Abstract
The mammalian organ of Corti of the inner ear is a highly sophisticated sensory end organ responsible for detecting sound. Noggin is a secreted glycoprotein, which antagonizes bone morphogenetic proteins 2 and 4 (Bmp2 and Bmp4). The lack of this antagonist causes increased rows of inner and outer hair cells in the organ of Corti. In mice, Bmp2 is expressed transiently in nascent cochlear hair cells. To investigate whether Noggin normally modulates the levels of Bmp2 for hair cell formation, we deleted Bmp2 in the cochlear hair cells using two cre strains, Foxg1cre/+ and Gfi1cre/+. Bmp2 conditional knockout cochleae generated using these two cre strains show normal hair cells. Furthermore, Gfi1cre/+;Bmp2lox/− mice are viable and have largely normal hearing. The combined results of Noggin and Bmp2 mutants suggest that Noggin is likely to regulate other Bmps in the cochlea such as Bmp4.
Keywords: Noggin, Bmp2, Foxg1-cre, Gfi1-cre, hair cell, cochlea, mouse, hearing
INTRODUCTION
The organ of Corti of the mammalian cochlear duct consists of one row of inner hair cells (IHCs), three rows of outer hair cells (OHCs), and several specialized types of supporting cells such as Deiters’ and pillar cells. Cells within the prosensory domain that give rise to the organ of Corti, start to exit from the cell cycle and express p27Kip1 at embryonic day 12.5 (E12.5; Chen and Segil, 1999). Proper differentiation of the various cell types within the organ of Corti requires diverse genetic pathways and interactions (Kelley, 2007; Doetzlhofer et al., 2009).
Bone morphogenetic proteins (Bmps) are diffusible molecules, which play important roles in the formation of multiple organs during embryogenesis such as limb, heart, kidney, brain, and eye (Dudley et al., 1995; Furuta et al., 1997; Delot et al., 2003; Bandyopadhyay et al., 2006). Bmps mediate cellular changes by binding to specific Bmp receptors, which lead to phosphorylation of cyotosolic Smad proteins. Phosphorylated Smads, in turn, form specific complexes and translocate to the nucleus to activate or repress transcription of Bmp target genes (von Bubnoff and Cho, 2001). Extracellularly, Bmp signaling pathways can be negatively regulated by antagonists such as Noggin and Chordin (Balemans and Van Hul, 2002). Both Noggin and Chordin are thought to interfere with Bmp signaling by directly binding Bmps and preventing their interactions with Bmp receptors (Holley et al., 1996, Piccolo et al., 1996). In fact, Noggin knockout (Nog−/−) mouse embryos are often used as an animal model for studying the role of Bmp functions in a gain-of-function context (Brunet et al., 1998; Choi et al., 2007; Davis and Camper, 2007).
Using Nog−/− mouse embryos, we showed that proper levels of Bmps are required for the formation of the stapes in the middle ear and cochlear duct in the inner ear (Bok et al., 2007; Hwang and Wu, 2008). Nog expression is not detected in the otic cup and otocyst stages, and the early defects of the mis-shapen cochlear duct in Nog−/− embryos is attributed to a misalignment of the hindbrain and inner ear primordia (Bok et al., 2007). Nevertheless, Nog expression is detected later in the developing cochlear duct starting at mid-gestation and its expression domain is associated with other Bmps (Bok et al., 2007). Here, we show that patterning of the organ of Corti within the Nog−/− cochlear duct is disrupted with an increase in the rows of IHC and OHC. While this hair cell phenotype in Nog−/− cochleae could be due to mis-regulation of multiple Bmps, we focused our attention on Bmp2, which we show to be transiently expressed in nascent cochlear hair cells. To address whether Bmp2 could be regulating hair cell numbers in the organ of Corti, we conditionally deleted Bmp2 expression in the mouse cochlear duct using Foxg1cre/+ and Growth Factor Independent-1-cre (Gfi1cre/+) strains. Our results indicate that Bmp2 is not required for proper hair cell formation and is unlikely to affect normal hearing in the mouse inner ear.
RESULTS
Increased Hair Cells in the Cochlea of Nog−/− Embryos
At E18.5, the length of Nog−/− cochleae was significantly shorter than cochleae from heterozygotes (Fig. 1A,B). While Nog+/− cochleae showed an organized single row of IHCs and three rows of OHCs along the entire duct (Fig. 1C,E,G), Nog−/− cochleae showed occasional extra IHCs at the base (Fig. 1D, arrowhead), and two rows of IHCs and four rows of OHCs in the middle and apical turns (Fig. 1F,H). There was no clear difference in hair bundle orientation between mutants and heterozygotes.
Fig. 1.
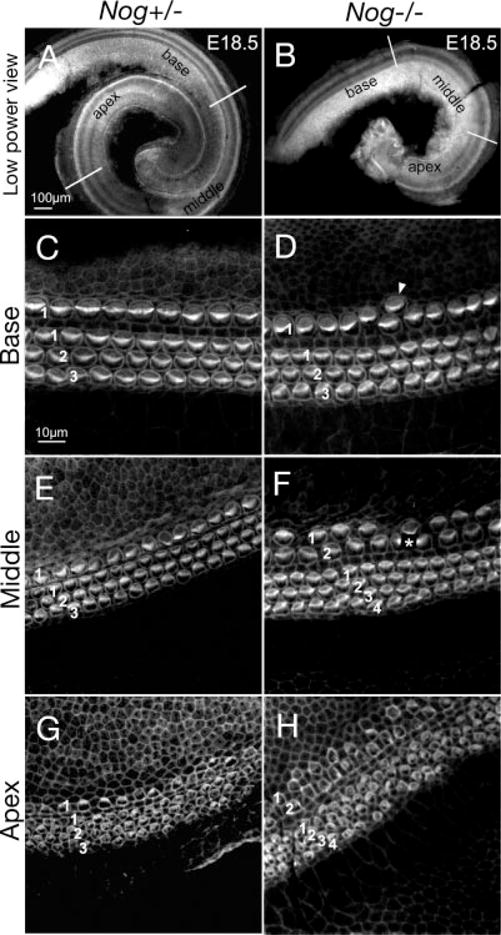
Increased inner and outer hair cells in Nog−/− cochlea. A–H: Phalloidin-labeled Nog+/− (A,C,E,G) and Nog−/− (B,D,F,H) cochleae at embryonic day (E) 18.5. A,B: Nog+/− cochlea shows one and three-fourth turns (A), whereas Nog−/− cochlea shows only one turn (B). Representative images from the base, middle, and apical regions are shown for Nog+/− (C,E,G) and Nog−/− cochleae (D,F,H). C,E,G: A Nog+/− cochlea showing the typical one row of inner hair cells (IHC; 1) and three rows of outer hair cells (OHC; 1,2,3), which are less organized at the apex. D,F,H: Nog−/− cochlea showing occasional extra IHC at the base (D) and two rows of IHC (1,2) and four rows of OHC (1,2,3,4) in the middle and apical turns (F,H). Asterisk in F indicates damaged hair cells. Scale bar in A applies to B. Scale bar in C applies to D–H.
Cochlear lengths and hair cell numbers in each turn were quantified and compared (see the Experimental Procedures section). No obvious difference between wild-type and heterozyotes (Supp. Fig. S1, which is available online) was observed and they were grouped together as controls for quantification. Nog−/− cochleae showed a 35% reduction in length, compared with controls (Fig. 2A; knockout mice [KO], 2932.8 ± 41.6 μm, n = 10; wild-type and heterozygotes [WT + Het], 4493.9 ± 94.7 μm, n = 7; P < 0.001). In the mutant cochlea, there was a significant increase in inner hair cell numbers in a given region, which was more pronounced in middle and apical turns (Fig. 2B, single asterisk; P < 0.05, double asterisks; P < 0.001). The OHC numbers were found to increase significantly throughout each turn of the cochlea as well (Fig. 2C). Despite the increase in hair cell counts in individual turns of the Nog−/− organ of Corti, the total estimated hair cell number based on the entire length of cochlear duct compared with wild-type and heterozygotes showed a 11% and 24% decrease in IHC and OHC, respectively (KO, IHC 572.7 ± 16.8; OHC 1587.2 ± 52.0; n = 7; WT + Het, IHC 642.6 ± 16.8, OHC 2085.7 ± 47.3; n = 6). Although these results indicate that the length of the mutant cochlear duct affected the total hair cell number, a shortened cochlear duct could not easily account for the lower ratios of outer versus inner hair cell number observed in the middle and apical turns (Fig. 2D). Therefore, it is likely that both longitudinal and radial patterning of the organ of Corti in Nog−/− cochleae are affected.
Fig. 2.
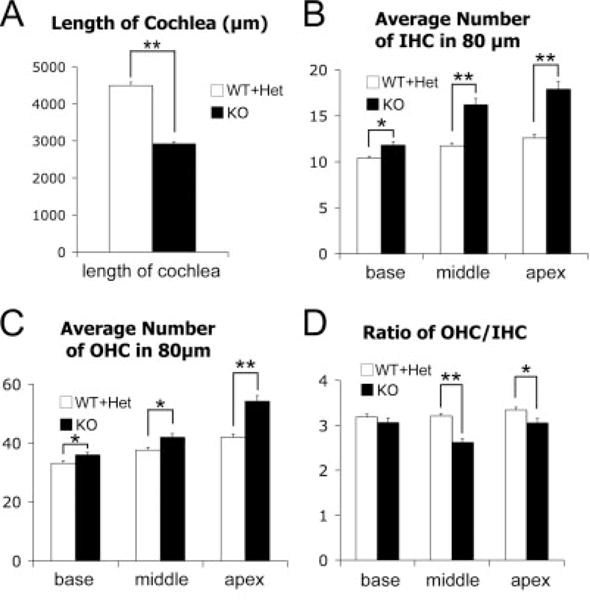
Quantification of the length and hair cell number in Nog−/− cochleae. A: Reduced cochlear lengths in Nog−/− cochleae (WT+Het, n = 7; KO, n = 10). B: Comparisons of the average IHC numbers from the base, middle, and apex of the cochlea between controls and mutants. The average IHC numbers are significantly increased throughout the Nog−/− cochlea (WT + Het, n = 6; KO, n = 12). C: Comparison of the average OHC numbers in each turn. Statistically significant increase of the average OHC number is also observed throughout the Nog−/− cochlea (WT+Het, n = 6; KO, n = 12). D: Comparison of the ratio of the average outer hair cell versus the average inner hair cell number. The ratio is significantly decreased in middle and apical turns, suggesting that IHCs are preferentially affected in middle and apical turns of Nog−/− cochlea (WT + Het n = 6, KO n = 12). Each error bar represents standard error of the mean. *P < 0.05, **P< 0.001. OHC, outer hair cell; IHC, inner hair cell; KO, knockout.
Normal Expression Patterns of Bmps in the Developing Cochlea
In other tissues where Noggin has been shown to modulate the levels of Bmps, its expression domain is intimately associated with those of Bmps (Brunet et al., 1998; Warren et al., 2003; Zhou et al., 2006). Similar relationships were observed in the developing cochlear duct (Morsli et al., 1998; Bok et al., 2007). At E16.5, Bmp4 expression is juxtaposed to the abneural side of the organ of Corti, a region presumed to give rise to Hensen’s and Claudius’ cells (Fig. 3A; Morsli et al., 1998; Bok et al., 2007). The expression of Nog overlaps partially with the Bmp4 expression domain (Fig. 3G; Bok et al., 2007). Bmp7 expression in the cochlear duct appears broad, but its strongest expression domain also colocalized in the Bmp4-positive region (Fig. 3C). In contrast, Bmp2 expression does not overlap with that of Bmp4 but coincides within the sensory region of the cochlea (Figs. 3E, 4A′; bracket), which is positive for Lunatic fringe (Lfng; Fig. 4A). Comparing adjacent sections probed for Myosin XVa (Myo15a) transcripts, which are localized in sensory hair cells, Bmp2 transcripts seem to be detected only in OHC and not IHC (Fig. 4A′, A″). Bmp2 expression in the hair cells appears transient, starting at E15.5 and is only detectable in the apical cochlear region by postnatal day 1 (data not shown). Nevertheless, the timing of this expression is at a critical developmental period for organ of Corti patterning (Shim et al., 2005; Puligilla et al., 2007).
Fig. 3.
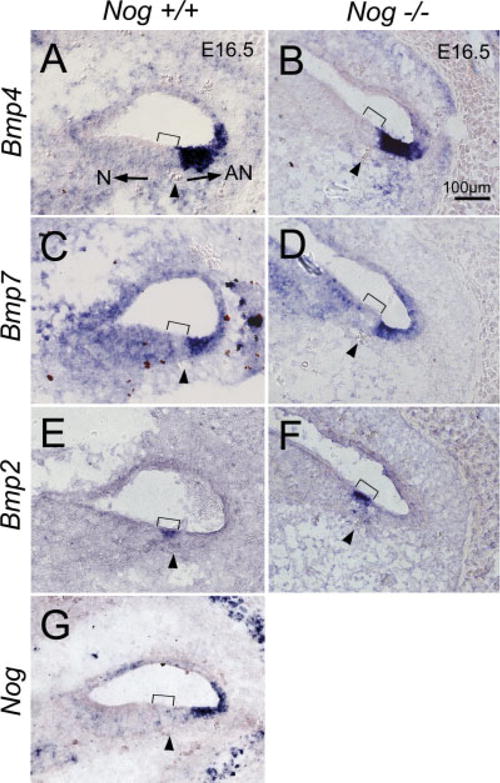
Expression patterns of Bmps and Nog in wild-type and Nog−/− cochleae at embryonic day (E) 16.5. A: Bmp4 is strongly expressed in the abneural region of wild-type cochlea. C: Bmp7 expression is broad but its strongest expression domain overlaps with the Bmp4-positive region. E: Bmp2 is expressed in the sensory region of the cochlea, not overlapping with the Bmp4-positive region. G: The expression domain of Nog partially overlaps with Bmp4 expression. B,D,F: There is no noticeable change in expression patterns of Bmp4, Bmp7, and Bmp2 in Nog−/− cochleae, respectively. Arrowhead indicates the spiral blood vessel beneath the prosensory domain (bracket). Arrows in A point toward neural (N) and abneural (AN) sides of the organ of Corti. Scale bar in B applies to A–G.
Fig. 4.
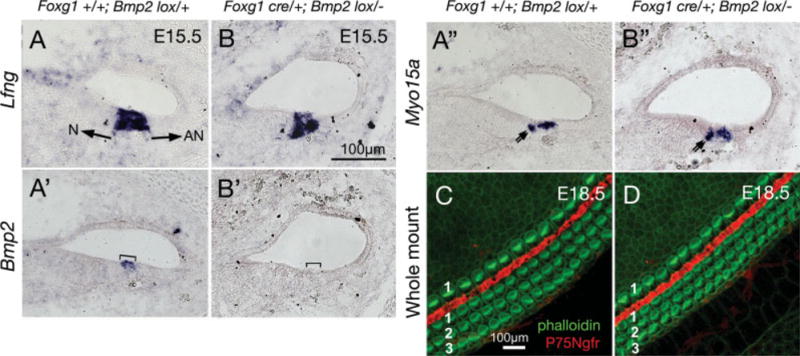
Targeted deletion of Bmp2 using Foxg1cre/+ mice. A–A″: Serial sections of a Foxg1+/+;Bmp2lox/+ cochlea at embryonic day (E) 15.5 showing Lfng expression in the organ of Corti (A), within which Bmp2 transcripts are detected in outer hair cell (OHC; A′, bracket), and Myo15a transcripts are detected in both OHC and inner hair cell (IHC; A″). B–B″: Serial sections of a Foxg1cre/+Bmp2lox/− cochlea at E15.5. Lfng and Myo15a expression patterns in the organ of Corti are similar to Foxg1+/+;Bmp2lox/+ cochlea, whereas Bmp2 expression is not detected (B′, bracket). Double arrows points to Myo15a-positive IHC in (A″) and (B″). Arrows in (A) point toward neural (N) and abneural (AN) sides of the organ of Corti. C,D: Phalloidin-labeled Foxg1+/+;Bmp2lox/+ (C) and Foxg1cre/+;Bmp2lox/− (D) cochleae at E18.5. Scale bar in B applies to A–A″,B′–B″, and C applies to D.
Targeted Deletion of Bmp2 Using Foxg1cre/+ Mice
Noggin could be functioning to regulate the levels of any one of the aforementioned Bmps in the cochlea, even though there was no obvious change in the expression patterns of Bmp2, Bmp4, and Bmp7 in Nog−/− cochleae (Fig. 3B,D,F). Because Bmps are specifically expressed in cochlear and not vestibular hair cells in both chicken and mouse (Oh et al., 1996; Morsli et al., 1998), we focused our attention on the role of Bmp2 in cochlear hair cell formation. We generated Bmp2 conditional knockout embryos using Foxg1cre/+ mice (Hebert and McConnell, 2000). In these mutant cochleae (Foxg1cre/+Bmp2lox/−), Bmp2 transcripts were not detectable, indicating that Bmp2 was successfully deleted (Fig. 4B′). Yet, the organ of Corti as well as the sensory hair cells within appear normal, based on the expression patterns of Lfng and Myo15a, respectively (Fig. 4B,B″). Phalloidin labeling of the whole-mount cochlea showed no obvious abnormality in Foxg1cre/+Bmp2lox/− mutants compared with controls (Fig. 4C,D), indicating that Bmp2 is not required for hair cell formation. Because Foxg1cre/+Bmp2lox/− mice die at birth, we used a different Bmp2 conditional mutant to evaluate the hair cell function in the absence of Bmp2.
Targeted Deletion of Bmp2 Expressed in Outer Hair Cells Using Gfi1cre/+ Mice
To evaluate the role of Bmp2 in hair cell function, we generated a more specific targeted deletion of Bmp2 using Gfi1cre/+ mice, in which cre is driven by the promoter of Gfi1. Gfi1 is expressed in hair cells in developing mouse inner ear starting from E14.5 (Wallis et al., 2003). Gfi1 is thought to function downstream of Math1 in sensory hair cells and contribute to the maintenance of Math1 function (Wallis et al., 2003; Hertzano et al., 2004). Our gene expression study showed that Gfi1 is strongly expressed in cochlear hair cells at E15.5 (data not shown). In Gfi1cre/+;Bmp2lox/− cochleae, Bmp2 expression was not detectable in sensory hair cells (Fig. 5B; bracket), which were Myo15a-positive (Fig. 5B′; bracket). Whole-mount cochlear preparations also showed no change in hair cell formation and patterning, confirming the results of Foxg1cre/+Bmp2lox/− embryos that Bmp2 is not required for hair cell formation (Fig. 5D).
Fig. 5.
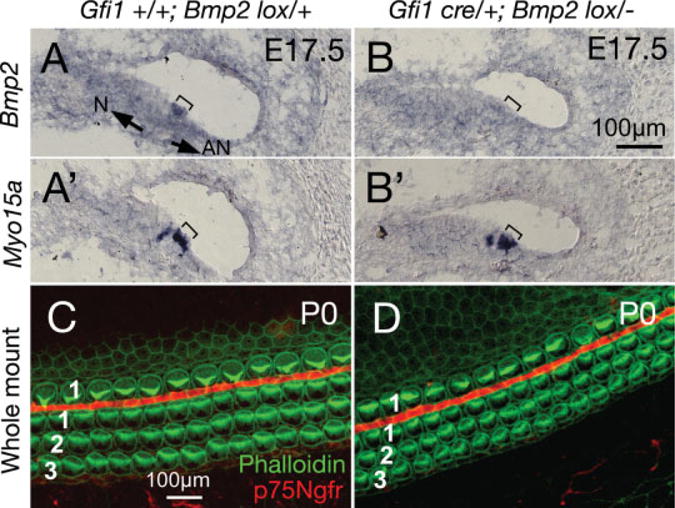
Targeted deletion of Bmp2 using Gfi1cre/+ mice. A–A′: Serial sections of a Gfi1+/+;Bmp2lox/+ cochlea at embryonic day (E) 17.5, showing Bmp2 expression overlapping with Myo15a in outer hair cell (OHC; bracket). B–B′: Serial sections of a Gfi1cre/+;Bmp2lox/− cochlea at E17.5 showing the absence of Bmp2 expression in OHC (compare bracketed regions). C,D: Whole-mounts of Gfi1+/+;Bmp2lox/+ (C) and Gfi1cre/+;Bmp2lox/− (D) cochleae at postnatal day (P) 0, showing the three rows of OHC and one row of inner hair cell (IHC; phalloidin labeling in green), separated by pillar cells (p75Ngfr staining in red). No difference in hair cell formation and organization are detected in Gfi1cre/+;Bmp2lox/− cochlea. Scale bar in B applies to A–A′,B′, and C applies to D. Arrows in A point toward neural (N) and abneural (AN) sides of the organ of Corti.
Hearing and Presbycusis of Gfi1cre/+;Bmp2lox/− Mice
Auditory brainstem response (ABR) measurements at click, 8 kHz, and 16 kHz were conducted on heterozygous and Gfi1cre/+;Bmp2lox/− mice at 3–6 months of age and they showed comparable hearing thresholds (Fig. 6A). Although the observed 6.5 dB threshold shift at 16 kHz is statistically significant, it is not clear if this minimal threshold shift noticeably compromises hearing because a 20-dB threshold shift is considered only a mild hearing loss (Zheng et al., 1999). To investigate the effect of lack of Bmp2 on hearing loss due to aging (presbycusis), ABR was measured at 9–10 months of age. At this age, heterozygous littermates started to show significant age-related hearing loss (Fig. 6B; P < 0.05), yet the hearing thresholds of Gfi1cre/+;Bmp2lox/− mice remained comparable to heterozygous littermates (Fig. 6B). These results suggest that Bmp2 is not required for proper hair cell function.
Fig. 6.
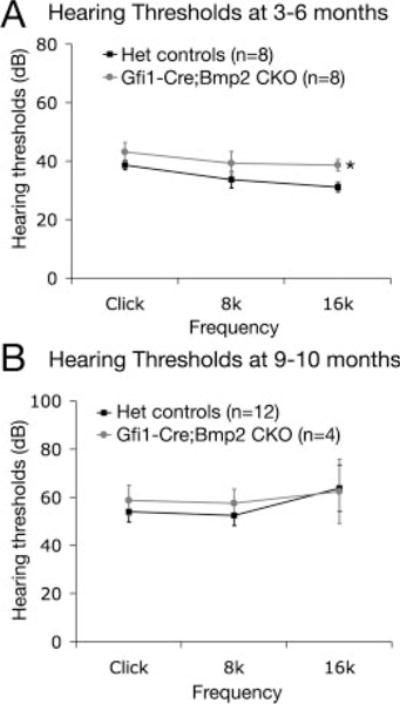
Hearing and presbycusis of Gfi1cre/+;Bmp2lox/− mice. The hearing thresholds at click, 8 kHz, 16 kHz are compared between heterozygotes (Gfi1cre/+;Bmp2lox/+ or Gfi1+/+;Bmp2lox/−) and Gfi1cre/+;Bmp2lox/− mice at 3–6 months and 9–10 months of age. Asterisk indicates a 6.5 dB threshold shift in Gfi1cre/+;Bmp2lox/− mice compared with heterozygotes at 16 kHz (P < 0.05). Both heterozygotes and conditional mutant mice start to show age-related hearing loss at 9–10 month (P < 0.05) but there is no significant difference in threshold shift between heterozygotes and Gfi1cre/+;Bmp2lox/− mice at the ages measured (P > 0.05).
DISCUSSION
Role of Noggin in the Growth of the Cochlear Duct and Hair Bundle Orientation
The anlagen of the cochlear duct in mice emerges from the ventral otocyst around E10.75 (Morsli et al., 1998). As the duct elongates, it starts to coil forming half a turn by E13.5 and reaches its final shape of one and three-quarter turns by E16.5 (Morsli et al., 1998). The elongation of the cochlear duct and patterning of the organ of Corti are thought to be mediated by the process of convergent extension whereby the cochlear duct lengthens along its longitudinal axis and at the same time narrows along the radial axis (Keller, 2002; McKenzie et al., 2004; Wang et al., 2005; Yamamoto et al., 2009). Thus, the prosensory domain begins as an epithelium of several cells thick, which thins to a layer of two cells (Wang et al., 2005). Genes involved in establishing planar cell polarity such as Vangl2, Scrb1, Dvl, and Wnt5 are important in dictating cochlear duct elongation and hair cell alignment (Montcouquiol and Kelley, 2003; Wang et al., 2005; Qian et al., 2007; Simons and Mlodzik, 2008). Lack of these gene products results in a similar phenotype of shortened cochlear duct with increased hair cell numbers in the apical region, as well as disrupted hair cell arrangement along the entire duct (Montcouquiol et al., 2003).
Cochlear ducts from Nog−/− mutants are shortened and misshapen. However, the planar cell polarity pathway does not appear to be affected in these mutants based on the relatively normal hair cell alignment in the organ of Corti, compared with Nog+/− cochlea (Fig. 1). In fact, the shortened cochlear duct in these mutants is thought to be indirect, due to misalignment of the rhombomeres and otocysts at an earlier stage of development (Bok et al., 2007). This misalignment presumably affects signaling molecules from the hindbrain, which confer the body plan to the inner ear and guide the proper outgrowth and patterning of the cochlear duct. Examples of other genes that are required for proper cochlear duct outgrowth but appear to be independent of the planar cell polarity pathway include Gli3 and Lmx1a (Driver et al., 2008; Koo et al., 2009).
Noggin Regulates Hair Cell Numbers in the Organ of Corti
The observed increase in the rows of sensory hair cells in Nog−/− cochleae could be due to defects in the longitudinal growth of the cochlear duct. Nevertheless, the preferential increase in IHC number in the middle and apical turns of the cochlea cannot be explained easily by the lengthening defects of the cochlear duct. It is likely that Noggin is involved in the radial patterning of the organ of Corti as well (Fig. 7). Targeted deletion of Noggin in the cochlear duct will address this issue. Those experiments are currently under way. The notion of Noggin being required for radial patterning of the organ of Corti is partially based on the expression patterns of Nog and Bmps in the developing cochlea. The relationships of these genes’ expression domains are conserved between chicken and mouse (Chang et al., 1999; Bok et al., 2007). Bmp4, Bmp7, and Nog are expressed in overlapping domains in the abneural side of the developing sensory region in both species (Oh et al., 1996; Chang et al., 1999; Bok et al., 2007). The intimate relationships among Bmps and Nog expression domains in the cochlea are similar to reports in other tissues where Noggin functions to regulate the levels of Bmps (Brunet et al., 1998; Warren et al., 2003; Zhou et al., 2006).
Fig. 7.
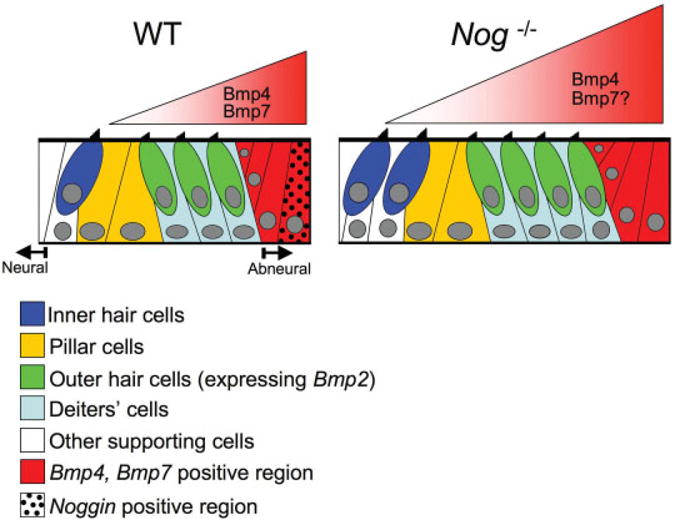
Model of the role of bone morphogenetic proteins (Bmps) in organ of Corti formation. In wild-type cochlea, Bmp4 and Bmp7 secreted from the abneural region regulate hair cell formation. The levels of Bmps are modulated by the antagonist, Noggin. In the absence of Noggin, the net level of Bmp4 and may be the level of Bmp7 as well across the prosensory domain are increased leading to extra hair cell formation in the organ of Corti.
Furthermore, several in vitro studies have implicated a role of Bmps in hair cell formation, which could be extrapolated to a role in vivo. For example, Bmp4 has been shown to induce hair cell formation in mouse cochlear explants as well as cultures of dissociated otic epithelial cells from chicken (Li et al., 2005; Puligilla et al., 2007). However, other studies using explants of chicken otocysts appear to arrive at an opposite conclusion showing that hair cell formation is induced by Noggin but inhibited by Bmp4 (Pujades et al., 2006). These various in vitro results are not necessarily in conflict with each other. In the chicken otocyst explants studies, the anterior and posterior cristae were the main sensory organs being analyzed, rather than an auditory sensory organ. Bmp4 has multiple functions in the sensory cristae promoting both sensory and nonsensory fates (Chang et al., 2008). It is possible that, at the developmental period of the otocyst cultures, Bmp4 is required to co-ordinate the nonsensory fate, which could account for the observed loss of sensory hair cells with exogenous Bmp4. While the nature of the increased hair cells in the dissociated cell culture studies is not clear (Li et al., 2005), the cochlear explant results are direct indications that Bmps promote cochlear hair cell formation (Puligilla et al., 2007). The expression patterns of Bmps and Noggin in the cochlea as well as the increased rows of auditory hair cells of Nog−/− mutants is consistent with the hypothesis that Bmps and Noggin are involved in radial patterning of the organ of Corti. The difference between the two gain-of-Bmp function models, cochlear explants, and Nog−/− mutants, is that increased IHC and OHC rows are observed in the Nog−/− cochlea, whereas only OHC number is observed to increase in cochlear explants treated with human Bmp4 (Puligilla et al., 2007). The lack of changes in IHC number in the explants could be related to the late timing of Bmp4 treatments.
A recent preliminary report showed that the lack of Bmp receptor Ia in the mouse cochlea caused a duplicated organ of Corti (T. Ohyama and A.K. Groves; personal communication). Further reduction of Bmp signaling in compound Bmp receptor Ia and Ib mutants resulted in loss of the prosensory domain. Thus, these authors suggested that organ of Corti development is sensitive to a gradient of Bmp levels across the prosensory region. Such levels of Bmps could be regulated by Noggin. Nevertheless, the various resulting phenotypes from the Bmp receptor knockouts or Nog mutants cannot be easily reconciled. It is possible that Bmps have multiple roles during the course of the organ of Corti formation. The profound sensory phenotypes obtained from knocking out the two Bmp receptors suggest an early role of Bmps in sensory organ specification. In contrast, the increase in rows of hair cells in Nog−/− cochlea or Bmp4 treated cochlear explants could be the consequence of a later, modulatory role of Bmps in promoting hair cell formation. This idea is consistent with the fact that Nog expression is not detected in the cochlea until mid-gestation (Bok et al., 2007).
Role of Bmp2 in Hair Cell Formation
It is not clear though which Bmps are being regulated by Noggin within the cochlear duct. The published data thus far do not exclude the possibility of Bmp2 playing a role in regulating hair cell formation in the cochlea. Bmp2 and Bmp4 are thought to have similar affinities in binding to Noggin and Bmp receptors (Rosen et al., 1996; Zimmerman et al., 1996). Thus, the increased OHC number in explant cultures using Bmp4 could be mimicking either one or both sources of Bmps in the cochlea. Even though expression of Bmp2 in the sensory hair cells is transient, it is at a developmental period when hair cell fates are amenable to change (Shim et al., 2005; Puligilla et al., 2007). Furthermore, expression of Bmps in auditory hair cells is conserved between chicken and mice, even though it is Bmp4 rather than Bmp2 that is expressed in auditory hair cells of chicken (Oh et al., 1996). Here, using two different cre expressing mouse strains to delete Bmp2 expression in auditory hair cells, we concluded that Bmp2 is not required for proper hair cell formation and is likely not needed for normal hearing in mice. Based on these results, Bmp4 expression in the abneural region seems to be the most likely candidate to be regulated by Noggin. Moreover, while there is no report of inner ear defects in Bmp7−/− embryos (Karsenty et al., 1996) and Noggin does not bind Bmp7 as effectively as Bmp2 and Bmp4 (Zimmerman et al., 1996), a role of Bmp7 in cochlear hair cell formation cannot be ruled out.
Interactions Between Fgfs and Bmps in the Organ of Corti
Multiple fibroblast growth factors are expressed in the developing organ of Corti such as Fgf8, Fgf10 and Fgf20 (Jacques et al., 2007; Pauley et al., 2003; Hayashi et al., 2008). Conditional deletion of Fgfr1 in the inner ear resulted in patches of sensory islands along the cochlear duct (Pirvola et al., 2002). Fgf20 expressed in the prosensory domain was proposed to be the main ligand activating Fgfr1 in the cochlea (Hayashi et al., 2008). In addition, loss of Fgfr3, which is also expressed in the prosensory domain, results in the absence of pillar cells and increased OHC (Puligilla et al., 2007; Hayashi et al., 2007). Fgf8 emanating from the IHC is thought to be the main ligand activating Fgfr3 (Jacques et al., 2007).
The interactions between Fgfs and Bmps in organ of Corti formation was implicated when Noggin treatment rescued the increase in OHC number in Fgfr3−/− cochlear explants (Puligilla et al., 2007). These results and the demonstration that Bmp4 induces OHC formation in cochlear explants led to the hypothesis that Fgfs interact with Bmp4 in the abneural region of the cochlear duct in specifying the number of OHC (Puligilla et al., 2007). While these results do not preclude the possibility that Bmp2 in the sensory hair cells could be interacting with Fgf signaling, our results here indicate that Bmp2’s function in the organ of Corti is dispensable and further implicate the importance of other Bmps expressed in the abneural region.
Given the antagonistic relationships between Fgf and Bmp signaling in other tissues (Pera et al., 2003; Cushing et al., 2008; Huang et al., 2009), the effects of a Bmp4 gradient, if it exists, could be further fine-tuned by Fgfs expressed in the prosensory region such as Fgf10 and Fgf20 or Fgf8 in the IHC (Jacques et al., 2007; Pauley et al., 2003; Hayashi et al., 2008). If this hypothesis is correct, one would predict a change in hair cell fate or number when either Bmp4 or Fgfs signaling is affected. On the contrary, only OHC and not IHC appear to be affected when Fgf signaling is perturbed in both Fgfr3−/− and Sprouty2−/− mutants (Shim et al., 2005; Hayashi et al., 2007; Puligilla et al., 2007). Functional redundancy among the Fgfs and their receptors could account for the lack of a more profound IHC phenotype in some of these mutants (Hayashi et al., 2007; Puligilla et al., 2007). Future studies will focus on the requirements of Fgfs and Bmps in establishing inner and outer hair cell fates.
EXPERIMENTAL PROCEDURES
Mice and Genotyping
The Nog+/tm1Amc (Nog+/−) mice (McMahon et al., 1998) on a congenic C57BL/6J background were obtained from Dr. Richard Harland (Univ. of California at Berkeley) and maintained on a C57BL/6J;FVB hybrid background. Genotyping for Nog−/− embryos was determined by PCR as previously described (McMahon et al., 1998). The Bmp2tm1.1Mis/tm1.1Mis (Bmp2lox/lox) and Bmp2+/tm1Brd (Bmp2+/−) mice, generated as previously described (Zhang and Bradley, 1996; Singh et al., 2008) were maintained on a 129SvEv; C57BL6/J mixed background and genotyped according to previous description (Singh et al., 2008). The generation of Gfi1cre/+ knock-in mouse, in which cre is driven by the promoter of Gfi1, will be described elsewhere (Lin Gan, Univ. of Rochester). Mice with conditional deletion of Bmp2 were generated by mating Foxg1cre/+;Bmp2+/− or Gfi1cre/+;Bmp2+/− with Bmp2lox/lox mice.
In Situ Hybridization, Immunohistochemistry, and Hair Cell Number Quantification
For in situ hybridization, RNA probes for Bmp4, Lfng (Morsli et al., 1998), Bmp2 (McMahon et al., 1998), Bmp7 (Bellusci et al., 1996), Nog (Brunet et al., 1998), Gfi1 (Yang et al., 2003), and Myo15a (Anderson et al., 2000) were prepared as previously described. Immunohistochemistry for whole-mount cochlear duct was done as previously described (Koo et al., 2009). To quantify the hair cell number, each cochlea was divided into the base, middle and apical turns of equal length demarcated in Figure 1A,B. In each turn, hair cell numbers were counted in three evenly spaced 80-μm segments when available, and the mean hair cell number was calculated and used to compute the average hair cell numbers among controls and mutants. Statistical analyses on hair cell numbers and hearing thresholds between mutants and controls were conducted using Student’s t-test.
Hearing Measurement
Auditory brainstem response (ABR) was recorded using Smart EP (Intelligent Hearing System, FL) as previously described (Hwang and Wu, 2008). Briefly, mice were anesthetized with an intra-peritoneal injection of the mixture of Ketamine (56 mg/kg body weight, Ketalar, Generamedix, USA) and Dexmedetomidine (0.375 mg/kg body weight, Dexdomitor, Pfizer Animal Health, USA) and kept on a heating pad in a soundproof chamber. Auditory stimuli used were click, 8 kHz, and 16 kHz tone pips and hearing of right ears was measured. After the procedure, mice were recovered by intra-peritoneal injection of Atipamezole (5 mg/kg body weight, Antisedan, Pfizer Animal Health, USA).
Supplementary Material
Acknowledgments
We thank Dr. Richard Harland for plasmids and Nog+/− mice, Dr. Brigid Hogan for plasmids, and Ms. Lydia Lui and Mr. Matthew Chang for technical assistance. We also thank Drs. Matthew Kelley, Chandrakala Puligilla, and Robert Morell for critical reading of the manuscript. D.K.W. was funded by the NIDCD intramural program, Y.M. was funded by an Intramural Research Program of the NIH/NIEH, and L.G. and S.H. were funded by NIH extramural programs.
Grant sponsor: NIDCD; Grant sponsor: NIH/NIEHS; Grant number: ES071003-11; Grant sponsor: NIH; Grant number: DC008856; Grant number: 1R01 AR054616.
Footnotes
Additional Supporting Information may be found in the online version of this article.
This article is a US Government work and, as such, is in the public domain of the United States of America
References
- Anderson DW, Probst FJ, Belyantseva IA, Fridell RA, Beyer L, Martin DM, Wu D, Kachar B, Friedman TB, Raphael Y, Camper SA. The motor and tail regions of myosin XV are critical for normal structure and function of auditory and vestibular hair cells. Hum Mol Genet. 2000;9:1729–1738. doi: 10.1093/hmg/9.12.1729. [DOI] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
- Bok J, Brunet LJ, Howard O, Burton Q, Wu DK. Role of hindbrain in inner ear morphogenesis: analysis of Noggin knockout mice. Dev Biol. 2007;311:69–78. doi: 10.1016/j.ydbio.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Chang W, Nunes FD, De Jesus-Escobar JM, Harland R, Wu DK. Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev Biol. 1999;216:369–381. doi: 10.1006/dbio.1999.9457. [DOI] [PubMed] [Google Scholar]
- Chang W, Lin Z, Kulessa H, Hebert J, Hogan BL, Wu DK. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 2008;4:e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Choi M, Stottmann RW, Yang YP, Meyers EN, Klingensmith J. The bone morphogenetic protein antagonist noggin regulates mammalian cardiac morphogenesis. Circ Res. 2007;100:220–228. doi: 10.1161/01.RES.0000257780.60484.6a. [DOI] [PubMed] [Google Scholar]
- Cushing MC, Mariner PD, Liao JT, Sims EA, Anseth KS. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J. 2008;22:1769–1777. doi: 10.1096/fj.07-087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305:145–160. doi: 10.1016/j.ydbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–220. doi: 10.1242/dev.00181. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver EC, Pryor SP, Hill P, Turner J, Ruther U, Biesecker LG, Griffith AJ, Kelley MW. Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J Neurosci. 2008;28:7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci. 2008;28:5991–5999. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Hertzano R, Montcouquiol M, Rashi-Elkeles S, Elkon R, Yucel R, Frankel WN, Rechavi G, Moroy T, Friedman TB, Kelley MW, Avraham KB. Transcription profiling of inner ears from Pou4f3(ddl/ddl) identifies Gfi1 as a target of the Pou4f3 deafness gene. Hum Mol Genet. 2004;13:2143–2153. doi: 10.1093/hmg/ddh218. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O’Connor MB, De Robertis EM, Ferguson EL. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Huang J, Dattilo LK, Rajagopal R, Liu Y, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. FGF-regulated BMP signaling is required for eyelid closure and to specify conjunctival epithelial cell fate. Development. 2009;136:1741–1750. doi: 10.1242/dev.034082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CH, Wu DK. Noggin heterozygous mice: an animal model for congenital conductive hearing loss in humans. Hum Mol Genet. 2008;17:844–853. doi: 10.1093/hmg/ddm356. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW. Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development. 2007;134:3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Luo G, Hofmann C, Bradley A. BMP 7 is required for nephrogenesis, eye development, and skeletal patterning. Ann N Y Acad Sci. 1996;785:98–107. doi: 10.1111/j.1749-6632.1996.tb56247.x. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Cellular commitment and differentiation in the organ of Corti. Int J Dev Biol. 2007;51:571–583. doi: 10.1387/ijdb.072388mk. [DOI] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin Z, Millen KJ, Wu DK. Lmx1a maintains proper neurogenic, sensory, and nonsensory domains in the mammalian inner ear. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Corrales CE, Wang Z, Zhao Y, Wang Y, Liu H, Heller S. BMP4 signaling is involved in the generation of inner ear sensory epithelia. BMC Dev Biol. 2005;5:16. doi: 10.1186/1471-213X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E, Krupin A, Kelley MW. Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev Dyn. 2004;229:802–812. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Kelley MW. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J Neurosci. 2003;23:9469–9478. doi: 10.1523/JNEUROSCI.23-28-09469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Johnson R, Wu DK. Differential expression of bone morphogenetic proteins in the developing vestibular and auditory sensory organs. J Neurosci. 1996;16:6463–6475. doi: 10.1523/JNEUROSCI.16-20-06463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Wright TJ, Pirvola U, Ornitz D, Beisel K, Fritzsch B. Expression and function of FGF10 in mammalian inner ear development. Dev Dyn. 2003;227:203–215. doi: 10.1002/dvdy.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Pujades C, Kamaid A, Alsina B, Giraldez F. BMP-signaling regulates the generation of hair-cells. Dev Biol. 2006;292:55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen V, Thies RS, Lyons K. Signaling pathways in skeletal formation: a role for BMP receptors. Ann N Y Acad Sci. 1996;785:59–69. doi: 10.1111/j.1749-6632.1996.tb56244.x. [DOI] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet. 2008;42:517–540. doi: 10.1146/annurev.genet.42.110807.091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Castranio T, Scott G, Guo D, Harris MA, Ray M, Harris SE, Mishina Y. Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex Dev. 2008;2:134–141. doi: 10.1159/000143431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, Zoghbi HY, Orkin SH, Bellen HJ. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SM, Brunet LJ, Harland RM, Economides AN, Longaker MT. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003;422:625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Okano T, Ma X, Adelstein RS, Kelley MW. Myosin II regulates extension, growth and patterning in the mammalian cochlear duct. Development. 2009;136:1977–1986. doi: 10.1242/dev.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liu HX, Mistretta CM. Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev Biol. 2006;297:198–213. doi: 10.1016/j.ydbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


