Abstract
Objective:
Idiopathic pulmonary fibrosis (IPF) is the most common parenchymal lung disease. Patients with IPF sometimes develop acute exacerbation (AE), which predicts a poor prognosis. To evaluate the predictors of 90-day mortality of AE in patients with IPF based on the new 2016 criteria.
Materials and Methods:
Sixty-five patients with AE were studied retrospectively between January 2001 and December 2016 at Okinawa Chubu Hospital.
Results:
The mean age of the patients was 74 years, with 40 (61.5%) men and 25 (38.5%) women. Among our cohort, 37 were current or ex-smokers, with a mean exposure of 32.4 pack-years. The mean grade of the modified Medical Research Council breathlessness scale was 2.8, and the mean duration of dyspnea prior to admission was 6.5 days. Clubbed fingernails were present in 29% of patients. Triggered AE occurred in 12 (18%) of patients. Patients with triggered AE had more extensive ground-glass opacity and higher consolidation scores than the idiopathic AE group (7.3 vs. 4.2, p=0.01). The triggered group had shorter survival than the idiopathic group (1.4 vs. 11.4 months, p=0.094). Serum lactate dehydrogenase (LDH), ΔLDH, and the ratio of partial pressure of oxygen to the fraction of inspiratory oxygen ratio were strong predictors of 90-day mortality. Hazard ratios were 1.003 (p=0.004), 1.004 (p=0.02), and 0.994 (p=0.010), respectively.
Conclusion:
Compared with idiopathic AE, triggered AE in patients with IPF had more extensive infiltration and tended toward shorter survival. Serial trends of serum LDH >2 weeks can help predict prognosis of AE in patients with IPF.
Keywords: Predictors, mortality, triggered, lactate dehydrogenase, partial pressure of oxygen/fraction of inspiratory oxygen ratio
Introduction
Idiopathic pulmonary fibrosis (IPF) is a relentless progressive parenchymal disease of unknown etiology [1]. Patients with IPF have variable clinical courses, from stable disease without significant symptoms to acute exacerbation (AE) [2, 3]. During AE in IPF, patients have acute progressive deterioration of their respiratory condition superimposed on a background of fibrosis [4, 5]. Significant decline in vital capacity (VC), smoking status, and reduced percent predicted forced vital capacity (%FVC) has been reported to be important risk factors for AE in IPF [6, 7]. We have previously described staging of AE in IPF [8]. The American Thoracic Society (ATS) released the new revised criteria last year, which categorized AE in patients with IPF as either triggered or idiopathic, depending upon whether a “trigger,” such as infection, drug toxicity, aspiration, surgery, or other procedural intervention, was felt to be present. Diagnosis of AE in IPF consists of first excluding alternative causes of acute respiratory deterioration and then determining whether the episode is associated with a trigger versus an idiopathic event [9]. The purpose of the current study is to evaluate the clinical characteristics and clinical course of the new categorization based on the revised criteria.
Material and Methods
Study subjects
This was a retrospective study. The study population consisted of all patients who presented with AE of IPF from January 2001 to December 2016 based on the 2016 ATS new criteria [9].
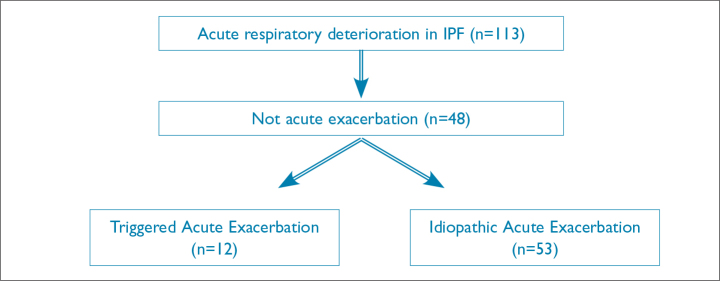
Patients were included if the 2016 criteria for AE were present, including acute respiratory deterioration for <1 month with new bilateral ground-glass opacity (GGO) and pulmonary consolidation on radiographs. Patients were excluded if diagnosed with simple bacterial pneumonia, pneumothorax, pulmonary embolism, or pleural effusion (Figure 1).
Figure 1.
Flow charr of AE of IPF patients at Okinawa Chubu Hospital
AE: acute exacerbation; IPF: Idiopathic pulmonary fibrosis
Diagnosis of IPF:
When surgical biopsies were performed, the typical pathological finding was a usual interstitial pneumonia (UIP) pattern. When a surgical biopsy was not performed, we made the diagnosis of IPF based on potential UIP patterns such as honeycombing, reticulation, traction bronchiectasis, and spatial heterogeneity, which are distinct from non-specific interstitial pneumonia.
Data collection
We identified 65 patients presenting with AE in IPF between January 1, 2001 and December 31, 2016 and reviewed their medical records retrospectively. Clinical parameters such as age, gender, dyspnea duration, modified Medical Research Council (mMRC) breathlessness scale, and presence or absence of clubbed fingernails were collected [10]. Duration of dyspnea was defined as the time between symptom onset and admission date. AE of IPF was defined as progressive dyspnea developing within the past 30 days, with new bilateral infiltrates in a patient with known IPF or evidence of honeycombing on chest high-resolution computed tomography (HRCT) [5]. We also collected inflammation markers such as white blood cell (WBC), C-reactive protein (CRP), and IPF activity markers such as lactate dehydrogenase (LDH) and Krebs von den Lungen-6 (KL-6) [11–13]. Serum KL-6 is measured by a latex agglutination method at our hospital. We also evaluated serial LDH, KL-6, and difference between admission and 2 weeks later, to evaluate the significance of trends in the levels of these IPF biomarkers. In addition, we reviewed pulmonary function tests mainly focusing on %FVC at baseline prior to admission [14, 15]. We checked the partial pressure of oxygen, partial pressure of carbon dioxide, and ratio of partial pressure of oxygen to the fraction of inspiratory oxygen (P/F) concentration.
New IPF categorization
We classified episodes of AE according to the ATS proposed new criteria, into triggered AE or idiopathic AE [9]. We excluded pneumothorax, pleural effusion, and pulmonary embolism by clinical history, chest X-ray, enhanced CT, and electrocardiogram. We further evaluated patients based on sputum or bronchoalveolar lavage fluid (BALF) culture. Sputum was sometimes obtained after induction with albuterol, delivered via a nebulizer. Purulent sputum samples were judged as good quality if a sputum culture showed three-plus (+++) organisms or ≥104 colony forming units for samples from BALF culture. If infection, post-procedure, post-operation, drug toxicity, or aspiration was diagnosed, the patient was classified as having triggered AE. If infection, medical procedures, surgery, and other underlying issues were excluded, then the patient was defined as having idiopathic AE.
Radiological findings
All patients underwent thin-section CT scans at end-inspiratory phase in the supine position, with images obtained at appropriate window settings for evaluation of lung parenchyma. We distinguished triggered AE from severe pneumonia by CT criteria, diagnosing pneumonia if there was local consolidation with peripheral reticular abnormality and idiopathic AE if there were no definite findings such as reticular abnormalities, traction bronchiectasis, or honeycombing. We evaluated the presence, distribution, and extent of baseline traction bronchiectasis, honeycombing, superimposed GGO and consolidation on admission [16, 17]. We set up a score for each finding.
The lungs were divided into six zones (upper, middle, and lower lung field of both sides). The upper lung fields were defined as the areas above the main carina, and the lower lung fields were defined as the areas between the inferior pulmonary vein and above the right hemidiaphragm. The middle lung fields were defined as all areas between the upper and lower lung fields [18]. Our chest physicians and radiologists reached a consensus on each radiograph with multidisciplinary discussions [19].
The extent of traction bronchiectasis, honeycombing, superimposed GGO, and consolidation was defined as follows: 0, none; 1, involving 1%–25%; 2, involving 26%–50%; and 3, involving >50%. The radiological finding scores of each zone were summed [18]. This calculated value was defined as total extension score. Therefore, the highest score was 18. We excluded uni-lateral new infiltrates in patients with IPF and categorized those as pneumonia in IPF.
Predictions of mortality
AE onset was defined as the date of hospital admission. AE in patients with IPF usually has a relentless clinical course, and long-term survival is not expected [20, 21]. Therefore, 3-month mortality is a reasonable primary outcome in daily practice. Three-month mortality was defined as being alive or dead within 3 months from onset of AE.
The Ethics Committee of Okinawa Chubu Hospital approved this study protocol (OCH 2017-12). Informed consent was waived for this retrospective study of deidentified data.
Statistical analysis
Continuous variables were presented as mean±SD, and categorical variables were presented as percentages. Unpaired t tests were used to compare continuous data between groups. Chi-squared tests and Fisher’s exact tests were used for categorical data. Cox regression analysis was used to identify significant predictors of 3-month mortality. The outcome was time to death, with all surviving patients censored at 90 days. We performed an initial univariate analysis to identify significant predictors of 3-month mortality with p<0.1. We then constructed multivariable models for the 3-month mortality outcome, including predictors in the model with p<0.05. All analyses were performed using STATA software version 11 (Stata Corporation, College Station, TX, USA).
Results
Clinical characteristics
We identified 65 patients with AE in IPF during the study period. The mean age of all patients was 74 years, with 40 (61.5%) men and 25 (38.5%) women. Among our cohort, 37 were current or ex-smokers with the mean exposure of 32.4 pack-years. Regarding clinical symptoms, the mean mMRC score was 2.8, and the mean duration of dyspnea prior to admission was 6.5 days. A total of 29% of patients had clubbing. The mean survival was 9.6 months (Table 1).
Table 1.
Clinical characteristics of patients with idiopathic pulmonary fibrosis with acute exacerbation
| Parameters | Mean±SD |
|---|---|
| Age (years) | 74.7±11.3 |
| Gender (male/female) | (40/25) |
| Pack-year (years) | 32.4±40.6 |
| mMRC | 2.8±1.0 |
| Dyspnea duration (days) | 6.5±5.8 |
| Clubbing (%) | 29 |
| Survival time (months) | 9.6±3.0 |
| Laboratory items | |
| WBC (mm3) | 11.369.2±5368.9 |
| CRP (mg/dl) | 8.2±7.3 |
| LDH (IU/l) | 392.2±144.6 |
| KL-6 (IU/l) | 1903.4±1720 |
| ΔLDH | −5.6±115.6 |
| ΔKL-6 | 288.4±698.8 |
| Pulmonary function | |
| Percent FVC (%) | 58.2±21.2 |
| Percent DLCO (%) | 32.2±11.6 |
| pH | 7.42±0.10 |
| PaO2 (mm Hg) | 62.6±17.5 |
| PaCO2 (mm Hg) | 43.8±15.9 |
| P/F ratio(value) | 159.1±91.0 |
| Imaging findings | |
| Traction bronchiectasis | 2.3±0.7 |
| Honeycombing | 1.7±0.6 |
| GGO+consolidation | 4.8±4.2 |
| Total extent | 9.5±6.8 |
mMRC: modified Medical Research Council; WBC: white blood cell; CRP: C-reactive protein; LDH: lactate dehydrogenase; KL-6: Krebs von den Lungen-6; FVC: forced vital capacity; PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide; P/F: ratio of partial pressure of oxygen to the fraction of inspiratory oxygen; GGO: ground-glass opacity
Laboratory findings
The mean serum WBC and CRP were 11,369 mm3 and 8.2 mg/dl. The mean interstitial lung disease (ILD) biomarkers such as LDH and KL-6 were 392 IU/l and 1903 IU/l, respectively. Dynamic data, such as mean ΔLDH and ΔKL-6, were −5.6 and 288, respectively (Table 1).
Physiologic measurements
The mean %FVC from pulmonary function testing was 58.2%. The initial arterial blood gas measurements included mean pH and the partial pressures of oxygen and carbon dioxide, which were 7.42, 62.6 mm Hg, and 43.8 mm Hg, respectively. In addition, the mean ratio between the partial pressure of oxygen to the inspired fraction of oxygen concentration at the time of admission was 159.1 (Table 1). A total of 34 of 65 patients (52%) required mechanical ventilation. We used high-flow nasal oxygen (HFNO) therapy for five of the most recent patients.
Radiological findings
In fibrotic area, the mean scores of traction bronchiectasis and honeycombing were 2.3 and 1.7, respectively. In superimposed area, the mean score of GGO and consolidation was 4.2. The mean overall score of total extent was 9.5 (Table 1).
Clinical characteristics by the new ATS criteria
The group with triggered AE had a trend toward higher mean pack-years (49.3 vs. 28.5 pack-years, p=0.06) and higher mMRC scores (3.2 vs. 2.7 points, p=0.08). WBC did not differ significantly between the triggered and the idiopathic group (11,008 mm3 vs. 11,450 mm3, p=0.6), nor did CRP (8.2 mg/dL vs. 8.2 mg/dL, p=0.5) or LDH (410 U/L vs. 389 U/L, p=0.3). KL-6 levels were significantly higher in the triggered group (2825 U/mL vs. 1700 U/mL, p=0.02). In addition, the triggered group tended to have shorter mean survival than the idiopathic group, although there was no significant statistical difference (1.4 months vs. 11.4 months, p=0.094; Table 2). Among 12 cultures from patients in the triggered group, four were Pseudomonas aeruginosa, three were Haemophilus influenzae, two were Stenotrophomonas maltophilia, one was Streptococcus pneumoniae, one was Haemophilus haemolyticus, and one was Mycobacterium tuberculosis. Nine cultures were obtained high-quality sputum, and three cultures were from BALF
Table 2.
Clinical characteristics of patients with idiopathic pulmonary fibrosis with acute exacerbation according to the ATS criteria
| Parameters | Triggered (n=12) Mean±SD | Idiopathic (n=53) Mean±SD | p |
|---|---|---|---|
| Age (years) | 77.1±8.4 | 74.2±11.8 | 0.214 |
| Gender (male/female) | 8/4 | 32/21 | 0.654 |
| Pack-year (years) | 49.3±51.3 | 28.5±37.3 | 0.056 |
| mMRC | 3.2±1.1 | 2.7±0.9 | 0.075 |
| Dyspnea duration (days) | 4.4±3.7 | 7.0±6.1 | 0.918 |
| Clubbing (%) | 42 | 26 | 0.151 |
| WBC (mm3) | 11,008±5116 | 11.450±5468 | 0.601 |
| CRP (mg/dL) | 8.2±6.5 | 8.2±7.5 | 0.504 |
| LDH (IU/l) | 410±153 | 389±144 | 0.332 |
| KL-6 (IU/l) | 2825±2354 | 1700±1502 | 0.024 |
| Survival time (months) | 1.4±0.4 | 11.4±3.6 | 0.094 |
| Imaging findings | |||
| Traction bronchiectasis | 2.3±0.7 | 2.3±0.1 | 0.395 |
| Honeycombing | 1.4±0.5 | 1.7±0.7 | 0.858 |
| GGO+consolidation | 7.3±6.7 | 4.2±3.3 | 0.010 |
| Total extent | 11.1±10.7 | 8.5±5.2 | 0.005 |
mMRC: modified Medical Research Council; WBC: white blood cell; CRP: C-reactive protein; LDH: lactate dehydrogenase; KL-6: Krebs von den Lungen-6; ATS: American Thoracic Society; GGO: ground-glass opacity
Radiological findings in the triggered group showed more extensive GGO and consolidation (7.3 vs. 4.2, p=0.01). In addition, the total extent of abnormal findings, including traction bronchiectasis and honeycombing, was more extensive in the triggered group (11.1 vs. 8.5%, p=0.005, Table 2).
Univariate analysis of predictors of 90-day mortality
Serum LDH, P/F ratio, ΔLDH, and ΔKL-6 were significant predictors associated with 90-day mortality (p<0.1) (Table 3).
Table 3.
Univariate and multivariable associations of predictors with 90-day mortality
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p | HR (95% CI) | p | |
| New criteria | 2.275 (0.874, 5.926) | 0.009 | 0.618 (0.312, 1.227) | 0.169 |
| LDH | 1.002 (1.000, 1.005) | 0.002 | 1.003 (1.001, 1.005) | 0.004 |
| ΔLDH | 1.009 (1.002, 1.016) | 0.013 | 1.004 (1.001, 1.008) | 0.017 |
| ΔKL-6 | 1.001 (1.000, 1.002) | 0.023 | 1.000 (0.999, 1.001) | 0.197 |
| P/F ratio | 1.001 (0.520, 1.728) | 0.080 | 0.994 (0.990, 0.999) | 0.010 |
HR: hazard ratio; LDH: lactate dehydrogenase; KL-6: Krebs von den Lungen-6; P/F: ratio of partial pressure of oxygen to the fraction of inspiratory oxygen
Multivariate analysis of predictors of 90-day mortality
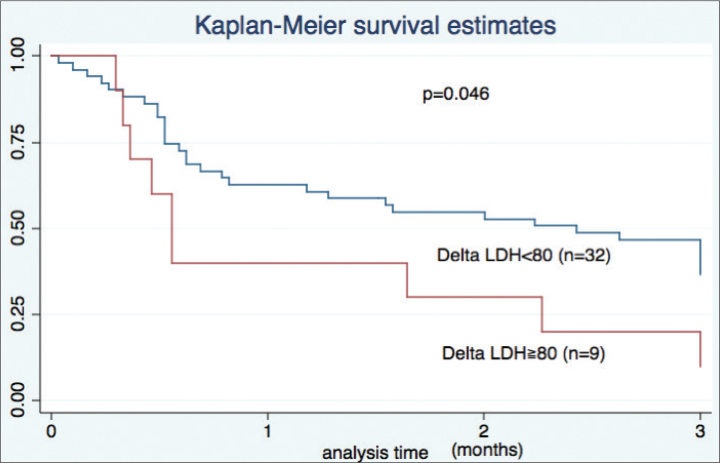
We observed significant associations with 90-day mortality for serum LDH (hazard ratio (HR) 1.003, p=0.004), ΔLDH (HR 1.004, p=0.02), and P/F ratio (HR 0.99, p=0.01) (Table 3). A receiver operating characteristic curve identified an increase of 80 IU/L as the optimal value to discriminate between survival and death, with ΔLDH >80 IU/L within 2 weeks associated with a poor prognosis (0.6 months vs. 1.5 months, p=0.05, Figure 2).
Figure 2.
Kaplan-Meier survival curve based on delta LDH
LDH: lactate dehydrogenase
Discussion
In this report, we showed that patients with IPF with triggered AE had more extensive fibrosis than those with idiopathic IPF and demonstrated the prognostic utility of the trend of the serum LDH levels. Based on the new diagnostic framework, our cohort had 12 patients with triggered AE, all of which were infection-related. We observed no cases of triggered AE due to drug toxicity, aspiration, surgery, or other invasive procedures [9].
Overall, 65 patients were elderly, and the mean dyspnea duration was 6.5 days. Therefore, our cohort tended to develop AE more acutely than previous reports [21, 22]. Among laboratory findings, KL-6 was more strongly associated with death than LDH in the triggered group. Patients with triggered AE might have strong expression of KL-6 in their bronchiolar epithelial cells due to local bacterial stimulus. Our patients had moderate restrictive disorders and severe respiratory failure and acute respiratory distress syndrome [22–24].Over half of our cohort required mechanical ventilation, yet survival was poor. Thus, the use of invasive ventilation in patients with IPF with AE is an important ethical issue [25, 26]. Recently, HFNO has become available in daily practice and has proven useful for management of AE among patients with IPF [27]. Five of our patients received HFNO, which is less invasive and more comfortable for the majority of patients [28].
Among patients with IPF with AE, those with triggered AE had more extensive GGO and consolidation. We compared the combined extent of both GGO and consolidation. In addition, the total areas of new infiltrates and fibrotic areas, such as traction bronchiectasis and honeycombing, were more extensive in the triggered group. Our results suggest that infectious processes were associated with the superimposed new infiltrates and contributed to widespread radiographic abnormalities.
We did not observe a significant difference in survival between the two groups, which may be partly explained by small number of patients with triggered AE. Papiris et al. [29] reported that a prior history of immunosuppression adversely influenced survival during AE. Song et al. [7] reported that age, reduced FVC, decreased DLCO, and corticosteroid exposure were significant predictors of poor survival. Our multivariable analysis identified three useful markers with significant associations with 90-day survival: LDH, ΔLDH, and P/F ratio. Ninety-day mortality is a reasonable threshold for AE of IPF because the short-term prognosis is so poor. This allowed us to evaluate the 2-week trends of candidate biomarkers. LDH is a classic biomarker for the activity of ILDs and is more sensitive in the short term. In our cohort, ΔLDH is a useful predictor of 90-day mortality compared with ΔKL-6. Based on our study result, an increase >80 IU/l of serum LDH within 2 weeks was associated with a poor prognosis at 3 months. KL-6 was associated with severity of IPF in our cohort, as in prior studies [30]. However, ΔKL-6 could not predict 90-day mortality in our study. KL-6 has a high-molecular weight, and elevation of KL-6 may be delayed and lag behind clinical activity of ILD in daily practice [31]. Therefore, ΔKL-6 may not reflect disease activity with timely fashion.
There are several limitations that should be acknowledged. First, this was a retrospective single-center study. Second, small sample size is an important limitation. Third, only five patients underwent surgical lung biopsy for definite diagnosis. Thus, our result cannot necessarily be generalized to the entire population of patients with IPF with AE. However, only 10% to 20% of patients with IPF typically undergo surgical lung biopsy, so the experience of our cohort is consistent with most IPF populations. Fourth, distinguishing AE of IPF from the bilateral severe pneumonia background of IPF may be difficult. We handled this uncertainty by strictly defining triggered AE in IPF, based on the new ATS criteria and performed comprehensive evaluations including detailed clinical history, BALF or sputum culture, and chest HRCT findings. Thus, our results are robust.
In conclusion, patients with IPF with triggered AE had more extensive infiltrates and had decreased short-term survival. Observing the serial trend of serum LDH over the first 2 weeks can predict future prognosis in patients with IPF with AE. Further studies, in multiple centers, are warranted to validate the observed associations.
Acknowledgments
We thank Dr. Rita McGill for statistical advice and English editing.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Okinawa Chubu Hospital (OCH 2017-12).
Informed Consent: Written informed consent was not recieved due to the retrospective natüre of the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.K.; Design - T.K., Y.N.; Supervision - T.K.; Data Collection and/or Processing -Y.N., M.M., H.N.; Analysis and/or Interpretation - T.K.; Literature Search - T.K., Y.N.; Writing Manuscript T.K., Y.N., S.Y.; Critical Review T.K., Y.N., S.Y.
Conflict of Interest: No conflict of interest was declared by the authors
Financial Disclosure: The authors declare that this study has received no financial support.
References
- 1.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. https://doi.org/10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–92. doi: 10.1513/pats.200601-005TK. https://doi.org/10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431–40. doi: 10.1164/rccm.201006-0894CI. https://doi.org/10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 4.Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest. 1993;103:1808–12. doi: 10.1378/chest.103.6.1808. https://doi.org/10.1378/chest.103.6.1808. [DOI] [PubMed] [Google Scholar]
- 5.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–43. doi: 10.1164/rccm.200703-463PP. https://doi.org/10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:103–10. https://doi.org/10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A1107. [PubMed] [Google Scholar]
- 7.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356–63. doi: 10.1183/09031936.00159709. https://doi.org/10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 8.Kishaba T, Tamaki H, Shimaoka Y, Fukuyama H, Yamashiro S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung. 2014;192:141–9. doi: 10.1007/s00408-013-9530-0. https://doi.org/10.1007/s00408-013-9530-0. [DOI] [PubMed] [Google Scholar]
- 9.Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An international working group report. Am J Respir Crit Care Med. 2016;194:265–75. doi: 10.1164/rccm.201604-0801CI. https://doi.org/10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 10.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6. doi: 10.1136/thx.54.7.581. https://doi.org/10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa N, Hattori N, Yokoyama A, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50:3–13. doi: 10.1016/j.resinv.2012.02.001. https://doi.org/10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Ohshimo S, Ishikawa N, Horimasu Y, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med. 2014;108:1031–9. doi: 10.1016/j.rmed.2014.04.009. https://doi.org/10.1016/j.rmed.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Hamai K, Iwamoto H, Ishikawa N, et al. Comparative study of circulating MMP-7, CCL18, KL-6, SP-A, and SP-D as disease markers of idiopathic pulmonary fibrosis. Dis Markers. 2016;2016:4759040. doi: 10.1155/2016/4759040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghu G, Wells AU, Nicholson AG, et al. Effect of Nintedanib in Subgroups of Idiopathic Pulmonary Fibrosis by Diagnostic Criteria. Am J Respir Crit Care Med. 2017;195:78–85. doi: 10.1164/rccm.201602-0402OC. https://doi.org/10.1164/rccm.201602-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taniguchi H, Xu Z, Azuma A, et al. Subgroup analysis of Asian patients in the INPULSIS® trials of nintedanib in idiopathic pulmonary fibrosis. Respirology. 2016;21:1425–30. doi: 10.1111/resp.12852. https://doi.org/10.1111/resp.12852. [DOI] [PubMed] [Google Scholar]
- 16.Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. Am J Roentgenol. 1997;168:79–83. doi: 10.2214/ajr.168.1.8976924. https://doi.org/10.2214/ajr.168.1.8976924. [DOI] [PubMed] [Google Scholar]
- 17.Akira M, Sakatani M, Ueda E. Idiopathic pulmonary fibrosis: progression of honeycombing at thin-section CT. Radiology. 1993;189:687–91. doi: 10.1148/radiology.189.3.8080483. https://doi.org/10.1148/radiology.189.3.8080483. [DOI] [PubMed] [Google Scholar]
- 18.Sumikawa H, Johkoh T, Colby TV, et al. Computed tomography findings in pathological usual interstitial pneumonia. Relationship to survival. Am J Respir Crit Care Med. 2008;177:433–9. doi: 10.1164/rccm.200611-1696OC. https://doi.org/10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 19.Raghu G, Collard HR, Egan JJ, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement. Idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. https://doi.org/10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryerson CJ, Cottin V, Brown KK, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur Respir J. 2015;46:512–20. doi: 10.1183/13993003.00419-2015. https://doi.org/10.1183/13993003.00419-2015. [DOI] [PubMed] [Google Scholar]
- 21.Atkins CP, Loke YK, Wilson AM. Outcomes in idiopathic pulmonary fibrosis: a meta-analysis from placebo controlled trials. Respir Med. 2014;108:376–87. doi: 10.1016/j.rmed.2013.11.007. https://doi.org/10.1016/j.rmed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 22.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33:2228–34. doi: 10.1097/01.ccm.0000181529.08630.49. https://doi.org/10.1097/01.CCM.0000181529.08630.49. [DOI] [PubMed] [Google Scholar]
- 24.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–40. doi: 10.1172/JCI60331. https://doi.org/10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern J-B, Mal H, Groussard O, et al. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest. 2001;120:213–9. doi: 10.1378/chest.120.1.213. https://doi.org/10.1378/chest.120.1.213. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Perez ER, Yilmaz M, Jenad H, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133:1113–9. doi: 10.1378/chest.07-1481. https://doi.org/10.1378/chest.07-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiruvoipati R, Lewis D, Haji K, Botha J. High-flow nasal oxygen vs. high-flow face mask: a randomized crossover trial in extubated patients. J Crit Care. 2010;25:463–8. doi: 10.1016/j.jcrc.2009.06.050. https://doi.org/10.1016/j.jcrc.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 28.Spoletini G, Alotaibi M, Blasi F, Hill NS. Heated humidified high-flow nasal oxygen in adults: Mechanisms of action and clinical implications. Chest. 2015;148:253–61. doi: 10.1378/chest.14-2871. https://doi.org/10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- 29.Papiris SA, Kagouridis K, Kolilekas L, et al. Survival in Idiopathic pulmonary fibrosis acute exacerbations: the non-steroid approach. BMC Pulm Med. 2015;15:162. doi: 10.1186/s12890-015-0146-4. https://doi.org/10.1186/s12890-015-0146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kishaba T. Practical management of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:90–8. [PubMed] [Google Scholar]
- 31.Ohnishi H, Yokoyama A, Kondo K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165:378–81. doi: 10.1164/ajrccm.165.3.2107134. https://doi.org/10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]