Abstract
The oral mucosal barrier is constantly exposed to a plethora of triggers requiring immune control, including a diverse commensal microbiome, ongoing damage from mastication, dietary and airborne antigens. However, how these tissue-specific cues participate in the training of immune responsiveness at this site is minimally understood. Moreover, the mechanisms mediating homeostatic immunity at this interface are not yet fully defined. Herein, we present basic aspects of the oral mucosal barrier and discuss local cues that may modulate and train local immune responsiveness. We particularly focus on the immune cell network mediating immune surveillance at a specific oral barrier, the gingiva; a constantly stimulated and dynamic environment where homeostasis is often disrupted resulting in the common inflammatory disease periodontitis.
Keywords: Oral Immunity, Oral mucosa, Periodontal Immunity, Gingival Barrier
The oral barrier – gateway into the human body
The oral mucosa is a site of first encounters. Commensal microbes, airborne antigens/allergens and food are all initially encountered here prior to entry in the gastrointestinal (GI), and often respiratory, tracts. As is the case with other barrier sites, the local immune system strikes a delicate balance in that it performs effective immune surveillance without exuberant inflammatory responses, while tolerating commensals and innocuous antigens [1]. However, although extensive work has focused on the regulation of barrier immunity at sites such as the gastrointestinal tract and skin, training and regulation of homeostatic immune responsiveness is much less explored at the oral barrier (see outstanding questions). In this current review, we will present unique aspects of the oral mucosal barrier and discuss current knowledge of the local immune cell network mediating immune homeostasis. Moreover, we will outline recent data on tissue-specific cues involved in training immune function at this site, with a focus on gingival barrier immunity.
Outstanding questions.
How do local, tissue-specific cues tailor immune responsiveness at the oral barrier?
Do oral microbial communities contribute to local training of immunity at the oral barrier?
What are the key mechanisms ensuring immune surveillance?
What regulatory networks mediate tolerance?
Does local priming of immune responses at the oral barrier affect distal and/or systemic immune responses?
Home to a rich and diverse microbiome
The oral barrier is one of the main ecological habitats of the human body [2]. Oral microbial communities are distinct from those at other barrier sites and are amongst the most diverse in terms of community membership [3]. Dominant phyla detected are Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes, with varying levels of representation in distinct ecological niches within the oral cavity, with the clearest distinction between shedding epithelia (oral mucosa) and non-shedding surfaces (tooth adherent biofilm) [4]. At the species level, over 600 prevalent taxa have been described in the oral cavity [5]. Interestingly, tooth adherent microbial communities are amongst the most rich and diverse [6] [7], and have been shown to form biofilms with complex structural organization [8]. However, despite, extensive characterization of the oral microbiome, the host-microbiome interplay at the oral mucosa is much less well understood than at the intestinal mucosa. In this section, we highlight three key areas of ongoing investigation.
Do microbial-signals train homeostatic immunity at the oral barrier?
Commensal microbiota have been shown to play critical roles in the development and conditioning of local immunity at barrier sites [1] [9], with specific microbes playing significant roles in tailoring immune cell function [10] [11]. At the oral barrier, the role of the microbiome, and its metabolites, in the training of local immune cells has not been fully elucidated, yet current data suggest both microbiome-dependent and -independent regulation of immune homeostasis. In this regard, germ free (GF) mice have been shown to have a broadly undisrupted immune cell network with comparable frequencies of CD45+ hematopoietic cells and T cells compared to specific pathogen free (SPF) controls [12]; suggesting microbiome-independent mechanisms support establishment of homeostatic immunity. However, clear roles for commensal colonization have been demonstrated in the induction of innate defenses at the oral barrier. While neutrophils have been detected in GF mice, they are found at much lower numbers compared to SPF counterparts suggesting both microbiome-dependent and -independent mechanisms promote steady-state neutrophil recruitment to the oral barrier [13]. Select innate epithelial antimicrobial oral defenses have also been reported to depend on commensal colonization, similar to the gastrointestinal tract [9]. Epithelial expression of Growth arrest specific 6 (GAS6), a ligand of the TYRO3–AXL–MERTK signaling system, was shown to be commensal-dependent and to play a role in the control inflammatory tone and host/microbiome symbiosis at the oral mucosa [14]. These insights indicate that at steady-state the oral microbiota locally influence immune function, yet it is becoming increasingly clear that immunological defense of this barrier is also mounted irrespective of commensal colonization.
What immunological mechanisms regulate commensalism in the oral environment?
Host-microbial coexistence at barriers depends on homeostatic mechanisms that safeguard commensalism. In this regard, IL-17 signaling mediates control of the commensal oral fungus Candida albicans. Elegant studies in animal models, as well as examination of patients with genetic defects in IL-17-signaling and Th17 differentiation, have revealed a vital role for IL-17 in oral anti-fungal immunity [15] [16]. Indeed, IL-17 secreting cells, both Th17 and TCRγδ T cells [17] [18], are implicated in protection against fungal infection. IL-17-signaling, particularly in epithelial cells, is critical for induction of innate anti-microbial defenses, with β-defensin 3 being best characterized for its role in surveillance of C. albicans [19, 20]. The importance of innate defenses in the control of oral microorganisms is also revealed in studies of patients with xerostomia (salivary dysfunction leading to low or lack of saliva) [21]. Saliva is the watery fluid which is produced by salivary glands and contains a plethora of innate antimicrobial agents (immunoglobulins IgA, IgM and IgG and antimicrobial peptides histatins, lysozyme, lactoferrin, peroxidases, SLPI) [22]. Patients with reduced or no saliva (and related reduction in innate anti-microbial defenses) present with increased susceptibility to oral candidiasis [23]. In addition these patients also present with rampant dental caries caused by increases in acid-producing oral microbes, revealing a role for innate defenses in the control of caries-associated oral bacteria [24]. However, specific immune mediators involved in the surveillance of commensals involved in the oral inflammatory disease periodontitis have not been defined. Therefore, besides specification of IL-17-mediated epithelial defenses for C.albicans surveillance, whether and how specific aspects of immune functionality participate in the constraint of given bacterial commensals or pathobionts has yet to be determined at the oral mucosa.
Can microbial stimuli prime local and systemic immunity?
Given that a plethora of commensal microbes, food and airborne antigens are encountered for the first time at the oral barrier, it is conceivable that immunological responses to microbes and antigens are primed at this site and may influence, not only local immunity, but subsequent recall responses at distant locations. Support for this concept arises from the fact that sublingual (and mucosal) delivery of antigens for vaccination produces efficient local and systemic protection in experimental models [25], indicating priming of systemic immune responses to orally encountered antigens. Whether oral commensals trigger specific local immunity in health is not well detailed, yet characterization of lymphocytes in healthy oral tissues reflects a predominance of memory populations [26], suggesting responsiveness to local antigens. Additional evidence for local responses to oral commensal comes from the detection of salivary IgA/IgG which are specific for oral commensals [27] [28]. It is also appreciated that oral commensals alter their functionality in the context of inflammatory periodontitis [29] and contribute to the local inflammatory reaction [30] [31]. As such, dysbiotic microbial communities arising in periodontitis are known to perpetuate local inflammatory pathology [32]. In the context of periodontitis, increased immune-responsiveness to oral commensals is evident by the presence of systemic antibody responses to periodontitis associated microbes [33]. Finally, oral microbes are known to gain access to the circulation [34] [35] (or enter the gastrointestinal tract) and have been recovered in disease lesions at distal locations (including atherosclerotic plaques, RA joints and colorectal cancer lesions) potentially contributing to disease pathology at sites away from the mouth [32] [36] [33].
A barrier surface exposed to a plethora of environmental stimuli
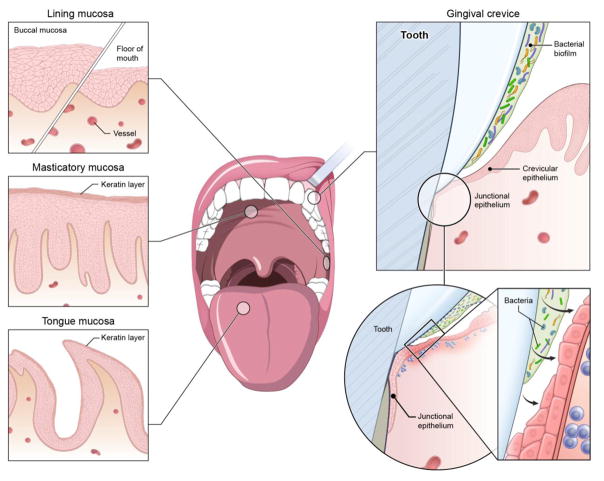
The oral mucosa is lined by stratified squamous epithelia (Figure 1), which are primarily non-keratinized (lining epithelium), allowing for direct interaction of microbes/antigens and environmental stimuli. Some areas are particularly thin such as the sublingual area and are highly vascularized serving as a target for vaccination, while sites subjected to the mechanical stimulation/injury of chewing (masticatory epithelia) bear a layer of keratin protection [37]. A particularly vulnerable site of the oral barrier is the epithelium of the gingival crevice, which lines the inside of the gingiva (the tissues surrounding teeth). The wall of the gingival sulcus is lined with non-keratinized epithelium (crevicular epithelium) that progressively thins towards the base of the sulcus. At the base of the sulcus where the mucosa meets the tooth, the epithelium transitions to an incompletely differentiated epithelium, the junctional epithelium (JE). The JE is considered a vulnerable point in an otherwise continuous epithelium. It tapers down to 3–5 layers of thickness and is attached to the tooth by hemi-desmosomes. The connection of the JE to the tooth is highly permeable, allowing for constant passage of tissue fluid termed Gingival Crevicular Fluid (GCF) which contains host factors including plasma proteins, cytokines, immunoglobulins and cells [38] [39]. In fact, it is through the JE that neutrophils continuously transmigrate into the oral cavity (see below for details). Importantly, the constituents of this local fluid reflect the inflammatory state of the neighboring gingiva [38] [39].
Figure 1. The oral and gingival barrier.
The oral mucosa is lined by stratified squamous epithelia of varying thickness and level of keratinization. Most the oral mucosa is covered by lining, non-keratinized epithelium (the floor of mouth being particularly thin and vascular). The areas related to mastication (hard palate, outer surface of gingiva) are partially keratinized epithelia and the tongue is covered with a specialized epithelium that incorporates the taste buds. The gingival crevice is a particularly open and vulnerable site. It is lined by the sulcular epithelium that is non-keratinized and becomes progressively thinner transitioning to the junctional epithelium, which connects to the tooth surface and is constantly exposed to the microbial biofilm and experiences trauma, leading to constant immune activation.
The gingival crevice; A site of constant immune activation
The barrier of the gingival crevice is constantly exposed to environmental stimulation by (i) the rich and diverse tooth adherent microbiome and (ii) the continuous barrier damage arising from chewing and hygiene regimens. These constant local triggers necessitate activation of immune mechanisms that can cope with the ongoing demands for surveillance, regulation and healing. In fact, histological studies reveal accumulations of inflammatory cells at steady state in the gingiva [40] and more recent flow cytometric analysis of oral mucosal tissue revealed an increased level of inflammatory cells at the gingiva compared to other oral mucosal sites in health [26]. As discussed above, a likely recruiter of immune cells is the oral microbiome. Indeed, control of the microbiome is vital for maintaining immune homeostasis. Numerous clinical studies have demonstrated that increases in microbial load will stimulate gingival inflammation and conversely, that reduction in microbial load (through antibiotics or oral hygiene) will reverse inflammatory states [41]. Early studies in which healthy volunteers abstained from oral hygiene have demonstrated that increases and shifts in microbial populations [42] led to clinically detectable gingival inflammation [43]. These inflammatory changes occurred over a period of days to a few weeks and were reversible, underscoring the ability of this site to continuously remodel and perform an active but delicate form of homeostasis.
The gingival barrier is also a site of constant mechanical damage due to mastication/hygiene. The vulnerable connection between the JE and tooth is routinely breached during physiologic functions such as chewing and brushing, allowing for transient microbial translocation [44]. In fact microbial translocation of oral microbes is reported after chewing and during dental procedures [34] [35]. Interestingly, one of the remarkable properties of the JE is that it readily regenerates if damaged or surgically excised in health [37] suggesting the presence of a unique immunological system tailored for both surveillance and repair programs. The delicate balance between microbiome/tissue injury and host responses at this interface is best reflected by the fact that this homeostasis is often lost, leading to destructive inflammation; specifically the development of the common inflammatory disease periodontitis. In periodontitis, a dysbiotic oral microbiome is considered the trigger of a chronic inflammatory response in the surrounding soft tissues [45], which causes destruction of supporting tissues and structures [46] [47]. In fact 40% of the adult population displays some level of periodontal immunopathology [48], suggesting that a state of mildly altered homeostasis is a norm [49]. Yet, approximately 10% of the general population will develop severe disease, linked to significant tissue destruction, reflecting increased host susceptibility [48] [49]. Moreover, severe periodontal destruction is associated with systemic translocation of periodontal microbes and is linked to numerous systemic inflammatory conditions [32] [33] [36], indicating that local immune/microbiome imbalance may affect distal inflammatory processes (either through increased microbial translocation, systemic inflammation or shared immune-pathological mechanisms) [36] [32]. The commonality of this disease and its potential for systemic inflammatory consequences renders understanding the immunological pathways implicated in gingival health and periodontitis of increased interest to the general community of immunologists and clinicians.
Homeostatic Immunity at the Gingival barrier
The immunological network at the gingival barrier
Despite our increasingly sophisticated understanding of the bacterial communities present at the oral/gingival barrier [6], how these microbes, alongside diet and other tissue-specific cues, tailor immune cell function at the gingiva remains minimally explored. Moreover, how local education combined with potentially distinct ontogenies of gingival resident immune populations participate in the generation of a unique mucosal immune system is poorly understood. Here we will outline the current understanding and outstanding questions regarding the local immuno-surveillance network operating at the gingival barrier in health (Figure 2).
Figure 2. The immunological network of the gingiva.
The gingiva houses a rich immunological network. Neutrophils continuously transmigrate through the junctional epithelium. Resident lymphocytes are predominately T cells, with some B cells and ILCs. Diverse mononuclear phagocytes are also present at this site.
Neutrophils; gatekeepers of oral immunity
The vast majority of cells recruited to the gingival crevice in health are neutrophils [50], constituting about 95% of total leukocytes, and increasing to even higher numbers during inflammation [51] [26]. Neutrophils constantly extravasate from the circulation into gingival tissue and traffic into the gingival crevice through the JE. An estimated 30,000 neutrophils undertake this journey every minute in humans [52] and can be found within the oral cavity, exhibiting varying levels of activation and functional states depending on the presence of oral inflammation [51, 53]. Whether the extravasated neutrophils, within and outside of the tissue, mediate roles in microbial surveillance through phagocytosis, degranulation, secretion of antimicrobial peptides and/or neutrophil extracellular traps is not yet fully defined [54] [55]. Yet, it has become clear that within the gingiva, neutrophils have functions beyond microbial surveillance. Neutrophils are present in the gingiva of GF mice in the absence of live microbes and studies in immune deficient patients suggest additional regulatory roles of tissue neutrophils beyond microbial killing.
The critical role of tissue neutrophils in oral/periodontal immunity has been revealed in patients with single gene mutations related to granulopoiesis or defective neutrophil recruitment/extravasation; including ELANE (neutrophil elastase), WAS (Wiskott-Aldrich syndrome), LYST (lysosomal trafficking regulator, mutation are responsible for Chediak-Higashi syndrome), CXCR4 (WHIM syndrome), and ITGB2 (integrin β2, involved in Leukocyte Adhesion Deficiency) mutations, all of which present with severe/aggressive periodontal immunopathology [56] [54]. Studies in such patients, have revealed critical functions for tissue neutrophils in periodontal homeostasis that extend beyond microbial killing. In fact, in patients with neutrophil defects a dysregulated IL17/Th17 response has been shown to drive immunopathology. In this context neutrophils, play roles beyond microbial surveillance and related to immunoregulation [57]. As such efferocytosis (uptake of apoptotic cells) of tissue neutrophils by macrophages, has been shown to be crucial in the resolution of inflammation [58] [59] by down-regulating production of IL-23, a critical trigger of Th17 responses [60]. In the context of tissue neutropenia, exacerbated IL-23/IL-17 axis responses becomes a pathogenic driver mediating immunopathology [61] [62] [59].
Importantly, a “goldilocks” balance of neutrophils must be established within the gingiva, with both too few and too many neutrophils contributing to periodontal immunopathology [63] [26] [64] [65]. However, the pathways leading to excessive neutrophil recruitment and activation in periodontitis are not completely detailed, neither are their specific functions that are critical in immune-regulation, suggesting that the gingival barrier is an ideal site of study to better understand neutrophil biology.
Other Granulocytes
Additional granulocytes reported to be present in the gingiva predominately include mast cells, with limited demonstrations that eosinophils or basophils are resident in the gingiva during health. Mast cells constitute just over 5% of granulocytes in health [26] and are expanded in settings of gingival inflammation [66]. Whether mast cells are poised for immune surveillance against pathogens [67], and/or contribute to immunopathology through degranulation and secretion of proinflammatory mediators is not conclusively defined.
Mononuclear Phagocytes
The gingival mononuclear phagocyte compartment is composed of an elaborate network of dendritic cells (DCs) [68], macrophages and recruited monocytes [26], each subset likely exhibiting contextual roles in defending the barrier and promoting immune-regulation. Monocytes expressing the chemokine receptor CX3CR1+ are recruited to the gingiva in response to bacterial infection [69], suggesting that these cells function similarly in the gingiva and other barrier sites, by being recruited in response to barrier inflammation. However, the relationship between recruited monocytes and resident macrophages has not been fully explored in the gingiva. Indeed, although tissue-resident macrophages are key gate-keepers of mucosal immunity at other barriers [70], the ontogeny, heterogeneity, functionality and niche location of macrophages in the gingiva remains to be elucidated. Given the importance of tissue location in shaping macrophage function [71], it is likely that gingival macrophages will exhibit distinct functional properties compared to other barriers. These cells likely mediate key antimicrobial functions at the gingiva, but may also participate in on-going wound healing and repair given the high levels of barrier damage occurring at this site and its potential for rapid healing [72]. Despite minimal information on gingival conditioning of macrophage populations, it has been reported that recruited monocytes can give rise to CD45+CD11c+CD11b+EpCAM+Langerin+ cells in the gingiva, which share transcriptional traits with skin-resident Langerhan cells (LC) [73]. Intriguingly, gingiva-resident LC-like cells with a similar phenotype to those derived from monocytes could also be generated from precursors to DCs [73], highlighting the impact of adopting gingival-residence on cell function and phenotype.
Within the DC network, multiple DC populations have been reported to reside in the gingiva and are increased during gingival inflammation [74] [75]. In mouse, CD11b+, CD103+ and, as already discussed, Langerin+ DC have been identified [68] [76]. Similarly, in human CD1c+, CD141+, and CD1a+EpCAM+ Langerhans-like cells constitute the gingiva DC network [26]. However, the transcription factor dependency and origin of these DC subsets has not yet been ascertained, and whether the transcriptional signatures and functional capabilities of gingival DC align with the more well-defined gastrointestinal and skin DC remains to be established [77] [78]. Moreover, in other barrier environments specific subsets of DCs have been shown to support particular T cell populations [79] [80] [81]. Whether these DC subsets perform similar functions within the gingiva remains to be explored.
Lymphocytes
T cells, B cells and innate-lymphoid cells (ILCs), as at other barriers, reside within the gingiva. B are present in gingiva in health and commensal specific IgA and IgG have been detected in oral fluids, however their contribution to maintenance of gingival immune homeostasis has not been fully elucidated [27]. Yet, in the context of periodontitis, B cells have been shown to have both protective and detrimental roles in settings of immunopathology [82] [83]. ILC populations have also been described in human gingiva (with a predominance of ILC1 and NK cells) although functions for ILCs in both health and disease remain to be established [26] and the ILC subsets resident in mouse gingiva await exploration. In contrast, much work has focused on gingival T cell populations, mainly due to the early demonstration in mouse models that T cells, particularly CD4+ T cells, were key mediators of periodontitis pathology [84] [85]. Indeed, both CD4+ and CD8+ T cells expand in settings of periodontitis. In health, in both mouse and man, CD4+ T cells dominate over CD8+ T cells, and most CD4+ and CD8+ T cells have a memory phenotype [26] [86]. In both cell populations, a proportion of gingiva resident T cells exhibit a resident terminally differentiated memory cell phenotype. Resident effector CD4+ and CD8+ T cell populations the gingiva in mice and humans produce the canonical type 1 and 17 effector cytokines IFNγ and IL-17 [26] [86]. Increased proportions of resident memory T cells are common at barrier sites, where they have been reported to support early/immediate defense mechanisms, providing site-specific protection from pathogen challenge [87].
Similar to effector T cell populations, gingival resident Foxp3+ regulatory T cells (Tregs) have also been described [26] [12]. However, little is known about specialization of Tregs in the gingiva, although studies in other tissues have shown that Treg phenotypes are typically exquisitely tailored by tissue location [88] [89]. Regardless of specialization, it is clear that Tregs play key roles in maintaining periodontal homeostasis [90] [76]. In the gastrointestinal tract, generation of Tregs toward orally-encountered antigens is a well described mechanism of tolerance [91], however it appears unlikely this would be a dominant tolerance mechanism at the gingiva. Indeed, fewer Tregs reside in the gingiva at steady-state compared to the gastrointestinal tract [92] [26], and in addition the DC subset preferentially supporting Treg generation, CD103+ DC, constitute the smallest proportion of gingival DCs [93] [68].
For all gingival resident lymphocyte populations, a key unanswered question is how do these cells traffic to this barrier. In the gastrointestinal tract, skin, lung and nasal mucosa, crucial chemokine and integrin interactions support trafficking of lymphocytes to, and residence at, the barrier site [94] [95]. Studies on gingival Tregs have indicated that CCR4-CCL22 supports Treg trafficking to the gingiva [96] [90]. Determining the recruitment and developmental requirements for residence of gingival lymphocytes in health and disease, is an important outstanding question with clear therapeutic implications. Beyond this, it is important to consider the vital functions mediated by these different lymphocytes at the gingiva. Gingival resident effector and memory T cells are present in both mouse and man [26] [86], their residence at the barrier suggesting effective, antigen-specific responses are mounted at this site. Yet no data is available on the specificity of any gingival resident T cell population and therefore understanding of their contribution to controlling and curtailing gingival inflammatory reactions during steady-state remains limited. However, a critical function for T/NKT cell immunity in oral viral protection has been documented in patients with combined immune-deficiencies and T/NKT cell defects [97] [56].
Tissue-specific training of gingival immune function: Ongoing damage due to mastication promotes local Th17 immunity
The gingiva is exposed to diverse signals; including the (i) commensal bacteria, viruses and fungi, (ii) metabolites, (iii) diet, and (iv) tissue injury (Figure 3). Yet the relative contribution of each of these signals to the induction and training of local immunity is poorly understood. Recently, the tissue-specific cues responsible for development of Th17 cells have been interrogated at the gingiva. Th17 cells have emerged as critical regulators of tissue homeostasis and immunopathology at the oral barrier [98] [99]. Physiologic roles of Th17 cells are well documented in fungal immune-surveillance (above), yet their deregulation has been associated with periodontal immunopathology in experimental periodontitis [63] [61] and in genetic forms of periodontitis in humans [61]. Interestingly, recent evidence from our group revealed that Th17 cells expand with age in the gingiva. This occurred independently of microbial colonization, with GF mice having comparable numbers of gingival Th17 cells to SPF mice [12]. These data starkly contrasted the developmental pathway for Th17 cells at other barrier sites such as the skin and gut, with Th17 cell development at these barriers dependent upon commensal colonization [10] [11]. Thus, residence of Th17 cells at the gingiva occurred via distinct mechanisms to those employed at other barrier sites. At the gingiva, accumulation of Th17 cells occurred in response to the physiological barrier damage that results from mastication/chewing [12]. Thus, ongoing mechanical damage was revealed as a tissue-specific cue capable of triggering immune responsiveness. Underscoring the vital role of mastication-induced barrier damage in the regulation of the local immune function, mice placed on a soft diet, and thus having reduced levels of gingival barrier damage, had reduced numbers of gingival Th17 cells. In contrast mice on a hardened diet, or following acute gingival injury, exhibited elevated frequencies of gingival Th17 cells. Damage-induced Th17 cells were shown to arise in an IL-6 and antigen dependent manner, and were, importantly, able to promote induction of protective innate barrier defenses [12, 100]. These data revealed the critical role of a physiological function such as mastication, as a central regulator of barrier immunity and highlight the importance of understanding unique local cues in the study of tissue immunity.
Figure 3. Tissue-specific cues tailoring immunity at the oral barrier.
Diverse microbial commensal communities and ongoing damage are established to educate immune function at the gingiva, while dietary elements and airborne allergens/particles are speculated to play a role in the training of local immunity.
Concluding Remarks
The oral mucosa is a barrier site constantly exposed to a multitude of environmental stimuli, yet the mechanisms that mediate immunological surveillance and tolerance, thus promoting tissue homeostasis are not well defined. As the immunological network of the oral barrier is starting to be deciphered, recent studies highlight some similarities in the cellular subsets detected compared to other barrier sites. However, in the oral and gingival environments the developmental requirements and functional roles of specific immune cell subsets remain largely unknown. Importantly, the unique aspects of the immune network policing the oral barrier, may be of importance and potentially reflect specialized functional capabilities required to maintain homeostasis in this this environment. As such, the continuous recruitment and extravasation of neutrophils within gingival tissues in health, and the severe inflammatory oral phenotypes in patients with neutrophil defects, highlights a vital role for this subset in oral homeostatic immunity. Indeed, both anti-microbial and anti-inflammatory functions of neutrophils appear to be key for periodontal homeostasis. More broadly, specific adaptations in the immune cell populations reflect the specialized tissue-specific cues operating in the oral environment. In this regard, the oral barrier is exposed to unique and diverse communities of commensal microbial communities that are known to play immune-stimulatory roles particularly in the setting of the oral inflammatory disease periodontitis. Moreover, an ongoing tissue-specific cue at the oral/gingival barrier is the continuous damage from mastication, which acts as a trigger for local immunity and tones homeostatic Th17-dependent barrier-protective immune responsiveness. Yet how the combination of these diverse signals participates in the regulation of homeostatic immunity at this important barrier and whether local responses influence systemic immune functioning awaits further exploration.
Trends.
Unique signals tailor immune functionality at the gingiva compared to other barrier sites.
Ongoing damage from mastication is a tissue-specific cue training immune function at the gingiva.
The oral microbiome provides key signals for the regulation of oral innate defenses.
Dysbiosis of the oral microbiome triggers the inflammatory disease periodontitis at the gingiva.
At the gingiva, a specialized immune cell network polices this dynamic barrier.
Better understanding of immune cell training and function at the gingival barrier will support development of barrier-targeted immune therapies.
Acknowledgments
The authors would like to thank Alan Hoofring, Lead Medical Illustrator, NIH Medical Arts for creating figure illustrations. We also would like to thank Dr. John Grainger, Dr. Eva Mezey and Ms. Teresa Wild for providing input on our manuscript. This work was funded in part by the Intramural Program of the National Institute of Dental and Craniofacial Research (to N.M.), by the BBSRC (BB/M025977/1 to J.E.K) and by the Manchester Collaborative Centre for Inflammation Research (to J.E.K).
Glossary of Terms
- Gingiva
Mucosal tissue that surrounds and supports teeth
- Gingival Sulcus
Space between the gingiva and teeth
- Crevicular Epithelium
Epithelium lining the gingival sulcus
- Dental Caries
Breakdown of tooth structure caused by acid-producing bacteria
- Junctional Epithelium
Epithelium connecting the oral mucosa directly with the tooth surface through hemidesmosome connections
- Lining mucosa
Stratified squamous epithelium (non-keratinized) covering the oral mucosa
- Masticatory epithelium
Oral epithelium (partially keratinized) that lines the areas subjected to mechanical forces of mastication
- Gingival Crevicular fluid
Fluid exiting the gingival tissues into the gingival sulcus containing local inflammatory mediators
- Saliva
Watery substance secreted by salivary glands and containing electrolytes, mucus, enzymes, glycoproteins and antimicrobial agents
- Periodontitis
Common chronic inflammatory disease that leads to destruction of tooth supporting structures, resulting in alveolar bone loss
- Dysbiosis
Imbalance of microbial communities and the host
- Th17 cells
T helper 17 cells, a subset of CD4+ T cells which produce the cytokine IL-17.
- Treg
Regulatory T cells, a subset of CD4+ T cells defined by their expression of the transcription factor Foxp3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belkaid Y, Harrison OJ. Homeostatic Immunity and the Microbiota. Immunity. 2017;46(4):562–576. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costello EK, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–7. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Human Microbiome Project, C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proctor DM, Relman DA. The Landscape Ecology and Microbiota of the Human Nose, Mouth, and Throat. Cell Host Microbe. 2017;21(4):421–432. doi: 10.1016/j.chom.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewhirst FE, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abusleme L, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7(5):1016–25. doi: 10.1038/ismej.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffen AL, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6(6):1176–85. doi: 10.1038/ismej.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mark Welch JL, et al. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A. 2016;113(6):E791–800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–73. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naik S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337(6098):1115–9. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutzan N, et al. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity. 2017;46(1):133–147. doi: 10.1016/j.immuni.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenobia C, et al. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell Microbiol. 2013;15(8):1419–26. doi: 10.1111/cmi.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nassar M, et al. GAS6 is a key homeostatic immunological regulator of host-commensal interactions in the oral mucosa. Proc Natl Acad Sci U S A. 2017;114(3):E337–E346. doi: 10.1073/pnas.1614926114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puel A, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lionakis MS, Netea MG, Holland SM. Mendelian genetics of human susceptibility to fungal infection. Cold Spring Harb Perspect Med. 2014;4(6) doi: 10.1101/cshperspect.a019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conti HR, et al. Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211(10):2075–84. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conti HR, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206(2):299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conti HR, et al. IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe. 2016;20(5):606–617. doi: 10.1016/j.chom.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conti HR, et al. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4(4):448–55. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross KF, Herzberg MC. Autonomous immunity in mucosal epithelial cells: fortifying the barrier against infection. Microbes Infect. 2016;18(6):387–98. doi: 10.1016/j.micinf.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85(2):162–9. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 23.Billings M, et al. Elucidating the role of hyposalivation and autoimmunity in oral candidiasis. Oral Dis. 2017;23(3):387–394. doi: 10.1111/odi.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathews SA, Kurien BT, Scofield RH. Oral manifestations of Sjogren’s syndrome. J Dent Res. 2008;87(4):308–18. doi: 10.1177/154405910808700411. [DOI] [PubMed] [Google Scholar]
- 25.Shim BS, et al. Sublingual delivery of vaccines for the induction of mucosal immunity. Immune Netw. 2013;13(3):81–5. doi: 10.4110/in.2013.13.3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutzan N, et al. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016;9(5):1163–72. doi: 10.1038/mi.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcotte H, Lavoie MC. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62(1):71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palm NW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajishengallis G, Lamont RJ. Dancing with the Stars: How Choreographed Bacterial Interactions Dictate Nososymbiocity and Give Rise to Keystone Pathogens, Accessory Pathogens, and Pathobionts. Trends Microbiol. 2016;24(6):477–89. doi: 10.1016/j.tim.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moutsopoulos NM, et al. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 2012;39(4):294–303. doi: 10.1016/j.jaut.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moutsopoulos NM, et al. Subgingival microbial communities in Leukocyte Adhesion Deficiency and their relationship with local immunopathology. PLoS Pathog. 2015;11(3):e1004698. doi: 10.1371/journal.ppat.1004698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konig MF, et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8(369):369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forner L, et al. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33(6):401–7. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 35.Geerts SO, et al. Systemic release of endotoxins induced by gentle mastication: association with periodontitis severity. J Periodontol. 2002;73(1):73–8. doi: 10.1902/jop.2002.73.1.73. [DOI] [PubMed] [Google Scholar]
- 36.Offenbacher S, Beck JD. Commentary: changing paradigms in the oral disease-systemic disease relationship. J Periodontol. 2014;85(6):761–4. doi: 10.1902/jop.2014.140115. [DOI] [PubMed] [Google Scholar]
- 37.Nanci A. Ten Cate’s Oral Histology: Development, Structure, and Function. 8. St Louis, Missouri: Elsevier; 2013. [Google Scholar]
- 38.FABJaC . Carranza’s Clinical Periodontology. St. Luis, Missouri: Elsevier; 2015. Defense Mechanisms of the Gingiva. [Google Scholar]
- 39.Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol. 1997;2(1):123–37. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- 40.Moskow BS, Polson AM. Histologic studies on the extension of the inflammatory infiltrate in human periodontitis. J Clin Periodontol. 1991;18(7):534–42. doi: 10.1111/j.1600-051x.1991.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 41.Feres M, et al. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000. 2015;67(1):131–86. doi: 10.1111/prd.12075. [DOI] [PubMed] [Google Scholar]
- 42.Theilade E, et al. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- 43.Loe H, Theilade E, Jensen SB. Experimental Gingivitis in Man. J Periodontol. 1965;36:177–87. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 44.Parahitiyawa NB, et al. Microbiology of odontogenic bacteremia: beyond endocarditis. Clin Microbiol Rev. 2009;22(1):46–64. doi: 10.1128/CMR.00028-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21(3):172–83. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):481–90. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 47.Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35(1):3–11. doi: 10.1016/j.it.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eke PI, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–20. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 49.Papapanou PN. The prevalence of periodontitis in the US: forget what you were told. J Dent Res. 2012;91(10):907–8. doi: 10.1177/0022034512458692. [DOI] [PubMed] [Google Scholar]
- 50.Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol. 2000;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. 2003. [DOI] [PubMed] [Google Scholar]
- 51.Rijkschroeff P, et al. Oral polymorphonuclear neutrophil characteristics in relation to oral health: a cross-sectional, observational clinical study. Int J Oral Sci. 2016;8(3):191–8. doi: 10.1038/ijos.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schiott CR, Loe H. The origin and variation in number of leukocytes in the human saliva. J Periodontal Res. 1970;5(1):36–41. doi: 10.1111/j.1600-0765.1970.tb01835.x. [DOI] [PubMed] [Google Scholar]
- 53.Fine N, et al. Distinct Oral Neutrophil Subsets Define Health and Periodontal Disease States. J Dent Res. 2016;95(8):931–8. doi: 10.1177/0022034516645564. [DOI] [PubMed] [Google Scholar]
- 54.Hajishengallis E, Hajishengallis G. Neutrophil homeostasis and periodontal health in children and adults. J Dent Res. 2014;93(3):231–7. doi: 10.1177/0022034513507956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amulic B, et al. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 56.Moutsopoulos NM, Lionakis MS, Hajishengallis G. Inborn errors in immunity: unique natural models to dissect oral immunity. J Dent Res. 2015;94(6):753–8. doi: 10.1177/0022034515583533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kruger P, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11(3):e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ley K. Breaking a Vicious Cycle. N Engl J Med. 2017;376(12):1172–1174. doi: 10.1056/NEJMe1615654. [DOI] [PubMed] [Google Scholar]
- 60.Stark MA, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Moutsopoulos NM, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med. 2014;6(229):229ra40. doi: 10.1126/scitranslmed.3007696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moutsopoulos NM, et al. Interleukin-12 and Interleukin-23 Blockade in Leukocyte Adhesion Deficiency Type 1. N Engl J Med. 2017;376(12):1141–1146. doi: 10.1056/NEJMoa1612197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eskan MA, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat Immunol. 2012;13(5):465–73. doi: 10.1038/ni.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74(1):66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 65.Matthews JB, et al. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin Exp Immunol. 2007;147(2):255–64. doi: 10.1111/j.1365-2249.2006.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steinsvoll S, Helgeland K, Schenck K. Mast cells--a role in periodontal diseases? J Clin Periodontol. 2004;31(6):413–9. doi: 10.1111/j.1600-051X.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 67.Urb M, Sheppard DC. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012;8(4):e1002619. doi: 10.1371/journal.ppat.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hovav AH. Dendritic cells of the oral mucosa. Mucosal Immunol. 2014;7(1):27–37. doi: 10.1038/mi.2013.42. [DOI] [PubMed] [Google Scholar]
- 69.Steinmetz O, et al. CX3CR1hi Monocyte/Macrophages Support Bacterial Survival and Experimental Infection-Driven Bone Resorption. J Infect Dis. 2016;213(9):1505–15. doi: 10.1093/infdis/jiv763. [DOI] [PubMed] [Google Scholar]
- 70.Grainger JR, et al. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. 2017;469(3–4):527–539. doi: 10.1007/s00424-017-1958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lavin Y, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–26. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sciubba JJ, Waterhouse JP, Meyer J. A fine structural comparison of the healing of incisional wounds of mucosa and skin. J Oral Pathol. 1978;7(4):214–27. doi: 10.1111/j.1600-0714.1978.tb01596.x. [DOI] [PubMed] [Google Scholar]
- 73.Capucha T, et al. Distinct Murine Mucosal Langerhans Cell Subsets Develop from Pre-dendritic Cells and Monocytes. Immunity. 2015;43(2):369–81. doi: 10.1016/j.immuni.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 74.Jotwani R, Cutler CW. Multiple dendritic cell (DC) subpopulations in human gingiva and association of mature DCs with CD4+ T-cells in situ. J Dent Res. 2003;82(9):736–41. doi: 10.1177/154405910308200915. [DOI] [PubMed] [Google Scholar]
- 75.Jotwani R, et al. Mature dendritic cells infiltrate the T cell-rich region of oral mucosa in chronic periodontitis: in situ, in vivo, and in vitro studies. J Immunol. 2001;167(8):4693–700. doi: 10.4049/jimmunol.167.8.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arizon M, et al. Langerhans cells down-regulate inflammation-driven alveolar bone loss. Proc Natl Acad Sci U S A. 2012;109(18):7043–8. doi: 10.1073/pnas.1116770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haniffa M, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37(1):60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bain CC, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6(3):498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schlitzer A, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38(5):970–83. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naik S, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520(7545):104–8. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliver-Bell J, et al. Periodontitis in the absence of B cells and specific anti-bacterial antibody. Mol Oral Microbiol. 2015;30(2):160–9. doi: 10.1111/omi.12082. [DOI] [PubMed] [Google Scholar]
- 83.Abe T, et al. The B Cell-Stimulatory Cytokines BLyS and APRIL Are Elevated in Human Periodontitis and Are Required for B Cell-Dependent Bone Loss in Experimental Murine Periodontitis. J Immunol. 2015;195(4):1427–35. doi: 10.4049/jimmunol.1500496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker PJ, et al. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67(6):2804–9. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teng YT, et al. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106(6):R59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park JY, et al. Phenotype and Tissue Residency of Lymphocytes in the Murine Oral Mucosa. Front Immunol. 2017;8:250. doi: 10.3389/fimmu.2017.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12(6):485–91. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gratz IK, Campbell DJ. Organ-specific and memory treg cells: specificity, development, function, and maintenance. Front Immunol. 2014;5:333. doi: 10.3389/fimmu.2014.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Konkel JE, et al. Transforming Growth Factor-beta Signaling in Regulatory T Cells Controls T Helper-17 Cells and Tissue-Specific Immune Responses. Immunity. 2017;46(4):660–674. doi: 10.1016/j.immuni.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 90.Glowacki AJ, et al. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proc Natl Acad Sci U S A. 2013;110(46):18525–30. doi: 10.1073/pnas.1302829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grainger JR, et al. Contextual functions of antigen-presenting cells in the gastrointestinal tract. Immunol Rev. 2014;259(1):75–87. doi: 10.1111/imr.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dutzan N, et al. Isolation, Characterization and Functional Examination of the Gingival Immune Cell Network. J Vis Exp. 2016;(108):53736. doi: 10.3791/53736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29(11):514–22. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 95.Danilova E, et al. A role for CCL28-CCR3 in T-cell homing to the human upper airway mucosa. Mucosal Immunol. 2015;8(1):107–14. doi: 10.1038/mi.2014.46. [DOI] [PubMed] [Google Scholar]
- 96.Araujo-Pires AC, et al. IL-4/CCL22/CCR4 axis controls regulatory T-cell migration that suppresses inflammatory bone loss in murine experimental periodontitis. J Bone Miner Res. 2015;30(3):412–22. doi: 10.1002/jbmr.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Betts K, et al. A 17-year old patient with DOCK8 deficiency, severe oral HSV-1 and aggressive periodontitis - a case of virally induced periodontitis? J Clin Virol. 2015;63:46–50. doi: 10.1016/j.jcv.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abusleme L, Moutsopoulos NM. IL-17: overview and role in oral immunity and microbiome. Oral Dis. 2016 doi: 10.1111/odi.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng WC, Hughes FJ, Taams LS. The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J Clin Periodontol. 2014;41(6):541–9. doi: 10.1111/jcpe.12238. [DOI] [PubMed] [Google Scholar]
- 100.Veldhoen M. Th17 Cells Require You to Chew before You Swallow. Immunity. 2017;46(1):8–10. doi: 10.1016/j.immuni.2016.12.016. [DOI] [PubMed] [Google Scholar]