Abstract
Background
Maternal asthma, uncontrolled asthma and low vitamin D levels during pregnancy have been individually linked to increased risk of preeclampsia.
Objective
To investigate the association of history of physician-diagnosed asthma and uncontrolled asthma status during pregnancy with risk of preeclampsia and the effects of early pregnancy vitamin D concentrations on this relationship.
Methods
816 subjects with available pregnancy outcome data and risk factors of interest were analyzed. A group of experienced obstetricians and gynecologists from three study centers validated the PE diagnoses. Vitamin D was measured using the DiaSorin method at 10–18 weeks of gestation. The Pregnancy Asthma Control Test (p-ACT) was used to assess asthma control during pregnancy. Criterion-based stepwise variable selection algorithm was applied to investigate the relationships of risk factors of interest (history of asthma diagnosis, uncontrolled asthma during pregnancy and vitamin D) to preeclampsia.
Results
The incidence of preeclampsia was not related to the presence of asthma diagnosis (8.9% with versus 7.4% without). The adjusted odds of PE controlled for maternal serum 25OHD concentrations was higher for women with a higher proportion of uncontrolled asthma months per visit during pregnancy (adjusted Odds Ratio (aOR): 3.55, 95% CI: 1.15–13.0). Adjusting for asthma control status during pregnancy, an additional decrease of the associated preeclampsia risk by 7% was observed for a 10 unit (ng/mL) increase of early pregnancy 25OHD levels (aOR10-unit: 0.60, 95% CI: 0.43–0.82) as compared to the prior risk estimate of preeclampsia associated with low maternal 25OHD unadjusted for asthma control status.
Conclusion
Uncontrolled asthma during pregnancy is associated with an increased risk of preeclampsia. Early pregnancy 25OHD contributes to the association of uncontrolled asthma status with preeclampsia.
Keywords: Asthma, Exacerbation, Pregnancy, Vitamin D, 25OHD, Preeclampsia
Introduction
Asthma is one of the most common serious medical problems during pregnancy affecting up to 8% pregnant women1. Some studies have shown that maternal history of asthma diagnosis or uncontrolled asthma during pregnancy might increase the risk of adverse pregnancy and prenatal outcomes such as low birth weight and preeclampsia, while other reports do not support these findings2–4. A few studies have particularly investigated the association of maternal history of asthma diagnosis or asthma control status during pregnancy on preeclampsia. Two studies showed that pregnant women with symptomatic asthma had 2–3 times higher odds of preeclampsia as compared to those without asthma symptoms5, 6. In contrast, the results from two earlier studies had been inconsistent with those two recent observations7, 8. The conflicting results in relating asthma and preeclampsia might be due to differences in study populations and inadequate control for confounding. None of the studies investigated the asthma-preeclampsia relationship adjusted for vitamin D status during pregnancy, which might be a potential moderating factor contributing to both risk of preeclampsia occurrence and asthma exacerbations9–12. This notion is principally conceived from systematic reviews and meta-analyses of clinical trials suggesting that vitamin D supplementation and higher serum levels of 25OHD might be associated with reduced risk of asthma exacerbations13, 14. The most recent meta-analysis study suggested that vitamin D supplementation could result in a reduced rate of exacerbations (rate ratio 0.63, 95% CI 0.45 to 0.88) and the risk of having a least one of exacerbation requiring emergency room visit or hospitalization (odds ratio (OR) 0.39, 95% CI 0.19 to 0.78)13. Furthermore, epidemiological studies have provided evidence on the association of lower serum vitamin D levels with increased asthma prevalence in general population samples15. Therefore, the main objective of this study was to investigate the association of asthma diagnosis and asthma control during pregnancy with preeclampsia development and determine the impact of maternal 25OHD levels on this relationship.
Methods
VDAART and Study Population
To attain the objective of the study, we used the data on the outcomes of pregnancy from the Vitamin D Antenatal Asthma Reduction Trial (VDAART). VDAART (www.vdaart.com) is a multicenter clinical trial conducted at three study centers across USA (Washington University in St. Louis, Boston Medical Center, Kaiser Health Care San Diego). The trial was sponsored by U01HL091528 from the National Heart, Lung, and Blood Institute (NHLBI) and registered at ClinicalTrials.gov (NCT00920621). Pregnant women were randomized between September 2009 and July 2011. The study population consisted of pregnant women with singleton pregnancies at 10–18 weeks who were enrolled in a multicenter, double-blind, randomized trial to investigate the effect of vitamin D supplementation during pregnancy. The VDAART aims were to investigate a reduction in risk of pre-defined pregnancy outcomes including preeclampsia as well as wheezing and asthma in the born children from mothers who received vitamin D supplementation. All the pregnant participants received prenatal vitamins containing 400 IU (10 μg/day) of cholecalciferol; thus, the vitamin D treatment arm received a total of 4400 IU/day (110 μg/day) and the placebo arm received 400 IU/day (10 μg/day). The participants were 18 up to 39 years old, who had either a personal history of physician-diagnosed asthma and/or allergies (i.e. allergic rhinitis and eczema) or a partner who had either asthma or allergies. The pregnant women’s clinical course was followed prospectively from randomization to the end of the pregnancy16, 17. Exclusion criteria for VDAART enrollment included current smoking and user of other nicotine products (e.g. nicotine patch for at least 1 month prior to enrollment), chronic conditions (i.e. diabetes mellitus and current treatment for chronic hypertension), multiple gestation pregnancy, pregnancy achieved by assisted reproduction techniques (e.g. IUI, IVF), parathyroid and thyroid disease, kidney stones and sarcoidosis, intake of vitamin D supplements more than 2000 IU/day, and taking illicit drugs in the last six months. The details of the trial design and clinical trial outcomes for the vitamin D supplementation effect on preeclampsia occurrence and wheezing in the offspring of the mothers have been published16–18. In brief, administration of 4000 IU vitamin D at 10–18 weeks of gestation did not reduce the risk of study outcomes, however, further analyses demonstrated pregnant women with higher 25OHD concentrations (≥30 ng/mL) at enrollment experienced lower risk of preeclampsia during their pregnancy and wheezing in the first three years of their infants’ lives16, 18.
Data Collection
Throughout the VDAART, the selected 10-member Data and Safety Monitoring Board (DSMB) monitored the trial. The enrollment visit occurred at the subjects’ pre-natal visit between 10 and 18 weeks of gestation, and serum 25OHD levels were measured at enrollment. A completed questionnaire at enrollment and monthly thereafter, in addition to medical record review, obtained information on maternal health characteristics during pregnancy.
Main Outcome Measure: Preeclampsia Diagnosis and Validation
The occurrence of preeclampsia was a pre-specified secondary outcome of the trial and was also an adverse event. Every month, study coordinators performed a medical record review for cases of preeclampsia as part of the protocol for identifying severe adverse events in the trial. After all participants completed their deliveries, medical records were abstracted for all participants. A committee of four board certified obstetricians reviewed 276 abstracted charts with any evidence of hypertension, proteinuria, or preeclampsia diagnosis in a blinded fashion to determine or validate preeclampsia status. The diagnosis of preeclampsia at the time of record reviews was based on the American Congress of Obstetricians and Gynecologists’ (ACOG) guidelines19: the identification of high blood pressure (BP) with proteinuria (≥300 mg per 24-hour collection or ≥1+ on urine dipstick) or the presence of elevated liver enzymes, high platelet count, headache or visual disturbances after 20 weeks of gestation. The blood pressure criteria included either systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or both, with a second elevated measurement at least six hours after the first measure in the medical record.
Primary and Secondary Exposure Variables: Maternal Asthma Status and Serum Vitamin D
I. Maternal asthma and its control status during pregnancy
Completed questionnaires at the first appointment (enrollment visit) and monthly maternal health questionnaires thereafter provided the information on absence or presence of maternal asthma and monitored asthma control throughout the study, from enrollment until delivery (Figure 1). A subject was considered to have asthma if she reported physician-diagnosed asthma at any time in her life. Thereafter, the monthly questionnaires prospectively collected all the information characterizing uncontrolled status of asthma, if any. The Pregnancy Asthma Control Test (p-ACT), a 5-item questionnaire included in the monthly questionnaires, was used to assess a multidimensional perspective of asthma control based on activity limitation, frequency of shortness of breath due to asthma, nighttime symptoms, use of rescue medication and self-perception of asthma control over a period of four weeks20. A p-ACT score ≤16 was considered the cut-off for discrimination of uncontrolled asthma from controlled asthma during pregnancy20–22. Accordingly, occurrence (p-ACT score ≤16) or non-occurrence (p-ACT score >16) of uncontrolled asthma month was abstracted from the monthly questionnaires of each subject. Thereafter, the number of uncontrolled asthma months during study time (from enrollment until delivery) in each woman was defined as the total number of p-ACT scores ≤16. To avoid any potential bias due to differences in subjects’ study time, the proportion of uncontrolled asthma months to the number of total study visits in each subject was used as the main exposure variable of interest for asthma control status during pregnancy (expressed as uncontrolled months per visit).
Figure 1.
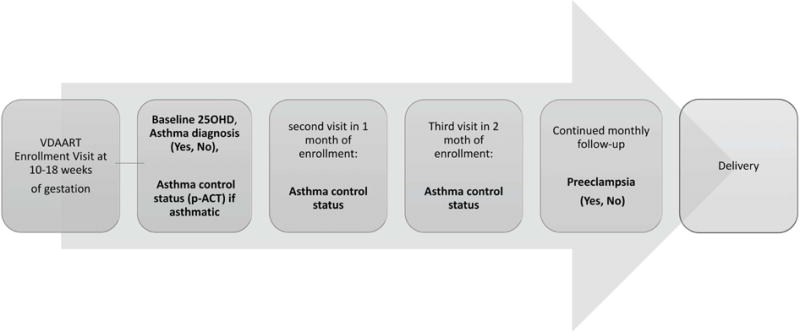
The follow-up scheme of VDAART study to collect the data on the variable of interest (history of physician-diagnosed asthma, asthma control status, vitamin D levels at enrollment and preeclampsia diagnosis).
II. Vitamin D Assay
Serum assays of 25OHD were performed at the Channing Division of Network Medicine. The applied method for quantitative determination of 25-hydroxyvitamin D is an FDA approved, direct, competitive chemiluminescence immunoassay (CLIA) using the DiaSorin LIAISON® 25-OH Vitamin D Total assay23. This assay is co-specific for 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2. All assays were performed at the data coordinating center blinded for treatment assignment, and for quality control, the laboratory used US National Institute of Standards and Technology (NIST) level 1 protocol.
Additional Variables
A priori covariates as risk factors for preeclampsia and potential confounders of the measure of association for the main variables of interest with preeclampsia were extracted from the study questionnaires and data records. These variables included: number of prior pregnancies, maternal age, gestational diabetes in the present pregnancy, race (African American, White, and other), treatment arm of the trial (placebo vs. vitamin D supplementation), serum 25OHD concentrations, body mass index (BMI) at first appointment, clinical centers, annual household income, marital status, and educational status (Table 1).
Table 1.
Pregnant women’s characteristics by maternal history of physician diagnosed asthma according to the intention to treat population of VDAART (N=816)*
| Features | Asthma (N=327) |
No asthma (N=489) |
|---|---|---|
|
| ||
| Preeclampsia (N, %) | ||
| Yes | 29 (8.9) | 38 (7.4) |
| No | 298 (91.1) | 451 (92.6) |
|
| ||
| Maternal monthly visits during pregnancy (N) | ||
| Median | 5 | 5 |
| Range | 1–7 | 1–7 |
|
| ||
| Gestational age (weeks) | ||
| Mean (SD)a | 14.2 (2.8) | 14.26 (2.8) |
| Median | 13.7 | 13.7 |
| Range | 10–18 | 10–18 |
|
| ||
| Age (years) | ||
| Mean (SD) | 25.67 (5.45) | 27.79 (5.49) |
| Range | 18.0–38.77 | 18–39.52 |
|
| ||
| Vitamin D level at 10–18 weeks (ng/ml) | ||
| Mean (SD) | 22.65 (10.3) | 23.15 (10.2) |
| Range | 4.6–65.3 | 4.5–80.8 |
| 95% central rangeb | 6.3–46.2 | 6.9–44.6 |
| Sufficiency status at 10–18 weeks (N, %) | ||
| <15 (ng/ml) | 74 (26.6) | 104 (21.3) |
| 15–29.9 (ng/ml) | 180(55) | 276 (56.4) |
| >30 (ng/ml) | 71 (21.7) | 106 (21.7) |
| N missing | 2 (3.4) | 3 (1.6) |
|
| ||
| Number of pregnancies | ||
| Median | 2 | 2 |
| Range | 1–11 | 1–13 |
| 95% central range | 1–6 | 1–6 |
| Category status (N, %) | ||
| 1st | 107 (32.7) | 179 (36.1) |
| 2nd | 81 (24.8) | 126 (25.8) |
| 3rd | 63 (19.3) | 100 (20.4) |
| >3 | 76 (23.2) | 84 (17.7) |
|
| ||
| Site (N, %) | ||
| Boston | 112 (34.2) | 165 (37.3) |
| San Diego | 96 (29.4) | 147 (30.3) |
| St. Louis | 119 (36.4) | 177 (32.4) |
|
| ||
| Trial arm (N, %) | ||
| Vitamin D intervention | 173 (52.9) | 235 (48.1) |
| Control | 154 (47.1) | 254 (51.9) |
|
| ||
| Gestational diabetes (N, %) | ||
| Yes | 19 (6.0) | 28 (5.7) |
| No | 307 (94.0) | 461 (94.3) |
|
| ||
| Body mass index at first appointment (kg/m2) | ||
| Mean (SD) | 30 (7.8) | 28.2 (7.1) |
| Range | 17.9–61.9 | 16.3–56.4 |
| 95% central range | 24.1–53.1 | 23.1–45.2 |
|
| ||
| Race (N, %) | ||
| African American | 152 (46.5) | 203 (42.5) |
| Non-African American | 175 (53.5) | 286 (58.5) |
| White | 125 (38.2) | 204 (41.7) |
| American Indian or Alaska | 5 (1.5) | 4 (0.8) |
| Asian | 9 (2.8) | 28 (5.7) |
| Native Hawaiian | 4 (1.2) | 7(1.4) |
| Other | 32 (9.8) | 43 (8.8) |
|
| ||
| Gestational age at delivery (weeks) | ||
| Mean (SD) | 38.75 (2.1) | 38.8 (2.4) |
| Range | 23.3–42.1 | 21–42.3 |
|
| ||
| Maternal marital status (N, %) | ||
| Not Married/Divorced | 190 (58.1) | 254 (51.1) |
| Married | 137 (51.9) | 235 (48.9) |
|
| ||
| Education completed (N, %) | ||
| less than high school | 49 (14.9) | 53 (10.8) |
| high school, technical school | 98 (30) | 146 (29.9) |
| some college | 84 (25.7) | 110 (22.5) |
| college graduate/graduate school | 96 (29.7) | 180 (36.8) |
|
| ||
| Annual Family Income | ||
| <$50,000 | 151 (46.2) | 193 (39.5) |
| >$50,000 | 95 (29.0) | 177 (36.2) |
| Unknown or Refused | 81 (24.8) | 119 (24.3) |
Standard deviation
95% central range is defined by the 2.5th and 97.5th percentiles of the values.
All P values of pairwise comparisons between characteristics of subjects with preeclampsia and without preeclampsia > 0.05
Statistical Analysis
We conducted a secondary analysis of the VDAART data using a cohort design. The cohort consisted of all randomized subjects included in the intent to treat analysis of the VDAART study (N=816, Supplemental Figure 1). Missing data were assumed to be completely at random as was confirmed by Little’s MCAR (Missing Completely at Random) test. R version 3.3.2 were used for all analyses. Differences in means of continuous variables for groups by the primary exposure of interest (asthma vs no asthma) were evaluated with t-tests, and differences in proportions were evaluated with chi-square tests (Table 1). Stepwise logistic regression analyses were used to explore the relationship of self-reported asthma, asthma control status (uncontrolled asthma months per visit) and early pregnancy vitamin D (25OHD) in association with the response variable (preeclampsia) after adjustment for demographic characteristics of pregnant women and additional potential confounding variables. Stepwise regression is a semi-automated process of building a model by successively adding or removing variables based solely on the t-statistics of their estimated coefficients and is especially useful for examining large numbers of potential independent variables. Prior studies to investigate the risk factors of preeclampsia considered either maternal asthma or vitamin D levels in different population samples or neither of them. Accordingly, we included asthma, asthma control status, baseline vitamin D and potential confounding variables and a priori known risk factors in the regression models using comparative approaches using the same study population to represent procedures in the prior studies. In these approaches, the key assumption is that one of the fitted models is true, in the sense that it contains all possible important predictors and no unimportant predictors. To this end, a criterion with a high probability of discovering that model is needed. The best-recommended procedure tailored for this goal is Bayesian Information Criterion (BIC). Accordingly, we used BIC as our model selection tool to determine the model resulting in lowest BIC over the whole set of included variables during the above steps. BIC decreases the risk of overfitting by introducing the penalty term d * log(N), which increases with the number of parameters resulting in more accurate estimation of several parameters on a given data set of size N24.
Univariate and comparative criterion based stepwise regression models were:
Univariate test of association to independently explore the relationship each exploratory variable with preeclampsia.
Assessing the relationship of maternal asthma, uncontrolled asthma months per visit during pregnancy and baseline 25OHD level with preeclampsia controlling for potential confounders or covariates (Kitchen Sink regression, Model 1).
Assessing the relationship of all exploratory variables with preeclampsia excluding asthma diagnosis, uncontrolled asthma months per visit and baseline 25OHD level using stepwise logistic regression (Model 2).
Assessing the relationship of asthma diagnosis and uncontrolled asthma months per visit along with other exploratory variables with exclusion of baseline 25OHD level using stepwise logistic regression (Model 3).
Assessing the association of baseline 25OHD level with preeclampsia including other exploratory variables with exclusion of asthma diagnosis and uncontrolled asthma months per visit using stepwise logistic regression (Model 4).
Assessing the relationship of asthma, uncontrolled asthma months per visit in presence of 25OHD and other exploratory variables and measure of their association with preeclampsia using stepwise logistic regression (Model 5: stepwise logistic regression of Model 1).
Model calibration was assessed with the use of Hosmer-Lemeshow chi-square test to avoid overfitting. Using the fitted model with lowest BIC adjusted for all other variables, we displayed the scaled risk of uncontrolled asthma months per visit in association with preeclampsia. We also tested for possible interactions by fitting interaction terms into the multivariable models. No significant interactions were identified. Continuous variables are presented as mean ± standard deviation (SD) and 95% confidence intervals (CI). Odds ratios (OR) are reported with 95% confidence intervals (CI). For interval variable, median and range was used. Tests with two-sided P-values <0.05 were considered to indicate statistical significance.
Results
The base-line characteristics of the women included in this study are shown in Table 1. 816 randomized subjects to the trial arms who were included in the intent to treat analysis for VDAART main outcomes were used in this analysis16, 18. Of these women, 67 developed preeclampsia. The mean of weeks of gestation at enrollment was 14.20 ± 2.8 and it was not different among women with or without asthma diagnosis (Table 1). Pairwise comparisons of groups’ characteristics (asthma vs. no asthma) were not statistically different (Table 1). There was no difference in distributions of preeclampsia among women with or without self-reported physician diagnosed of asthma (8.87% and 7.36%, respectively). Among 327 asthmatic women, 31.5% experienced at least one episode of uncontrolled asthma status during their pregnancy25.
Table 2 demonstrates the results from univariate associations of study variables (asthma diagnosis, uncontrolled asthma months per visit, early pregnancy 25OHD and other potential confounders) with preeclampsia and adjusted measure of associations including all variables in a multivariable logistic regression model with lowest BIC. The details of results from other compared models and BIC values have been provided in Supplement Table 1. In univariate analysis, both uncontrolled asthma months per visit and maternal serum 25OHD at enrollment and but not self-reported physician diagnosed asthma were associated with preeclampsia (OR: 0.95, CI: 0.92–0.98; OR: 4.15, CI: 1.70–12.53; and OR: 1.15, CI: 0.69–1.90, respectively; Table 2 and Supplement Table 1).
Table 2.
Univariate and multivariable logistic regression to investigate the relationship of asthma diagnosis, uncontrolled asthma status during pregnancy and early pregnancy 25OHD (10–18 weeks of gestation) with preeclampsia including other potential confounders (Nsubjects=816). Bayesian Information Criterion was applied to identify the model with important variables (lowest BIC) using a step-by-step inclusion and exclusion of confounders and main variable of interests (please see supplemental Table 1 for the addtional comparative models).
| Variables | Univariate model ORa (CIb) |
Multivariable model including all variables§ (with lowest BIC) adjusted OR (CI) |
|---|---|---|
|
| ||
| Physician-diagnosed asthma | ||
| No | Ref | --------- |
| Yes | 1.15 (0.69–1.90) | |
|
| ||
| Uncontrolled asthma month per monthly visitc | 4.15 (1.2–12.53) | 3.75 (1.15–13.0) |
|
| ||
| Serum 25OHD at enrollment (10–18 weeks, ng/ml) | 0.95 (0.92–0.98)* | 0.95 (0.92–0.98)* |
|
| ||
| Age>35 (years) | ||
| No | Ref | --------- |
| Yes | 0.78 (0.23–1.99) | |
|
| ||
| Number of pregnanciesd | 0.79 (0.64–0.96) | 0.69 (0.55–0.86) |
|
| ||
| Site | ||
| Boston | Ref | |
| San Diego | 0.99 (0.48–2.0) | --------- |
| St. Louis | 2.0 (1.10–3.90) | |
|
| ||
| Trial arm | ||
| Control | Ref | |
| Vitamin D intervention | 0.97 (0.57–1.60) | --------- |
|
| ||
| Gestational diabetes | ||
| No | Ref | |
| Yes | 1.35 (0.46–3.25) | --------- |
|
| ||
| BMIe at first appointment (kg/m2) | 1.047 (1.02–1.08) | 1.04 (1.006–1.073) |
|
| ||
| Race | ||
| White | Ref | |
| African- American | 1.65 (0.95–2.94) | --------- |
| Other | 1.20 (0.53–2.57) | |
|
| ||
| Maternal marital status | ||
| Not Married/Divorced | Ref | --------- |
| Married | 0.95 (0.92–0.99) | |
|
| ||
| Education completed | ||
| college graduate/graduate school | Ref | |
| less than high school | 3.83 (1.68–8.94) | |
| high school, technical school | 2.75 (1.35–5.94) | |
| some college | 2.31 (1.07–5.20) | --------- |
|
| ||
| Annual Family Income | ||
| <$50,000 | Ref | |
| >$50,000 | 0.98 (0.94–1.02) | --------- |
| Unknown or Refused | 1.03 (0.98–1.08) | |
Odds Ratio
Confidence Interval
Total number of uncontrolled asthma months during study time until delivery/number of monthly visits during study time until delivery.
Total number of previous and current pregnancies
Body Mass index
P=0.51 By Hosmer-Lemeshow test for goodness of fit (GOF).
Values were rounded by two digits.
After adjustment for all variables except baseline vitamin D (25OHD) and applying stepwise multivariable logistic-regression, BMI at first appointment and number of pregnancies but not uncontrolled asthma months per visit were associated with preeclampsia (aOR: 1.04, CI: 1.01–1.08; aOR: 0.71, CI: 0.70, CI: 0.57–0.87; and aOR: 2.97, CI: 0.85–10.40, respectively). Addition of baseline vitamin D to the model, re-running the algorithm did not change the associated odds of preeclampsia with BMI and number of pregnancies. However, this adjustment substantially increased the associated odds of PE with uncontrolled asthma months per visit by 27% and the association became significant (aOR: 3.75, CI: 1.15–13.0, P=0.038). This model demonstrated the lowest BIC as compared to all other models and showed no change in associated odds of preeclampsia with vitamin D as compared to other models (aOR: 0.95, CI: 0.92–0.98, P=0.002) as well as those of number of pregnancies and BMI at first appointment (Supplement Table 1). Furthermore, Hosmer-Lemeshow test indicated an acceptable fit for the model with lowest BIC (P=0.51). Of note, the results of comparative models using BIC was also consistent with those attained applying AIC (Supplement Table 1).
Adjusting for the variables derived from the final best-fit logistic regression model, we demonstrated the probability of preeclampsia affected by uncontrolled asthma months per visit among asthmatic pregnant women during pregnancy. The plot showed a trend in increased risk of preeclampsia by the increase in uncontrolled asthma months (Figure 2). The results from this model showed that a 10-unit increase in maternal serum 25OHD (ng/mL) at 10–18 weeks of gestation decreased the odds of preeclampsia by 40% (aOR10-unit: 0.60, CI: 0.43–0.82). The associated adjusted odds of preeclampsia for 20 and 30-units increase of early pregnancy vitamin D were 0.36 (CI: 0.19–0.68) and 0.22 (CI: 0.10–0.56), respectively.
Figure 2.
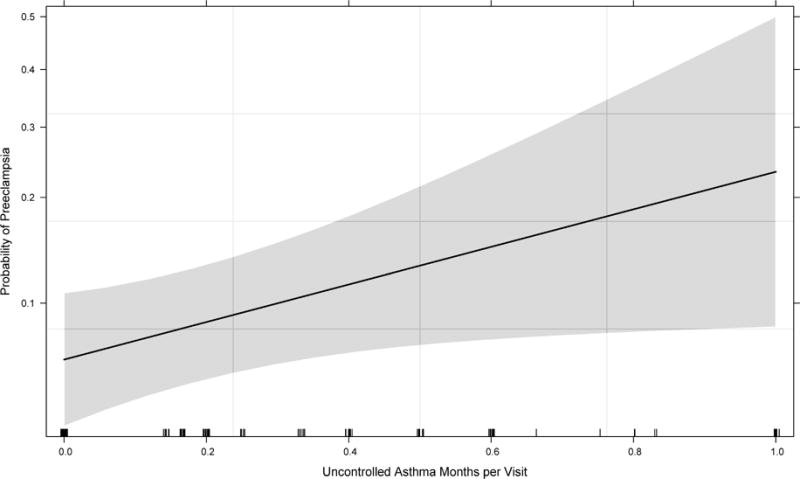
The associated effect of asthma control during pregnancy after approximately 10 weeks of gestation with risk of preeclampsia controlling for other covariates and plausible confounders derived from criterion based stepwise logistic regression. The model with lowest Bayesian Information Criterion (BIC) was used for construction of the effect plot representing the relationship of uncontrolled asthma month per visit with risk of preeclampsia occurrence (Table 2 and Supplemental Table 1). The solid line demonstrates the point risk estimate and the gray shade its 95% point-wise confidence band. The ticks on horizontal axis represent the subjects with uncontrolled asthma status measured by proportion of uncontrolled asthma months to number of visits in each subject.
Discussion
In this secondary analysis of VDAART cohort, we showed that self-reported physician diagnosed asthma was not associated with an increased risk of PE and that pregnant women with controlled asthma during pregnancy do not have a higher risk of PE as compared to non-asthmatic pregnant women. We also showed that the increased frequency of uncontrolled asthma status during pregnancy was associated with an increased risk of PE (Figure 2). Our investigation suggests that controlled status of asthma during pregnancy might reduce the risk of preeclampsia. Our study also suggests that maternal 25ODH status might have a moderating role in the association of maternal asthma control status with risk of preeclampsia. This observation implicates the potential role of higher vitamin D levels in reducing the risk of uncontrolled asthma during pregnancy and subsequent risk of preeclampsia development.
The lack of association of physician-diagnosed asthma with preeclampsia is consistent with other reports5, 6. Our adjusted risk assessment of preeclampsia controlling for maternal vitamin D status is in contrast with some researchers’ findings of no association between asthma control status and preeclampsia2, 9. There are two important points to be considered regarding prior investigations. Firstly, most of these studies had a retrospective case-control design and used ICD-9 codes to identify the asthmatics and preeclamptic subjects. Secondly, while these studies considered varying risk factors or potential confounders, none of them included maternal 25OHD status. In the current study, the association of uncontrolled asthma status during pregnancy with an increased risk of preeclampsia after controlling for maternal vitamin D status agrees with several other studies demonstrating that asthmatic women who were symptomatic during pregnancy had a higher risk of preeclampsia5, 6. The relatively recent meta-analysis investigating adverse perinatal outcomes in women with asthma concluded that there could be at least 50% increased risk of preeclampsia development in asthmatic pregnant women9. Furthermore, the subgroup analysis by active asthma management showed a higher imposed risk of maternal asthma on preeclampsia occurrence in studies without active management (RR: 1.70, 95% CI 1.11–2.59). In our recent work, we demonstrated the inverse dose-response effect of early pregnancy 25OHD on risk of preeclampsia adjusted for other covariates and confounders18. In contrast with our prior report from VDAART, controlling for the effect of uncontrolled asthma status in this study not only did not change the prior observed inverse association of maternal early pregnancy 25OHD serum concentrations with risk of preeclampsia, but also resulted in an additional reduction in the associated preeclampsia risk by 7%, 6% and 8% for 10, 20 and 30 unit increase of early pregnancy 25OHD levels, respectively. Accordingly, our findings suggest that maternal vitamin D status during pregnancy might play an important role in the association of uncontrolled asthma status with an increased risk of preeclampsia and should be considered along with uncontrolled asthma status during pregnancy as risk factors of preeclampsia. This finding also highlights the potential effect of vitamin D on asthma control during pregnancy, which has been widely investigated in non-pregnant adult asthmatics and children15, 26. In a recent study, Confino-Cohen et al. investigated this association in a cohort of 308,000 adults with known vitamin D status. According to their study, while there was no significant association between vitamin D status and physician-diagnosed asthma, low vitamin D status was associated with 25% higher odds of asthma exacerbations as compared to vitamin D sufficiency (20–40 ng/mL). The authors also found an inverse linear association between the proportion of asthmatics with exacerbations and vitamin D levels26. It is noted that the approximate 30–40 ng/mL circulating vitamin D level has been suggested by some experts as the cutoff for vitamin D deficiency in adults and during pregnancy27–29. However, the 2011 US Institute of Medicine (IOM) report defines values above 20 ng/mL (50 nmol/L) as the adequate level, which was based solely on skeletal integrity, and not considering any extra-skeletal effects of vitamin D during pregnancy (2011). VDAART was not specifically designed to investigate the effect of vitamin D supplementation on asthma control status during pregnancy as one of the outcomes of trial. However, we have shown a graduated inverse relationship of 25OHD with risk of preeclampsia development and uncontrolled asthma status at enrollment with maximum effect for values above 30 ng/mL18, 25. Accordingly, it is advisable that clinicians pay attention to the current recommendation by the IOM for vitamin D concentrations of at least 20 ng/mL in adults, particularly at preconception in women until further studies define the optimal concentration of vitamin D during pregnancy.
A few studies provide information on the potential mechanisms involved in the association of asthma control during pregnancy and PE, but the mechanisms linking uncontrolled asthma to preeclampsia requires further investigation. Tamasi and colleagues observed a higher proliferation of IFN-γ+ and IL-4+ producing T cells (Th1 and Th2) in asthmatic pregnant women who developed PE than the values in pregnant asthmatics without PE30. There is substantial evidence that 1,25(OH)2D directly affects both Th1 and Th2 cells and modulates the production of IFN-γ+ and IL-4+, two cytokines that are involved in asthma severity. In addition, vitamin D controls regulatory T-cells (Tregs), which are pivotal for T-cell balance31–33. The potential effects of vitamin D sufficiency on reducing respiratory infections as a trigger of uncontrolled asthma and better response to inhaled corticosteroids should also be added to the potential effect modulation of vitamin D on asthma and its severity34, 35.
The VDAART cohort is a unique population in which to investigate the relationship of maternal asthma diagnosis, asthma control status and vitamin D status with preeclampsia. However, VDAART inclusion criteria, a maternal or paternal history of allergies or asthma, might limit the generalizability of the findings to pregnant women with these conditions rather than all pregnant women. As clinical control of asthma varies in different clinical settings due to its multidimensional and heterogeneous nature36, we considered the cutoff of p-ACT score ≤16 as the threshold for any occurrence of uncontrolled asthma. This cutoff has demonstrated an objectively validated higher accuracy in discriminating controlled and uncontrolled asthma during pregnancy as compared to the recommended cutoff score of 19 for asthmatic adults22 and is consistent with findings and applied cut-off in two other studies20, 21. However, asthmatic sample size, low occurrence of uncontrolled asthma during pregnancies and lack of study predefined objective assessment of asthma control limited our ability to assess the risk domain of asthma control. Adherence to treatment with inhaled corticosteroid may be one of the contributors to asthma exacerbations in asthmatic patients, particularly pregnant women2, 37.
Conclusion
Our data indicate that physician diagnosed asthma does not increases the risk of preeclampsia development. However, pregnant women with uncontrolled asthma might have 3 times higher risk of preeclampsia than asthmatic women with well-controlled asthma or non-asthmatic pregnant women. Pregnant women with asthma that is not well controlled and at high risk of preeclampsia may benefit from increased surveillance to achieve and maintain optimal control of asthma. Early pregnancy vitamin D (25OHD) might contribute to the association of uncontrolled asthma status with preeclampsia such that early pregnancy correction of 25OHD deficiency in pregnant women might decrease the risk of uncontrolled asthma and the degree of its effect on the subsequent associated risk of preeclampsia. Our study further highlights the potential application of early pregnancy p-ACT (as early as 10 weeks of gestation) as a clinical tool with easy applicability and interpretability for evaluation of asthma control during pregnancy. A study that considers vitamin D supplementation at early pregnancy and asthma control intervention during pregnancy might be beneficial to women who are at risk of adverse pregnancy outcomes including preeclampsia. Such an investigation also helps to understand the mechanisms by which asthma control influences pregnancy complications.
Supplementary Material
Fig E1. VDAART Preeclampsia Consort Diagram.
Significance.
What is already known about this topic?
History of a diagnosis of maternal asthma and asthma control during pregnancy have been linked to increased risk of preeclampsia. Vitamin D status during pregnancy might be a potential factor moderating the risk of asthma exacerbations and subsequent risk of preeclampsia.
What does this article add to our knowledge?
The current study found the association of uncontrolled asthma during pregnancy with risk of preeclampsia and revealed that maternal vitamin D status at early pregnancy could possibly affect the extent to which uncontrolled asthma may be associated with a higher risk of preeclampsia
How does this study impact current management guidelines?
Attention to vitamin D levels in pregnant asthmatic women as well as optimal asthma control may decrease the risk of preeclampsia. Future study is warranted to define the optimal level of vitamin D concentrations during pregnancy for maternal-fetal health.
Acknowledgments
The authors thank the women who participated in this trial and all the study staff of the clinical centers for their contribution to this research. Special thanks go to the deceased Dr. Robert Strunk for the contribution and sharing his wisdom with us during this trial.
Funding: VDAART was supported by U01HL091528 from the National Heart, Lung, and Blood Institute; and National Research Service Award T32-HL007427. Additional support was provided by U54TR001012 from the National Centers for Advancing Translational Sciences (NCATS) for participant visits at Boston Medical Center.
Role of the Sponsor: NHLBI monitored the conduct of the trial and selected the membership of the Data and Safety Monitoring Board (DSMB). All communication between the investigators and the DSMB coursed through the staff of the NHLBI. All manuscripts, including this current one, during the trial are presented to the DSMB for approval prior to submission for peer review. NHLBI had no role in the design and the conduct of the study, in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript, other than what pertained to the DSMB.
Abbreviations
- VDAART
Vitamin D Antenatal Asthma Reduction Trial
- IU
International Unit
- IUI
Intrauterine Insemination
- IVF
Intrauterine Fertilization
- 25OHD
25-Hydroxyvitamin D
- ACOG
American Congress of Obstetricians and Gynecologists
- DSMB
Data and Safety Monitoring Board
- BP
Blood Pressure
- p-ACT
Pregnancy-Asthma Control Test
- CLIA
Chemiluminescence Immunoassay
- BMI
Body Mass Index
- BIC
Bayesian Information Criterion
- AIC
Akaike Information Criterion
- CI
Confidence Interval
- OR
Odds Ratio
- MCAR
Missing Completely at Random
- SD
Standard Deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of interests: The authors report no conflicts of interest.
Ethical approval: The Institutional Review Boards (IRB) of the participating Clinical Centers and the data coordinating center in Brigham Women’s Hospital (Partners Health Care, Boston, MA) approved VDAART, and written consent was obtained from all the participating pregnant women at the first enrollment visit.
Contribution of authorship: AAL, TFM, GO, RI, ALP, RCS, LBB, GAM, RSZ, MS, BWH, VJC, and STW contributed to design, coordination and conduction of the VDAART study. HM conceptualize the project, performed statistical analyses and wrote the manuscript. NL and VC assisted in the data preparation and analyses. AAL and STW supervised the trial. STW supervised and guided the trial and project. TFM provided important advice on the clinical aspect of project and supervised validation of preeclampsia cases. All authors reviewed and assisted in the editing of the manuscript.
References
- 1.Schatz M, Dombrowski MP. Clinical practice. Asthma in pregnancy. N Engl J Med. 2009;360:1862–9. doi: 10.1056/NEJMcp0809942. [DOI] [PubMed] [Google Scholar]
- 2.Murphy VE, Clifton VL, Gibson PG. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61:169–76. doi: 10.1136/thx.2005.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namazy JA, Murphy VE, Powell H, Gibson PG, Chambers C, Schatz M. Effects of asthma severity, exacerbations and oral corticosteroids on perinatal outcomes. Eur Respir J. 2013;41:1082–90. doi: 10.1183/09031936.00195111. [DOI] [PubMed] [Google Scholar]
- 4.Rejno G, Lundholm C, Gong T, Larsson K, Saltvedt S, Almqvist C. Asthma during pregnancy in a population-based study–pregnancy complications and adverse perinatal outcomes. PLoS One. 2014;9:e104755. doi: 10.1371/journal.pone.0104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudra CB, Williams MA, Frederick IO, Luthy DA. Maternal asthma and risk of preeclampsia: a case-control study. J Reprod Med. 2006;51:94–100. [PubMed] [Google Scholar]
- 6.Triche EW, Saftlas AF, Belanger K, Leaderer BP, Bracken MB. Association of asthma diagnosis, severity, symptoms, and treatment with risk of preeclampsia. Obstet Gynecol. 2004;104:585–93. doi: 10.1097/01.AOG.0000136481.05983.91. [DOI] [PubMed] [Google Scholar]
- 7.Schatz M, Zeiger RS, Hoffman CP, Harden K, Forsythe A, Chilingar L, et al. Perinatal outcomes in the pregnancies of asthmatic women: a prospective controlled analysis. Am J Respir Crit Care Med. 1995;151:1170–4. doi: 10.1164/ajrccm/151.4.1170. [DOI] [PubMed] [Google Scholar]
- 8.Stenius-Aarniala BS, Hedman J, Teramo KA. Acute asthma during pregnancy. Thorax. 1996;51:411–4. doi: 10.1136/thx.51.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy VE, Namazy JA, Powell H, Schatz M, Chambers C, Attia J, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118:1314–23. doi: 10.1111/j.1471-0528.2011.03055.x. [DOI] [PubMed] [Google Scholar]
- 10.Hypponen E, Cavadino A, Williams D, Fraser A, Vereczkey A, Fraser WD, et al. Vitamin D and pre-eclampsia: original data, systematic review and meta-analysis. Ann Nutr Metab. 2013;63:331–40. doi: 10.1159/000358338. [DOI] [PubMed] [Google Scholar]
- 11.Tabesh M, Salehi-Abargouei A, Tabesh M, Esmaillzadeh A. Maternal vitamin D status and risk of pre-eclampsia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:3165–73. doi: 10.1210/jc.2013-1257. [DOI] [PubMed] [Google Scholar]
- 12.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 13.Martineau AR, Cates CJ, Urashima M, Jensen M, Griffiths AP, Nurmatov U, et al. Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511. doi: 10.1002/14651858.CD011511.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassim R, Russell MA, Lodge CJ, Lowe AJ, Koplin JJ, Dharmage SC. The role of circulating 25 hydroxyvitamin D in asthma: a systematic review. Allergy. 2015;70:339–54. doi: 10.1111/all.12583. [DOI] [PubMed] [Google Scholar]
- 15.Mirzakhani H, Al-Garawi A, Weiss ST, Litonjua AA. Vitamin D and the development of allergic disease: how important is it? Clin Exp Allergy. 2015;45:114–25. doi: 10.1111/cea.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315:362–70. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. 2014;38:37–50. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirzakhani H, Litonjua AA, McElrath TF, O’Connor G, Lee-Parritz A, Iverson R, et al. Early pregnancy vitamin D status and risk of preeclampsia. J Clin Invest. 2016;126:4702–15. doi: 10.1172/JCI89031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Obstetricians and Gynecologists, Pregnancy in TFoHi. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 20.Palmsten K, Schatz M, Chan PH, Johnson DL, Chambers CD. Validation of the Pregnancy Asthma Control Test. J Allergy Clin Immunol Pract. 2016;4:310–5 e1. doi: 10.1016/j.jaip.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Araujo GV, Leite DF, Rizzo JA, Sarinho ES. Asthma in pregnancy: association between the Asthma Control Test and the Global Initiative for Asthma classification and comparisons with spirometry. Eur J Obstet Gynecol Reprod Biol. 2016;203:25–9. doi: 10.1016/j.ejogrb.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Monteiro de Aguiar M, Rizzo JA, de Melo Junior EF, Pires Lins ESLME, Cavalcanti Sarinho ES. Validation of the Asthma Control Test in pregnant asthmatic women. Respir Med. 2014 doi: 10.1016/j.rmed.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr, Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37:867–74. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6(2):461–464. [Google Scholar]
- 25.Mirzakhani H, O’Connor G, Bacharier LB, Zeiger RS, Schatz MX, Weiss ST, et al. Asthma Control Status in Pregnancy, BMI, and Maternal Vitamin D Levels. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Confino-Cohen R, Brufman I, Goldberg A, Feldman BS. Vitamin D, asthma prevalence and asthma exacerbations: a large adult population-based study. Allergy. 2014;69:1673–80. doi: 10.1111/all.12508. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher JC, Sai AJ. Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab. 2010;95:2630–3. doi: 10.1210/jc.2010-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352:515–6. doi: 10.1056/NEJM200502033520521. author reply -6. [DOI] [PubMed] [Google Scholar]
- 29.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamasi L, Bohacs A, Pallinger E, Falus A, Rigo J, Jr, Muller V, et al. Increased interferon-gamma- and interleukin-4-synthesizing subsets of circulating T lymphocytes in pregnant asthmatics. Clin Exp Allergy. 2005;35:1197–203. doi: 10.1111/j.1365-2222.2005.02322.x. [DOI] [PubMed] [Google Scholar]
- 31.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 32.Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52:12–8. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11:29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charan J, Goyal JP, Saxena D, Yadav P. Vitamin D for prevention of respiratory tract infections: A systematic review and meta-analysis. J Pharmacol Pharmacother. 2012;3:300–3. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali Z, Ulrik CS. Incidence and risk factors for exacerbations of asthma during pregnancy. J Asthma Allergy. 2013;6:53–60. doi: 10.2147/JAA.S43183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schatz M, Leibman C. Inhaled corticosteroid use and outcomes in pregnancy. Ann Allergy Asthma Immunol. 2005;95:234–8. doi: 10.1016/S1081-1206(10)61219-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig E1. VDAART Preeclampsia Consort Diagram.


