Abstract
Prior studies have reported that orthostatic hypotension (OH) is associated with increased risk of atrial fibrillation (AF). We sought to determine whether the association persists after adjusting for hypertension and other cardiovascular risk factors. We studied the Framingham Heart Study Original cohort participants evaluated between 1981 and 1984 without baseline AF. OH was defined as drop in standing systolic blood pressure (BP) of at least 20 mm Hg or standing diastolic BP of at least 10 mm Hg from their supine values after standing for 2 minutes. We estimated Cox proportional hazards regression models to calculate multivariable-adjusted hazards ratios (HR) for association between OH and risk of incident AF, adjusting for age, sex, seated systolic BP and diastolic BP, resting heart rate, height, weight, current tobacco use, hypertension treatment, diabetes, and history of myocardial infarction and heart failure. Of 1,736 participants (mean age, 71.7±6.5 years, 60% women) 256 (14.8%) had OH at baseline. During 10 years follow-up, 224 participants developed new AF. In our multivariable-adjusted model, OH (HR 1.61, 95% CI 1.17 to 2.20) and greater orthostatic decrease in mean arterial pressure (MAP) (HR 1.11, 95% CI 1.02 to 1.22 per 8.6 mmHg change in MAP) were both associated with higher risk of new AF. In conclusion, in our longitudinal community-based sample, OH and orthostatic decline in MAP were significantly associated with increased risk of incident AF after adjustment for systolic BP, diastolic BP, and hypertension treatment.
Keywords: Atrial Fibrillation, Orthostatic Hypotension, Risk Factors
There is growing evidence that orthostatic hypotension (OH) is independently associated with cardiovascular diseases including coronary heart disease, heart failure, stroke, and all-cause mortality.1 Prior studies have reported that OH is associated with the risk of incident atrial fibrillation (AF). In a community-based study from Malmö, Sweden, during 24 years of follow-up, OH was associated with increased risk of AF in individuals with hypertension [hazard ratio (HR) 1.30, 95% confidence interval (CI) 1.05 to 1.61], but not in normotensive individuals (p value for interaction=0.06).2 The Atherosclerosis Risks in Communities (ARIC) study also reported a significant association between OH and AF after multivariable adjustments (HR 1.40, 95% CI 1.15 to 1.71) during 18 years of follow-up; the measure of association did not significantly differ among several subgroups including those with and without hypertension.3 Therefore, whether OH is associated with risk of AF accounting for cardiovascular risk factors including hypertension needs to be examined further. In the present study, we examined if OH and postural change in mean arterial pressure (MAP) were associated with increased risk of incident AF including in individuals with and without hypertension. Our aim was to investigate the role of OH on the long-term risk of incident AF in a community-based cohort. We hypothesized that OH and drop in MAP with standing are associated with increased risk of incident AF.
Methods
The participant selection and study design for the Framingham Heart Study Original cohort have been described previously.4 We studied the Original cohort participants evaluated during examination 17 from 1981 to 1984 (n=2,179). We excluded participants with pre-existing AF at the time of study entry (n=133) and participants with missing hemodynamic measurements (n=178) or covariates (n=132). Institutional Review Boards at Boston University Medical Center approved the protocols. All participants provided written informed consent.
OH was defined as drop in standing systolic BP of at least 20 mm Hg or standing diastolic BP of at least 10 mm Hg. MAP was calculated as MAP = [diastolic BP + (systolic BP − diastolic BP)/3]. Postural change in hemodynamic values was calculated by subtracting the supine value from the standing value. BP for supine position was measured after participants rested for approximately 4–5 minutes. Participants were then asked to sit up and stand. Standing BP was measured approximately 2 minutes later, which is enough time for BP to reach the new equilibrium with orthostatic stress.5 We measured auscultatory BP at the level of brachial artery by using a mercury sphygmomanometer. Orthostatic change in heart rate was not recorded.
Participants were diagnosed with AF if they had AF or atrial flutter documented on electrocardiography from routine biennial examination at the Framingham Heart Study research center, or outpatient clinic or inpatient hospital records. The cardiologists at the Framingham Heart Study validated the electrocardiographic evidence of incident AF.
Hypertension was defined as sitting systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, or receiving treatment for hypertension. Tobacco and medication use were ascertained using self-report. Current tobacco use was defined as smoking 1 or more cigarettes per day within 1 year before the Framingham Heart Study examination. Diabetes mellitus was defined as having overnight fasting serum glucose ≥126 mg/dL or as use of hypoglycemic agents. Myocardial infarction and heart failure were determined by examination of medical records by a panel of 3 physicians using previously reported criteria.6
We described the demographic and hemodynamic characteristics of the study sample using mean ± standard deviation (SD) for continuous variables and count (%) for dichotomous variables. We excluded participants with prevalent AF from the analysis. We conducted Cox proportional hazards regression analyses to calculate age- and sex-adjusted and multivariable-adjusted hazards ratios (HR) for association between orthostatic change in BP parameters and 10-year risk of incident AF. The proportional hazards assumption was verified by examining a plot of ln[−ln(survival probability)] versus ln(time) for parallelism and it was determined that the assumption was met (data not shown). Participants were followed for the development of incident AF after exam 17 until AF diagnosis, death, date of last contact, or December 31, 2014, whichever occurred first. We adjusted for standard risk factors associated with AF in the multivariable model, including age, sex, seated systolic and diastolic BPs, height, weight, current tobacco use, antihypertensive medication use, diabetes, heart rate, and history of myocardial infarction or heart failure.7 Our primary analysis examined 10-year risk of AF; in a secondary analysis, we expanded the time frame to include all available follow-up for the participants. In addition, in our secondary analyses, we assessed the interaction between OH and the following AF risk factors: age (≥70 versus <70 years), sex, hypertension, hypertension treatment, diabetes, prevalent myocardial infarction, and prevalent heart failure. A cross-product term for each of the AF risk factors and OH was included in the multivariable-adjusted model and its significance was assessed using a Chi-square test. Two-sided p values <0.05 were considered statistically significant. All our analyses were conducted using SAS, version 9.4; SAS Institute, Cary, North Carolina.
Results
Baseline characteristics and hemodynamic variables of the 1736 study participants are summarized in Table 1. Our study sample consisted of older adults, 45% of whom were taking antihypertensive medication. On average, supine systolic BP in the full sample was in the hypertensive range. Upon standing, there was a modest decrease in systolic BP and a modest increase in diastolic BP and MAP. Pulse pressure dropped substantially. Of participants studied, 256 (14.8%) met the diagnostic criteria for OH; of these participants, 17% (n=43) had a drop in diastolic BP of 10 mm Hg or more, 70% (n=180) had drop in systolic BP of 20 mm Hg or more, and 13% (n=33) had both. During follow-up (primary analysis, mean 8.3 years, maximum 10 years), 224 participants developed incident AF.
Table 1.
Study sample baseline characteristics and hemodynamic variables
| Variable | Total population (n=1736) |
|---|---|
| Age (years) | 71.7±6.5 |
| Women | 1045 (60.2%) |
| Height (cm) | 161.8±9.7 |
| Weight (kg) | 69.6±13.8 |
| Body mass index (kg/m2) | 26.5±4.4 |
| Current smoker | 312 (18.0%) |
| Systolic blood pressure (mm Hg) | 142±19 |
| Diastolic blood pressure (mm Hg) | 78±10 |
| Resting heart rate (beats/min) | 71±13 |
| Antihypertensive medication use | 774 (44.6%) |
| Diabetes mellitus | 115 (6.6%) |
| Prevalent heart failure | 32 (1.8%) |
| Prevalent myocardial infarction | 98 (5.7%) |
| Orthostatic hypotension* | 256 (14.8%) |
| Supine hemodynamics | |
| Systolic blood pressure (mm Hg) | 144±21 |
| Diastolic blood pressure (mm Hg) | 79±10 |
| Mean arterial pressure (mm Hg) | 101±12 |
| Pulse pressure (mm Hg) | 65±18 |
| Standing hemodynamics | |
| Systolic blood pressure (mm Hg) | 141±21 |
| Diastolic blood pressure (mm Hg) | 84±11 |
| Mean arterial pressure (mm Hg) | 103±12 |
| Pulse pressure (mm Hg) | 57±18 |
| Change on standing, median (25th, 75th percentiles) | |
| Systolic blood pressure (mm Hg) | −4 (−12, 6) |
| Diastolic blood pressure (mm Hg) | 4 (0, 10) |
| Mean arterial pressure (mm Hg) | 2 (−3.3, 8) |
| Pulse pressure (mm Hg) | −8 (−16, 0) |
Drop in systolic blood pressure ≥20 mm Hg or diastolic blood pressure ≥10 mm Hg from supine to standing (standing – supine)
Values are n (%), mean ± SD, or median (25th, 75th percentile)
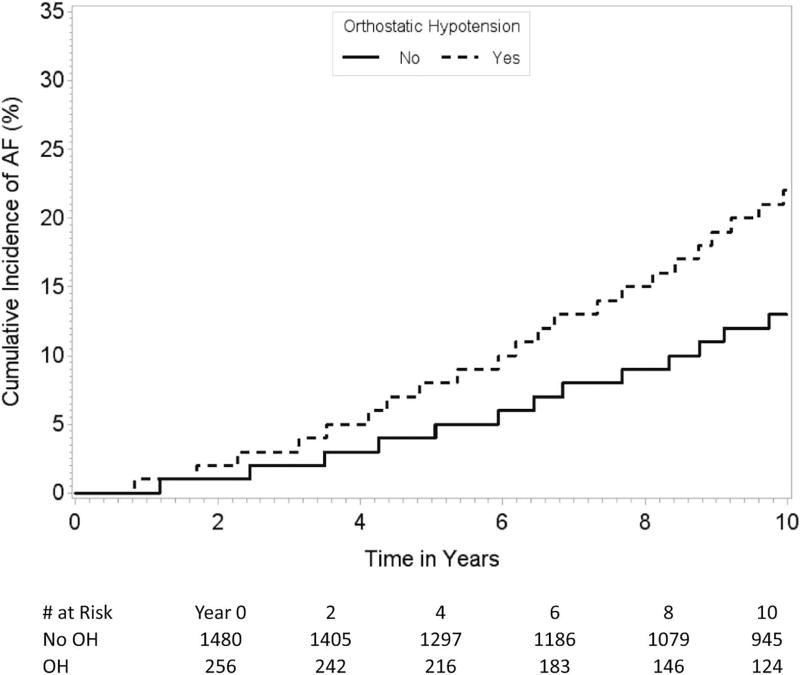
Results of our Cox proportional hazards analysis for association between OH and the risk of incident AF are summarized in Table 2. In a multivariable model that adjusted for age, sex, seated systolic and diastolic BPs, resting heart rate, height, weight, current tobacco use, antihypertensive medication use, diabetes, and history of myocardial infarction or heart failure, OH was associated with 1.6-fold higher risk of incident AF. Orthostatic decline in MAP was associated with greater risk of incident AF. We found similar associations for full follow-up (mean 13.5 years, maximum 33.0 years, Table 2). Cumulative incidence curves demonstrate higher AF incidence in participants with compared to those without OH (Figure A).
Table 2.
Associations of postural hemodynamics with incident atrial fibrillation
| Variable | Model | 10 Year Hazard ratio (95% confidence interval) |
p Value | Full Follow-up Hazard ratio (95% confidence interval) |
p Value |
|---|---|---|---|---|---|
| Orthostatic Hypotension* | |||||
| Age- and sex-adjusted | 1.76 (1.29–2.41) | 0.0004 | 1.65 (1.32–2.07) | <0.0001 | |
| Multivariable-adjusted‡ | 1.61 (1.17–2.20) | 0.003 | 1.51 (1.20–1.90) | 0.0004 | |
| Change in Mean Arterial Pressure† | |||||
| Age- and sex-adjusted | 1.17 (1.03–1.34) | 0.02 | 1.14 (1.05–1.25) | 0.004 | |
| Multivariable-adjusted‡ | 1.14 (1.00–1.30) | 0.048 | 1.11 (1.02–1.22) | 0.02 | |
Drop in systolic blood pressure ≥20 mm Hg or diastolic blood pressure ≥10 mm Hg from supine to standing (Yes vs. No)
1 standard deviation (SD) change = 8.6 mm Hg; hazard ratio expressed per 1 SD decrease in mean pressure from supine to standing.
Adjusted for age, sex, seated systolic and diastolic blood pressure, resting heart rate, height, weight, current tobacco use, antihypertensive medication use, diabetes, heart rate, and history of myocardial infarction or heart failure.
Figure A.
Age- and sex-adjusted cumulative incidence of AF in participants with and without OH. The figure demonstrates divergence of the cumulative incidence curves in participants with and without OH over time.
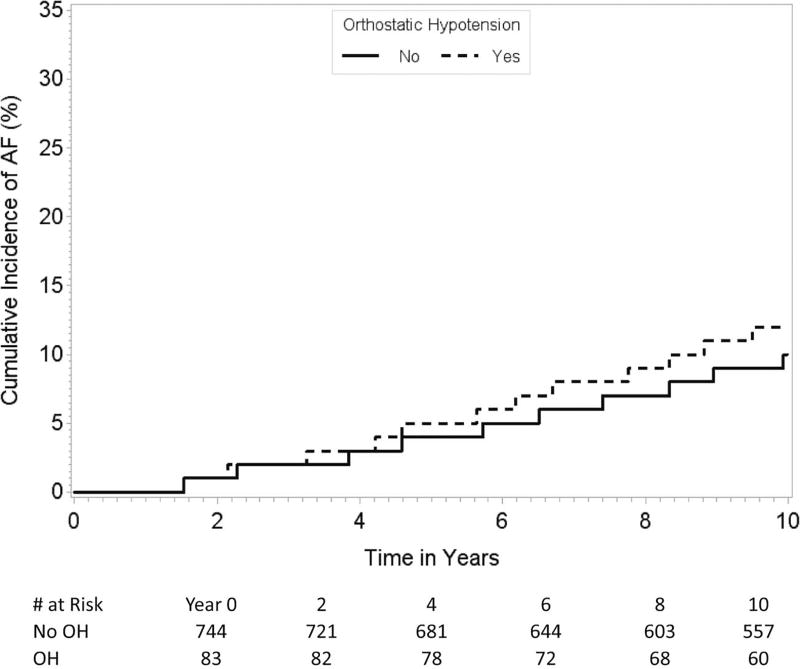
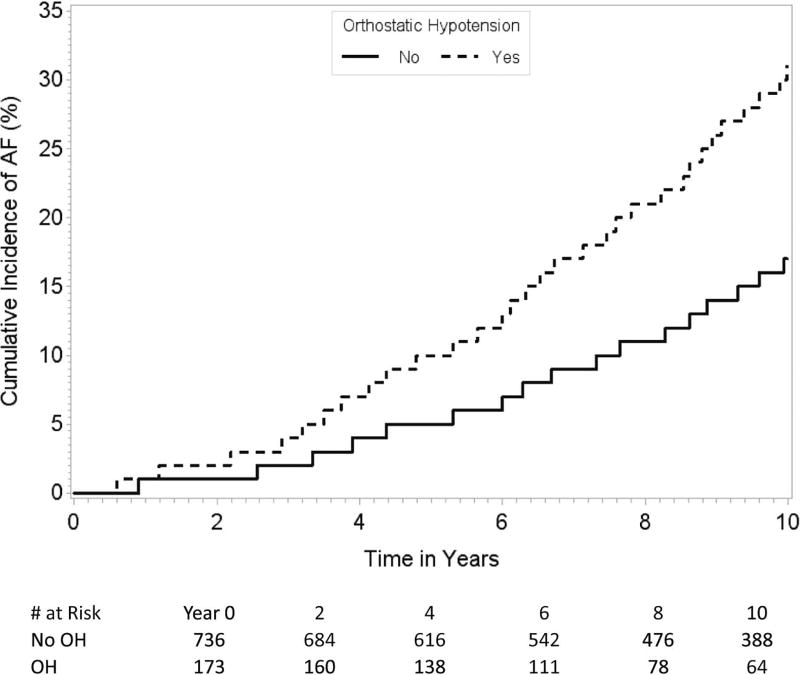
The association between OH and increased risk of incident AF was generally consistent throughout all subgroups except for the participants below 70 years of age compared to those aged 70 years or above (p value for interaction=0.009, Table 3, Figures B–C). We did not find any statistically significant interaction between presence of hypertension and the association between OH and the risk of AF (p value for interaction=0.26). There also was no interaction with hypertension treatment.
Table 3.
Association between orthostatic hypotension and incident atrial fibrillation among subgroups (10-year follow-up)
| Variable | No. events / No. participants |
HR (95% confidence interval) |
p Value | p Value for interaction |
|---|---|---|---|---|
| Age (years) | ||||
| < 70 | 83/827 | 1.24 (0.63–2.44) | 0.54 | 0.009* |
| ≥ 70 | 141/909 | 1.98 (1.37–2.86) | 0.0003 | |
| Sex | ||||
| Male | 107/691 | 1.55 (0.95–2.54) | 0.08 | 0.71 |
| Female | 117/1045 | 1.58 (1.04–2.40) | 0.03 | |
| Hypertension | 180/1195 | 1.76 (1.26–2.47) | 0.0009 | 0.26 |
| Hypertension treatment | 133/774 | 1.53 (1.03–2.28) | 0.03 | 0.56 |
| Diabetes mellitus | 20/115 | 1.24 (0.36–4.30) | 0.73 | 0.25 |
| Prevalent myocardial infarction | 19/98 | 0.99 (0.24–4.15) | 0.99 | 0.05 |
| Prevalent heart failure | 8/32 | 24.5 (0.59–1009.8) | 0.09 | 0.43 |
P-value for interaction performed using age as a continuous variable
All models are adjusted for age, sex, systolic and diastolic blood pressure, resting heart rate, height, weight, current tobacco use, antihypertensive medication use, diabetes, heart rate and history of myocardial infarction and heart failure.
Figures B and C.
Cumulative incidence of AF in participants < 70 years (Figure B) and ≥ 70 years of age (Figure C) with and without OH. The figures demonstrate that the association between OH and the risk of incident AF differs by the 2 age groups.
Discussion
In our longitudinal analysis of the association between postural change in BP and risk of incident AF, we report that presence of OH and an orthostatic decline in MAP were both associated with higher risk of incident AF in models that adjusted for various established risk factors for AF.
Our results are consistent with the population-based longitudinal study results from Malmö2 and the ARIC study.3 In aggregate, the results from these 3 prospective cohort studies demonstrate that relations of OH with risk of AF are consistent in different study samples with different mean ages. The Malmö study included Swedish urban participants; the ARIC study enrolled individuals from 4 cities in the U.S., 25% of whom were African Americans. The mean ages of the participants were 46, 54, and 72 years for the Malmö, ARIC, and Framingham Heart Study cohorts, respectively.
We found that the association between AF and OH persisted after adjusting for systolic and diastolic BP and hypertension treatment. In addition, we did not find any statistically significant interaction with presence of hypertension or with hypertension treatment. It is possible that we did not have adequate statistical power to detect interaction with hypertension or hypertension treatment. Alternatively, interaction by hypertension status may have been less relevant in our cohort whose mean age was much higher than the mean age of the Malmö study cohort. In our study, there was a significant interaction by age ≥70 vs. <70 years. The finding may reflect higher likelihood of developing AF and OH in parallel later in life. Alternatively, our result may represent a common underlying mechanism, such as autonomic dysfunction or arterial stiffness, which is more common in older adults and contributes to risk for developing AF and OH.
The association between OH and development of AF is likely complex. Arterial stiffness and increased pulse pressure are associated with endothelial dysfunction,8 OH,9 and AF.10–13 Arterial stiffness also is associated with autonomic dysfunction,14,15 which contributes to OH and AF.16,17 Conversely, endothelial dysfunction may contribute to arterial stiffness and OH in parallel, leading to AF.10 In the present study, we did not have detailed measures of arterial stiffness in this cohort at the specific examination when OH was assessed. However, by adjusting for systolic and diastolic BP together in our multivariable models, we effectively adjusted for pulse pressure and still observed relations between orthostatic change in BP and AF risk. Hence, our finding suggests that a component of the latter association is separate from effects of arterial stiffness on AF risk.
Orthostatic blood pressure stabilization is a complex process mainly regulated by tone in the sympathetic component of the autonomic nervous system. Upon standing, there is a volume-dependent drop in arterial BP, which is detected by baroreceptors in the carotid sinuses and aortic arch.18 The baroreceptors then activate the sympathetic nervous system, which in turn increases heart rate, myocardial contractility, and peripheral resistance.18 With older age or neurodegenerative disease, sensitivity of the baroreceptors and sympathetic response to baroreceptor activity may be reduced, resulting in inadequate increase or even a decrease in MAP on standing, which in severe cases may be associated with categorical OH. Findings from multiple cohort studies demonstrating that OH is associated with increased risk of incident AF warrant future studies to understand the underlying mechanisms.
Our study has several limitations. First, data on heart rate variability with postural changes was not collected. Second, tonometry was not performed for our study sample at examination 17, and therefore, we were not able to calculate MAP using an arterial pressure wave form. Third, we may have had limited statistical power to detect a significant interaction. Fourth, because we measured standing BP at a fixed time point after standing, we may have missed transient BP changes. Fifth, incidence of new onset or paroxysmal AF may have been underestimated because AF is often undetected. Sixth, we did not adjust for other covariates that may affect AF risk such as obstructive sleep apnea, autonomic dysfunction, left atrial remodeling, and diastolic function. Hence, we cannot exclude residual confounding, and cannot establish causal relations. Finally, our study included predominantly older adults of European descent; therefore, the results may not be generalizable to other age groups or ethnicities. Major strengths of our study include prospective and longitudinal follow-up, careful validation of AF by cardiologists, and availability of extensive information on the covariates.
Acknowledgments
Funding. This work was supported by the Boston University School of Medicine and the National Heart, Lung, and Blood Institute’s Framingham Heart Study (contract N01-HC-25195; HHSN268201500001I) and the Division of Intramural Research of the National Heart, Lung, and Blood Institute. Additional support for this project was from the National Institutes of Health (NIH) 5T32HL007224-41 (Ko), K23HL114724 (Lubitz), KL2RR031981, 1R01HL126911-01A1 (McManus), 2R01HL092577, 1R01HL128914, 1P50HL120163 (Benjamin); 4R01HL115391 (Hamburg); 5R01HL107385 and R01HL126136 (Vasan); grant 2014105 from the Doris Duke Charitable Foundation (Lubitz)
Disclosures
Dr. Lubitz has received grant funding from Boehringer Ingelheim to test electronic notification methods to improve adherence to guideline-directed anticoagulation and has received consulting support from St. Jude Medical for the use of implantable atrial fibrillation detection technologies. Dr. McManus has consulted and/or received grant from Bristol-Myers Squibb, Pfizer, Philips, Samsung Semiconductor, and Biotronik, Inc. He is an equity stakeholder in MobileSense Technologies, Inc. Dr. Mitchell has consulted for Servier, Merck, Philips Healthcare, and Novartis. He is an owner of Cardiovascular Engineering, Inc, a company that designs and manufactures devices that measure vascular stiffness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, Zimarino M, De Caterina R. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. 2015;36:1609–1617. doi: 10.1093/eurheartj/ehv093. [DOI] [PubMed] [Google Scholar]
- 2.Fedorowski A, Hedblad B, Engstrom G, Gustav Smith J, Melander O. Orthostatic hypotension and long-term incidence of atrial fibrillation: the Malmo Preventive Project. J Intern Med. 2010;268:383–389. doi: 10.1111/j.1365-2796.2010.02261.x. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal SK, Alonso A, Whelton SP, Soliman EZ, Rose KM, Chamberlain AM, Simpson RJ, Jr, Coresh J, Heiss G. Orthostatic change in blood pressure and incidence of atrial fibrillation: results from a bi-ethnic population based study. PLoS One. 2013;8:e79030. doi: 10.1371/journal.pone.0079030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of Atrial Fibrillation on the Risk of Death: The Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Finucane C, O'Connell MD, Fan CW, Savva GM, Soraghan CJ, Nolan H, Cronin H, Kenny RA. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA) Circulation. 2014;130:1780–1789. doi: 10.1161/CIRCULATIONAHA.114.009831. [DOI] [PubMed] [Google Scholar]
- 6.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 7.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellien J, Favre J, Iacob M, Gao J, Thuillez C, Richard V, Joannidès R. Arterial Stiffness Is Regulated by Nitric Oxide and Endothelium-Derived Hyperpolarizing Factor During Changes in Blood Flow in Humans. Hypertension. 2010;55:674–680. doi: 10.1161/HYPERTENSIONAHA.109.142190. [DOI] [PubMed] [Google Scholar]
- 9.Torjesen A, Cooper LL, Rong J, Larson MG, Hamburg NM, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of Arterial Stiffness With Postural Change in Mean Arterial Pressure in Middle-Aged Adults: The Framingham Heart Study. Hypertension. 2017;69:685–690. doi: 10.1161/HYPERTENSIONAHA.116.08116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaikh AY, Wang N, Yin X, Larson MG, Vasan RS, Hamburg NM, Magnani JW, Ellinor PT, Lubitz SA, Mitchell GF, Benjamin EJ, McManus DD. Relations of Arterial Stiffness and Brachial Flow-Mediated Dilation With New-Onset Atrial Fibrillation: The Framingham Heart Study. Hypertension. 2016;68:590–596. doi: 10.1161/HYPERTENSIONAHA.116.07650. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell GF, Vasan RS, Keyes MJ, Parise H, Wang TJ, Larson MG, D'Agostino RB, Sr, Kannel WB, Levy D, Benjamin EJ. Pulse pressure and risk of new-onset atrial fibrillation. J Am Med Assoc. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 12.Roetker NS, Chen LY, Heckbert SR, Nazarian S, Soliman EZ, Bluemke DA, Lima JA, Alonso A. Relation of systolic, diastolic, and pulse pressures and aortic distensibility with atrial fibrillation (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2014;114:587–592. doi: 10.1016/j.amjcard.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larstorp AC, Ariansen I, Gjesdal K, Olsen MH, Ibsen H, Devereux RB, Okin PM, Dahlof B, Kjeldsen SE, Wachtell K. Association of pulse pressure with new-onset atrial fibrillation in patients with hypertension and left ventricular hypertrophy: the Losartan Intervention For Endpoint (LIFE) reduction in hypertension study. Hypertension. 2012;60:347–353. doi: 10.1161/HYPERTENSIONAHA.112.195032. [DOI] [PubMed] [Google Scholar]
- 14.Milazzo V, Maule S, Di Stefano C, Tosello F, Totaro S, Veglio F, Milan A. Cardiac Organ Damage and Arterial Stiffness in Autonomic Failure: Comparison With Essential Hypertension. Hypertension. 2015;66:1168–1175. doi: 10.1161/HYPERTENSIONAHA.115.05913. [DOI] [PubMed] [Google Scholar]
- 15.Huijben AM, Mattace-Raso FU, Deinum J, Lenders J, van den Meiracker AH. Aortic augmentation index and pulse wave velocity in response to head-up tilting: effect of autonomic failure. J Hypertens. 2012;30:307–314. doi: 10.1097/HJH.0b013e32834f09ee. [DOI] [PubMed] [Google Scholar]
- 16.Shen MJ, Choi EK, Tan AY, Lin SF, Fishbein MC, Chen LS, Chen PS. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. 2011;9:30–39. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- 17.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci F, De Caterina R, Fedorowski A. Orthostatic Hypotension: Epidemiology, Prognosis, and Treatment. J Am Coll Cardiol. 2015;66:848–860. doi: 10.1016/j.jacc.2015.06.1084. [DOI] [PubMed] [Google Scholar]