highlighting that point, in one study [7], extracellular expansion and edema has been shown in the RVIP LGE region.
This observation might well explain the results obtained by Freed, et al. 2012 where the presence of RVIP LGE remarkably failed to hold up as a significant independent prognosticator on multivariate analysis. Moreover, in the first attempt of linking the extent of RVIP LGE to outcome; Swift et al. concluded that the more extensive form of RVIP LGE involving the IVS, was not associated with an increase in overall mortality [11]. On the other hand, in the context of structural adaptations (and later maladaptations) to altered load (the remodeling hypothesis of heart failure), collagen and the interstitial are normally in a steady state but increase during hypertrophy and after loss of myocytes because of myocardial injury. In heart failure, the interstitial space includes reparative and interstitial fibrosis. Contrary to previous concepts, the interstitial is a very dynamic structure, with a homeostatic process between collagen synthesis and collagen degradation by matrix metalloproteinase [21–23].
After abnormal loading conditions are removed, connective tissue hypertrophy regresses more slowly than myocyte hypertrophy. This, again, might explain why a single point assessment of the connective tissue status (via LGE in our case) will not be an accurate reflection of the course of the disease per se [24–26]. Furthermore, as pointed out by Weber et al., myocardial mechanical properties are not only determined by the relative proportions of the muscular and collage nous compartments, but also by the viscoelasticity and proportionality of types I and III collagen. Tiedemann et al. have also emphasized that the physical arrangement of collagen fibrils to myocytes is more relevant to myocardial mechanics than the quantity of collagen. Previous observations, however, indicate that a realignment of muscle fibers does not occur during the compensatory or decompensatory phases of LVH in the human myocardium [27,28].
It is noteworthy that in their work, Weber et al. detected a heterogeneous collagen concentration within the left ventricular mid wall at 88 weeks of induced hypertension, which might denote the varying rates of ventricular collagen remodeling between individuals subject to the same duration of a pressure-overload [27].
Moreover, during the “evolutionary” phase of LVH in their experiment (4 weeks of pressure overload), collagen matrix remodeling was associated with an increase in “embryonic” type III collagen [29]. The relationship between the relative proportions of type I and III collagen and the Gd-based contrast kinetics has not been explored before. This, however, might be modulating the extent of RV LGE on CMR throughout various stages of PH. This calls for “molecular” MRI, which indeed, was used by Polasek et al. where they employed a type I collagen-targeted, Gd-based contrast agent “EP-3533” to image liver fibrosis [30].
Another explanatory framework of our results can be constructed on the background of Weber et al.’s identification of the evolution of the remodeling process in the non-human primate pressure-overloaded model. It began with evidence of collagen fiber degradation, edematous-appearing inter muscular spaces and an increased formation of type III collagen. These findings had disappeared with the established phase of hypertrophy. This could form a basis to explain the tendency for better outcomes (although not statistically significant) seen in patients with higher RVIP LGE percentages; if only assessed at a single point of time. In other words, this group of patients might be going through an early stage of disease (with increased volume of distribution for LGE) unlike the others with more advanced disease. However, persistence of this pattern over time with no regression of LGE could signify failure of resolution of a state of injury, which portends a worse outcome [31,32]. When taken together, this explanation incorporating animal, human and CMR imaging constructs, has validity in many authors’ opinions including some of our prior work [33–46].
On the other hand, the results of this study concur with the already conceptualized path physiological interaction of RV and chronic pressure overload in PH. The effective arterial elastance (Ea) describes the ability of the vessel to accomodate pulsatile flow [12].
The ratio of Ea to the ventricular end-systolic elastance (Ees) respresents a measure of pump efficiency in expelling blood into the vasculature. As Ea increases initially, the arteries accomodate greater blood flow, permitting a greater stroke work. If Ea were to continue to increase, the stroke work would reach a maximal plateau value where arterial and ventricular properties are equal, meaning that Ea= Ees. Ventricular efficiency is maximal at Ea= Ees/2, meaning that the most favorable ventriculo-arterial coupling occurs when the Ea/Ees ratio lies in the range 0.5–1.0 [13].
Therefore, it follows in PH that the RV teliologically must increase its contractility and stroke work to maintain its SV (homeometric adaptation) until such a point is reached where SV is maintained at the distinct cost of increased EDV (heterometric adaptation). The true intrinsic representation of this increased RV myocardial work is the RVIW, which herein was a significant predictor of MACE. The Ea/E max, though, has the advantage of being an entirely non-invasive MRI-derivation, which would be very convenient for practitioners [18].
Theoretic considerations and experimental data suggest that end-systolic fiber length closely reflects the contractile performance of the ventricular myocardium; since end-systolic fiber length is virtually independent of preload and is dependent only upon contractile state and afterload (a direct linear function of afterload within the physiologic range). It has been shown by Grossman et al. that end-systolic volume alone (albeit cine-fluorography-based) was a useful measure of myocardial function, whereas end-systolic pressure-volume relationships exhibited considerable overlap [20,21]. Of note, dividing RVESV by RVSV was prognostic ally inferior to RVESV alone. This could be explained by the confounding presence of significant tricuspid regurgitation in many of the study’s patients; where the RV is substantially unloaded during early systole prior to pulmonary valve opening, since during the normally isovolumic phase of pressure development volume can diminish as blood is ejected into the low pressure RA. In keeping with the above, when removing the contemporary metric of RVIW from our multivariate logistic regression model; the most significant traditional clinical metric predicting MACE was RVESVI (OR=1.016, CI=1.004 to 1.029, p=0.009).
This might explain why in Vanderpool’s et al work, surprisingly, the volume measurement of Emax/Ea outperformed three other pressure-incorporating methods of assessing the adequacy of RV-afterload coupling. The methods that failed to predict survival were all single-beat-based estimations of Ees/Emax (and not based on “families” of P-V loops) [19]. Other reasons for this outperformance of the simple non-invasive Emax/Ea calculation are not clear [19]. It remains our unique observation that LGE as a universal marker in PH patients does not retain the prognostic value that has universally been observed in other left (and right) sided diseases.
Study limitations
This study is subject to all limitations of a retrospective design. Follow-up studies with gadolinium contrast could not be obtained for approximately 65% of patients due to the clinical nature of this study. Due to the retrospective nature, RHC and CMR were not performed simultaneously and while not the optimal situation, RHC was, however, performed on average within 1±2 of CMR. The study included patients from all WHO clinical PH subgroups which introduces some heterogeneity to the study population but retains its ‘real world’. The actual duration of PH is not accounted for (nor clinically feasible); follow-up started at the index CMR for each patient and this arbitrary time point was set as the baseline. It is yet to be investigated whether particular therapies/interventions controlled the change of RV LGE over time but is theoretically not likely in the short/intermediate term. While recognizing the low odds ratio for RVIW as a predictor of MACE, its dramatic statistical significance suggests its overall importance yet permits the more clinically feasible and easily obtained traditional metric of RVESVI to be alternatively used for risk prognostication.
Conclusions
Neither a single time-point RVIP nor whole IVS LGE% can predict outcome in PH patients. However, RV IW remains as an important predictor of outcome in PH patients and superior to volumetric Ea/Emax, as well as standard clinical metrics such as RVEF. RVESVI could be used as an alternative clinically feasible metric for risk prognostication. These observations, for the first time, in the largest population of PH patients studied expressly for this purpose is contrary to current dogma based on far smaller populations and may impact our contemporary understanding and interpretation of CMR-LGE in PH patients.
Figure 1.
WHO Categorizations for the study population.
Acknowledgments
This work was, in part, supported by NHLBI Grant (HH-SN268201400008C)
Abbreviations and acronyms list
- BMI
Body Mass Index
- BMPR2
Bone Morphogenetic Protein Receptor Type II
- CMR
Cardiovascular Magnetic Resonance
- DGE
Delayed Gadolinium Enhancement
- DRVP
Diastolic Right Ventricular Pressure
- DSI
Delayed Signal Intensity
- Ea
Arterial Elastance
- Emax
Ventricular End-Systolic Elastance
- IVS
Inter Ventricular Septum
- LGE
Late Gadolinium Enhancement
- M
Mean
- MACE
Major Adverse Cardiovascular Events
- M PAP
Mean Pulmonary Arterial Pressure
- M RAP
Mean Right Atrial Pressure
- MVO2
Mixed Venous Oxygen Saturation
- PVRI
Pulmonary Vascular Resistance Index
- RHC
Right Heart Catheterization
- RVEF
Right Ventricular Ejection Fraction
- RVESV
Right Ventricular End-Systolic Volume
- RVIW
RV Internal Mechanical Work
- RVM
Right Ventricular Mass
- RVSV
Right Ventricular Stroke Volume
- SA
Short Axis
- SD
Standard Deviation
- S RVP
Systolic Right Ventricular Pressure
- TE
Echo Time
- TR
Repetition Time
References
- 1.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;10:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 2.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest. 2007;6:1906–1912. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 3.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;24:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Kang KW, Chang HJ, Yoo YP, Yoon HS, Kim YJ, et al. Cardiac magnetic resonance-derived right ventricular outflow tract systolic flow acceleration: a novel index of right ventricular function and prognosis in patients with pulmonary arterial hypertension. Int J Cardiovasc Imaging. 2013;8:1759–1767. doi: 10.1007/s10554-013-0262-2. [DOI] [PubMed] [Google Scholar]
- 5.Swift AJ, Rajaram S, Campbell MJ, Hurdman J, Thomas S, et al. Prognostic value of cardiovascular magnetic resonance imaging measurements corrected for age and sex in idiopathic pulmonary arterial hypertension. Circ Cardiovasc Imaging. 2014;1:100–106. doi: 10.1161/CIRCIMAGING.113.000338. [DOI] [PubMed] [Google Scholar]
- 6.Blyth KG, Groenning BA, Martin TN, Foster JE, Mark PB, et al. Contrast enhanced-cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur Heart J. 2005;19:1993–1999. doi: 10.1093/eurheartj/ehi328. [DOI] [PubMed] [Google Scholar]
- 7.McCann GP, Gan CT, Beek AM, Niessen HW, Vonk Noordegraaf A, et al. Extent of MRI delayed enhancement of myocardial mass is related to right ventricular dysfunction in pulmonary artery hypertension. AJR Am J Roentgenol. 2007;2:349–355. doi: 10.2214/AJR.05.1259. [DOI] [PubMed] [Google Scholar]
- 8.Sanz J, Dellegrottaglie S, Kariisa M, Sulica R, Poon M, et al. Prevalence and correlates of septal delayed contrast enhancement in patients with pulmonary hypertension. Am J Cardiol. 2007;4:731–735. doi: 10.1016/j.amjcard.2007.03.094. [DOI] [PubMed] [Google Scholar]
- 9.Freed BH, Gomberg-Maitland M, Chandra S, Mor-Avi V, Rich S, et al. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2012;1:14–11. doi: 10.1186/1532-429X-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Junqueira FP, Macedo R, Coutinho AC, Loureiro R, De Pontes PV, et al. Myocardial delayed enhancement in patients with pulmonary hypertension and right ventricular failure: evaluation by cardiac MRI. Br J Radiol. 2009;982:821–826. doi: 10.1259/bjr/28241773. [DOI] [PubMed] [Google Scholar]
- 11.Swift A, Rajaram S, Capener D. LGE patterns in pulmonary hypertension do not impact overall mortality. J Am Coll Cardiol Img. 2014;12:1209–1217. doi: 10.1016/j.jcmg.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Vest A, Heupler F., Jr . Chapter 2: Afterload. In: Anwaruddin S, Marin J, Stephens J, Askari A, editors. Cardiovascular Hemodynamic An Introductory Guide. Humana Press; 2013. p. 40. [Google Scholar]
- 13.Robyn J, Lewis J. Chapter 71: Pulmonary Hypertension. In: Fuster V, Walsh R, editors. Hurst’s the Heart. 13. Harrington: McGraw Hill; 2011. [Google Scholar]
- 14.Downey J, Heusch G. Sequence of Cardiac Activation and Ventricular Mechanics. In: Kurachi Y, Terzic A, Cohen M, editors. Heart Physiology and Path physiology. 2000. [Google Scholar]
- 15.Kuehne T, Sevim Y, Steendijk P, Moore P, et al. Magnetic Resonance Analysis of Right Ventricular Pressure-Volume Loops: In Vivo Validation and Clinical Application in Patients with Pulmonary Hypertension. Circulation. 2004;14:2010–2016. doi: 10.1161/01.CIR.0000143138.02493.DD. [DOI] [PubMed] [Google Scholar]
- 16.Simonneau G, Gatzoulis M, Adatia I, et al. Updated Clinical Classification of Pulmonary Hypertension. J Am Coll Cardiol. 2013;62:34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Moravsky G, Ofek E, Rakowski H, et al. Myocardial Fibrosis in Hypertrophic Cardiomyopathy. J Am Coll Cardiol Img. 2013;6:587–96. doi: 10.1016/j.jcmg.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Sanz J, Garcia-Alvarez A, Fernandez-Friera L, Nair A, et al. Evaluation of right ventriculoarterial coupling in pulmonary hypertension: a magnetic resonance study. Journal of Cardiovascular Magnetic Resonance. 2011;1:O73. [Google Scholar]
- 19.Vanderpool R, Pinsky M, Naeije R. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart published online first. 2014;10:1136. doi: 10.1136/heartjnl-2014-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borow K, Green L, Mann T, et al. End-Systolic Volume as a Predictor of Postoperative Left Ventricular Performance in Volume Overload from Valvular Regurgitation. Am J Med. 1980;5:655–663. doi: 10.1016/0002-9343(80)90251-x. [DOI] [PubMed] [Google Scholar]
- 21.Grossman W, Braunwald E, Mann T, McLaurin L, Green L. Contractile State of the Left Ventricle in Man as Evaluated from End-systolic Pressure-Volume Relations. Circulation. 1977;5:845–852. doi: 10.1161/01.cir.56.5.845. [DOI] [PubMed] [Google Scholar]
- 22.Bradlow WM, Assomull R, Kilner PJ, Gibbs JSR, Sheppard MN, et al. Understanding Late Gadolinium Enhancement in Pulmonary Hypertension. Circ Cardiovasc Imaging. 2010;3:501–503. doi: 10.1161/CIRCIMAGING.109.919779. [DOI] [PubMed] [Google Scholar]
- 23.Kehr E, Sono M, Chugh S, Jerosch-Herold M. Gadolinium-enhanced magnetic resonance imaging for detection and quantification of fibrosis in human myocardium in vitro. Int J Cardiovasc Imaging. 2008;24:61–68. doi: 10.1007/s10554-007-9223-y. [DOI] [PubMed] [Google Scholar]
- 24.Weber K. Monitoring tissue repair and fibrosis from a distance. Circulation. 1997;8:2488–2492. [PubMed] [Google Scholar]
- 25.Weber K, Brilla C, Janicki J. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;3:341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 26.Thomas C, Coker M, Zellner J. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;17:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 27.Weber K, Brilla C. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 28.Harada M, Itoh H, Nakagawa O. significance of ventricular myocytes and nonmyocytes interaction during cardiocyte hypertrophy: evidence for endothelin-1 as a paracrine hypertrophic factor from cardiac nonmyocytes. Circulation. 1997;96:3737–3744. doi: 10.1161/01.cir.96.10.3737. [DOI] [PubMed] [Google Scholar]
- 29.Biederman R, Magovern J, Grant S. LV reverse remodeling imparted by aortic valve replacement for severe aortic stenosis; is it durable? A cardiovascular MRI study sponsored by the American Heart Association. Journal of Cardiothoracic Surgery. 2011;6:53. doi: 10.1186/1749-8090-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber K, Janicki J, Shroff S, Pick R, Chen R, et al. Collagen remodeling of the pressure-overloaded hypertrophied non-human primate myocardium. Circ Res. 1988;62:757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 31.Thiedemann K, Holubarsch C, Medugorac I, Jacob R. Connective tissue content and myocardial stiffness in pressure overload hypertrophy. A combined study of morphologic, morphometric, biochemical and mechanical parameters. Basic Res Cardiol. 1983;78:140–155. doi: 10.1007/BF01906668. [DOI] [PubMed] [Google Scholar]
- 32.Medugorac IRJ. Characterization of left ventricular collagen in the rat. Cardiovasc Res. 1983;17:15–21. doi: 10.1093/cvr/17.1.15. [DOI] [PubMed] [Google Scholar]
- 33.Polasek M, Schühle D, Fuchs B, Alford J, Borra1 R, et al. Molecular MR Imaging of Liver Fibrosis with a Collagen-Targeting Gadolinium-Based Contrast Agent. Proc. Intl. Soc. Mag. Reson. Med. 2011:19. [Google Scholar]
- 34.Laine G. Microvascular changes in the heart during chronic arterial hypertension. Circ Res. 1988;62:953–960. doi: 10.1161/01.res.62.5.953. [DOI] [PubMed] [Google Scholar]
- 35.Bhan R, Giacomelli F, Wiener J. Ultrastructure of coronary arteries and myocardium in experimental hypertension. Exp Mol Pathol. 1978;29:66–81. doi: 10.1016/0014-4800(78)90027-8. [DOI] [PubMed] [Google Scholar]
- 36.Benza R, Biederman R, Murali S, Gupta H. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;21:1683–1692. doi: 10.1016/j.jacc.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Biederman RWW. Cardiovascular magnetic resonance imaging as applied to patients with pulmonary arterialhypertension. Int J Clin Pract. 2009;162:20–35. doi: 10.1111/j.1742-1241.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 38.Menon PG, Adhypak SM, Williams RB, Doyle M, Biederman RW. Investigating cardiac MRI based right ventricular contractility as a novel non-invasive metric of pulmonary arterial pressure. Clin Med Insights Cardiol. 2015:45–50. doi: 10.4137/CMC.S15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber K. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;7:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 40.Clore J, Cohen I, Diegelmann R. quantification of collagen types I and III during wound healing in rat skin. Proc Soc Exp Biol Med. 1979;161:337–340. doi: 10.3181/00379727-161-40548. [DOI] [PubMed] [Google Scholar]
- 41.Dorfman A. Polysaccharides of connective tissue. J Histochem Cytochem. 1983;11:2–13. [Google Scholar]
- 42.von Knorring J. Analysis of myocardial mucopolysaccharides in hyper- and hypothyroid rats and guinea pigs. Ann Med Exp Biol Fenn. 1970;48:8–15. [PubMed] [Google Scholar]
- 43.Judd J, Wexler B. Myocardial connective tissue metabolism in response to injury. Histological and chemical studies of mucopolysaccharide and collagen in rat hearts after isoproterenol-induced infarction. Circ Res. 1969;25:201–214. doi: 10.1161/01.res.25.2.201. [DOI] [PubMed] [Google Scholar]
- 44.Judd J, Wexler B. Prolyl hydroxylase and collagen metabolism after experimental myocardial infarction. Am J Physiol. 1975;228:212–216. doi: 10.1152/ajplegacy.1975.228.1.212. [DOI] [PubMed] [Google Scholar]
- 45.Giacomelli F, Anversa PJW. Effect of angiotensin-induced hypertension on rat coronary arteries and myocardium. Am J Pathol. 1976;1:111–138. [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, et al. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. 2001;38:1137–1142. doi: 10.1016/s0735-1097(01)01496-6. [DOI] [PubMed] [Google Scholar]