Abstract
Objective:
The aim of the present study is to determine the presence of iron (Fe) deficiency and Fe deficiency anemia in children with zinc (Zn) deficiency.
Materials and Methods:
This retrospective study comprised 560 patients aged 6 months to 16 years in whom Zn levels in hair samples were measured concurrently with serum levels of ferritin, Fe, Fe-binding capacity, and blood count analysis. For all patients, we retrospectively assessed serum ferritin, serum Fe, Fe-binding capacity, transferrin saturation index, red blood cell count, hemoglobin levels, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and red cell distribution width (RDW). Patients were divided into two groups according to the level of the hair Zn level as Zn deficiency (hair Zn level <100 µg/g) and without Zn deficiency (hair Zn level >100 µg/g). Data were analyzed to determine whether there was a significant difference between any of these parameters and the presence of Fe deficiency and Fe deficiency anemia between patients with and without Zn deficiency.
Results:
A total of 238 patients had Zn levels <100 µg/g, and 322 patients had Zn levels >100 µg/g. The median ferritin level was 16.2 (9.8–24.9) ng/mL in the Zn-deficient group and 18.7 (12–29.3) ng/mL in those without Zn deficiency group. The presence of Fe deficiency was higher in the Zn deficiency group (60.1%) than in the without Zn deficiency group (50%; p<0.05). The presence of Fe deficiency anemia was significantly higher in the Zn deficiency group (20.2%) than in the without Zn deficiency group (12.7%; p<0.05). There were very weak negative significant correlation between hair Zn and RDW level (r=−0.24; p<0.001) and weak positive correlation between hair Zn and MCV (r=0.31; p<0.001).
Conclusion:
Fe deficiency and Fe deficiency anemia increased in patients with zinc deficiency.
Keywords: Ferritin, Zinc deficiency, Iron deficiency, Iron deficiency anemia
Introduction
Zinc (Zn) deficiency is an important health issue in developing countries. It is most commonly seen in preschool-age children. In general, it is caused by decreased Zn ingestion or Zn-binding fibers and phytates in diet [1].
Deficiencies in other trace elements can accompany Zn deficiency. Iron (Fe) and Zn are most frequently encountered element deficiencies in developing countries. Fe and Zn deficiencies are more commonly associated with individuals at lower socioeconomic level. Zn deficiency is frequently reported in individuals with Fe deficiency and Fe deficiency anemia [2].
In the present study, we aimed to assess the development of Fe deficiency and Fe deficiency anemia in pediatric patients with Zn deficiency. We hypothesize that the frequency of Fe deficiency and Fe deficiency anemia is higher in patients with Zn deficiency.
Materials and Methods
Study design, study groups, and setting
This was a retrospective study in Zn-deficient children aged 6 months to 16 years. It was conducted at Pediatric Clinic of Kayseri Training and Research Hospital. This is a 162-bed pediatrics clinic providing healthcare to 175,000 patients per year. The study was approved by the local ethics committee. Patient consent was not required because of its retrospective nature.
The present study enrolled patients attending to hospital in which vitamin deficiency was analyzed based on code E56.9 of the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) between January and March 2016.
The inclusion criteria were age between 6 months and 16 years and available complete blood count, serum ferritin, Fe, and Fe-binding capacity values studied simultaneously with hair Zn analysis. The exclusion criteria were age <6 months or >16 years and having a ferritin value >200 ng/dL.
Study groups
The study included all patients presented to pediatrics outpatient clinics in which hair Zn analysis was ordered with ICD-10 code of E56.9 (vitamin deficiency). The patients included were stratified into two groups according to hair Zn analysis: patients with and without Zn deficiency.
Primary and secondary outcomes
The primary outcome of the present study was to determine whether there was a difference in ferritin level and presence of Fe deficiency and Fe deficiency anemia in children aged 6 months–16 years with Zn deficiency. The secondary outcome was whether there was a difference in red blood cell count (RBC), hemoglobin (Hb), hematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red cell distribution width (RDW) between patients with Zn deficiency and controls.
Determination of primary and secondary outcomes
Serum ferritin levels and complete blood counts were retrospectively recorded in patients with and without Zn deficiency. We determined whether there was a significant difference in the presence of Fe deficiency and Fe deficiency anemia between groups.
In addition, we determined whether there was a significant difference in RBC, Hb, Hct, MCV, MCH, and MCHC values between groups with and without Zn deficiency.
Definitions
Fe deficiency and anemia were diagnosed according to the World Health Organization criteria. Ferritin levels <12 ng/dL and <15 ng/dL were defined as Fe deficiency in children aged <5 years and >5 years, respectively. Patients having transferrin saturation index <16% were also determined as Fe deficiency. Patients with Hb value at the lowest limit of normal according to age (11 g/dL for <60 months of age, 11.5 g/dL for 5–11 years of age, 12.0 g/dL for 12–15 years of age, and 13 g/dL for ≥15 years of age) were considered to have Fe-deficiency anemia [3].
There is no cut-off value for hair Zn level to define a Zn deficiency. Hair Zn levels can change within a population due to the analytical method or the pre-analytical variables. Hair Zn levels <100 µg/g were defined as Zn deficiency according to a study about Zn deficiency in an Anatolian population and reference values for the test method [4].
Laboratory studies
From all patients, 2 mL blood sample was collected into a K3 EDTA tube (Vacuette; Greiner Bio-One, Kremsmünster, Austria) for a complete blood count, whereas 3 mL blood sample was drawn into standard tubes for ferritin, Fe and Fe-binding capacity assays.
A complete blood cell count was performed using a cytometry (BC-6800 Auto Hematology Analyzer; Mindray, Shenzhen, China). Ferritin concentration was analyzed by immuno-assay using an automated chemiluminescence (Immulite 2000; Diagnostic Products Corporation, LA, USA). Serum Fe and total Fe-binding capacity were measured by an auto-analyzer (Olympus AU 1600; Mishima, Japan). Transferrin saturation was calculated from the formula: (serum Fe/total Fe-binding capacity) ×100.
Before analyzing hair Zn, all participants were asked not to process their hair chemically for approximately 8 weeks. This included dyeing, perming, straightening, or frosting. The hair also had to be free of all gels, oils, and hair creams before sample collection. Approximately 150 mg of the proximal portion of hair was obtained from four or five different locations on the posterior vertex and occipital regions of the scalp using stainless steel scissors. A 2 cm length of the root part of the hair was used for analyses. After weighing, the hair samples were burned in an oven at 530 °C for 2 h. Then, they were diluted in 0.5 N HCl to a defined volume.
Zn measurements were analyzed in sample pairs of hair concurrently by atomic absorption spectrometry using a Thermo Scientific™ iCE™ 3000 Series (Rockford, IL, USA). The Zn hollow-cathode lamp current was 5 mA, wavelength was 213.9 nm, and slit width was 0.5 nm. Standard solutions were run before the analysis, and a 5-point calibration curve was drawn. Results were calculated according to the absorbance–concentration calibration curve fit and expressed as µg/g hair. Internal quality control samples were used to verify assay accuracy and maintain the quality of the standard solutions. Coefficient of variation was <0.999 with a stock standard concentration of 1000 µg/mL. Reproducibility was 3.1%, and the lower limit of linearity was 0.0277 µg/mL. The results are expressed in µg/g.
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences for Windows ver. 21.0 (IBM Corp., Armonk, NY, USA). Normality was assessed using the Kolmogorov–Smirnov test. For independent samples, the Mann–Whitney U-test was used to analyze non-parametrical continuous variables. Categorical variables were analyzed using the chi-square test. Spearman’s correlation test was used to assess correlations among non-parametrical data. Nonparametrical data are presented as medians (25th–75th percentile). In all tests, a p-value <0.05 was considered as statistically significant.
Results
The hair Zn level was <100 µg/g in 238 patients and >100 µg/g in 322 patients. No significant difference was detected between groups regarding gender (p>0.05; Table 1). The median age was significantly lower in the Zn deficiency group than in the without Zn deficiency group (p<0.05; Table 1).
Table 1.
Comparison of groups regarding demographic characteristics, complete blood count, serum Fe, ferritin, Fe-binding capacity values, and presence of Fe deficiency and Fe deficiency anemia
| Group 1 (Zn>100 µg/g) (n=322) | Group 2 (Zn<100 µg/g) (n=238) | p | |
|---|---|---|---|
| Gender | |||
| Female/male (n) | 203/119 | 155/83 | 0.612 |
| Female/male (%) | 63/37 | 65.1/34.9 | |
| Age | |||
| Month | 85 (54–120) | 40 (25–58) | <0.001 |
| Hair Zn µg/g | 163.8 (125–211.3) | 61.7 (46.1–83.1) | <0.001 |
| Serum ferritin ng/mL | 18.7 (12–29.3) | 16.2 (9.8–24.9) | 0.007 |
| Serum Fe μg/dL | 65 (41–91.2) | 53.5 (32–83) | <0.001 |
| Total Fe-binding capacity μg/dL | 286 (250–335) | 300 (257–339) | 0.481 |
| Transferrin saturation index % | 22.2 (12.9–34.3) | 17.1 (9.6–29) | 0.001 |
| Presence of Fe deficiency n (%) | 161 (50) | 143 (60.1) | 0.018 |
| Presence of Fe deficiency anemia n (%) | 41 (12.7) | 48 (20.2) | 0.017 |
| RBC ×103/µL | 4.83 (4.6–5.1) | 4.82 (4.59–5.09) | 0.496 |
| PLT ×103/µL | 330 (282–378) | 348 (294–406) | 0.011 |
| Hb g/dL | 12.9 (12–13.7) | 12.3 (11.4–12.9) | <0.001 |
| Hct % | 38.7 (36.7–40.6) | 36.9 (35–38.8) | <0.001 |
| MCV fL | 80.6 (77.4–83.4) | 77.7 (73.8–80.2) | <0.001 |
| MCH Pg | 26.9 (25.5–28) | 25.8 (24.2–26.9) | <0.001 |
| MCHC g/dL | 33.3 (32.5–33.9) | 33 (33.2–33.8) | 0.071 |
| RDW % | 13.3 (12.9–14.1) | 13.8 (13.2–14.9) | <0.001 |
Data are presented as medians (25th–75th percentile)
Fe: iron; Zn: zinc; RBC: red blood cell count; WBC: white blood cell count; PLT: platelet count; Hb: hemoglobin; Hct: hematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; RDW: red cell distribution width
Median serum ferritin level was found to be 16.2 ng/dL (9.8–24.9) in the group with Zn deficiency, whereas 18.7 ng/dL (12–29.3) in the group without Zn deficiency. Serum Fe and transferrin saturation index were lower and Fe-binding capacity was higher in Zn deficiency. Hb, Hct, MCV, MCH, and MCHC were lower, and RDW was higher in the Zn deficiency group than in the without Zn deficiency group (p<0.05; Table 1). Fe deficiency and Fe deficiency anemia rates were higher in the Zn deficiency group than in the without Zn deficiency group (p>0.05; Table 1).
There was a very weak negative correlation between tissue Zn level and RDW (r=−0.24; p<0.001), whereas a weak positive correlation between tissue Zn level and MCV (r=0.31; p<0.001).
Discussion
In our study, the presence of Fe deficiency and Fe deficiency anemia was higher in children with Zn deficiency. It was found that there was a significant negative correlation between hair Zn level and RDW, whereas a positive correlation with MCV.
Deficiencies of many trace elements can accompany Zn deficiency, with Fe deficiency being the most frequent. Zn is a cofactor for many enzymes involved in Fe metabolism [2, 5]. Thus, Zn deficiency has been intensively investigated in children with Fe deficiency anemia. Prevel et al. [6] reported that Fe deficiency was frequently observed with Zn deficiency. Motadi et al. [7] revealed the rate of association of Fe and Zn deficiencies as 2% among preschool-age children. Tural et al. [8] noted that there was a strict relationship between Fe and Zn, attributing this relationship to the fact that Zn is a cofactor for several enzymes involved in Fe metabolism. Kelkitli et al. [9] found markedly low serum Zn level in patients with Fe deficiency anemia when compared with controls, suggesting that Zn levels should be assessed in patients with Fe deficiency anemia. In the study by Bains et al. [5], Zn deficiency was detected in 17.9% of children aged 6 months–5 years, and rates of low Fe and ferritin level were 84.6% and 71.8% among children with Zn deficiency, respectively. In the studies of Tural et al. [8] and Kelkitli et al. [9] from Turkey, Zn deficiency has been frequently detected in patients with Fe deficiency. In the study by Erdoğan et al. [10], serum Zn level was markedly lower in patients with Fe deficiency anemia than in controls. In the study by Arcagök et al. [11], serum Zn level was found to be lower in children with Fe deficiency anemia than those in controls, and rate of Zn deficiency was reported as 9.2% in children with Fe deficiency anemia. Prasad et al. [12] reported that there might be moderate anemia as well as growth retardation, geophagia, and delayed puberty in children with Zn deficiency.
Although Zn deficiency has been frequently investigated in children with Fe deficiency in the literature, there is limited number of study about the development of Fe deficiency in patients with Zn deficiency. In our study, we assessed Fe deficiency and Fe deficiency anemia in patients with Zn deficiency since Zn is a cofactor in many enzymes involved in Fe metabolism. Thus, it is likely that patients with Zn deficiency would have Fe deficiency. Arcagök et al. [11] investigated Zn levels in children with Fe deficiency and found that serum Fe level and saturation index were lower in the group with Zn deficiency than in controls. Several authors interpreted that findings of Fe deficiency were seen before the onset of anemia in Zn deficiency.
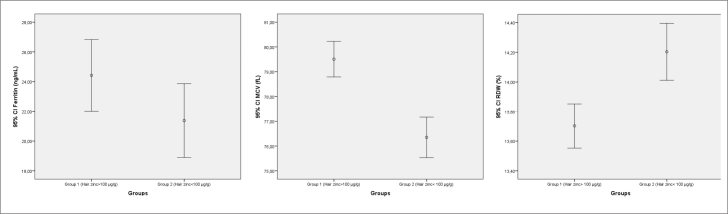
In our study, serum ferritin level was assessed for diagnosis of Fe deficiency in children with Zn deficiency (Figure 1). Ferritin is a major storage protein for Fe and has a significant role in Fe homeostasis; thus, it is an important biomarker for assessment of total Fe stores in clinical practice [13]. However, it is influenced by many physiological and pathological conditions including inflammation. Ferritin is increased in inflammation as it is also an acute phase reactant. Thus, transferrin saturation index was used in addition to ferritin level to diagnose Fe deficiency in our study. Based on our study, a significant difference was detected in serum ferritin value and incidence of Fe deficiency and Fe deficiency anemia in the group with Zn deficiency as presumed. Fe deficiency and Fe deficiency anemia were higher in patients with Zn deficiency.
Figure 1.
Comparison of ferritin, RDW, and MCV levels between groups
RDW: red cell distribution width; MCV: mean corpuscular volume
The increase in RDW value is one of the earliest findings of Fe deficiency. In Fe deficiency, increased RDW value can be observed before the onset of change in other parameters of complete blood count. In Fe deficiency, decreased Hb, MCV, and MCH values are other findings that could be detected in complete blood count [3]. In our study, MCV was lower and RDW was higher in the Zn deficiency group than in the without Zn deficiency group (Figure 1). Tissue Zn level was negatively correlated with RDW, whereas it was positively correlated with MCV in our study. This finding could be interpreted as early findings of Fe deficiency developed in Zn deficiency. Zn deficiency resulting in Fe deficiency and Fe deficiency anemia could be associated with severity of Zn deficiency.
The limitation of our study is its retrospective design. Development of Fe deficiency in children with Zn deficiency can be evaluated in a larger, prospective study that assesses Fe deficiency together with severity of Zn deficiency.
In conclusion, Fe deficiency and Fe deficiency anemia appear to be increased in individuals with Zn deficiency. There is a need for prospective studies assessing Fe deficiency together with severity of Zn deficiency.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Erciyes University School of Medicine (number: 2017/446).
Informed Consent: Written informed consent was not obtained from the parents of the patients who participated in this study due to retrospective design of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.B.E.; Design - A.B.E., Y.A.T., S.K.; Supervision - A.B.E., Y.A.T., S.K.; Resources - C.T.; Materials - C.T., A.B.E.; Data Collection and/or Processing - A.B.E., C.T.; Analysis and/or Interpretation - A.B.E.; Literature Search - A.B.E.; Writing Manuscript -A.B.E.; Critical Review - A.B.E., Y.T., S.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ozden TA, Gokcay G, Cantez MS, et al. Copper, zinc and iron levels in infants and their mothers during the first year of life: a prospective study. BMC Pediatr. 2015;15:157. doi: 10.1186/s12887-015-0474-9. https://doi.org/10.1186/s12887-015-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedraza DF, Rocha AC. Micronutrient deficiencies in Brazilian children attending daycare centers: a review of the literature. Cien Saude Colet. 2016;21:1525–44. doi: 10.1590/1413-81232015215.20712014. https://doi.org/10.1590/1413-81232015215.20712014. [DOI] [PubMed] [Google Scholar]
- 3.Sazawal S, Dhingra U, Dhingra P, et al. Efficiency of red cell distribution width in identification of children aged 1–3 years with iron deficiency anemia against traditional hematological markers. BMC Pediatr. 2014;14:8. doi: 10.1186/1471-2431-14-8. https://doi.org/10.1186/1471-2431-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donma O, Günbey S, Taş MA, Donma MM. Zinc, copper, and magnesium concentrations in hair of children from southeastern Turkey. Biol Trace Elem Res. 1990;24:39–47. doi: 10.1007/BF02789139. https://doi.org/10.1007/BF02789139. [DOI] [PubMed] [Google Scholar]
- 5.Bains K, Kaur H, Bajwa N, Kaur G, Kapoor S, Singh A. Iron and Zinc Status of 6-Month to 5-Year-Old Children From Low-Income Rural Families of Punjab, India. Food Nutr Bull. 2015;36:254–63. doi: 10.1177/0379572115597396. https://doi.org/10.1177/0379572115597396. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Prevel Y, Allemand P, Nikiema L, et al. Biological Status and Dietary Intakes of Iron, Zinc and Vitamin A among Women and Preschool Children in Rural Burkina Faso. PLoS One. 2016;11:e0146810. doi: 10.1371/journal.pone.0146810. https://doi.org/10.1371/journal.pone.0146810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motadi SA, Mbhenyane XG, Mbhatsani HV, Mabapa NS, Mamabolo RL. Prevalence of iron and zinc deficiencies among preschool children ages 3 to 5 y in Vhembe district, Limpopo province, South Africa. Nutrition. 2015;31:452–8. doi: 10.1016/j.nut.2014.09.016. https://doi.org/10.1016/j.nut.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Tural E, Meral C, Suleymanoglu S, et al. Renal zinc clearance/glomerular filtration rate ratio as an indicator of marginal zinc deficiency associated with iron deficiency in childhood. J Am Coll Nutr. 2010;29:107–12. doi: 10.1080/07315724.2010.10719823. https://doi.org/10.1080/07315724.2010.10719823. [DOI] [PubMed] [Google Scholar]
- 9.Kelkitli E, Ozturk N, Aslan NA, et al. Serum zinc levels in patients with iron deficiency anemia and its association with symptoms of iron deficiency anemia. Ann Hematol. 2016;95:751–6. doi: 10.1007/s00277-016-2628-8. https://doi.org/10.1007/s00277-016-2628-8. [DOI] [PubMed] [Google Scholar]
- 10.Erdoğan S, Akyol B, Önal H, Önal Z, Keleş ES. Demir eksikliği anemisinde serum çinko düzeylerinin değerlendirilmesi. Çocuk Dergisi. 2003;3:49–55. [Google Scholar]
- 11.Arcagök B, Özdemir N, Yıldız İ, Celkan T. The association between iron deficiency and serum zinc levels. Cocuk Sagligi ve Hastaliklari Der. 2013;56:2. [Google Scholar]
- 12.Prasad AS, Miale A, Jr, Farid Z, Sandstead HH, Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypogonadism. J Lab Clin Med. 1963;61:537–549. [PubMed] [Google Scholar]
- 13.Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23:95–104. doi: 10.1016/j.blre.2008.08.001. https://doi.org/10.1016/j.blre.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]