Abstract
The aim of the present study was to evaluate the validity of potential prognostic parameters of clear cell renal cell carcinoma (ccRCC) recommended by the 2012 International Society of Urological Pathology (ISUP) Consensus Conference in the Japanese population. We reviewed 406 Japanese patients with localized or locally advanced ccRCC who underwent curative surgery during 2004–2014 at Tokai University Hospital (Isehara, Japan) and were followed up for >2 years after surgery. A single pathologist reviewed all the histological slides. Morphological subtype and pathological T stage were reassigned according to the 2016 World Health Organization and TNM classifications. Sarcomatoid differentiation (SD), rhabdoid differentiation (RD), tumor necrosis (TN) and microvascular invasion (MVI) were assessed according to the 2012 ISUP recommendations. Nuclear grade was reclassified according to both the Fuhrman and the ISUP grading systems. Recurrence-free survival (RFS) and cancer-specific survival (CSS) were assessed through univariate and multivariate analyses. According to the Fuhrman grading system (group Fuhrman), TN and MVI were independent risk factors for postoperative recurrence in the multivariate analysis using the Cox proportional hazards model. According to the ISUP grading system (group ISUP), TN and MVI were independent risk factors for postoperative recurrence. In group Fuhrman, age, Fuhrman grade and TN were independent risk factors for CSS. In group ISUP, age, ISUP grade, and TN were independent risk factors for CSS. Furthermore, the group that was upgraded from Fuhrman grade 2 to ISUP grade 3 exhibited poorer CSS compared with the group that was reclassified from Fuhrman grade 2 to ISUP grade 2 (non-upgraded). Regardless of the nuclear grade, TN remained an independent predictor of RFS and CSS. To the best of our knowledge, this is the first report to prove the correlation between the 2012 ISUP recommendations and clinical outcomes in a Japanese ccRCC cohort. TN and upgrading to ISUP grade 3 were found to be potentially useful independent indicators of postoperative prognosis.
Keywords: renal cell carcinoma, clear cell renal cell carcinoma, International Society of Urological Pathology, coagulative tumor necrosis
Introduction
Kidney cancer is the ninth and fourteenth most common cancer in men and women, respectively, and the sixteenth most common cause of cancer-related mortality worldwide. In Japan, the estimated age-standardized incidence of kidney cancer among both sexes was 5.3 per 100,000 population (http://gco.iarc.fr/today/online-analysis-map?mode=cancer&mode_population=continents&population=900&sex=0&cancer=29&type=0&statistic=0&prevalence=0&color_palette=default&projection=natural-earth) (1,2). Renal cell carcinoma (RCC) is the most common type of adult epithelial kidney cancer (2). The 2016 World Health Organization (WHO) classification describes 13 distinct morphotypes (including unclassified ones) in RCC. Among those subtypes, clear cell RCC (ccRCC) is the most common type of renal parenchymal tumor, accounting for 63–83% of RCC cases, and its outcome is significantly poorer when compared with the outcome of other subtypes, such as papillary RCC or chromophobe RCC (3–5). The International Society of Urological Pathology (ISUP) 2012 Consensus Conference also reported new recommendations for classification. There was a strong consensus (98%) that the main morphotypes of RCC have prognostic significance, and that the cancer-specific survival (CSS) rate of ccRCC is significantly lower compared with that of papillary or chromophobe RCC at comparable stages (6).
In addition to tumor morphotype, other pathological parameters were suggested as being potential prognostic factors at the 2012 ISUP Conference, including sarcomatoid differentiation (SD), rhabdoid differentiation (RD), tumor necrosis (TN), novel nuclear ISUP grade and microvascular invasion (MVI) (6). In the present study, we focused on TN, ISUP grade and MVI as potential prognostic factors. TN is often encountered in RCC and has been reported in 27–32% of ccRCC cases (7,8). The presence of TN has been correlated with not only high-risk clinicopathological parameters, but also poor disease-specific survival (9–13). The 2012 ISUP Conference recommended the use of the ISUP grading system rather than the Fuhrman grading system. The ISUP grading system for ccRCC is defined only by nucleolar prominence and has exhibited a stronger association with patient outcome compared with that exhibited by the Fuhrman grading system (14). MVI is controversial as a prognostic factor in some studies.
To date, there have been several reports on the assessment of the ISUP recommendations in Western countries; however, there are few reports from East Asia, and no reports on the Japanese population. As the Japanese are a homogeneous race, the validity of the research results in Western countries require verification in a Japanese cohort.
The aim of the present study was to review all pathological slides of localized RCC from Japanese patients who underwent radical surgery at our institution over the past 10 years, and reclassify them by morphotype, TN, ISUP grade and MVI, according to the consensus of the 2012 ISUP Conference and, subsequently, on the basis of these results, to identify strong predictive factors affecting recurrence-free survival (RFS) and CSS. To the best of our knowledge, this study is the first to report on a Japanese population with postoperative localized or locally advanced ccRCC assessed according to the 2012 ISUP recommendations.
Materials and methods
Patient selection
The postoperative prognostic and clinicopathological data were retrospectively analyzed in accordance with a protocol approved by Tokai University Institutional Review Board. Between January 2004 and December 2014, a total of 631 patients with localized or locally advanced RCC underwent radical or partial nephrectomy at the Department of Urology of Tokai University Hospital (Isehara, Japan). Patients with synchronous bilateral renal tumors or synchronous metastases (to lymph nodes or other organs) were excluded from the analysis. All pathological slides of the patients were revised on the basis of the 2016 WHO classification and the TNM system by a single pathologist (C.I.). Among all 631 tumors, 514 were confirmed as being of the morphological ccRCC type. Finally, 406 patients who could be observed for >2 years after surgery were included in the analysis. The median age of the 406 patients was 62 years (range, 27–85 years). The male:female ratio was ~3:1. Radical nephrectomy was performed in 268 patients, and partial nephrectomy in 138 patients. The median postoperative follow-up period was 59 months (range, 3–137 months). A total of 48 patients had recurrent tumors during the follow-up period. The characteristics of the patients are summarized in Table I.
Table I.
Clinicopathological characteristics of the 406 patients with ccRCC.
| Characteristics | No. |
|---|---|
| Median age, years (range) | 62 (27–85) |
| Sex | |
| Male | 309 |
| Female | 97 |
| Side | |
| Left | 192 |
| Right | 214 |
| Nephrectomy type | |
| Radical | 268 |
| Partial | 138 |
| Median postoperative follow-up, months (range) | 59 (3–137) |
| Pathological T stage | |
| T1a | 272 |
| T1b | 76 |
| T2a | 12 |
| T2b | 3 |
| T3a | 16 |
| T3b | 26 |
| T4 | 1 |
| Tumor size, median mm (range) | 32 (9–250) |
| Fuhrman grade | |
| 1 | 3 |
| 2 | 343 |
| 3 | 38 |
| 4 | 22 |
| ISUP grade | |
| 1 | 4 |
| 2 | 227 |
| 3 | 153 |
| 4 | 22 |
| Microvascular invasion | |
| Positive | 77 |
| Negative | 329 |
| Tumor necrosis | |
| Positive | 43 |
| Negative | 363 |
| Sarcomatoid differentiation | |
| Positive | 9 |
| Negative | 397 |
| Rhabdoid differentiation | |
| Positive | 17 |
| Negative | 389 |
ccRCC, clear cell renal cell carcinoma; ISUP, International Society of Urological Pathology.
Pathological review
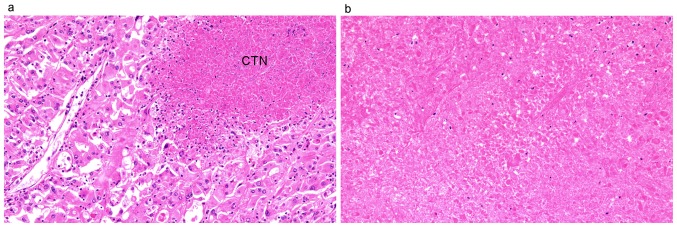
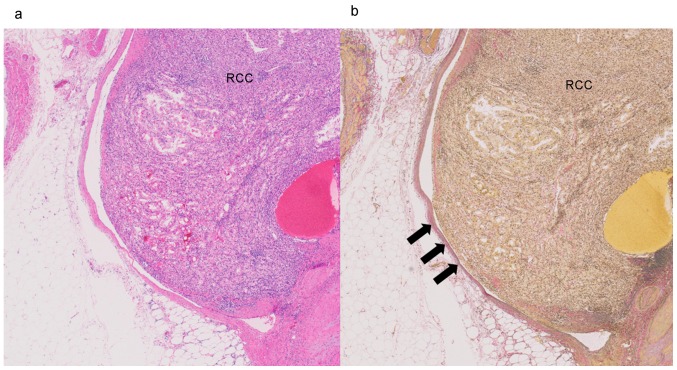
A single pathologist (C.I.) reviewed all tumors of the 631 patients and reassigned the tumors by histological subtype, pathological T stage, nuclear grade, TN, SD, RD and MVI. Histological subtype and pathological T stage were reassigned according to the 2016 WHO classification. TN, SD, RD, and MVI were assessed according to the 2012 ISUP Consensus Conference recommendations (6). The presence of TN was defined as microscopic coagulative tumor necrosis in the tumor section (Fig. 1a). Fibrosis, hyalinization, hemorrhage and ischemic necrosis (Fig. 1b) within a tumor were distinguished from coagulative tumor necrosis to avoid misdiagnosis of tumor necrosis. MVI was defined as a lump of tumor cells inside a vessel lined by one or more layers of vascular endothelial cells. If the presence of endothelial cells made the image unclear, elastin staining was performed to identify the vessel wall (Fig. 2a and b). Nuclear grade was reassigned according to both the Fuhrman and ISUP grading systems. The group that was reclassified by the Fuhrman grading system was named group Fuhrman, and the group that was reclassified by the ISUP grading system was named group ISUP. Cases with questionable pathological diagnoses were reviewed by another pathologist (H.K.). If the two pathologists had different opinions regarding the diagnosis, they examined the slides together and consensus was reached through discussion. The pathologists were blinded to the clinical outcome. The pathological findings are summarized in Table I. The Fuhrman and ISUP grading systems are defined as in Table II.
Figure 1.
Photomicrograph of coagulative tumor necrosis. (a) Example of coagulative tumor necrosis (CTN) adjacent to eosinophilic tumor cells (H&E staining; magnification, ×20). (b) Histopathological slide showing ischemic necrosis, which should be distinguished from CTN (H&E staining; magnification, ×20). H&E, hematoxylin and eosin.
Figure 2.
Photomicrograph of microvascular invasion. (a) Renal cell carcinoma (RCC) is seen protruding into the vessel (hematoxylin and eosin staining; magnification, ×20). (b) The tumor cells broke through the vessel collagen wall and invaded into the vessel (arrows). Elastica Van Gieson staining; magnification, ×20.
Table II.
Fuhrman and ISUP grading systems.
| Fuhrman grading system | ISUP grading system |
|---|---|
| Grade 1 | Grade 1 |
| Tumors were composed of cells with small (~10 µm) round uniform nuclei with inconspicuous or absent nucleoli | Nucleoli are inconspicuous or absent at a magnification of ×400 |
| Grade 2 | Grade 2 |
| Tumor cells had larger (~15 µm) nuclei that exhibited irregularities in the outline and nucleoli when examined under high-power magnification (×400) | Nucleoli are clearly visible at high-power magnification, but are not prominent |
| Grade 3 | Grade 3 |
| Tumor cells had even larger nuclei (~20 µm) with an obviously irregular outline and prominent large nucleoli even at low-power magnification (×100) | Nucleoli are prominent and are easily visualized at low-power magnification |
| Grade 4 | Grade 4 |
| Tumor cells exhibit characteristics similar to those of grade 3 tumors with the addition of bizarre, often multilobed nuclei and heavy chromatin clumps. These tumors often display areas of spindled-shaped cells resembling sarcomas | Presence of tumor giant cells and/or marked nuclear pleomorphism and/or with rhabdoid or sarcomatoid differentiation |
ISUP, International Society of Urological Pathology.
Statistical analysis
The RFS interval was defined as the time from the day of surgery until detection of recurrence. The CSS interval was defined as the time from the day of surgery until death from RCC. Data from patients who remained alive without recurrence at the last evaluation or who died of other causes were censored. RFS and CSS for clinical and pathological factors were calculated according to the Kaplan-Meier method and compared with the log-rank test. Clinical and pathological factors were defined as follows: Age (≤62 vs. >62 years), sex (male vs. female), pT stage (≤pT2 vs. ≥pT3), Fuhrman grade (1/2 vs. 3/4), ISUP grade (1/2 vs. 3/4), TN (absence or presence) and MVI (absence or presence). SD and RD were excluded, as these factors were included in one of the factors of grade 4 of the ISUP grading system. The median age of 62 years was used to separate younger from older patients. Multivariate analyses were performed using a Cox proportional hazards model. P-values of <0.05 were considered to indicate statistically significant difference. Clinical outcomes (RFS and CSS) were separately calculated in group Fuhrman and group ISUP. To discriminate the predictive accuracy for clinical outcomes between groups Fuhrman and ISUP, the concordance index (c-index) was used. A value of 1.0 represents perfect predictive models, and a value of 0.5 is equivalent by chance.
We also specifically evaluated the clinical outcomes for patients whose cancers were reclassified to a different grade by the ISUP grading system. All the statistical analyses were performed with JMP® version 12.0.1 (SAS Institute, Cary, NC, USA) and Stata 14 (StataCorp LP, College Station, TX, USA).
Results
Results of pathological review based on the 2016 WHO classification and the 2012 ISUP Consensus Conference
Table I shows the histopathological characteristics of all 406 patients with ccRCC reviewed on the basis of the 2016 WHO classification and the 2012 ISUP recommendations. The nuclear grade according to the Fuhrman grading system was 1 in 3 tumors, 2 in 343 tumors, 3 in 38 tumors, and 4 in 22 tumors. In the same population, the ISUP grade was 1 in 4 tumors, 2 in 227 tumors, 3 in 153 tumors, and 4 in 22 tumors. The number of cases whose nuclear grading differed between the Fuhrman and the ISUP grading systems are presented in Table III. The percentage of positivity of the adverse pathological characteristics of TN and MVI was 10.6 and 19.0%, respectively.
Table III.
Number of cases whose nuclear grading changed between the Fuhrman and ISUP systems.
| ISUP grade | Fuhrman grade
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| 1 | 2 | 2 | 0 | 0 | 4 |
| 2 | 1 | 224 | 2 | 0 | 227 |
| 3 | 0 | 117 | 36 | 0 | 153 |
| 4 | 0 | 0 | 0 | 22 | 22 |
| Total | 3 | 343 | 38 | 22 | 406 |
ISUP, International Society of Urological Pathology.
Statistical analysis of the impact of clinicopathological factors on RFS and CSS
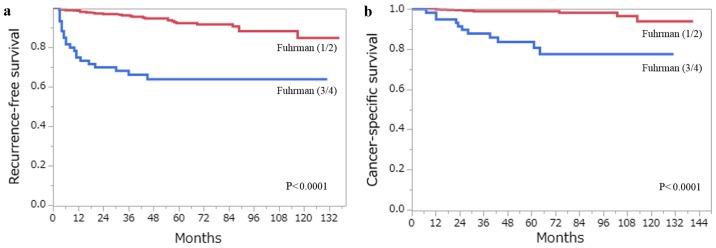
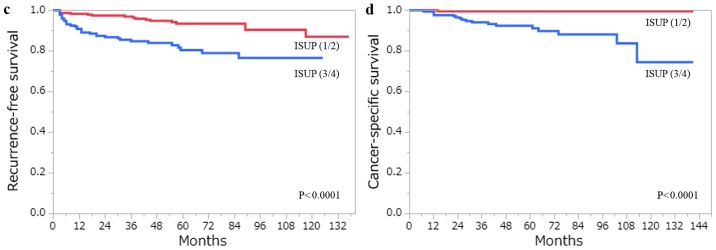
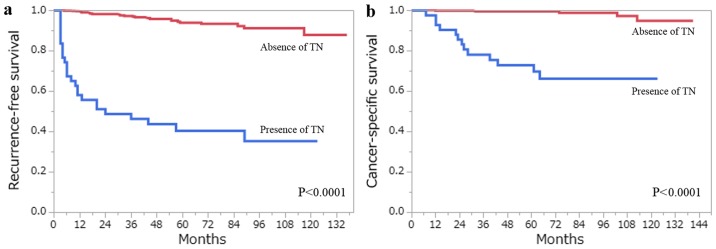
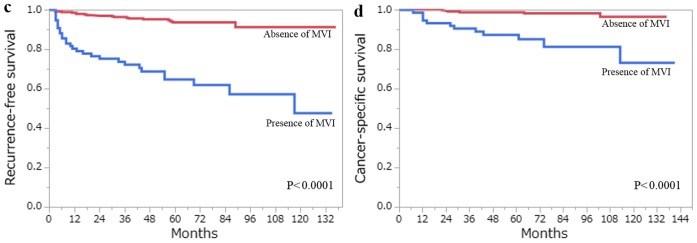
The 5-year RFS and CSS rates for all 406 patients with ccRCC estimated by the Kaplan- Meier method were 88.1 and 96.6%, respectively. A total of 48 patients developed recurrence, and 18 patients had succumbed to RCC at the last follow-up. Among the clinicopathological factors that were assessed by the log-rank test, age (>62 years), pT stage (≥pT3), Fuhrman grade (3/4), ISUP grade (3/4), the presence of TN and the presence of MVI, were all associated with RFS and CSS on the univariate analysis (Figs. 3 and 4). Using multivariate analysis, we analyzed independent prognostic factors for RFS and CSS for the Fuhrman grade (group Fuhrman) and ISUP grade (group ISUP). The former group, consisting of age, pT stage, Fuhrman grade, TN and MVI, was incorporated into a multivariate analysis using the Cox proportional hazards model. The latter group, consisting of age, pT stage, ISUP grade, TN and MVI, was incorporated into a multivariate analysis using the Cox proportional hazards model. In group Fuhrman, TN (P<0.0001) and MVI (P=0.0057) were independent risk factors for postoperative recurrence, while age (P=0.0059), Fuhrman grade (P=0.0497) and TN (P=0.0007) were independent risk factors for CSS (Table IV). In group ISUP, TN (P<0.0001) and MVI (P=0.0057) were independent risk factors for postoperative recurrence, while age (P=0.0327), ISUP grade (P=0.0355) and TN (P=0.0003) were independent risk factors for CSS (Table V). The predictive accuracy for RFS using group Fuhrman and group ISUP was 0.8637 and 0.8642, respectively, whereas for CSS using group Fuhrman and group ISUP was 0.9366 and 0.9478, respectively.
Figure 3.
(a) RFS and (b) CSS following curative surgery for tumors classified as Fuhrman grade 1/2 (red line) vs. Fuhrman grade 3/4 (blue line). (c) RFS and (d) CSS following curative surgery for tumors classified as ISUP grade 1/2 (red line) vs. ISUP grade 3/4 (blue line). RFS, recurrence-free survival; CSS, cancer-specific survival; ISUP, International Society of Urological Pathology.
Figure 4.
(a) RFS and (b) CSS following curative surgery for tumors without TN (red line) vs. those with TN (blue line). (c) RFS and (d) CSS following curative surgery for tumors without MVI (red line) vs. those with MVI (blue line). RFS, recurrence-free survival; CSS, cancer-specific survival; TN, tumor necrosis; MVI, microvascular invasion.
Table IV.
Risk factors for predicting postoperative recurrence and cancer-specific survival in ccRCC using the Fuhrman grading system.
| Factors | Recurrence-free survival
|
Cancer-specific survival
|
||||
|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
|||
| P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value | |
| Age (years) | 0.0246 | 0.0580 | 0.0018 | 0.0059 | ||
| ≤62 | ||||||
| >62 | 1.78 (0.99–3.31) | 4.74 (1.51–20.9) | ||||
| Sex | 0.5708 | 0.4909 | ||||
| Female | ||||||
| Male | ||||||
| pT stage | <0.0001 | 0.4687 | <0.0001 | 0.8612 | ||
| ≤pT2 | ||||||
| ≥pT3 | 1.35 (0.61–3.10) | 1.12 (0.33–4.42) | ||||
| Fuhrman grade | <0.0001 | 0.1666 | <0.0001 | 0.0497 | ||
| 1/2 | ||||||
| 3/4 | 1.62 (0.82–3.23) | 2.97 (1.00–9.37) | ||||
| TN | <0.0001 | <0.0001 | <0.0001 | 0.0007 | ||
| Absence | ||||||
| Presence | 6.62 (3.11–13.9) | 8.90 (2.45–34.8) | ||||
| MVI | <0.0001 | 0.0057 | <0.0001 | 0.2171 | ||
| Absence | ||||||
| Presence | 2.96 (1.34–6.27) | 2.41 (0.58–9.21) | ||||
ccRCC, clear cell renal cell carcinoma; TN, tumor necrosis; MVI, microvascular invasion; HR, hazard ratio; CI, confidence interval.
Table V.
Risk factors for predicting postoperative recurrence and cancer-specific survival in ccRCC using the ISUP grading system.
| Factors | Recurrence-free survival
|
Cancer-specific survival
|
||||
|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
|||
| P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value | |
| Age (years) | 0.0246 | 0.0630 | 0.0018 | 0.0327 | ||
| ≤62 | ||||||
| >62 | 1.77 (0.98–3.28) | 3.91 (1.27–17.0) | ||||
| Sex | 0.5708 | 0.4909 | ||||
| Female | ||||||
| Male | ||||||
| pT stage | <0.0001 | 0.4095 | <0.0001 | 0.8970 | ||
| ≤pT2 | ||||||
| ≥pT3 | 1.41 (0.63–3.26) | 1.09 (0.32–4.33) | ||||
| ISUP grade | <0.0001 | 0.8596 | <0.0001 | 0.0355 | ||
| 1/2 | ||||||
| 3/4 | 1.07 (0.53–2.19) | 9.31 (1.73–172) | ||||
| TN | <0.0001 | <0.0001 | <0.0001 | 0.0003 | ||
| Absence | ||||||
| Presence | 8.01 (3.91–16.5) | 8.56 (2.80–29.9) | ||||
| MVI | <0.0001 | 0.0057 | <0.0001 | 0.2185 | ||
| Absence | ||||||
| Presence | 2.97 (1.33–6.30) | 2.32 (0.56–8.67) | ||||
ccRCC, clear cell renal cell carcinoma; ISUP, International Society of Urological Pathology; TN, tumor necrosis; MVI, microvascular invasion; HR, hazard ratio; CI, confidence interval.
Clinical outcomes of ccRCC with Fuhrman grade 2
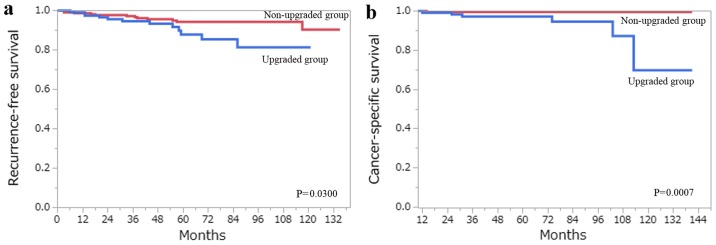
A total of 343 cases with Fuhrman grade 2 were identified, which accounted for the majority of our study population. The accuracy of the ISUP grading system in predicting clinical outcomes was compared with that of the Fuhrman grading system. On reclassifying the 343 Fuhrman grade 2 cases using the ISUP grading system, 224 were classified as ISUP grade 2, while 117 were upgraded to ISUP grade 3. The RFS at 10 years was 90.4% in tumors that were revised from Fuhrman grade 2 to ISUP grade 2 (non-upgraded group), and 81.5% in the tumors that were upgraded from Fuhrman grade 2 to ISUP grade 3 (upgraded group) (Fig. 5a). The CSS at 10 years was 99.6 and 69.9% in the non-upgraded and upgraded groups, respectively (Fig. 5b). Multivariate analysis using this cohort demonstrated that upgrading from Fuhrman grade 2 to ISUP grade 3 was an independent predictor of CSS (Table VI).
Figure 5.
(a) RFS and (b) CSS following curative surgery for tumors classified from Fuhrman grade 2 to ISUP grade 2 (red line) vs. those upgraded from Fuhrman grade 2 to ISUP grade 3 (blue line). RFS, recurrence-free survival; CSS, cancer-specific survival; ISUP, International Society of Urological Pathology.
Table VI.
Risk factors for predicting postoperative recurrence and cancer-specific survival in ccRCC with Fuhrman grade 2.
| Factors | Recurrence-free survival
|
Cancer-specific survival
|
||||
|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
|||
| P-value | HR (95% CI) | P-value | P-value | HR (95% CI) | P-value | |
| Age (years) | 0.2070 | 0.0325 | 0.2167 | |||
| ≤62 | ||||||
| >62 | 3.45 (0.52–67.8) | |||||
| Sex | 0.6169 | 0.5200 | ||||
| Female | ||||||
| Male | ||||||
| pT stage | <0.0001 | 0.0582 | 0.0283 | 0.1785 | ||
| ≤pT2 | ||||||
| ≥pT3 | 3.06 (0.96–9.55) | 0.23 (0.02–1.94) | ||||
| Fuhrman grade 2 | 0.0300 | 0.7038 | 0.0007 | 0.0389 | ||
| Non-upgraded group | ||||||
| Upgraded group | 1.19 (0.49–2.88) | 7.20 (1.09–143) | ||||
| TN | <0.0001 | 0.0349 | <0.0001 | 0.0444 | ||
| Absence | ||||||
| Presence | 3.33 (1.09–9.75) | 7.58 (1.06–47.2) | ||||
| MVI | <0.0001 | 0.0047 | 0.0001 | 0.0168 | ||
| Absence | ||||||
| Presence | 4.65 (1.63–12.8) | 9.48 (1.54–74.1) | ||||
ccRCC, clear cell renal cell carcinoma; TN, tumor necrosis; MVI, microvascular invasion; HR, hazard ratio; CI, confidence interval.
Discussion
At the ISUP 2012 Consensus Conference, tumor morphotype, SD, RD, TN, the ISUP grading system and MVI were recommended as potential prognostic factors of RCC. Among the major morphological subtypes, namely ccRCC, papillary RCC and chromophobe RCC, ccRCC accounts for ~60–70% of malignant kidney epithelial tumors and has the poorest prognosis (3–5,15). Therefore, in the present study, these recommended factors were evaluated with the focus being ccRCC. To evaluate the validity of these factors in Japanese patients with localized ccRCC, 406 ccRCC pathological slides were reviewed and reassigned pT stage, SD, RD, TN, nuclear grade and MVI, according to the 2012 ISUP recommendations and the 2016 WHO classification. For each potential risk factor, postoperative RFS and CSS were calculated by the Kaplan-Meier method; independent associations with RFS and CSS were calculated by the Cox regression analysis. Nuclear grade was classified by the Fuhrman and ISUP grading systems and then RFS and CSS were calculated for each classified group. Multivariate analysis indicated that TN and MVI were significantly associated with RFS, while age, nuclear grade and TN were significantly associated with CSS in both groups Fuhrman and ISUP. The c-index for RFS using group ISUP (c-index, 0.8642) indicated that its predictive ability is equivalent to that using group Fuhrman (c-index, 0.8637), whereas there was a possibility that the predictive ability for CSS using group ISUP (c-index, 0.9478) may be superior to that using group Fuhrman (c-index, 0.9366).
In the present study, the presence of TN affected both RFS and CSS as an independent risk factor. Although a number of studies have reported the correlation of TN with established clinicopathological parameters, such as high T stage, large tumor size, poor tumor grade, and CSS or overall survival, there are only a few reports on the association of the presence of TN with RFS, particularly in the East Asian population (3,10,13,16–18). In our study population of Japanese patients with localized ccRCC, TN, one of the new potential prognostic factors, was found to be the strongest predictive prognostic factor for both RFS and CSS.
The Fuhrman grading system has been widely adopted worldwide, including Japan; however, certain problems have been identified in the literature, such as the inclusion of various tumor types that differ morphologically and genetically, and poor reproducibility due to multiple parameters, including nuclear size and appearance (19–23). The ISUP grading system assesses only nucleolar prominence, is simple and reproducible, and is correlated with clinical outcome (14,24). In our study cohort, multivariate analysis revealed that the ISUP grade was not significantly associated with RFS, but it was significantly associated with CSS. In particular, cases that were subsequently upgraded to ISUP grade 3 from the Fuhrman grade 2 group exhibited a worse prognosis compared with those in the non-upgraded group. The ISUP grading system was found to be superior to the Fuhrman grading system due to its diagnostic reproducibility and its ability to predict clinical outcomes.
The predictive ability of MVI is controversial. Some reports have shown MVI to be correlated with prognosis; however, others have reported no such correlation (25–30). In the present study, the multivariate analysis demonstrated that MVI was independently associated with RFS of both group Fuhrman and group ISUP.
To the best of our knowledge, this study is the first to prove the correlation between coagulative TN and clinical outcome (RFS and CSS), as well as demonstrate the advantages of the ISUP grading system when compared with Fuhrman grading in Japanese ccRCC patients. Based on the results of our study, as well as the report of the 2012 ISUP Consensus Conference, we recommend closer surveillance or adjuvant therapy for patients with the presence of TN and higher ISUP grade (ISUP grade >3) (6).
The present study is limited by its retrospective design. Another limitation is that the majority of our cases lacked clinical data on risk factors that are considered to be associated with the prognosis of RCC, such as smoking and hypertension. Moreover, some patients dropped out of follow-up shortly after surgery, resulting in loss of prognostic data. In the present study, the unclear histological subtypes were excluded, but we included various stages of ccRCC, which may have resulted in confounding bias. To strengthen the reliability of the results of this study, prospective multicenter studies must be designed to confirm these pathological parameters in a larger population of consecutive patients with RCC.
In conclusion, to the best of our knowledge, this retrospective study is the first to report on a Japanese population with RCC to investigate the correlations between the postoperative prognosis of ccRCC and the pathological parameters recommended by the 2012 ISUP Consensus Conference. Adapting the ISUP recommendations and the 2016 WHO classification for a Japanese ccRCC population is likely to be of value in clinical practice.
Acknowledgments
Not applicable.
Footnotes
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
HKi, CI and TU collected the data and wrote the manuscript. CI and HKa reviewed the slides and confirmed the pathological diagnosis. HF and TK analyzed and interpreted the patient data regarding the clinical outcomes. HKo, NN and AM provided the study concept and design, and revised the manuscript. The final version of the manuscript was read and approved by all authors.
Ethics approval and consent to participate
The postoperative prognostic and clinicopathological data were retrospectively analyzed in accordance with a protocol approved by the Tokai University Institutional Review Board (15R-065).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Moch H, Gasser T, Amin MB, Torhorst J, Sauter G, Mihatsch MJ. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: A Swiss experience with 588 tumors. Cancer. 2000;89:604–614. doi: 10.1002/1097-0142(20000801)89:3<604::AID-CNCR16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Amin MB, Amin MB, Tamboli P, Javidan J, Stricker H, de-Peralta Venturina M, Deshpande A, Menon M. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: An experience of 405 cases. Am J Surg Pathol. 2002;26:281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V, Martignoni G, Galfano A, Novara G, Gobbo S, Brunelli M, Pea M, Zattoni F, Artibani W. Prognostic role of the histologic subtypes of renal cell carcinoma after slide revision. Eur Urol. 2006;50:786–793. doi: 10.1016/j.eururo.2006.04.009. discussion 793–794. [DOI] [PubMed] [Google Scholar]
- 6.Delahunt B, Cheville JC, Martignoni G, Humphrey PA, Magi-Galluzzi C, McKenney J, Egevad L, Algaba F, Moch H, Grignon DJ, et al. Members of the ISUP Renal Tumor Panel: The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol. 2013;37:1490–1504. doi: 10.1097/PAS.0b013e318299f0fb. [DOI] [PubMed] [Google Scholar]
- 7.Lee SE, Byun SS, Oh JK, Lee SC, Chang IH, Choe G, Hong SK. Significance of macroscopic tumor necrosis as a prognostic indicator for renal cell carcinoma. J Urol. 2006;176:1332–1337. doi: 10.1016/j.juro.2006.06.021. discussion 1337–1338. [DOI] [PubMed] [Google Scholar]
- 8.Katz MD, Serrano MF, Grubb RL, III, Skolarus TA, Gao F, Humphrey PA, Kibel AS. Percent microscopic tumor necrosis and survival after curative surgery for renal cell carcinoma. J Urol. 2010;183:909–914. doi: 10.1016/j.juro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 10.Pichler M, Hutterer GC, Chromecki TF, Jesche J, Kampel-Kettner K, Rehak P, Pummer K, Zigeuner R. Histologic tumor necrosis is an independent prognostic indicator for clear cell and papillary renal cell carcinoma. Am J Clin Pathol. 2012;137:283–289. doi: 10.1309/AJCPLBK9L9KDYQZP. [DOI] [PubMed] [Google Scholar]
- 11.Lam JS, Shvarts O, Said JW, Pantuck AJ, Seligson DB, Aldridge ME, Bui MH, Liu X, Horvath S, Figlin RA, et al. Clinicopathologic and molecular correlations of necrosis in the primary tumor of patients with renal cell carcinoma. Cancer. 2005;103:2517–2525. doi: 10.1002/cncr.21127. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta S, Lohse CM, Leibovich BC, Frank I, Thompson RH, Webster WS, Zincke H, Blute ML, Cheville JC, Kwon ED. Histologic coagulative tumor necrosis as a prognostic indicator of renal cell carcinoma aggressiveness. Cancer. 2005;104:511–520. doi: 10.1002/cncr.21206. [DOI] [PubMed] [Google Scholar]
- 13.Ficarra V, Martignoni G, Lohse C, Novara G, Pea M, Cavalleri S, Artibani W. External validation of the Mayo Clinic Stage, Size, Grade and Necrosis (SSIGN) score to predict cancer specific survival using a European series of conventional renal cell carcinoma. J Urol. 2006;175:1235–1239. doi: 10.1016/S0022-5347(05)00684-1. [DOI] [PubMed] [Google Scholar]
- 14.Delahunt B, Sika-Paotonu D, Bethwaite PB, William Jordan T, Magi-Galluzzi C, Zhou M, Samaratunga H, Srigley JR. Grading of clear cell renal cell carcinoma should be based on nucleolar prominence. Am J Surg Pathol. 2011;35:1134–1139. doi: 10.1097/PAS.0b013e318220697f. [DOI] [PubMed] [Google Scholar]
- 15.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Cho NH, Kim DS, Kwon YM, Kim EK, Rha SH, Park YW, Shim JW, Lee SS, Lee SN, et al. Genitourinary Pathology Study Group of the Korean Society of Pathologists: Renal cell carcinoma in South Korea: A multicenter study. Hum Pathol. 2004;35:1556–1563. doi: 10.1016/j.humpath.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RH, Leibovich BC, Lohse CM, Cheville JC, Zincke H, Blute ML, Frank I. Dynamic outcome prediction in patients with clear cell renal cell carcinoma treated with radical nephrectomy: The D-SSIGN score. J Urol. 2007;177:477–480. doi: 10.1016/j.juro.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 18.Isbarn H, Patard JJ, Lughezzani G, Rioux-Leclercq N, Crépel M, Cindolo L, de la Taille A, Zini L, Villers A, Shariat SF, et al. Limited prognostic value of tumor necrosis in patients with renal cell carcinoma. Urology. 2010;75:1378–1384. doi: 10.1016/j.urology.2009.07.1221. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Delahunt B. Advances and controversies in grading and staging of renal cell carcinoma. Mod Pathol. 2009;22(Suppl 2):S24–S36. doi: 10.1038/modpathol.2008.183. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein NS. Grading of renal cell carcinoma. Urol Clin North Am. 1999;26:637–642, vii. doi: 10.1016/S0094-0143(05)70204-4. [DOI] [PubMed] [Google Scholar]
- 22.Lang H, Lindner V, de Fromont M, Molinié V, Letourneux H, Meyer N, Martin M Jacqmin D. Multicenter determination of optimal interobserver agreement using the Fuhrman grading system for renal cell carcinoma: Assessment of 241 patients with > 15-year follow-up. Cancer. 2005;103:625–629. doi: 10.1002/cncr.20812. [DOI] [PubMed] [Google Scholar]
- 23.Lanigan D, Conroy R, Barry-Walsh C, Loftus B, Royston D, Leader M. A comparative analysis of grading systems in renal adenocarcinoma. Histopathology. 1994;24:473–476. doi: 10.1111/j.1365-2559.1994.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 24.Delahunt B, Bethwaite PB, Nacey JN. Outcome prediction for renal cell carcinoma: Evaluation of prognostic factors for tumours divided according to histological subtype. Pathology. 2007;39:459–465. doi: 10.1080/00313020701570061. [DOI] [PubMed] [Google Scholar]
- 25.Pichler M, Hutterer GC, Chromecki TF, Jesche J, Groselj-Strele A, Kampel-Kettner K, Pummer K, Zigeuner R. Prognostic value of the Leibovich prognosis score supplemented by vascular invasion for clear cell renal cell carcinoma. J Urol. 2012;187:834–839. doi: 10.1016/j.juro.2011.10.155. [DOI] [PubMed] [Google Scholar]
- 26.Cho HJ, Kim SJ, Ha US, Hong SH, Kim JC, Choi YJ, Hwang TK. Prognostic value of capsular invasion for localized clear-cell renal cell carcinoma. Eur Urol. 2009;56:1006–1012. doi: 10.1016/j.eururo.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Sevinç M, Kirkali Z, Yörükoğlu K, Mungan U, Sade M. Prognostic significance of microvascular invasion in localized renal cell carcinoma. Eur Urol. 2000;38:728–733. doi: 10.1159/000020370. [DOI] [PubMed] [Google Scholar]
- 28.Van Poppel H, Vandendriessche H, Boel K, Mertens V, Goethuys H, Haustermans K, Van Damme B, Baert L. Microscopic vascular invasion is the most relevant prognosticator after radical nephrectomy for clinically nonmetastatic renal cell carcinoma. J Urol. 1997;158:45–49. doi: 10.1097/00005392-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Roos FC, Weirich J, Victor A, Elsässer A, Brenner W, Biesterfeld S, Hampel C, Thüroff JW. Impact of several histopathological prognosticators and local tumour extension on oncological outcome in pT3b/c N0M0 renal cell carcinoma. BJU Int. 2009;104:461–469. doi: 10.1111/j.1464-410X.2009.08489.x. [DOI] [PubMed] [Google Scholar]
- 30.Lang H, Lindner V, Letourneux H, Martin M, Saussine C, Jacqmin D. Prognostic value of microscopic venous invasion in renal cell carcinoma: Long-term follow-up. Eur Urol. 2004;46:331–335. doi: 10.1016/j.eururo.2004.03.020. [DOI] [PubMed] [Google Scholar]