Abstract
Introduction
Treatment and prevention are of critical importance in patients with cutaneous lupus erythematosus (CLE), as the disease can have a devastating effect on patient well-being and quality of life.
Areas Covered
We conducted a selective search of the PubMed database for articles published between December 2010 and November 2015. This review encompasses both non-pharmaceutical (photoprotection, smoking cessation, drug withdrawal, and vitamin D replacement) and pharmaceutical (topicals, antimalarials, immunosuppressives, biologics, etc.) interventions used in the treatment of CLE.
Expert Commentary
Recent work has expanded our understanding of established therapies as well as introduced new treatments for consideration, though existing medications still prove inadequate for a subset of patients. Changes in trial design may help to alleviate this issue.
Keywords: cutaneous lupus erythematosus, topical calcineurin inhibitors, antimalarials, immunosuppressives, immunomodulators, biologics, Cutaneous Lupus Erythematosus Disease Area and Severity Index™ (CLASITM)
1. Introduction
Lupus erythematosus (LE) is an autoimmune disease characterized by a wide range of cutaneous (CLE) and/or systemic (SLE) symptoms. CLE can be seen with or without SLE (i.e. as part of a systemic disease or as a separate entity with primarily cutaneous manifestations), and the latter may present or develop cutaneous manifestations. As such, evaluating for potential systemic involvement is a critical component of initial clinical staging and subsequent monitoring of disease progression.
Current theories regarding the pathogenesis of CLE emphasize a multifactorial etiology involving genetic polymorphisms and susceptibility loci, environmental factors such as UV exposure, and induction of innate and adaptive autoimmune responses. Standard treatment of CLE involves preventative measures, such as sunscreen use and smoking cessation, coupled with topical corticosteroids or calcineurin inhibitors. For more severe cases, antimalarials are implemented as first-line systemic treatment. In patients unresponsive to or unable to tolerate antimalarial therapy, alternative options include immunosuppressive, immunomodulatory, or biologic agents. Recent work over the past five years has been successful in both reinforcing the efficacy of established treatments as well as introducing new options for consideration.
2. Methods
We conducted this review through a selective search of the PubMed database for articles published between December 2010 and November 2015 using two overlapping search strategies with the following keywords: 1) “lupus erythematosus” and “treatment” and 2) “cutaneous lupus erythematosus” and “treatment”. We selected articles based on their relevance and contribution to recent advances in the treatment of CLE and incorporated additional articles published prior to the designated time frame as needed.
3. Classification of CLE
CLE is divided into acute (ACLE), subacute (SCLE), and chronic (CCLE) subtypes, with lupus erythematosus tumidus (LET) either grouped with chronic CCLE, its own category, or in lupus nonspecific skin findings. CCLE includes discoid lupus erythematosus (DLE) and lupus erythematosus panniculitis (LEP), as well as LET depending on which of the classifications are used [1].
The 1997 American College of Rheumatology (ACR) SLE classification criteria are comprised of 11 criteria, 4 of which must be met for classification as SLE [2]. However, the first 4 criteria (malar rash, discoid rash, photosensitivity, and oral ulcers) could all be present in a patient with skin-predominant disease who is otherwise healthy, raising issues regarding the potential inaccuracy of this classification system. Another system has been developed by the Systemic Lupus International Collaborating Clinics (SLICC) and includes a total of 17 clinical and immunological criteria. The SLICC criteria do not include photosensitivity and are able to account for additional cutaneous manifestations. Classification of SLE by this system also needs a minimum of 4 criteria to be met, but with the added requirement of at least one criterion present in each category [3]. Though this therefore avoids the aforementioned issue with the ACR criteria, the requirement of at least one immunological criterion excludes all seronegative patients, even those with extensive skin disease and other significant systemic features. This may limit the number and breadth of patients included in SLE studies.
4. Evaluation of Disease Severity
Disease damage and activity can be measured with the CLE Disease Area and Severity Index™ (CLASI™), a quantitative scoring tool in which a 4-point or 20% decrease in activity score indicates a clinically significant improvement [4]. The CLASI™ can be used to follow patients in a specialty clinic, but its main role is for cohort or therapeutic studies. The CLASI™ has been validated against physician- and patient-reported outcomes, including measures of cutaneous damage [5] and quality of life [4]. The latter is especially important to consider in the assessment of these patients, as individuals with CLE have demonstrated a poorer quality of life than those with other common conditions affecting the skin [6] across multiple geographic populations [7]. Worse quality of life in CLE patients is associated with a number of factors including female gender, younger age, presence of facial lesions, and non-responsiveness to treatment [6, 8]. There are a number of other measures that have not been fully validated or utilized in international trials [9, 10].
5. Assessment for Systemic Involvement
Patients with indications of systemic disease, including proteinuria, low complements, and/or high-dsDNA titer are at an increased risk for progression to systemic disease. Such patients should receive careful follow-up testing, with complete blood counts and urinalyses every two to three months. In contrast, in patients with stable, long-standing CLE, monitoring may be performed annually. Anti-Smith antibody titers may also assist with patient diagnosis and serve as a high-sensitivity, low-specificity test for SLE. In patients with a history of clots or livedo pattern, an anti-phospholipid antibody panel should be ordered. Patients with CLE or minor SLE can be treated and continually evaluated by a dermatologist, whereas those with active significant SLE should be referred to rheumatology for co-management.
6. Non-Pharmaceutical Interventions
6.1 Photoprotection
Recent studies have reaffirmed the role of UV irradiation in the development and progression of CLE lesions. In a multicenter study of 47 subjects with CLE, a standardized photoprovocation protocol caused UV-induced lesions in about half of all patients, with the highest rates observed in those with SCLE and LET [11]. An updated retrospective analysis revealed even higher rates of positive photoprovocation (61.7%) [12]. Photoprotection thus represents a key component of preventative therapy in CLE, and the consistent use of sunscreens is commonly recommended for these patients [13]. Application of a photoprotective sunscreen was shown to limit UV-induced inflammatory responses through a decrease in CD11c- and CD123-positive dendritic cells, with a corresponding decrease in MxA expression and interferon levels [14]. Another study assessing the efficacy of a broad-spectrum sunscreen reported the complete absence of lesions in sunscreen-treated portions of the skin following UV irradiation [15]. Similar results were obtained in an open-label study involving a liposomal sunscreen, with CLE lesions observed only in untreated, UV-irradiated areas of the skin [16].
Unfortunately, most patients with CLE fail to remain consistent in their use of sunscreen and other photoprotective methods [17, 18]. A cross-sectional survey of 100 CLE subjects identified only a third of the group as daily sunscreen users, with over half of the remaining individuals not using sunscreen at all. Poor levels of adherence were most often attributed to simple factors such as forgetfulness or presumed ineffectiveness [18]. A separate study also revealed that patients between 31 to 50 years of age and/or with medium to dark skin were least likely to engage in photoprotective habits [17]. Outside of traditional sun exposure, additional sources of UV irradiation exist which should also be limited whenever possible. A pilot study investigating the effect of different lighting types on patient symptoms showed that UV-emitting bulbs (compact fluorescent lamp and energy-efficient halogen) were erythema-inducing. These two light sources should be avoided in favor of light-emitting diode bulbs, which were identified as a safer, non-UV-emitting alternative [19]. Surgical lighting was also recently reported to induce flares in a photosensitive LE patient [20].
Given the exacerbative effect of UV exposure on patient symptoms, it is imperative that individuals with CLE put their best effort toward the prevention of UV-induced disease progression. Therefore, in addition to recommending and educating patients on proper and routine sunscreen use, it is equally important to monitor and assess the photoprotective habits of these patients throughout the course of treatment. Though sunscreens are not yet recognized as therapeutic drugs and therefore not covered by most health insurances worldwide, this may change with the increasing emphasis on preventative care.
6.2 Smoking cessation
Smoking cessation is also recommended in controlling CLE symptoms. A prospective cohort study of CLE patients showed greater disease severity and worse quality of life measurements in current smokers [21]; a separate, larger-scale study also identified smoking as a risk factor for increased disease severity [22], though other analyses have suggested that the association is restricted to select CLE subtypes such as LET and DLE [23]; and baseline data from a recently completed randomized trial has demonstrated significantly increased CLASI™ scores in current smokers [24]. Previously, many reports concerning smoking in CLE patients have emphasized its negative impact on antimalarial treatment efficacy [25]. In the case of hydroxychloroquine (HCQ), smoking was observed to counteract the drug’s proposed inhibition of Toll-like receptor-mediated signaling [26]. However, recent studies have revealed the absence of any significant relationship between smoking and patient response to HCQ [27] and other antimalarials [24], suggesting a treatment-independent effect of smoking on disease severity.
6.3 Drug withdrawal
Drug-induced SCLE (DI-SCLE) has been observed and some cutaneous features may distinguish it from idiopathic SCLE [28]. A case-control study of SCLE patients identified terbinafine, tumor necrosis factor-alpha inhibitors, antiepileptics, and proton pump inhibitors as the most common offending agents, with DI-SCLE accounting for over a third of all SCLE cases. As DI-SCLE symptoms are reversible, discontinuation of the drug is an effective method of treatment and demonstrates the importance of active medication screening in SCLE patients [29]. Other drugs previously implicated in DI-SCLE include calcium-channel blockers, angiotensin-converting enzyme inhibitors, and thiazide diuretics [30].
6.4 Vitamin D replacement
Vitamin D monitoring and treatment may also represent a valid option for CLE symptom control. A recent analysis revealed a greater prevalence of vitamin D deficiency among CLE patients, especially with increasing age and disease duration. Disease severity was noted to improve in the treatment group, suggesting a therapeutic role for vitamin D replacement [31]. Oral vitamin D supplementation has also been observed to decrease T-cell production of IFN-γ and IL-17 [32], and certain polymorphisms in the vitamin D receptor gene were associated with cutaneous, arthritic, and immunological manifestations of SLE [33]. Therefore, in patients with low vitamin D levels, especially with sunscreen use, vitamin D replacement should be considered and recommended as part of the overall treatment plan.
7. Pharmaceutical Interventions
7.1 Topical corticosteroids
Topical corticosteroids serve as first-line treatments for mild or local cases of CLE. Though they have therefore been applied in all CLE subtypes, the only randomized controlled trial to date supporting their use in CLE involved 78 DLE patients. The 12-week crossover study revealed a greater response rate with 0.05% fluocinomide than with 1% hydrocortisone, suggesting that higher-potency topical corticosteroids are more effective for treatment [34]. However, the potency and duration of topical steroid use should generally be kept to a minimum due to side-effects including atrophy and telangiectasia. Treatment of facial lesions should be limited to low-potency steroids such as hydrocortisone butyrate, while high-potency steroids such as clobetasol proprionate should be reserved for lesions involving thicker areas of the skin or cases of severe disease activity [35].
7.2 Topical calcineurin inhibitors
With their greatly reduced side-effect profile, the calcineurin inhibitors tacrolimus and pimecrolimus have emerged as effective alternatives to corticosteroids in the topical treatment of CLE [36]. A study of 38 patients showed both tacrolimus and pimecrolimus to be effective in improving symptoms of erythema, desquamation, and edema, independent of disease type [37]. In a comparison between 0.1% tacrolimus and 0.05% clobetasol propionate ointments in 21 DLE patients, negative effects of tacrolimus were limited to transient pruritus and burning, whereas telangiectasia was observed in 61% of patients following clobetasol treatment. Though tacrolimus had a lower overall efficacy, both treatments led to significant decreases in disease severity [38]. In a randomized, vehicle-controlled trial, similar improvements were seen in patients treated with 0.1% tacrolimus ointment, especially in those with LET [39]. More recently, treatment with a 0.3% tacrolimus lotion was also observed to relieve symptoms in three patients with antimalarial-resistant DLE [40].
7.3 Topical vitamin D derivatives
In addition to its frequent deficiency among LE patients, vitamin D has also demonstrated immunomodulatory effects that further support its use in the treatment of autoimmune diseases [41]. Topical derivatives of vitamin D have been developed that achieve similar immunomodulatory activity while limiting subsequent risk of hypercalcemic toxicity [42, 43]. Calcipotriene, for instance, is an FDA-approved treatment for psoriasis that has also been reported to improve LE skin lesions. Topical application of the drug may therefore be considered in CLE [44–46].
7.4 Antimalarials
Antimalarials such as HCQ, quinacrine, and chloroquine continue to serve as first-line systemic treatments for more severe cases of CLE and are administered according to ideal and/or real body weight [47]. Despite recommended dosing, chloroquine is associated with a higher risk of eye toxicity [48, 49]. HCQ is typically the treatment of choice [50], though its utility as a monotherapy is limited due to variations in patient response. Factors most strongly associated with a lack of response include smoking, disease severity, and presence of SLE. In a retrospective cohort study of 200 DLE patients, 60% showed a response to HCQ in the first six months of treatment [27]. Further analysis of the same population revealed an overall decline in response rate over time, with the long-term HCQ response rate dropping to 45% [51].
In such cases, quinacrine can be added to improve the response to HCQ. In a prospective study, 67% of HCQ non-responders showed a significant improvement in disease activity following the addition of quinacrine [52]. Similarly, patients that had failed to maintain an initial response to HCQ were often observed to regain symptom control through this combination of antimalarial agents [51]. Though quinacrine remains a valid therapeutic option in the treatment of recalcitrant CLE, it has declined in use and is currently only available from compounding pharmacies, including those in the United States, United Kingdom, and Germany [35].
Rates of adherence to antimalarial treatment have been studied and reported in LE patients, though much more so in SLE. Treatment maintenance can be assessed by monitoring serum HCQ concentrations, with low levels in the blood indicative of poor adherence [53]. Among 203 patients with SLE, 7% were found to have a low mean HCQ concentration and later confirmed their non-adherence. Similarly, a prospective, multicenter study of 300 refractory CLE patients identified 10% of subjects as non-adherent, and higher HCQ concentrations were associated with partial or complete remission. In both studies, a 200 ng/ml blood HCQ concentration was proposed and applied as a minimum cutoff threshold for adherence [54, 55].
7.5 Retinoids
In patients failing to tolerate or show improvement with other treatments, retinoids may be employed as second-line therapeutic agents. Successful off-label use of alitretinoin was reported in a case series of three patients with recalcitrant SLE, DLE, and SCLE. Alitretinoin was well tolerated and led to a complete clearance of lesions in all three patients [56]. Similarly, in a patient receiving oral isotretinoin for SCLE, significant symptom reduction was observed within the first month with no evidence of recurrence after six months [57]. A rare case of lupus/lichen planus overlap syndrome also demonstrated improvement following treatment with acitretin [58]. As retinoids are known teratogens, women of child-bearing age are required to take contraceptives before, during, and after treatment. In such cases, isotretinoin is typically preferred for its shorter half-life [59].
Immunosuppressives
Immunosuppressives such as methotrexate and mycophenolate mofetil (MMF) can also be used as second-line treatments for CLE. Past retrospective analyses have supported the safety and efficacy of methotrexate with refractory CLE [60, 61], with successfully treated cases of SCLE [62, 63] and DLE [64] also reported in the literature. More recently, in a prospective, open-label study of 41 patients comparing low-dose methotrexate to chloroquine, both treatments were shown to be equally effective in treating cutaneous symptoms [65]. When combined with the calcineurin inhibitor cyclosporine, methotrexate was also observed to improve symptom control in two cases of recalcitrant SCLE. However, the long-term safety of this combination is in need of further study, and both drugs have been associated with nephro- and hepatotoxicity [66]. In SLE patients, methotrexate treatment has been shown to be highly effective, leading to significant reductions in SLEDAI-measured disease activity and overall steroid burden [67–69].
Like methotrexate, MMF can also be used in combination therapies targeting recalcitrant CLE. In a retrospective analysis of 24 patients with antimalarial-resistant CLE, many were able to achieve complete symptom control following the addition of MMF to the established drug regimen [70]. Similarly, a combination of MMF and HCQ was reported to induce either partial or full remission in three patients with recalcitrant CLE [71].
In pregnancy, the immunosuppressant azathioprine may be used if there are no suitable alternatives for the treatment of skin disease.
7.6 Immunomodulators
Though it is more commonly used as an antimicrobial agent, dapsone has been successfully implemented in the treatment of various subtypes of CLE, with an overall response rate of 55% across multiple case series [72]. The immunomodulatory antibiotic was also shown to be effective in treating a rare case of pediatric, corticosteroid-resistant bullous SLE [73]. Dapsone should not be given to patients with glucose-6-phosphate dehydrogenase deficiency due to an increased risk of hemolysis and methemoglobinemia [59].
Thalidomide is an anti-inflammatory agent that prevents UV-induced apoptosis of keratinocytes. A prospective study of 60 patients with recalcitrant CLE showed a 98% response rate with thalidomide, though many experienced disease recurrence following withdrawal of the drug. This relapse was especially common among DLE patients, whereas SCLE patients tended to maintain symptom control even after withdrawal [74]. Thalidomide is also known to cause multiple neuropathic side-effects in those receiving treatment, as confirmed by a recent retrospective analysis [75]. Though treatment dosage and duration are typically held to a minimum, neither of these factors has been shown to have an impact on resulting rates of thalidomide-associated neuropathy [74, 76]. As neurotoxic and teratogenic side effects may be observed even at low doses of the drug, thalidomide use should therefore be limited to more severe cases of recalcitrant CLE [59, 76].
Lenalidomide is a thalidomide derivative that has demonstrated similar utility in the treatment of recalcitrant CLE. Of 15 such patients enrolled in a single-center pilot trial, 85% showed a complete response to lenalidomide therapy, and no neuropathic effects occurred as a result of treatment [77]. In a smaller open-label study, four out of five patients experienced meaningful improvement in their cutaneous symptoms [78]. Long-term follow-up of this same group revealed a clinically significant decline in CLASI™ score at 12 weeks for all five patients. Again, neuropathy was not observed following treatment, suggesting that lenalidomide may be preferred over thalidomide in the treatment of recalcitrant CLE [79]. In either case, it should be noted that the off-label use of thalidomide or its derivatives for CLE can be very costly, making it difficult for some patients to receive treatment with these drugs.
7.7 Biologics
Much of the recent work involving the use of biologics in LE has focused on the treatment of SLE rather than CLE. In addition, many of these studies did not closely evaluate the skin with established indices but were instead limited to general observations of skin manifestations.
Rituximab is a monoclonal antibody that acts against human CD20, leading to B cell death and depletion. A systematic review of the literature supported the short-term efficacy of rituximab in the treatment of recalcitrant SLE, though relapse was frequently observed [80]. A retrospective analysis of 17 patients showed similar results [81], and a prospective study further demonstrated a steroid-sparing effect with early treatment [82]. For CLE patients, studies suggest that rituximab may only be helpful in treating those with ACLE [83], though treatment of other subtypes has been reported in a few cases [84–87].
Belimumab, a monoclonal antibody specific to B lymphocyte stimulator, has consistently shown positive results in clinical trials involving SLE patients and was the first biologic to be approved for SLE treatment [88]. Its safety and efficacy in CLE patients have yet to be studied, though it was successfully implemented in a case of refractory SCLE [89]. In the BLISS-52 and -76 randomized controlled trials, belimumab doses of 1 and 10 mg/kg were evaluated and compared to placebo plus standard therapy in SLE patients, with both trials demonstrating the safety and therapeutic efficacy of the drug [90, 91]. Subsequent analyses revealed significant musculoskeletal and mucocutaneous improvement [92] as well as increased health-related quality of life [93] in those receiving treatment, and a long-term continuation study of a separate trial showed effective disease control over a seven year treatment period [94]. Data from the two BLISS trials was also used to show that factors such as increased disease activity, low complement levels, anti-dsDNA positivity, and corticosteroid use were associated with an increased benefit from treatment. These characteristics may therefore be helpful in the decision-making process and, when identified in patients, support the use of belimumab therapy [95].
Other biologic agents have also been reported to improve outcomes in patients with drug-resistant LE. Ustekinumab is a monoclonal antibody that binds and sequesters IL-12 and IL-23, thereby inhibiting pathways of Th1 and Th17 differentiation. 45 mg injections of the drug successfully treated a case of recalcitrant SCLE, with sustained remission through seven months of follow-up [96]. Similar results were seen in a separate patient with DLE [97]. Ustekinumab was also used to treat a rare case of coexistent psoriasis and DLE. Slight reductions in DLE symptoms were observed following a series of 45 mg injections, with significant improvement upon switching to a 90 mg dose [98].
Sirukumab and tocilizumab are monoclonal antibodies that act as inhibitors of the IL-6 pathway. Sirukumab was well tolerated in a phase I trial of 46 LE patients and led to dose-dependent decreases in white blood cell counts and acute phase reactant levels [99]. Tocilizumab was successfully implemented in the treatment of an LET patient with elevated IL-6 levels [100]. Positive results were also initially observed in two other SLE patients, though subsequent flares required treatment with belimumab for long-term symptom control [101].
Anifrolumab and sifalimumab are another pair of biologics that target human interferon-alpha and interferon-alpha receptor, respectively. 300 and 1000 mg doses of anifrolumab were shown to lead to equally dramatic reductions in disease activity and severity in a phase II randomized controlled trial of 385 patients with drug-resistant SLE [102]. Sifalimumab was well-tolerated by patients and showed an acceptable safety profile in two phase I trials [103, 104], and a recent phase II trial of 431 SLE patients demonstrated significant improvements in disease activity following treatment with the drug [105].
The anti-T cell therapies abatacept (CTLA4-Ig) and AMG 811 (anti-IFN- γ) have also been evaluated in randomized controlled trials, though their results did not demonstrate efficacy. In both studies, participants failed to demonstrate an adequate response to treatment. However, these outcomes may be attributed to issues in endpoint determination or overall trial design [106, 107].
7.8 Laser therapy
Pulsed dye laser (PDL) therapy may be used in cases of refractory CLE. Though PDL therapy has not yet been reported to induce skin lesions, lasers should still be employed with caution in LE patients, and spot testing is recommended prior to treatment. In a prospective study of nine CLE patients, clinical and histological improvements were observed four weeks after 595-nm PDL treatment [108]. PDL therapy has also been reported to successfully treat individual cases of refractory LET [109] and DLE [110, 111].
8. Conclusion
Studies concerning the treatment and prevention of lupus erythematosus have led to significant advances in the field over the past five years. Topical steroids and oral antimalarials continue to serve as first-line treatments with methotrexate and systemic steroids as second-line options. In cases of recalcitrant disease, other agents such as dapsone, retinoids, immunosuppressives, and targeted biologic therapies may be also be implemented. As research continues to unveil the underlying mechanisms of LE pathogenesis, novel therapeutic options will surely follow, and it will be interesting to observe the role that pharmacoepigenetics and genetic analysis play in the development of future treatments.
9. Expert commentary
As the list of available treatments for CLE continues to grow, implications for clinical practice and decision-making abound. Though treatment of patients tends to follow a basic pattern, individual options should still be considered in the context of disease subtype and severity, as many of the aforementioned studies have demonstrated the impact of these factors on treatment response. Maintenance of treatment should be regularly assessed and closely monitored to avoid unnecessary escalation or alteration of treatment in cases of non-adherence. Finally, a holistic approach to the evaluation and treatment of these patients is key, as patient well-being and quality of life are especially impacted by CLE.
Still, the need for novel therapeutic options remains evident. Older drugs such as quinacrine and chloroquine are becoming increasingly difficult to obtain, and existing regimens are often inadequate for patients who present with recalcitrant CLE or are unable to tolerate otherwise-effective medications. Unfortunately, not a single drug has yet been approved for the treatment of CLE (if defined as separate entity), and belimumab remains the only medication approved for SLE in the past 50 years. This disparity between the increasing need for new medications and the near-complete lack of formal drug approval can in part be attributed to challenges in trial design, as studies of LE patients often involve background treatments that lead to inflated placebo response rates. This then decreases the accuracy of results and may prevent identification of a clinically significant response in the treatment arm, especially when evaluating medications with a smaller therapeutic effect. In order to alleviate these issues, it may therefore be helpful to identify individuals who are less responsive to background therapies and assess treatment efficacy separately in that subset of patients.
10. Five-year view
In spite of certain challenges, significant progress has already been made in the treatment of CLE, and we anticipate the identification and development of additional therapeutic options in the near future. Recent treatments that have been reported but are in need of further study include lenalidomide, a thalidomide derivative [77–79]; octreotide, a peptide analog of somatostatin [112]; mizoribine, an immunosuppressive [113]; and blisibimod, a biologic BAFF inhibitor [114], among others. Likewise, treatments such as intravenous immunoglobulin therapy [115], mesenchymal stem cell transplantation [116], and regulatory T cell therapy [117] that have shown success in other diseases are now being applied to CLE in exploratory studies. Table 2 provides a summary of ongoing clinical trials [117–144].
Table 2.
Current clinical trials for lupus erythematosus treatment.
| Treatment | Condition(s) | Phase | Enrollment | Start | Completion |
|---|---|---|---|---|---|
| Biologics | |||||
|
| |||||
| ALX-0061 (anti-IL-6R) | SLE | 2 | 300 | Jul ’15 | Mar ’18 |
| Anifrolumab (anti-IFNAR1) | SLE | 3 | 360 | Jul ’15 | Oct ’18 |
| BIIB059 (anti-BDCA-2) | SLE | 1 | 108 | Apr ’14 | Jun ’16 |
| Brentuximab vedotin (anti-CD30) | SLE | 2 | 40 | Jul ’15 | Apr ’17 |
| BT063 (anti-IL-10) | SLE | 2 | 36 | Aug ’15 | Aug ’17 |
| Lulizumab pegol (anti-CD28) | SLE | 2 | 350 | Nov ’14 | Mar ’17 |
| Milatuzumab (anti-CD74) | CLE, DLE, SLE | 1–2 | 30 | Jan ’15 | Jan ’17 |
| Omalizumab (anti-IgE) | SLE | 1 | 30 | Oct ’12 | Jul ’20 |
| SAR113244 (anti-CCR5) | SLE | 1 | 24 | Jul ’15 | Sep ’16 |
| Ustekinumab (anti-IL-12, -23) | SLE | 2 | 100 | Oct ’15 | Dec ’17 |
|
| |||||
| Fusion Proteins | |||||
|
| |||||
| Atacicept (TACI:Fc5) | SLE | 2 | 306 | Dec ’13 | Mar ’16 |
| Etanercept (TNFR:Fc) | CCLE, CLE, DLE | 2 | 25 | Feb ’16 | Aug ’17 |
| RSLV-132 (RNase:Fc) | SLE | 2 | 50 | Jan ’16 | Jun ’17 |
|
| |||||
| Immunomodulators | |||||
|
| |||||
| AMG 570 | SLE | 1 | 40 | Mar ’16 | Apr ’17 |
| CC-220 | SLE | 2 | 140 | Sep ’14 | Aug ’16 |
| Cenerimod | SLE | 1–2 | 64 | Jun ’15 | Jan ’17 |
|
| |||||
| Other | |||||
|
| |||||
| Allogeneic mesenchymal stem cells | SLE | 2 | 81 | Jul ’16 | Jun ’21 |
| Amiselimod (immunosuppressant) | SLE | 1 | 18 | Feb ’15 | Jan ’17 |
| Autologous EBV-specific cytotoxic T cells | SLE | 1–2 | 10 | Jan ’16 | Jan ’19 |
| Autologous polyclonal Tregs | CLE, DLE, SLE | 1 | 18 | Jul ’15 | Dec ’17 |
| Bortezomib (proteosome inhibitor) | SLE | 2 | 18 | Oct ’14 | Dec ’16 |
| Dipyridamole | SLE | Unavailable | 50 | Feb ’13 | Feb ’16 |
| IL-2 | SLE | 2 | 132 | Jan ’14 | Jan ’16 |
| MSC2364447C (Btk inhibitor) | SLE | 1 | 24 | Nov ’15 | Sep ’16 |
| Nelfinavir | SLE | 2 | 43 | Sep ’14 | Dec ’16 |
| Rigerimod (T cell inhibitor) | SLE | 3 | 200 | Dec ’15 | Jun ’17 |
| Tofacitinib (JAK inhibitor) | SLE | 1 | 38 | Aug ’15 | May ’20 |
| UVA1 radiation | CLE | Unavailable | 15 | Sep ’12 | Sep ’17 |
SLE = systemic lupus erythematosus; CLE = cutaneous lupus erythematosus; DLE = discoid lupus erythematosus; CCLE = chronic cutaneous lupus erythematosus
As our understanding of CLE pathogenesis matures, novel therapeutic targets may be identified that lead to the development of new treatments. For instance, in the STING-interferon-beta pathway, signaling begins with the binding of dsDNA by cyclic GMP-AMP synthase (cGAS) and ultimately results in the upregulation of interferon response genes [145]. Antimalarials were recently observed to prevent the initial cGAS-dsDNA binding interaction, suggesting that other inhibitors of this pathway could be designed as alternative treatments for CLE [146]. Antimalarial inhibition of endosomal TLRs, which was recently demonstrated to occur through nucleic acid binding, may provide a similar opportunity for treatment development [147]. Proteins involved in apoptotic signaling such as TWEAK [148, 149], TRAIL [150], and Fas/FasL [151, 152] have also been implicated in the pathogenesis of CLE. Similarly, elevated levels of corticotropin-releasing hormone [153], anti-C1q antibodies [154], and serum cytokine CXCL16 [155] have been detected in CLE patients and suggest the wide array of molecules that could eventually serve as potential biomarkers for disease.
Pharmacoepigenetics may also be involved in future treatment development. Aberrant demethylation of B and T cell DNA has been suggested to play a role in SLE pathogenesis, and DNA methyltransferase inhibitors currently being implemented in the treatment of cancer may soon be applied to LE as well [156, 157]. In addition, CLE susceptibility loci in multiple antigen presentation, apoptosis, RNA processing, and interferon response genes have been identified by genome-wide analysis. Identification of these CLE-associated SNPs in patients would then have a significant impact on counseling and preventative treatment practices [158].
In addition, there are likely different pathways activated, leading to similar phenotypes. Dissecting these pathways and individualizing approaches to treatment are likely to be fruitful approaches in the future.
Figure 1.
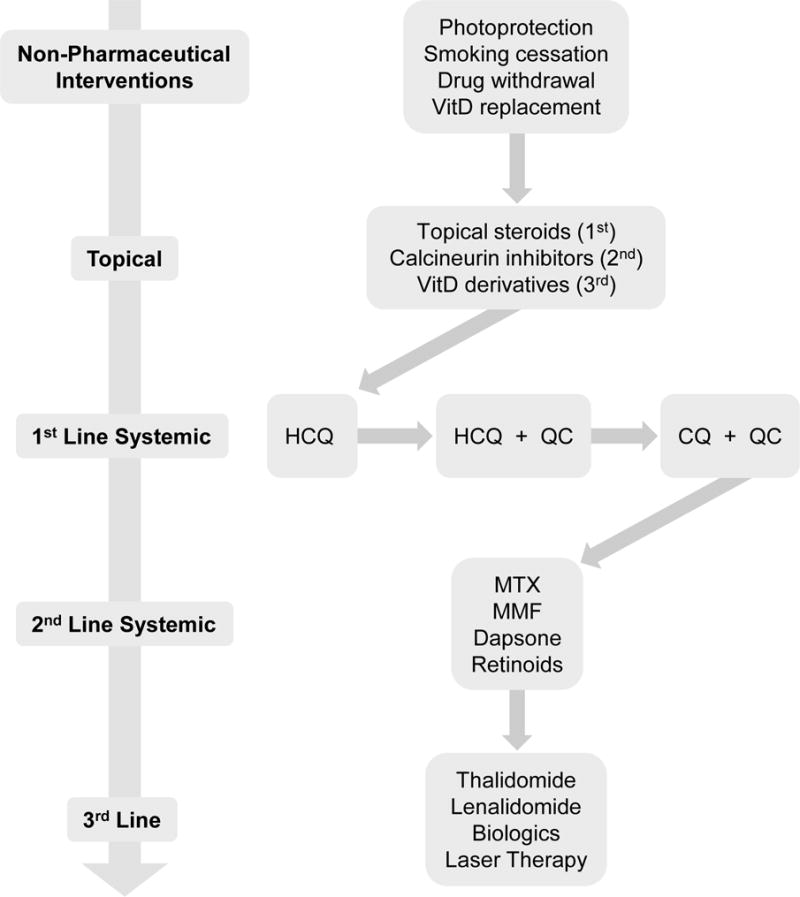
Cutaneous lupus erythematosus treatment algorithm. Treatment of mild or local disease begins with topical therapies, whereas treatment of severe or widespread disease begins with systemic therapies. If the response to therapy is inadequate, arrows indicate the direction of treatment progression. In many cases, combining therapies from multiple classes (e.g. first- and second-line systemic treatments) may be necessary.
HCQ = hydroxychloroquine; QC = quinacrine; CQ = chloroquine; MTX = methotrexate; MMF = mycophenolate mofetil
Table 1.
Level and subtype-specific distribution of evidence for cutaneous lupus erythematosus treatments. Evidence level: +/− = weak or controversial support; + = support limited to case reports or similar; ++ = support limited to non-randomized studies or similar; +++ = support limited to randomized trials or similar. Evidence distribution: check mark = corresponding literature explicitly demonstrates favorable response to the treatment in this subtype; question mark = corresponding literature only provides weak or controversial support for treatment in this subtype; x mark = corresponding literature explicitly demonstrates little or no response to the treatment in this subtype; blank = majority of literature does not explicitly address effects of the treatment in this subtype.
| Treatment | Evidence Level | Evidence Distribution | |||||
|---|---|---|---|---|---|---|---|
| ACLE | SCLE | DLE | LEP | LET | Bullous LE | ||
| Topicals | |||||||
|
| |||||||
| Topical corticosteroids | +++ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Calcineurin inhibitors | +++ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Vitamin D derivatives | + | ✓ | ✓ | ✓ | ✓ | ✓ | |
|
| |||||||
| Antimalarials | |||||||
|
| |||||||
| Hydroxychloroquine | +++ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Chloroquine | +++ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Quinacrine | ++ | ✓ | ✓ | ✓ | ✓ | ✓ | |
|
| |||||||
| Retinoids | |||||||
|
| |||||||
| Acitretin | +++ | ✓ | ✓ | ✓ | |||
| Isotretinoin | ++ | ✓ | ✓ | ✓ | |||
| Alitretinoin | + | ✓ | ✓ | ✓ | |||
|
| |||||||
| Immunosuppressives | |||||||
|
| |||||||
| Methotrexate | ++ | ✓ | ✓ | ✓ | ✓ | ||
| Mycophenolate mofetil | ++ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Azathioprine* | +/− | ? | ? | ? | ? | ? | |
|
| |||||||
| Immunomodulators | |||||||
|
| |||||||
| Dapsone | ++ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Thalidomide | ++ | ✓ | ✓ | ✓ | ✓ | ? | |
| Lenalidomide | ++ | ✓ | ✓ | ✓ | |||
|
| |||||||
| Biologics | |||||||
|
| |||||||
| Rituximab | ++ | ✓ | ✗ | ✗ | ✗ | ✓ | |
| Belimumab | + | ✓ | |||||
| Ustekinumab | + | ✓ | ✓ | ||||
| Tocilizumab | + | ✓ | |||||
|
| |||||||
| Laser Therapy | |||||||
|
| |||||||
| Pulsed dye laser | ++ | ✓ | ✓ | ✓ | ✓ | ✓ | |
Though evidence supporting its use in CLE is weak, azathioprine may be used in pregnancy if no alternatives are available.
ACLE = acute cutaneous lupus erythematosus; SCLE = subacute cutaneous lupus erythematosus; DLE = discoid lupus erythematosus; LEP = lupus erythematosus panniculitis; LET = lupus erythematosus tumidus
Key Issues.
Increased CLASI™-measured disease severity is associated with worse quality of life in CLE.
Preventative practices such as photoprotection and smoking cessation should always be recommended. Drug withdrawal or vitamin D replacement can also be helpful in patients with drug-induced lesions or vitamin D deficiency.
Depending on the severity of disease, first-line treatment of CLE lesions may involve topical corticosteroids or oral antimalarials.
Alternative treatment options include methotrexate, mycophenolate mofetil, dapsone, and retinoids.
Biologics have emerged as a major class of drugs in the treatment of recalcitrant CLE.
There is not yet an FDA-approved drug for CLE. Belimumab has been approved for use in patients with SLE.
Continued research on CLE pathogenesis has identified multiple potential therapeutic targets and may contribute to the development of novel treatments in the coming years.
Certain genetic polymorphisms and epigenetic modifications have been suggested to contribute to LE susceptibility and disease. Pharmacoepigenetics may play an important role in future treatment development.
Acknowledgments
This project is supported by the Department of Veterans Affairs Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development 5 I01 BX000706-04 and NIH 1R21AR066286 to VP Werth. VP Werth has grants from Celgene, Biogen, Janssen, Amgen. She consults for Biogen, Medimmune, Celgene, Pfizer, Janssen, GSK, and Sanofi. VP Werth developed the CLASI, and the copyright is owned by the University of Pennsylvania.
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. Journal of autoimmunity. 2014 Feb-Mar;48–49:14–9. doi: 10.1016/j.jaut.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997 Sep;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 3.Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis and rheumatism. 2012 Aug;64(8):2677–86. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Moghadam-Kia S, LoMonico J, et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Archives of dermatology. 2011 Feb;147(2):203–8. doi: 10.1001/archdermatol.2010.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolly M, Kazmi N, Mikolaitis RA, et al. Validation of the Cutaneous Lupus Disease Area and Severity Index (CLASI) using physician- and patient-assessed health outcome measures. Journal of the American Academy of Dermatology. 2013 Apr;68(4):618–23. doi: 10.1016/j.jaad.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Moghadam-Kia S, Taylor L, et al. Quality of life in cutaneous lupus erythematosus. Journal of the American Academy of Dermatology. 2011 May;64(5):849–58. doi: 10.1016/j.jaad.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasquez R, Wang D, Tran QP, et al. A multicentre, cross-sectional study on quality of life in patients with cutaneous lupus erythematosus. The British journal of dermatology. 2013 Jan;168(1):145–53. doi: 10.1111/j.1365-2133.2012.11106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 *.Chang AY, Ghazi E, Okawa J, et al. Quality of life differences between responders and nonresponders in the treatment of cutaneous lupus erythematosus. JAMA dermatology. 2013 Jan;149(1):104–6. doi: 10.1001/2013.jamadermatol.467. This prospective study demonstrated a strong association between patient response to antimalarial therapy and improved quality of life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wahie S, McColl E, Reynolds NJ, et al. Measuring disease activity and damage in discoid lupus erythematosus. The British journal of dermatology. 2010 May;162(5):1030–7. doi: 10.1111/j.1365-2133.2010.09656.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn A, Meuth AM, Bein D, et al. Revised Cutaneous Lupus Erythematosus Disease Area and Severity Index (RCLASI): a modified outcome instrument for cutaneous lupus erythematosus. The British journal of dermatology. 2010 Jul;163(1):83–92. doi: 10.1111/j.1365-2133.2010.09799.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn A, Wozniacka A, Szepietowski JC, et al. Photoprovocation in cutaneous lupus erythematosus: a multicenter study evaluating a standardized protocol. The Journal of investigative dermatology. 2011 Aug;131(8):1622–30. doi: 10.1038/jid.2011.101. [DOI] [PubMed] [Google Scholar]
- 12.Ruland V, Haust M, Stilling RM, et al. Updated analysis of standardized photoprovocation in patients with cutaneous lupus erythematosus. Arthritis care & research. 2013 May;65(5):767–76. doi: 10.1002/acr.21867. [DOI] [PubMed] [Google Scholar]
- 13.Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best practice & research Clinical rheumatology. 2013 Jun;27(3):391–404. doi: 10.1016/j.berh.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahn S, Graef M, Patsinakidis N, et al. Ultraviolet light protection by a sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Experimental dermatology. 2014 Jul;23(7):516–8. doi: 10.1111/exd.12428. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn A, Gensch K, Haust M, et al. Photoprotective effects of a broad-spectrum sunscreen in ultraviolet-induced cutaneous lupus erythematosus: a randomized, vehicle-controlled, double-blind study. Journal of the American Academy of Dermatology. 2011 Jan;64(1):37–48. doi: 10.1016/j.jaad.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 16.Patsinakidis N, Wenzel J, Landmann A, et al. Suppression of UV-induced damage by a liposomal sunscreen: a prospective, open-label study in patients with cutaneous lupus erythematosus and healthy controls. Experimental dermatology. 2012 Dec;21(12):958–61. doi: 10.1111/exd.12035. [DOI] [PubMed] [Google Scholar]
- 17.Yang SY, Bernstein I, Lin DQ, et al. Photoprotective habits of patients with cutaneous lupus erythematosus. Journal of the American Academy of Dermatology. 2013 Jun;68(6):944–51. doi: 10.1016/j.jaad.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutmark EL, Lin DQ, Bernstein I, et al. Sunscreen use in patients with cutaneous lupus erythematosus. The British journal of dermatology. 2015 Sep;173(3):831–4. doi: 10.1111/bjd.13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenton L, Dawe R, Ibbotson S, et al. Impact assessment of energy-efficient lighting in patients with lupus erythematosus: a pilot study. The British journal of dermatology. 2014 Mar;170(3):694–8. doi: 10.1111/bjd.12719. [DOI] [PubMed] [Google Scholar]
- 20.Tiao J, Werth VP. Cutaneous lupus erythematosus flare following exposure to surgical light during a dental procedure. BMJ case reports. 2015;2015 doi: 10.1136/bcr-2015-212864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piette EW, Foering KP, Chang AY, et al. Impact of smoking in cutaneous lupus erythematosus. Archives of dermatology. 2012 Mar;148(3):317–22. doi: 10.1001/archdermatol.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhn A, Sigges J, Biazar C, et al. Influence of smoking on disease severity and antimalarial therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. The British journal of dermatology. 2014 Sep;171(3):571–9. doi: 10.1111/bjd.13006. [DOI] [PubMed] [Google Scholar]
- 23.Bockle BC, Sepp NT. Smoking is highly associated with discoid lupus erythematosus and lupus erythematosus tumidus: analysis of 405 patients. Lupus. 2015 Jun;24(7):669–74. doi: 10.1177/0961203314559630. [DOI] [PubMed] [Google Scholar]
- 24.Werth VP, Khamashta MA, Illei GG, et al. Smoking is associated with more severe skin disease in subjects with moderate to severe systemic lupus erythematosus (SLE) Arthritis and rheumatism. 2013;65(S10):2512. [Google Scholar]
- 25.Chasset F, Frances C, Barete S, et al. Influence of smoking on the efficacy of antimalarials in cutaneous lupus: a meta-analysis of the literature. Journal of the American Academy of Dermatology. 2015 Apr;72(4):634–9. doi: 10.1016/j.jaad.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 26.Kwatra SG. Toll-like receptor-9 signaling and decreased efficacy of antimalarial drugs in smokers with cutaneous lupus erythematosus. Journal of the American Academy of Dermatology. 2015 Aug;73(2):e79. doi: 10.1016/j.jaad.2015.04.068. [DOI] [PubMed] [Google Scholar]
- 27 *.Wahie S, Daly AK, Cordell HJ, et al. Clinical and pharmacogenetic influences on response to hydroxychloroquine in discoid lupus erythematosus: a retrospective cohort study. The Journal of investigative dermatology. 2011 Oct;131(10):1981–6. doi: 10.1038/jid.2011.167. This retrospective cohort study observed that smoking was associated with increased disease severity rather than response to HCQ. [DOI] [PubMed] [Google Scholar]
- 28.Marzano AV, Lazzari R, Polloni I, et al. Drug-induced subacute cutaneous lupus erythematosus: evidence for differences from its idiopathic counterpart. The British journal of dermatology. 2011 Aug;165(2):335–41. doi: 10.1111/j.1365-2133.2011.10397.x. [DOI] [PubMed] [Google Scholar]
- 29.Gronhagen CM, Fored CM, Linder M, et al. Subacute cutaneous lupus erythematosus and its association with drugs: a population-based matched case-control study of 234 patients in Sweden. The British journal of dermatology. 2012 Aug;167(2):296–305. doi: 10.1111/j.1365-2133.2012.10969.x. [DOI] [PubMed] [Google Scholar]
- 30.Liakou AI, Brunner M, Theodorakis MJ, et al. Recurrent subacute cutaneous lupus erythematosus following exposure to different drugs. Acta dermato-venereologica. 2011 Sep;91(5):586–7. doi: 10.2340/00015555-1115. [DOI] [PubMed] [Google Scholar]
- 31.Cutillas-Marco E, Marquina-Vila A, Grant WB, et al. Vitamin D and cutaneous lupus erythematosus: effect of vitamin D replacement on disease severity. Lupus. 2014 Jun;23(7):615–23. doi: 10.1177/0961203314522338. [DOI] [PubMed] [Google Scholar]
- 32.Drozdenko G, Heine G, Worm M. Oral vitamin D increases the frequencies of CD38+ human B cells and ameliorates IL-17-producing T cells. Experimental dermatology. 2014 Feb;23(2):107–12. doi: 10.1111/exd.12300. [DOI] [PubMed] [Google Scholar]
- 33.de Azevedo Silva J, Monteiro Fernandes K, Tres Pancotto JA, et al. Vitamin D receptor (VDR) gene polymorphisms and susceptibility to systemic lupus erythematosus clinical manifestations. Lupus. 2013 Oct;22(11):1110–7. doi: 10.1177/0961203313500549. [DOI] [PubMed] [Google Scholar]
- 34.Roenigk HH, Jr, Martin JS, Eichorn P, et al. Discoid lupus erythematosus. Diagnostic features and evaluation of topical corticosteroid therapy. Cutis. 1980 Mar;25(3):281–5. [PubMed] [Google Scholar]
- 35.Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. Journal of the American Academy of Dermatology. 2011 Dec;65(6):e179–93. doi: 10.1016/j.jaad.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 36.Sticherling M. Update on the use of topical calcineurin inhibitors in cutaneous lupus erythematosus. Biologics : targets & therapy. 2011;5:21–31. doi: 10.2147/BTT.S9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avgerinou G, Papafragkaki DK, Nasiopoulou A, et al. Effectiveness of topical calcineurin inhibitors as monotherapy or in combination with hydroxychloroquine in cutaneous lupus erythematosus. Journal of the European Academy of Dermatology and Venereology : JEADV. 2012 Jun;26(6):762–7. doi: 10.1111/j.1468-3083.2011.04161.x. [DOI] [PubMed] [Google Scholar]
- 38.Pothinamthong P, Janjumratsang P. A comparative study in efficacy and safety of 0.1% tacrolimus and 0.05% clobetasol propionate ointment in discoid lupus erythematosus by modified cutaneous lupus erythematosus disease area and severity index. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2012 Jul;95(7):933–40. [PubMed] [Google Scholar]
- 39.Kuhn A, Gensch K, Haust M, et al. Efficacy of tacrolimus 0.1% ointment in cutaneous lupus erythematosus: a multicenter, randomized, double-blind, vehicle-controlled trial. Journal of the American Academy of Dermatology. 2011 Jul;65(1):54–64. 64 e1–2. doi: 10.1016/j.jaad.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 40.Milam EC, Ramachandran S, Franks AG., Jr Treatment of Scarring Alopecia in Discoid Variant of Chronic Cutaneous Lupus Erythematosus With Tacrolimus Lotion, 0.3. JAMA dermatology. 2015 Oct 1;151(10):1113–6. doi: 10.1001/jamadermatol.2015.1349. [DOI] [PubMed] [Google Scholar]
- 41.May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Current drug targets Inflammation and allergy. 2004 Dec;3(4):377–93. doi: 10.2174/1568010042634596. [DOI] [PubMed] [Google Scholar]
- 42.Nagpal S, Lu J, Boehm MF. Vitamin D analogs: mechanism of action and therapeutic applications. Current medicinal chemistry. 2001 Nov;8(13):1661–79. doi: 10.2174/0929867013371950. [DOI] [PubMed] [Google Scholar]
- 43.Brown AJ, Slatopolsky E. Vitamin D analogs: therapeutic applications and mechanisms for selectivity. Molecular aspects of medicine. 2008 Dec;29(6):433–52. doi: 10.1016/j.mam.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Callen JP. Cutaneous lupus erythematosus: a personal approach to management. The Australasian journal of dermatology. 2006 Feb;47(1):13–27. doi: 10.1111/j.1440-0960.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 45.Knott HM, Martinez JD. Innovative management of lupus erythematosus. Dermatologic clinics. 2010 Jul;28(3):489–99. doi: 10.1016/j.det.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Callen JP. Management of skin disease in patients with lupus erythematosus. Best practice & research Clinical rheumatology. 2002 Apr;16(2):245–64. doi: 10.1053/berh.2001.0224. [DOI] [PubMed] [Google Scholar]
- 47.Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011 Feb;118(2):415–22. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 48.Levy GD, Munz SJ, Paschal J, et al. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis and rheumatism. 1997 Aug;40(8):1482–6. doi: 10.1002/art.1780400817. [DOI] [PubMed] [Google Scholar]
- 49.Finbloom DS, Silver K, Newsome DA, et al. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. The Journal of rheumatology. 1985 Aug;12(4):692–4. [PubMed] [Google Scholar]
- 50.Rainsford KD, Parke AL, Clifford-Rashotte M, et al. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015 Oct;23(5):231–69. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 51.Wahie S, Meggitt SJ. Long-term response to hydroxychloroquine in patients with discoid lupus erythematosus. The British journal of dermatology. 2013 Sep;169(3):653–9. doi: 10.1111/bjd.12378. [DOI] [PubMed] [Google Scholar]
- 52.Chang AY, Piette EW, Foering KP, et al. Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis. Archives of dermatology. 2011;147(11):1261–7. doi: 10.1001/archdermatol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farhangian ME, Huang WW, Feldman SR. Adherence to Oral and Topical Medications in Cutaneous Lupus Erythematosus is not Well Characterized. Dermatology and therapy. 2015 Jun;5(2):91–105. doi: 10.1007/s13555-015-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costedoat-Chalumeau N, Pouchot J, Guettrot-Imbert G, et al. Adherence to treatment in systemic lupus erythematosus patients. Best practice & research Clinical rheumatology. 2013 Jun;27(3):329–40. doi: 10.1016/j.berh.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 55 *.Frances C, Cosnes A, Duhaut P, et al. Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: a French multicenter prospective study. Archives of dermatology. 2012 Apr;148(4):479–84. doi: 10.1001/archdermatol.2011.2558. This prospective study revealed a 10% rate of patient non-adherence to antimalarial therapy, highlighting the importance of monitoring HCQ levels during treatment. [DOI] [PubMed] [Google Scholar]
- 56.Kuhn A, Patsinakidis N, Luger T. Alitretinoin for cutaneous lupus erythematosus. Journal of the American Academy of Dermatology. 2012 Sep;67(3):e123–6. doi: 10.1016/j.jaad.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 57.D’Erme AM, Milanesi N, Difonzo EM, et al. Treatment of refractory subacute cutaneous lupus erythematosus with oral isotretinoin: a valid therapeutic option. Dermatologic therapy. 2012 May-Jun;25(3):281–2. doi: 10.1111/j.1529-8019.2012.01461.x. [DOI] [PubMed] [Google Scholar]
- 58.Lospinoso DJ, Fernelius C, Edhegard KD, et al. Lupus erythematosus/lichen planus overlap syndrome: successful treatment with acitretin. Lupus. 2013 Jul;22(8):851–4. doi: 10.1177/0961203313492243. [DOI] [PubMed] [Google Scholar]
- 59.Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part II. Journal of the American Academy of Dermatology. 2011 Dec;65(6):e195–213. doi: 10.1016/j.jaad.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 60.Wenzel J, Brahler S, Bauer R, et al. Efficacy and safety of methotrexate in recalcitrant cutaneous lupus erythematosus: results of a retrospective study in 43 patients. The British journal of dermatology. 2005 Jul;153(1):157–62. doi: 10.1111/j.1365-2133.2005.06552.x. [DOI] [PubMed] [Google Scholar]
- 61.Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatology international. 1998;18(2):59–62. doi: 10.1007/s002960050058. [DOI] [PubMed] [Google Scholar]
- 62.Kuhn A, Specker C, Ruzicka T, et al. Methotrexate treatment for refractory subacute cutaneous lupus erythematosus. Journal of the American Academy of Dermatology. 2002 Apr;46(4):600–3. doi: 10.1067/mjd.2002.114608. [DOI] [PubMed] [Google Scholar]
- 63.Bohm L, Uerlich M, Bauer R. Rapid improvement of subacute cutaneous lupus erythematosus with low-dose methotrexate. Dermatology. 1997;194(3):307–8. doi: 10.1159/000246141. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein E, Carey W. Discoid lupus erythematosus: successful treatment with oral methotrexate. Archives of dermatology. 1994 Jul;130(7):938–9. [PubMed] [Google Scholar]
- 65.Islam MN, Hossain M, Haq SA, et al. Efficacy and safety of methotrexate in articular and cutaneous manifestations of systemic lupus erythematosus. International journal of rheumatic diseases. 2012 Feb;15(1):62–8. doi: 10.1111/j.1756-185X.2011.01665.x. [DOI] [PubMed] [Google Scholar]
- 66.Klein A, Vogt T, Wenzel SM, et al. Cyclosporin combined with methotrexate in two patients with recalcitrant subacute cutaneous lupus erythematosus. The Australasian journal of dermatology. 2011 Feb;52(1):43–7. doi: 10.1111/j.1440-0960.2010.00689.x. [DOI] [PubMed] [Google Scholar]
- 67.Miyawaki S, Nishiyama S, Aita T, et al. The effect of methotrexate on improving serological abnormalities of patients with systemic lupus erythematosus. Modern rheumatology / the Japan Rheumatism Association. 2013 Jul;23(4):659–66. doi: 10.1007/s10165-012-0707-9. [DOI] [PubMed] [Google Scholar]
- 68.Sakthiswary R, Suresh E. Methotrexate in systemic lupus erythematosus: a systematic review of its efficacy. Lupus. 2014 Mar;23(3):225–35. doi: 10.1177/0961203313519159. [DOI] [PubMed] [Google Scholar]
- 69.Fortin PR, Abrahamowicz M, Ferland D, et al. Steroid-sparing effects of methotrexate in systemic lupus erythematosus: a double-blind, randomized, placebo-controlled trial. Arthritis and rheumatism. 2008 Dec 15;59(12):1796–804. doi: 10.1002/art.24068. [DOI] [PubMed] [Google Scholar]
- 70.Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. Journal of the American Academy of Dermatology. 2011 Oct;65(4):717–21. doi: 10.1016/j.jaad.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Sadlier M, Kirby B, Lally A. Mycophenolate mofetil and hydroxychloroquine: an effective treatment for recalcitrant cutaneous lupus erythematosus. Journal of the American Academy of Dermatology. 2012 Jan;66(1):160–1. doi: 10.1016/j.jaad.2011.08.036. author reply 61-2. [DOI] [PubMed] [Google Scholar]
- 72.Chang AY, Werth VP. Treatment of cutaneous lupus. Current rheumatology reports. 2011 Aug;13(4):300–7. doi: 10.1007/s11926-011-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu KL, Shen JL, Yang CS, et al. Bullous systemic lupus erythematosus in a child responding to dapsone. Pediatric dermatology. 2014 Jul-Aug;31(4):e104–6. doi: 10.1111/pde.12340. [DOI] [PubMed] [Google Scholar]
- 74.Cortes-Hernandez J, Torres-Salido M, Castro-Marrero J, et al. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. The British journal of dermatology. 2012 Mar;166(3):616–23. doi: 10.1111/j.1365-2133.2011.10693.x. [DOI] [PubMed] [Google Scholar]
- 75.Baret I, De Haes P. Thalidomide: Still an important second-line treatment in refractory cutaneous lupus erythematosus? The Journal of dermatological treatment. 2015 Apr;26(2):173–7. doi: 10.3109/09546634.2014.906036. [DOI] [PubMed] [Google Scholar]
- 76.Frankel HC, Sharon VR, Vleugels RA, et al. Lower-dose thalidomide therapy effectively treats cutaneous lupus erythematosus but is limited by neuropathic toxicity. International journal of dermatology. 2013 Nov;52(11):1407–9. doi: 10.1111/j.1365-4632.2011.05200.x. [DOI] [PubMed] [Google Scholar]
- 77.Cortes-Hernandez J, Avila G, Vilardell-Tarres M, et al. Efficacy and safety of lenalidomide for refractory cutaneous lupus erythematosus. Arthritis research & therapy. 2012;14(6):R265. doi: 10.1186/ar4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braunstein I, Goodman NG, Rosenbach M, et al. Lenalidomide therapy in treatment-refractory cutaneous lupus erythematosus: histologic and circulating leukocyte profile and potential risk of a systemic lupus flare. Journal of the American Academy of Dermatology. 2012 Apr;66(4):571–82. doi: 10.1016/j.jaad.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okon L, Rosenbach M, Krathen M, et al. Lenalidomide in treatment-refractory cutaneous lupus erythematosus: Efficacy and safety in a 52-week trial. Journal of the American Academy of Dermatology. 2014 Mar;70(3):583–4. doi: 10.1016/j.jaad.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cobo-Ibanez T, Loza-Santamaria E, Pego-Reigosa JM, et al. Efficacy and safety of rituximab in the treatment of non-renal systemic lupus erythematosus: a systematic review. Seminars in arthritis and rheumatism. 2014 Oct;44(2):175–85. doi: 10.1016/j.semarthrit.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Hofmann SC, Leandro MJ, Morris SD, et al. Effects of rituximab-based B-cell depletion therapy on skin manifestations of lupus erythematosus–report of 17 cases and review of the literature. Lupus. 2013 Aug;22(9):932–9. doi: 10.1177/0961203313497115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ezeonyeji AN, Isenberg DA. Early treatment with rituximab in newly diagnosed systemic lupus erythematosus patients: a steroid-sparing regimen. Rheumatology. 2012 Mar;51(3):476–81. doi: 10.1093/rheumatology/ker337. [DOI] [PubMed] [Google Scholar]
- 83 *.Vital EM, Wittmann M, Edward S, et al. Brief report: responses to rituximab suggest B cell-independent inflammation in cutaneous systemic lupus erythematosus. Arthritis & rheumatology. 2015 Jun;67(6):1586–91. doi: 10.1002/art.39085. This prospective study identified variable rituximab response rates in patients with different subtypes of CLE. [DOI] [PubMed] [Google Scholar]
- 84.Fanto M, Salemi S, Socciarelli F, et al. A case of subacute cutaneous lupus erythematosus in a patient with mixed connective tissue disease: successful treatment with plasmapheresis and rituximab. Case reports in rheumatology. 2013;2013:857694. doi: 10.1155/2013/857694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cieza-Diaz DE, Aviles-Izquierdo JA, Ceballos-Rodriguez C, et al. Refractory subacute cutaneous lupus erythematosus treated with rituximab. Actas dermo-sifiliograficas. 2012 Jul-Aug;103(6):555–7. doi: 10.1016/j.ad.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 86.Moreno-Suarez F, Pulpillo-Ruiz A. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatologic therapy. 2013 Sep-Oct;26(5):415–8. doi: 10.1111/dth.12014. [DOI] [PubMed] [Google Scholar]
- 87.Alsanafi S, Kovarik C, Mermelstein AL, et al. Rituximab in the treatment of bullous systemic lupus erythematosus. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2011 Apr;17(3):142–4. doi: 10.1097/RHU.0b013e318214f30c. [DOI] [PubMed] [Google Scholar]
- 88.Borba HH, Wiens A, de Souza TT, et al. Efficacy and safety of biologic therapies for systemic lupus erythematosus treatment: systematic review and meta-analysis. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2014 Apr;28(2):211–28. doi: 10.1007/s40259-013-0074-x. [DOI] [PubMed] [Google Scholar]
- 89.Husein-ElAhmed H, Callejas-Rubio JL, Rios-Fernandez R, et al. Refractory subacute cutaneous lupus erythematous responding to a single course of belimumab: a new anti-BLyS human monoclonal antibody. Indian journal of dermatology, venereology and leprology. 2014 Sep-Oct;80(5):477–8. doi: 10.4103/0378-6323.140335. [DOI] [PubMed] [Google Scholar]
- 90 **.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011 Feb 26;377(9767):721–31. doi: 10.1016/S0140-6736(10)61354-2. The BLISS-52 trial. Data from this and the BLISS-76 trial demonstrated the safety and efficacy of belimumab, the first and only drug approved for the treatment of SLE. [DOI] [PubMed] [Google Scholar]
- 91 **.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2011 Dec;63(12):3918–30. doi: 10.1002/art.30613. The BLISS-76 trial. Data from this and the BLISS-52 trial demonstrated the safety and efficacy of belimumab, the first and only drug approved for the treatment of SLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manzi S, Sanchez-Guerrero J, Merrill JT, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Annals of the rheumatic diseases. 2012 Nov;71(11):1833–8. doi: 10.1136/annrheumdis-2011-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strand V, Levy RA, Cervera R, et al. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Annals of the rheumatic diseases. 2014 May;73(5):838–44. doi: 10.1136/annrheumdis-2012-202865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ginzler EM, Wallace DJ, Merrill JT, et al. Disease control and safety of belimumab plus standard therapy over 7 years in patients with systemic lupus erythematosus. The Journal of rheumatology. 2014 Feb;41(2):300–9. doi: 10.3899/jrheum.121368. [DOI] [PubMed] [Google Scholar]
- 95.van Vollenhoven RF, Petri MA, Cervera R, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Annals of the rheumatic diseases. 2012 Aug;71(8):1343–9. doi: 10.1136/annrheumdis-2011-200937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Souza A, Ali-Shaw T, Strober BE, et al. Successful treatment of subacute lupus erythematosus with ustekinumab. Archives of dermatology. 2011 Aug;147(8):896–8. doi: 10.1001/archdermatol.2011.185. [DOI] [PubMed] [Google Scholar]
- 97.Dahl C, Johansen C, Kragballe K, et al. Ustekinumab in the treatment of refractory chronic cutaneous lupus erythematosus: a case report. Acta dermato-venereologica. 2013 May;93(3):368–9. doi: 10.2340/00015555-1467. [DOI] [PubMed] [Google Scholar]
- 98.Winchester D, Duffin KC, Hansen C. Response to ustekinumab in a patient with both severe psoriasis and hypertrophic cutaneous lupus. Lupus. 2012 Aug;21(9):1007–10. doi: 10.1177/0961203312441982. [DOI] [PubMed] [Google Scholar]
- 99.Szepietowski JC, Nilganuwong S, Wozniacka A, et al. Phase I, randomized, double-blind, placebo-controlled, multiple intravenous, dose-ascending study of sirukumab in cutaneous or systemic lupus erythematosus. Arthritis and rheumatism. 2013 Oct;65(10):2661–71. doi: 10.1002/art.38091. [DOI] [PubMed] [Google Scholar]
- 100.Makol A, Gibson LE, Michet CJ. Successful use of interleukin 6 antagonist tocilizumab in a patient with refractory cutaneous lupus and urticarial vasculitis. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2012 Mar;18(2):92–5. doi: 10.1097/RHU.0b013e31823ecd73. [DOI] [PubMed] [Google Scholar]
- 101.Juptner M, Zeuner R, Schreiber S, et al. Successful application of belimumab in two patients with systemic lupus erythematosus experiencing a flare during tocilizumab treatment. Lupus. 2014 Apr;23(4):428–30. doi: 10.1177/0961203314520844. [DOI] [PubMed] [Google Scholar]
- 102.Furie R, Merrill JT, Werth VP, et al. Anifrolumab, an Anti-Interferon Alpha Receptor Monoclonal Antibody, in Moderate to Severe Systemic Lupus Erythematosus. Arthritis & rheumatology. 2015;67(suppl 10) doi: 10.1002/art.39962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merrill JT, Wallace DJ, Petri M, et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon alpha monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Annals of the rheumatic diseases. 2011 Nov;70(11):1905–13. doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- 104.Petri M, Wallace DJ, Spindler A, et al. Sifalimumab, a human anti-interferon-alpha monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis and rheumatism. 2013 Apr;65(4):1011–21. doi: 10.1002/art.37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khamashta M, Merrill JT, Werth VP, et al. Safety and Efficacy of Sifalimumab, an Anti IFN-Alpha Monoclonal Antibody, in a Phase 2b Study of Moderate to Severe Systemic Lupus Erythematosus. Arthritis & rheumatology. in press. [Google Scholar]
- 106.Werth V, Fiorentino D, Cohen S, et al. A phase I single-dose crossover study to evaluate the safety, toelrability, pharmacokinetics, pharmacodyanmics, and clinical efficacy of AMG 811 (anti-IFN-gamma) in subjects with discoid lupus erythematosus [abstract] Arthritis and rheumatism. 2013;65(Suppl 10):1608. [Google Scholar]
- 107.Belmont HM. Treatment of systemic lupus erythematosus - 2013 update. Bulletin of the Hospital for Joint Disease. 2013;71(3):208–13. [PubMed] [Google Scholar]
- 108.Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2011 Jul;37(7):971–81. doi: 10.1111/j.1524-4725.2011.02041.x. [DOI] [PubMed] [Google Scholar]
- 109.Truchuelo MT, Boixeda P, Alcantara J, et al. Pulsed dye laser as an excellent choice of treatment for lupus tumidus: a prospective study. Journal of the European Academy of Dermatology and Venereology : JEADV. 2012 Oct;26(10):1272–9. doi: 10.1111/j.1468-3083.2011.04281.x. [DOI] [PubMed] [Google Scholar]
- 110.Yelamos O, Roe E, Baselga E, et al. Pediatric cutaneous lupus erythematosus treated with pulsed dye laser. Pediatric dermatology. 2014 Jan-Feb;31(1):113–5. doi: 10.1111/pde.12248. [DOI] [PubMed] [Google Scholar]
- 111.Park KY, Lee JW, Li K, et al. Treatment of refractory discoid lupus erythematosus using 1,064-nm long-pulse neodymium-doped yttrium aluminum garnet laser. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2011 Jul;37(7):1055–6. doi: 10.1111/j.1524-4725.2011.02019.x. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y, Huang W, Li F, et al. Octreotide for the treatment of systemic lupus erythematosus: clinical effects and an in vitro study on its therapeutic mechanism. Lupus. 2011 Oct;20(11):1172–81. doi: 10.1177/0961203311409268. [DOI] [PubMed] [Google Scholar]
- 113.Sugita K, Kabashima R, Kawakami C, et al. Therapeutic efficacy of mizoribine for discoid lupus erythematosus with normalized frequency of circulating T helper 17 cells. Clinical and experimental dermatology. 2011 Apr;36(3):315–7. doi: 10.1111/j.1365-2230.2010.03971.x. [DOI] [PubMed] [Google Scholar]
- 114.Stohl W, Merrill JT, Looney RJ, et al. Treatment of systemic lupus erythematosus patients with the BAFF antagonist “peptibody” blisibimod (AMG 623/A-623): results from randomized, double-blind phase 1a and phase 1b trials. Arthritis research & therapy. 2015;17:215. doi: 10.1186/s13075-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ky C, Swasdibutra B, Khademi S, et al. Efficacy of Intravenous Immunoglobulin Monotherapy in Patients with Cutaneous Lupus Erythematosus: Results of Proof-of-Concept Study. Dermatology reports. 2015 Mar 16;7(1):5804. doi: 10.4081/dr.2015.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Collins E, Gilkeson G. Hematopoetic and mesenchymal stem cell transplantation in the treatment of refractory systemic lupus erythematosus–where are we now? Clinical immunology. 2013 Sep;148(3):328–34. doi: 10.1016/j.clim.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 117.National Institute of Allergy and Infectious Diseases (NIAID) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Autologous Polyclonal Tregs for Lupus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02428309. NLM Identifier: NCT02428309. [Google Scholar]
- 118.Ablynx. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. A Phase II Study to Evaluate Safety and Efficacy of ALX-0061 in Subjects With Systemic Lupus Erythematosus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02437890?term=“cutaneous+lupus”&recr=Open&rank=16. NLM Identifier: NCT02437890. [Google Scholar]
- 119.Actelion. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Clinical Trial to Evaluate the PK, PD, Biological Activity and Safety of ACT-334441 in SLE. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02472795?term=NCT02472795&rank=1. NLM Identifier: NCT02472795. [Google Scholar]
- 120.Amgen. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2016. Single Ascending Dose Study of AMG 570 in Healthy Subjects. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02618967?term=NCT02618967&rank=1. NLM Identifier: NCT02618967. [Google Scholar]
- 121.Assistance Publique - Hôpitaux de Paris; Iltoo Pharma. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2014. Induction of Regulatory t Cells by Low Dose il2 in Autoimmune and Inflammatory Diseases (TRANSREG) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT01988506?term=NCT01988506&rank=1. NLM Identifier: NCT01988506. [Google Scholar]
- 122.AstraZeneca; PRA Health Sciences. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Efficacy and Safety of Anifrolumab Compared to Placebo in Adult Subjects With Active Systemic Lupus Erythematosus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02446899?term=NCT02446899&rank=1. NLM Identifier: NCT02446899. [Google Scholar]
- 123.Ben Chong; Daavlin Corporation. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2012. Low-dose UVA1 Radiation in Cutaneous Lupus Patients. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT01776190?term=“cutaneous+lupus”&recr=Open&rank=2. NLM Identifier: NCT01776190. [Google Scholar]
- 124.Biogen. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2014. Study Evaluating the Safety, Tolerability, and Pharmacokinetics of Single Doses and Multiple Doses of BIIB059 in Healthy Volunteers and Participants With Systemic Lupus Erythematosus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02106897?term=NCT02106897&rank=1. NLM Identifier: NCT02106897. [Google Scholar]
- 125.Biotest. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Proof-of-Concept Study With BT063 in Subjects With Systemic Lupus Erythematosus (BT063 in SLE) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02554019?term=NCT02554019&rank=1. NLM Identifier: NCT02554019. [Google Scholar]
- 126.Bristol-Myers Squibb. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2014. Safety and Efficacy Study of a Biologic to Treat Systemic Lupus Erythematosus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT02265744?term=“cutaneous+lupus”&recr=Open&rank=17. NLM Identifier: NCT02265744. [Google Scholar]
- 127.Celgene Corporation. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2014. A Pilot Study of CC-220 to Treat Systemic Lupus Erythematosus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02185040?term=“cutaneous+lupus”&recr=Open&rank=12. NLM Identifier: NCT02185040. [Google Scholar]
- 128.Charite University, Berlin, Germany; Prof Dr med Falk Hiepe (Charité, Internal Medicine / Rheumathology) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2014. Therapy of Antibody-mediated Autoimmune Diseases by Bortezomib (TAVAB) (TAVAB) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02102594?term=NCT02102594&rank=1. NLM Identifier: NCT02102594. [Google Scholar]
- 129.EMD Serono. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2013. Efficacy and Safety of Atacicept in Systemic Lupus Erythematosus (ADDRESS II) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT01972568?term=NCT01972568&rank=1. NLM Identifier: NCT01972568. [Google Scholar]
- 130.EMD Serono. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. MSC2364447C Phase 1b in Systemic Lupus Erythematosus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02537028?term=NCT02537028&rank=1. NLM Identifier: NCT02537028. [Google Scholar]
- 131.Immunomedics, Inc.; United States Department of Defense. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Phase Ib Study of SC Milatuzumab in SLE. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT01845740?term=lupus&recr=Open&no_unk=Y&cond=lupus+erythematosus&rank=7. NLM Identifier: NCT01845740. [Google Scholar]
- 132.ImmuPharma. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. A 52-Week, Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Study to Evaluate the Efficacy and Safety of a 200-mcg Dose of IPP-201101 Plus Standard of Care in Patients With Systemic Lupus Erythematosus (LUPUZOR) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02504645?term=NCT02504645&rank=1. NLM Identifier: NCT02504645. [Google Scholar]
- 133.Janssen Research Development LLC. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. A Phase 2a, Efficacy and Safety Study of Ustekinumab in Systemic Lupus Erythematosus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02349061?term=NCT02349061&rank=1. NLM Identifier: NCT02349061. [Google Scholar]
- 134.Medical University of South Carolina. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2016. A Controlled Trial of Allogeneic Mesenchymal Stem Cells for the Treatment of Refractory Lupus (MscISLE) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02633163?term=NCT02633163&rank=1. NLM Identifier: NCT02633163. [Google Scholar]
- 135.Mitsubishi Tanabe Pharma Corporation. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Exploratory Study of MT-1303 in Systemic Lupus Erythematosus Patients. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02307643?term=NCT02307643&rank=1. NLM Identifier: NCT02307643. [Google Scholar]
- 136.Nantes University Hospital. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2016. Autologous EBV-specific Cytotoxic T Cells for the Treatment of Systemic Lupus Erythematosus (SLE) (LUPUS CTL EBV) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02677688?term=NCT02677688&rank=1. NLM Identifier: NCT02677688. [Google Scholar]
- 137.National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2012. Omalizumab for Lupus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT01716312?term=NCT01716312&rank=1. NLM Identifier: NCT01716312. [Google Scholar]
- 138.National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Safety of Tofacitinib, an Oral Janus Kinase Inhibitor, in Systemic Lupus Erythematosus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02535689?term=NCT02535689&rank=1. NLM Identifier: NCT02535689. [Google Scholar]
- 139.Northwell Health; National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2014. Nelfinavir in Systemic Lupus Erythematosus (NISLE) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02066311?term=NCT02066311&rank=1. NLM Identifier: NCT02066311. [Google Scholar]
- 140.Oklahoma Medical Research Foundation. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2013. Dipyridamole Assessment for Flare Reduction in Systemic Lupus Erythematosus (SLE) (DARE) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/record/NCT01781611?term=“cutaneous+lupus”&recr=Active%2C+not+recruiting&rank=1. NLM Identifier: NCT01781611. [Google Scholar]
- 141.Resolve Therapeutics. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2016. A Phase 2a of RSLV-132 in Subjects With Systemic Lupus Erythematosus (SLE) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02660944?term=“cutaneous+lupus”&recr=Open&rank=13. NLM Identifier: NCT02660944. [Google Scholar]
- 142.Sanofi. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Pharmacodynamics Assessment Study After Single Subcutaneous Dose Of SAR113244 Versus Placebo In Lupus Male And Female Patients. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02331810?term=NCT02331810&rank=1. NLM Identifier: NCT02331810. [Google Scholar]
- 143.Seattle Genetics, Inc. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2015. Dose Ranging Study of Brentuximab Vedotin in Adults With Lupus. [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02533570?term=NCT02533570&rank=1. NLM Identifier: NCT02533570. [Google Scholar]
- 144.University of Leeds; National Institute for Health Research, United Kingdom; Pfizer; Clinical Trials Research Unit, Leeds. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2016. Targeted Therapy Using Intradermal Injection of Etanercept for Remission Induction in Discoid Lupus Erythematosus (TARGET-DLE) [cited 2016 Mar 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT02656082?term=lupus&recr=Open&no_unk=Y&cond=lupus+erythematosus&rank=1. NLM Identifier: NCT02656082. [Google Scholar]
- 145.Liu Y, Jesus AA, Marrero B, et al. Activated STING in a vascular and pulmonary syndrome. The New England journal of medicine. 2014 Aug 7;371(6):507–18. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146 *.An J, Woodward JJ, Sasaki T, et al. Cutting edge: Antimalarial drugs inhibit IFN-beta production through blockade of cyclic GMP-AMP synthase-DNA interaction. Journal of immunology. 2015 May 1;194(9):4089–93. doi: 10.4049/jimmunol.1402793. This study revealed the mechanism by which antimalarials downregulate interferon gene expression and identified the STING-interferon-beta pathway as a potential target for future treatment development. [DOI] [PubMed] [Google Scholar]
- 147.Kuznik A, Bencina M, Svajger U, et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. Journal of immunology. 2011 Apr 15;186(8):4794–804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 148.Friedman A, Putterman C, Blecher K, et al. The TWEAK/Fn14 as a novel therapeutic target for cutaneous lupus erythematosus. Journal of the American Academy of Dermatology. 2012;66(4):AB134. [Google Scholar]
- 149.Doerner JL, Wen J, Xia Y, et al. TWEAK/Fn14 Signaling Involvement in the Pathogenesis of Cutaneous Disease in the MRL/lpr Model of Spontaneous Lupus. The Journal of investigative dermatology. 2015 Aug;135(8):1986–95. doi: 10.1038/jid.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zahn S, Rehkamper C, Ferring-Schmitt S, et al. Interferon-alpha stimulates TRAIL expression in human keratinocytes and peripheral blood mononuclear cells: implications for the pathogenesis of cutaneous lupus erythematosus. The British journal of dermatology. 2011 Nov;165(5):1118–23. doi: 10.1111/j.1365-2133.2011.10479.x. [DOI] [PubMed] [Google Scholar]
- 151.Saenz-Corral CI, Vega-Memije ME, Martinez-Luna E, et al. Apoptosis in chronic cutaneous lupus erythematosus, discoid lupus, and lupus profundus. International journal of clinical and experimental pathology. 2015;8(6):7260–5. [PMC free article] [PubMed] [Google Scholar]
- 152.Toberer F, Sykora J, Gottel D, et al. Apoptotic signal molecules in skin biopsies of cutaneous lupus erythematosus: analysis using tissue microarray. Experimental dermatology. 2013 Oct;22(10):656–9. doi: 10.1111/exd.12216. [DOI] [PubMed] [Google Scholar]
- 153.Schmitz MK, Botte DA, Sotto MN, et al. Increased corticotropin-releasing hormone (CRH) expression in cutaneous lupus lesions. Lupus. 2015 Jul;24(8):854–61. doi: 10.1177/0961203315569335. [DOI] [PubMed] [Google Scholar]
- 154.Hegazy A, Barakat AF, El Gayyar MA, et al. Prevalence and clinical significance of anti-C1q antibodies in cutaneous and systemic lupus erythematosus. Egyptian Journal of Medical Human Genetics. 2012;13(2):167–71. [Google Scholar]
- 155.Qin M, Guo Y, Jiang L, et al. Elevated levels of serum sCXCL16 in systemic lupus erythematosus; potential involvement in cutaneous and renal manifestations. Clinical rheumatology. 2014 Nov;33(11):1595–601. doi: 10.1007/s10067-014-2741-9. [DOI] [PubMed] [Google Scholar]
- 156.Miao CG, Yang JT, Yang YY, et al. Critical role of DNA methylation in the pathogenesis of systemic lupus erythematosus: new advances and future challenges. Lupus. 2014 Jul;23(8):730–42. doi: 10.1177/0961203314527365. [DOI] [PubMed] [Google Scholar]
- 157.Guo Y, Sawalha AH, Lu Q. Epigenetics in the treatment of systemic lupus erythematosus: potential clinical application. Clinical immunology. 2014 Nov;155(1):79–90. doi: 10.1016/j.clim.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 158.Kunz M, Konig IR, Schillert A, et al. Genome-wide association study identifies new susceptibility loci for cutaneous lupus erythematosus. Experimental dermatology. 2015 Jul;24(7):510–5. doi: 10.1111/exd.12708. [DOI] [PubMed] [Google Scholar]


