Abstract
BACKGROUND/OBJECTIVES
Poorer motor development is reported in infants with iron deficiency (ID). The role of timing, duration and severity is unclear. We assessed relations between ID timing, duration, and severity and gross motor scores, neurological integrity, and motor behavior quality at 9 months.
METHODS
Iron status was determined at birth and 9 months in otherwise healthy term Chinese infants. The 9-month motor evaluation included the Peabody Developmental Motor Scale (PDMS-2), Infant Neurological International Battery (INFANIB), and motor quality factor. Motor outcomes were analyzed by ID timing (fetal-neonatal, infancy), duration, and severity. For severity, we also considered maternal iron status.
RESULTS
Data were available for 1194 infants. Iron status was classified as fetal-neonatal and infancy ID (n=253), fetal-neonatal ID (n=256), infancy ID (n=288), and not ID (n=397). Compared with not ID, infants with fetal-neonatal or infancy ID had lower locomotion scores (effect size ds=0.19, 0.18) and those with ID in both periods (longer duration) had lower locomotion and overall PDMS-2 gross motor scores (ds=0.20, 0.18); ID groups did not differ. More severe ID in late pregnancy was associated with lower INFANIB Vestibular function (p=0.01), and total score (p=0.03). More severe ID in infancy was associated with lower scores for locomotion (p=0.03), overall gross motor (p=0.05).
CONCLUSIONS
Fetal-neonatal and/or infancy ID was associated with lower overall gross motor development and locomotion test scores at 9 months. Associations with ID severity varied by ID timing: more severe ID in late pregnancy, poorer neurological integrity; more severe ID in infancy, poorer gross motor development.
Keywords: infant, motor development, iron deficiency, iron deficiency anemia
INTRODUCTION
Motor development plays an important role in children’s overall development, since motor skills and activity can also affect cognitive and social-emotional development.1–3 Iron deficiency (ID), which is common among infants and pregnant women,4 causes nutritional anemia and may also contribute to poor neuropsychomotor development.5 Relevant effects on brain areas and processes related to motor function are demonstrated in rodent models, especially striatum and hippocampus during the human equivalent of late pregnancy, frontal cortex and basal ganglia during the equivalent of infancy/toddlerhood, and myelination throughout.6
This study examined effects of timing and duration of ID (fetal-neonatal, infancy) and severity of ID on different aspects of motor development at 9 months in over 1100 Chinese infants. To our knowledge, no prior human study has jointly considered developmental effects of ID in the fetal-neonatal and infancy periods. We identified only three studies that assessed motor development impacts of ID during gestation. A randomized controlled trial (RCT) of iron supplementation during pregnancy found no effects on Bayley motor scores at 3–24 months.7 However, one study found that anemia in late pregnancy was associated with lower Bayley motor scores at 6 months,8 and another found that more severe ID in late pregnancy was associated with poorer newborn neurobehavioral integrity.9 There has been more research on postnatal/infancy ID, especially ID anemia (IDA). Most studies report poorer motor functioning not only during infancy but also later on.10–12
This analysis used data from two linked RCTs of pregnancy and/or infancy iron supplementation. We previously reported supplementation effects on iron status, growth, and motor outcomes. 1,13 Here, we addressed a different question: how ID timing, duration, and severity relate to motor development, regardless of iron supplementation. We made the following predictions: 1) ID in infancy affects motor development more negatively than ID during gestation; 2) the poorest outcome is with ID in both late gestation and early infancy; and 3) more severe ID is associated with poorer motor outcome.
MATERIALS AND METHODS
Study setting and design
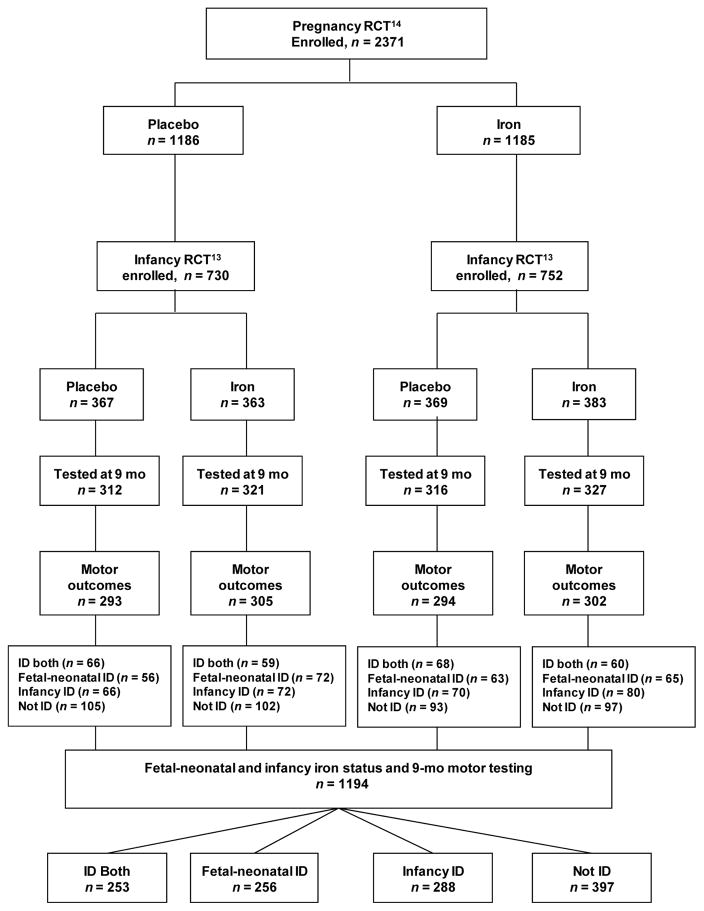
The study was conducted in Hebei Province, China. University of Michigan and Peking University First Hospital Institutional Review Boards approved the study, and all participants (mothers) signed informed consent. The analysis of ID timing, duration, and severity regarding motor outcomes was observational in design. However, participants came from a combined RCT of iron supplementation (an RCT of infancy iron supplementation13 connected to an RCT of pregnancy iron supplementation14), which was designed to support causal inferences regarding the developmental effects of reducing ID in the fetus, young infant, or during both periods (see Figure 1).
Figure 1.
Flowchart of study participants
Participants (Figure 1)
Participants were infants born to women in the pregnancy RCT.14 The pregnancy RCT enrolled 2371 women with uncomplicated singleton pregnancies between June 2009 and December 2011. They were randomized to receive iron/folate or placebo/folate, specifically daily folate (0.40 mg) and either iron (300 mg ferrous sulfate) or placebo from enrollment to birth. Most attrition was due to mothers giving birth in a nonparticipating hospital. In the infancy RCT,13 1482 healthy term infants were enrolled between December 2009 and June 2012. They were randomly assigned to receive placebo (carrier only) (n = 730) or supplemental iron (a single daily dose of ~1 mg/kg of elemental iron as an iron protein succinylate oral solution [Ferplex, Italfarmico, S.A., Madrid, Spain]) (n = 752) from 6 weeks to 9 months. At 9 months, 1194 infants provided motor outcome data (September 2010–March 2013). Their iron status was classified as below, yielding four groups: 1) ID both periods (n=253), 2) fetal-neonatal ID only (n=256), 3) infancy ID only (n=288), and 4) not ID (n=397) (See Figure 1).
Measures
Iron status
Iron measures in maternal blood in mid- and late pregnancy, cord blood, and infant blood (finger stick) at 9 months included hemoglobin (Hb), mean corpuscular volume (MCV), serum ferritin (SF), zinc protoporphyrin/heme (ZPP/H), and serum transferrin receptor (sTfR). Fetal-neonatal ID was defined as cord SF <75 μg/L or ZPP/H > 118 μmol/mol.14 At 9 months, infancy ID was defined as body iron (BI) <0 mg/kg, calculated using SF and sTfR15 and anemia as Hb <110 g/L.16 We characterized ID severity as a continuum using BI and a standardized iron status composite (IC). BI was calculated for all time points. IC was created based on a factor analysis of MCV, Hb, SF, and inverted ZPP and sTfR. Mid-pregnancy and cord-blood iron measures did not yield a good composite – relations among measures were weak. Therefore, IC was created only for mothers in late pregnancy and infants at 9 months. Iron status definitions were determined prior to data analysis.
Infant motor development
Gross motor development was assessed by Peabody Developmental Motor Scale 2nd edition (PDMS-2),17 Chinese translation. Overall 9-month gross motor scores are derived from Reflexes, Stationary, and Locomotion subscales. Reflexes cover automatic reactions to environmental events (e.g., righting reflex). Stationary assesses postural control within the center of gravity and equilibrium (e.g., sitting while manipulating objects). Locomotion covers moving from one place to another (e.g., crawling). Passed items above ceiling were included for each subscale.18 Results are expressed as raw subscale and total gross motor scores.
Early neurological function
Early neurological function was evaluated by the Infant Neurological International Battery (INFANIB).19 This 20-item assessment yields a total score based on five factors (Spasticity/muscle tone, Head and Trunk control, Vestibular function, Legs/lower limb function, and hip/joint angles). Results are expressed as raw scores for subscales and total (overall neurological integrity, also called neuromotor assessment).
Quality of motor behavior
The motor quality factor of the Bayley II Behavior Rating Scale (BRS)20 is based on 8 examiner ratings (gross and fine motor movement required by tasks, control of movement, hypotonicity, hypertonicity, tremulousness, slow/delayed movement, frenetic movement). Results are expressed as factor raw scores.
Developmental testing occurred at Maternity and Child Health Care Center (MCHC). Infants were accompanied by a parent/guardian and given frequent breaks. US and Chinese investigators trained Chinese supervisory personnel, who then trained examiners. Reliability for all motor tests was assessed before testing, along with weekly supervision by Chinese researchers. Examiners reached ≥90% reliability and were blind to children’s iron status.
Statistical analysis
Motor outcome variables were first checked for normality. The following motor outcomes were substantially left skewed and therefore required logarithmic transformation (x′ = log (k−x), where k = the constant, largest score +1): INFANIB spasticity/muscle tone score, INFANIB head and trunk score, INFANIB legs/lower limb function, and BRS motor quality factor. Logistic regression, χ2, and ANOVA in SAS were used to compare groups on infant and family characteristics. We used ANCOVA to compare groups on motor outcomes, with age at testing and RCT group as covariates. SAS PROC GLMSELECT with stepwise inclusion was used to consider other potential covariates, namely, sex, gestational age, birth weight, 9-month weight-for-age z-score, family income, parental education, lead levels at different time points, feeding pattern at 9 months, and maternal mood. None contributed significantly in final models and were therefore not included. We conducted post-hoc pair-wise comparisons when iron status group was a significant predictor in the ANCOVA model. For effects of ID timing, group comparisons were a) fetal-neonatal ID vs. not ID, b) infancy ID vs. not ID, and c) fetal-neonatal ID vs. infancy ID. For effects of ID duration, contrasts were a) ID both periods vs. fetal-neonatal ID, b) ID both periods vs. infancy ID, and c) ID both periods vs. not ID. We used multiple regression to assess effects of ID severity (late-pregnancy maternal IC and BI and 9-month infant IC and BI) on gross motor outcomes. In addition, we used a unified multiple regression model including all 4 time points (mid-pregnancy, late-pregnancy, birth [cord blood], and infancy) to evaluate associations between ID severity at different time points and motor outcomes. This approach was possible only for BI, since IC was not applicable for mid-pregnancy or cord-blood iron measures. Statistical significance was set at p<0.05 (two-tailed).
RESULTS
Participant characteristics (Table 1)
Table 1.
Infant and family characteristics
| ID both periods | Fetal-neonatal ID | Infancy ID | Not ID | Significant Contrastsc | pb | |
|---|---|---|---|---|---|---|
|
| ||||||
| Group number | 1 | 2 | 3 | 4 | ||
| n | 253 | 256 | 288 | 397 | ||
| Infant Characteristics | ||||||
| Age of testing, mo | 9.25 (0.41) | 9.32 (0.42) | 9.30 (0.40) | 9.31 (0.47) | 0.27 | |
| Male sex, n (%) | 150 (59.3%) | 135 (52.7%) | 157 (54.5%) | 157 (39.6%) | 4 < 1, 2, 3 | < 0.001 |
| Birth weight, g | 3365.9 (389.9) | 3374.9 (399.9) | 3331.5 (349.9) | 3384.7 (351.8) | 0.3 | |
| Gestational age, wk | 39.5 (1.2) | 39.6 (1.1) | 39.7 (1.1) | 39.9 (1.0) | 4 > 1, 2; 3 > 1 | 0.0003 |
| First/Only child, n (%) | 191 (76.7%) | 199 (79.6%) | 214 (74.6%) | 319 (82.0%) | 0.105 | |
| Breastfed at 9mo, n (%)d | 180 (96.3%) | 129 (66.5%) | 221(97.4%) | 231 (74.0%) | 1, 3 > 2, 4 | < 0.001 |
| 9mo Weight-for-age z-score | 1.04 (0.99) | 0.77 (1.01) | 1.11 (1.08) | 0.73 (0.99) | 1, 3 > 2, 4 | < 0.001 |
| Family Characteristics | ||||||
| Maternal age, y | 24.8 (3.7) | 25.3 (4.0) | 24.5 (3.8) | 24.5 (3.8) | 0.07 | |
| Maternal education, ≥ high school, n (%) | 91 (36.1%) | 94 (37.3%) | 74 (26.1%) | 142 (36.0%) | 3 < 1,2,4 | 0.016 |
| Net family income, ≤ 50,000 yuan/year, n (%) | 204 (82.3%) | 210 (83.0%) | 239 (86.3%) | 323 (83.9%) | 0.612 | |
| Maternal Mood total score (9mo), max=30, possible depression >=10 | 5.8 (4.6) | 5.8 (4.2) | 6.2 (4.5) | 6.2 (4.4) | 0.471 | |
| HOME (9m) total score (max=45) | 31.3 (4.3) | 31.5 (4.1) | 31.5 (3.8) | 31.5 (3.8) | 0.882 | |
ID both periods: Fetal-neonatal and infancy iron deficiency (ID), Fetal-neonatal ID, Infancy ID, Not ID. Fetal-neonatal ID was defined as ferritin < 75 μg/l or ZPP/H > 118 μmol/mol. Infancy ID was defined as body iron (BI) < 0 mg/kg. Ns vary due to missing data.
Values are means (SD) for continuous variables and n (%) for categorical.
Anova or x2.
Infants breastfed received breast milk as the sole source of milk or any breast milk in addition to formula or another source of milk. Breastfeeding data was collected via a feeding questionnaire that was not available to the whole sample.
The proportion of males was lowest in the Not ID group. The groups differed in gestational age (GA), but since all averaged >39 wk, the difference is unlikely to be clinically meaningful. There were no differences in birth weight (~3,300g). Over 75% of infants were first-born.
Infants were relatively heavy for age: 9-month WAZ was close to 1.0 for ID both and Infancy ID groups and between 0.74–0.79 for Fetal-neonatal ID and Not ID groups. Most infants were breastfed at 9 months but more so for ID both and Infancy ID (96.3% and 97.4%, respectively), compared to Fetal-neonatal ID or Not ID (65.6% and 74.0%, respectively).
Maternal age averaged 24–25y for all groups. About a third of mothers completed high school; the proportion was lower in the infancy ID group (26%). Most families (>80%) reported household incomes below the county threshold for public housing assistance.21 Maternal depressive symptoms and family support for child development were similar across groups.
Iron status (Table 2)
Table 2.
Iron status measures in late pregnancy, birth, and 9 months
| ID both periods | Fetal-neonatal ID | Infancy ID | Not ID | Significant Contrastsc | p b | |
|---|---|---|---|---|---|---|
|
| ||||||
| Group number | 1 | 2 | 3 | 4 | ||
| n | 253 | 256 | 288 | 397 | ||
| Late pregnancy | ||||||
| Body iron (BI)d, mg/kg | −0.2 (−0.6, 0.2) | 0.3 (−0.1, 0.8) | 0.1 (−0.3,0.5) | 0.6 (0.3, 1.0) | 1, 3 < 4 | 0.02 |
| Iron compositee, z score | −0.1 (−0.2, 0.1) | 0.0 (−0.1, 0.1) | 0.0 (−0.1, 0.1) | 0.2 (0.1, 0.3) | 1, 2, 3 < 4 | 0.0003 |
| Birth (cord blood) | ||||||
| Hemoglobin, g/L | 153.9 (152.0, 155.8) | 153.5 (151.6, 155.3) | 151.7 (149.9, 153.5) | 149.6 (148.1, 151.1) | 4 < 1, 2 | 0.001 |
| Serum ferritin (SF), μg/L | 83.2 (73.6, 92.9) | 106.4 (96.8, 116.0) | 136.1 (127.1, 145.2) | 175.8 (168.1, 183.5) | 1 < 2 < 3 < 4 | <.0001 |
| Zinc protoporphyrin/heme (ZPP/H), μmol/mol heme | 120.3 (116.8, 125.2) | 121.5 (117.9, 125.2) | 80.6 (78.3, 83.9) | 80.6 (78.3, 82.3) | 1, 2 > 3, 4 | <.0001 |
| Serum transferrin receptor (sTfR), nmol/L | 31.7 (30.5, 33.0) | 31.5 (30.3, 32.7) | 29.3 (28.2, 30.3) | 29.3 (28.4, 30.2) | 1, 2 > 3, 4 | 0.0005 |
| 9 Months (fingerstick) | ||||||
| Hemoglobin, g/L | 103.6 (102.3, 104.9) | 117.6 (116.3, 118.9) | 108.9 (107.7, 110.1) | 116.3 (115.3) | 1 < 3 < 2, 4 | <.0001 |
| Serum ferritin (SF), μg/L | 4.9 (4.6, 5.2) | 21.4 (20.0, 22.8) | 5.5 (5.1, 5.8) | 23.4 (22.2, 24.6) | 1 < 3 < 2 < 4 | <.0001 |
| Zinc protoporphyrin/heme (ZPP/H), μmol/mol heme | 103.0 (97.2, 109.1) | 68.2 (64.4, 72.2) | 81.2 (76.9, 85.7) | 59.7 (57.0, 62.5) | 1 > 3 > 2 > 4 | <.0001 |
| Serum transferrin receptor (sTfR), nmol/L | 32.1 (31.1, 33.0) | 20.8 (19.9, 21.7) | 29.0 (28.1, 29.8) | 20.8 (20.1, 21.5) | 1 > 3 > 2, 4 | <.0001 |
| Mean corpuscular volume (MCV), fL | 66.3 (65.9, 66.7) | 70.6 (70.1, 71.0) | 67.7 (67.3, 68.1) | 70.6 (70.3, 71.0) | 1 < 3 < 2, 4 | <.0001 |
| BId, mg/kg | −3.9 (−4.2, −3.6) | 2.8 (5.6, 3.1) | −3.2 (−3.4, −2.9) | 3.2 (3.0, 3.4) | 1 < 3 < 2 < 4 | <.0001 |
| Iron compositee, z-score | −0.8 (−0.9, −0.7) | 0.5 (0.4, 0.6) | −0.4 (−0.5, −0.3) | 0.6 (0.5, 0.6) | 1 < 3 < 2, 4 | <.0001 |
| Anemia, Hb < 110 g/L, n (%) | 172 (68.0%) | 53 (20.7%) | 153 (53.1%) | 94 (23.7%) | 1 > 3 > 2, 4 | <.0001 |
ID both periods: Fetal-neonatal and infancy iron deficiency (ID), Fetal-neonatal ID, Infancy ID, Not ID. Fetal-neonatal ID was defined as ferritin < 75 μg/l or ZPP/H > 118 μmol/mol. Infancy ID was defined as body iron (BI) < 0 mg/kg. Ns vary due to missing data.
Values are means (95% CI) for continuous variables and n (%) for categorical ones.
Anova or Logistic regression. Cord (ZPP/H, sTfR) and 9mo (SF, ZPP/H) were log-transformed to normalize the distribution.
Group differences indicated, p <0.05
Body iron (BI) was calculated by using SF and sTfR according to the formula in Cook et al. 2003: BI (mg/kg) = 2[log10(sTfR 3 1000/ferritin) − 2.8229]/0.1207.
Iron composite (IC), z-score was based on a factor analysis of iron measures (Hb, MCV, log(ZPP), log(sTfR), log(sf)).
As expected by definition, groups differed in iron status at each time point except mid-pregnancy (enrollment). In late pregnancy, mothers of infants in ID both and Infancy ID groups had lower BI than the Not ID group; IC was lower for all ID groups compared to Not ID. At birth, ID both and Fetal-neonatal ID groups had higher cord Hb than the Not ID group but lower SF and higher ZPP/H and sTfR than infants in the other groups. At 9 months, the ID both group had worse values for Hb, SF, ZPP/H, sTfR, MCV and iron status (BI, IC, and anemia) than the Infancy ID group; ID both and Infancy ID were worse than Fetal-Neonatal ID and Not ID. Anemia without ID (<2 abnormal iron measures) was observed in 23% of Not ID infants.
ID timing and duration (Table 3)
Table 3.
Fetal-neonatal and infancy iron status and gross motor development at 9 months
| n | Mean (95% CI) | pa | Effect size d | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| ID both periods 253 |
Fetal-neonatal ID 256 |
Infancy ID 288 |
Not ID 397 |
Timing | Duration | ||||||
|
|
|
||||||||||
| Fetal-neonatal vs. Not ID | Infancy vs. Not ID | Fetal-neonatal vs. Infancy | ID both vs. Fetal-neonatal | ID both vs. Infancy | ID both vs. Not ID | ||||||
| Reflexes | 14.5 (14.4, 14.7) | 14.5 (14.3, 14.6) | 14.6 (14.4, 14.7) | 14.6 (14.4, 14.7) | 0.88 | 0.05 | 0.01 | 0.06 | 0.03 | 0.04 | 0.03 |
| Stationary | 33.6 (33.4, 33.8) | 33.6 (33.4, 33.8) | 33.7 (33.5, 33.9) | 33.8 (33.7, 34.0) | 0.22 | 0.13 | 0.08 | 0.05 | 0.01 | 0.07 | 0.14 |
| Locomotion | 40.0 (39.2, 40.8) | 39.9 (39.1, 40.7) | 40.1 (39.3, 40.8) | 41.2 (40.6, 41.9) | 0.02 | 0.19b | 0.18b | 0.01 | 0 | 0.02 | 0.20b |
| Overall gross motor | 88.2 (87.2, 89.2) | 88.0 (87.0, 89.0) | 88.5 (87.5, 89.4) | 89.7 (88.9, 90.6) | 0.04 | 0.19b | 0.15 | 0.04 | 0.01 | 0.03 | 0.18b |
ID both periods: Fetal-neonatal and infancy iron deficiency (ID), Fetal-neonatal ID, Infancy ID, Not ID. Fetal-neonatal ID was defined as ferritin < 75 μg/l or ZPP/H > 118 μmol/mol. Infancy ID was defined as body iron (BI) < 0 mg/kg. Ns vary slightly due to missing data.
Ancova with covariates (RCT groups, age at testing). Pairwise comparisons expressed as Effect size d (difference between the two means divided by the pooled SD).
Significant difference between groups, p<0.05.
Iron status was a significant predictor for models of PDMS-2 Locomotion (F(3, 1183) = 3.20, p=0.02), and overall gross motor (F(3, 1154) = 2.95, p=0.04). Fetal-neonatal ID and Infancy ID groups did not differ; both had lower scores than Not ID (ds= 0.19, 0.18) for Locomotion skills. Fetal-neonatal ID and infancy ID had lower scores than Not ID (ds=0.19, 0.15) for overall gross motor. There were no significant differences for Reflexes or Stationary subscales. Regarding INFANIB results, iron status was a significant predictor only for Spasticity (F(3, 1170) = 2.79, p=0.04) with fetal-neonatal ID showing higher values than Not ID. No other INFANIB factor showed significant effects for ID timing or duration.
Regarding ID duration, ID both had lower scores than Not ID for PDMS-2 Locomotion (d=0.20) and overall gross motor (d=0.18); Fetal-neonatal ID and Infancy ID did not differ from ID both. There were no significant group differences that indicated effects of ID timing or duration on motor quality ratings.
ID severity (Table 4)
Table 4.
Relations between severity of ID in fetal-neonatal and infancy periods, and motor development tests at 9 months
| Late pregnancy iron status | Infancy iron status (9 months) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| BI | IC | BI | IC | |||||
|
|
|
|||||||
| pa | Estimateb | pa | Estimateb | pa | Estimateb | pa | Estimateb | |
| PDMS-2 at 9 months | ||||||||
| Locomotion | 0.09 | 0.10 | 0.07 | 0.42 | 0.03 | 0.46 | ||
| Overall gross motor | 0.05 | 0.54 | ||||||
| INFANIB at 9 months | ||||||||
| Factor 1/Spasticity | 0.07 | 0.06 | ||||||
| Factor 2/Head and Trunk | 0.09 | 0.02 | ||||||
| Factor 3/Vestibular function | 0.01 | 0.07 | ||||||
| INFANIB total score | 0.03 | 0.14 | ||||||
| BRS motor quality factor at 9 months | ||||||||
| BRS total motor | 0.09 | −0.02 | ||||||
GLMSELECT model with age at testing and RCT group as covariate. Suggestive trends (p<0.10) and significant associations are reported (p<0.05).
Estimate: change in the developmental test score for each unit increase in the body iron (BI) or iron composite (IC).
Maternal BI in mid-pregnancy (enrollment) did not predict any motor outcome. Severity of maternal ID in late pregnancy (BI or IC) did not predict PDMS-2 Reflexes, Stationary or overall gross motor score. However, there were suggestive trends that poorer maternal iron status in late pregnancy was associated with lower Locomotion scores. For each unit increase in maternal IC, there was a 0.42-unit increase in Locomotion score (p=0.07); for each unit increase in maternal BI, there was a 0.10-unit increase in Locomotion score (p=0.09).
Maternal late pregnancy BI predicted 9-month neurological integrity for INFANIB Vestibular function (p=0.01) and total score (p=0.03). A one-unit increase in late pregnancy BI was associated with a 0.07-unit increase in Vestibular function and a 0.14-unit increase in total score. Maternal late pregnancy BI showed a suggestion of slight inverse association (estimate= −0.02, p=0.09) with tester observations of motor behavior quality. There were no significant associations with maternal IC.
For infancy ID severity, IC related to 9-month PDMS-2 Locomotion (p=0.03) and overall gross motor score (p=0.05). A unit increase in infancy IC was associated with a 0.46-unit increase in Locomotion score and a 0.54-unit increase in overall gross motor score.. Infancy BI and IC were not related to Reflexes or Stationary scores.
In the unified model including BI for mid- and late pregnancy, fetal-neonatal and infancy time points, the only time point at which ID severity related to 9-month neuromotor development was late pregnancy. Lower maternal BI in late pregnancy was associated with poorer neuromotor outcome at 9 months, specifically INFANIB vestibular function (p=0.04) and total score (suggestive trend, p=0.06) and poorer motor behavior quality by tester assessment (suggestive trend, p=0.06). There were no significant associations with PDMS scores. For other time points, there were no significant associations between BI and any motor outcomes.
DISCUSSION
This study examined ID timing, duration, and severity in relation to infant gross motor development, neurological integrity, and quality of motor behavior at 9 months. We considered ID in the fetus/neonate and infant separately and jointly in a single cohort. We predicted worse motor outcomes when ID occurred during infancy (timing), in both fetal and infancy periods (duration), and was more severe.
Our prediction regarding timing was not supported. Infants with ID in the fetal-neonatal or infancy period had similar locomotor and overall gross motor scores, which were lower than Not ID infants. Our results for ID in infancy are congruent with previous studies. However, no prior study allows comparisons regarding our finding of similar negative impacts with ID in either period. Our findings for fetal-neonatal ID are consistent with two observational studies,8,9 though direct comparisons are limited by differences in assessment age, tests, and maternal iron measures. The INFANIB neurological integrity results also suggested minimal differences between iron status groups, except for Spasticity in fetal-neonatal ID, and those results are difficult to interpret since they suggest more normal tone in fetal-neonatal ID.
Regarding duration, it seems logical that motor development would be poorest with ID in both fetal-neonatal and infancy periods. However, we found ID in only one period was as detrimental as a longer duration of ID, i.e., in both periods. We were unable to identify any comparable studies and thus cannot relate this unexpected finding to prior research.
These results about ID timing and duration point to a different conclusion than our previous findings for timing and duration of iron supplementation. In the linked RCT analysis,1 we found that iron supplementation during infancy resulted in better gross motor scores, while iron supplementation during pregnancy had no impact. Yet in the present analysis, we found lower motor scores with indications of fetal-neonatal ID. This apparent contradiction may be explained by the fact that the groups were different in the two analyses. Despite a statistically significant reduction in risk for ID with iron supplementation in infancy, ID was common in all RCT groups at 9 months. Consequently, the groups in this ID timing, duration, and severity analysis were relatively independent of supplementation group. Another possible explanation for the differing results is the potential limited sensitivity of the assessments used to detect subtle motor differences.
Regarding severity, we attempted to utilize all available iron measures and avoid the arbitrariness inherent in cutoff approaches. We analyzed continuous measures, BI and IC, to index ID severity across the full spectrum. Neither is standard for pregnant women or infants, although BI is increasingly used. Both severity measures were related to functional motor outcomes but in different ways. Maternal IC in late gestation showed stronger associations with motor outcomes than maternal BI, yet only maternal BI was significantly associated with neurological integrity (INFANIB total score and vestibular function). In contrast, infant IC at 9 months showed stronger associations than BI to functional outcomes: it was associated with PDMS-2 Locomotion and overall motor scores. Thus, BI and IC showed different sensitivity to motor outcomes, depending on developmental period. It is possible that IC showed stronger associations than BI in infancy due to inclusion of more iron measures, whereas this was not the case in pregnancy when many iron measures are difficult to interpret. The results from the unified model with BI at all 4 time points agreed with those of individual BI regression models. This indicates the robustness of the findings about ID severity in late pregnancy and infant neurological integrity and motor quality.
Results of the ID severity analysis suggest differential effects of timing, even though the timing analysis did not. Severity of ID in pregnancy (maternal BI) was associated with neurological integrity (or neuromotor assessment) at 9 months, while ID severity in infancy (9-month IC) was associated with gross motor outcomes. The INFANIB has been shown to be a reliable and valid predictor of neurodevelopmental outcomes in Chinese infants at 10 month age.22
We suggest that this pattern of findings can be interpreted with respect to neurodevelopment, especially myelination. ID severity in late pregnancy related to vestibular function, which develops early in humans;23 the structure and connectivity of the reflex and central vestibular pathways are in place at or around birth.24 Activation of the vestibular system, cerebellum, and reticular system are integrated through vestibulo-spinal pathways to provide balance and coordinate locomotion. Recently, Beraneck et al. found that exposure to hypergravity in a mouse model caused changes in the vestibulo-spinal track and/or muscle tone that, in turn, affected locomotion.25 They showed that such exposure prior to the onset of locomotion produced later locomotor alterations. Neonatal-fetal ID may act similarly as a developmentally-altering factor impacting the vestibulo-spinal track. ID severity in infancy may relate more to the development and maturation of the cortico-spinal and cortico-striatal pathways, which are not completely myelinated at birth.26 Their progressive postnatal myelination directly impacts gross motor skills, which might make them particularly vulnerable to effects of ID severity in infancy. Myelination of efferent neural pathways in the cerebellum and basal ganglia is also necessary for the onset of directed locomotion.27
Our findings on locomotion may be relevant to other aspects of infant development. Pollitt and Gorman proposed that understanding associations between motor and cognitive development should focus on gross motor milestones that foster developmentally meaningful actions.28 Acquiring locomotor milestones facilitates further exploratory behaviors. Conversely, delays in the onset of locomotion skills may delay the acquisition of perceptual abilities, spatial orientation and memory, and socio-emotional behaviors.3,29,30 Thus, ID-related effects on locomotion may contribute to poorer development in other areas.
Although the pattern of findings we observed is consistent with the time course of different motor-related neurodevelopmental processes, another potential explanation relates to caregiver-infant interaction.31 Maternal BI is an indirect index of fetal iron status but a direct measure of maternal iron status. Poor maternal iron status may interfere with maternal caregiving such that the mother provides less developmental support for the infant. There is some evidence of this effect, though not for motor outcomes.32 There is also evidence that mothers of ID children are less responsive to their children’s everyday activities than mothers of iron-sufficient infants,33 and children who had chronic ID in infancy are more likely to display lower levels of physical activity.34 It is possible that other ID-related alterations in infant behavior influence caregiver interaction in ways that are less developmentally supportive. This mechanism seems plausible as a factor in gross motor outcomes but less so for neurological integrity.
This study has important limitations. It cannot support causal inferences or isolate ID as the sole factor in our results. Although we considered numerous background characteristics and controlled for those that contributed to the models, ID often goes along with other biological and psychosocial risks. Nonetheless, the findings indicate that negative effects of inadequate iron to the fetus and neonate should not be ruled out, even though analysis by the RCT design did not show motor effects of iron supplementation in pregnancy.1 For the fetal-neonatal period, we examined iron measures in cord blood and maternal blood in late pregnancy. One prior study suggested that ID earlier in pregnancy may also be important,9 but we did not have maternal 1st trimester iron measures. However, maternal BI in mid-pregnancy did not relate to motor development in our study, whereas BI in late pregnancy did. Pregnancy and fetal-neonatal are times when iron measures are hard to interpret. Perhaps reflecting this, IC components did not make strong factors at mid-pregnancy or in the fetus/neonate (cord-blood). Also, our results might not generalize to populations where ID is not as widespread or infants are not as healthy. As in other studies in the field, blood measures of iron status provide little information about the iron status of the brain and its pertinent regions. Therefore, interpretations regarding the central nervous system should be considered with caution.
CONCLUSIONS
ID in the fetal-neonatal or infancy periods was associated with similar detrimental motor outcomes, which were not worse with ID in both periods. Not having ID in either time period was associated with the best motor development. It could be concluded that timing and duration of ID were not critically relevant regarding locomotor and motor scores. However, ID severity in late pregnancy was associated with neurological integrity at 9 months, suggesting that adverse effects of inadequate iron during gestation should not be dismissed. More severe ID in infancy was associated with poorer overall gross motor development.
Acknowledgments
Funding source: A grant from the US National Institutes of Health (R01 HD052069), which included funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Office of Dietary Supplements, provided support for the infancy study and laboratory measures of iron status for mothers and infants, Betsy Lozoff, Principal Investigator. Vifor Pharma, Ltd. provided financial support for the pregnancy study. São Paulo Research Foundation – FAPESP/Brazil (2014/00018-0) and Methodist University of Piracicaba – UNIMEP/Brazil provided financial support for Denise CC Santos. The content is solely the responsibility of the authors and does not necessarily represent the official views of funding sources. Authors had full control of primary data and did not have an agreement with the funders that limited their ability to complete the research as planned.
Financial Disclosure: Betsy Lozoff was an unpaid speaker at 2 seminars supported by Lee’s Pharmaceutical Holdings Limited. The topic was iron deficiency and child development (Shanghai, April 11, 2010, and Beijing, May 15, 2011). The company covered hotel accommodations and, for the 2011 seminar, internal airfare between Hangzhou and Beijing.
The authors have indicated they have no other financial relationships relevant to this article.
Footnotes
CONFLICT OF INTEREST: Denise CC Santos, Rosa M Angulo-Barroso, Ming Li, Yang Bian, Julie Sturza, Blair Richards, and Betsy Lozoff have no conflicts of interest.
References
- 1.Angulo-Barroso RM, Li M, Santos DCC, Bian Y, Sturza J, Jiang Y, et al. Iron supplementation in pregnancy or infancy and motor development: a randomized controlled trial. Pediatr. 2016;137(4):e20153547. doi: 10.1542/peds.2015-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray GK, Veijola J, Moilanen K, Miettunen J, Glahn DC, Cannon TD, et al. Infant motor development is associated with adult cognitive categorisation in a longitudinal birth cohort study. J Child Psychol Psychiatry. 2006;47:25–29. doi: 10.1111/j.1469-7610.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- 3.Clearfield MW. Learning to walk changes infants’ social interactions. Infant Beh Dev. 2011;34:15–25. doi: 10.1016/j.infbeh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: A systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgieff MK, Brunette KE, Tran PV. Early life nutrition and neural plasticity. Dev Psychopathol. 2015;27:411–423. doi: 10.1017/S0954579415000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics. 2013;131:e755–e763. doi: 10.1542/peds.2011-3513. [DOI] [PubMed] [Google Scholar]
- 8.Tran TD, Tran T, Simpson JA, Tran HT, Nguyen TT, Hanieh S, et al. Infant motor development in rural Vietnam and intrauterine exposures to anaemia, iron deficiency and common mental disorders: a prospective community-based study. BMC Pregnancy Childbirth. 2014;14:8. doi: 10.1186/1471-2393-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez-Martinez C, Canals J, Aranda N, Ribot B, Escribano J, Arija V. Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum Dev. 2011;87:165–169. doi: 10.1016/j.earlhumdev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 11.Gunnarsson BS, Thorsdottir I, Palsson G, Gretarsson SJ. Iron status at 1 and 6 years versus developmental scores at 6 years in a well-nourished affluent population. Acta Paediatr. 2007;96:391–395. doi: 10.1111/j.1651-2227.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 12.Shafir T, Angulo-Barroso R, Calatroni A, Jimenez E, Lozoff B. Effects of iron deficiency on patterns of motor development over time. Hum Mov Sci. 2006;25:821–838. doi: 10.1016/j.humov.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lozoff B, Jiang Y, Li X, Zhou M, Richards B, Xu G, et al. Low-dose iron supplementation in infancy modestly increases infant iron status at 9 months without decreasing growth or increasing illness in a randomized clinical trial in rural China. J Nutr. 2016;146:612–621. doi: 10.3945/jn.115.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao G, Xu G, Zhou M, Jiang Y, Richards B, Clark KM, et al. Prenatal iron supplementation reduces maternal anemia, iron deficiency, and iron deficiency anemia in a randomized clinical trial in rural China, but iron deficiency remains widespread in mothers and neonates. J Nutr. 2015;145:1916–1923. doi: 10.3945/jn.114.208678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011. (WHO/NMH/NHD/MNM/11.1) [Google Scholar]
- 17.Folio MK, Fewell R. Peabody Developmental Motor Scales: Examiner’s Manual. 2. Austin, TX: PRO-ED, Inc; 2000. [Google Scholar]
- 18.Zhao G, Bian Y, Li M. Impact of passing items above the ceiling on the assessment results of Peabody developmental motor scales. Beijing Da Xue Bao (Journal of Peking University Health Sciences) 2013;45:928–932. [PubMed] [Google Scholar]
- 19.Ellison PH, Horn JL, Browning CA. Construction of an Infant Neurological International Battery (INFANIB) for the assessment of neurological integrity in infancy. Physical Therapy. 1985;65:1326–1331. doi: 10.1093/ptj/65.9.1326. [DOI] [PubMed] [Google Scholar]
- 20.Bayley N. Bayley Scales of Infant Development. 2. San Antonio: The Psychological Corporation; 1993. [Google Scholar]
- 21.Sanhe City People’s Government. Sanhe City People’s Government on the issuance of “Sanhe 2013 annual public housing security plan”. 2013 http://www.he.xinhuanet.com/zfwq/sanhe/zhengwu/zhengwu/2013-10/21/c_117803056.htm. [cited 1 Jun 2015]
- 22.Liao W, Wen EY, Li C, Chang Q, Lv KL, Yang W, He ZM, Zhao CM. Predicting neurodevelopmental outcomes for at-risk infants: reliability and predictive validity using a Chinese version of the INFANIB at 3, 7 and 10 months. BMC Pediatr. 2012;12:72. doi: 10.1186/1471-2431-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blayney AW. Vestibular disorders. In: Adams DA, Cinnamond MJ, editors. Paediatric Otolarngology (Sixth Edition). Scott-Brown’s Otolaryngology: Vol. 6. Chapter 12. CRC Press; 1997. pp. 1–29. [Google Scholar]
- 24.O’Reilly R, Grindle C, Zwicky EF, Morlet T. Development of the vestibular system and balance function: Differential diagnosis in the pediatric population. Otolaryngol Clin North Am. 2011;44:251–71. vii. doi: 10.1016/j.otc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Beraneck M, Lambert FM, Sadeghi SG. Functional development of the vestibular system: Sensorimotor pathways for stabilization of gaze and posture. In: Varelanieto R, Romand I, editors. Development of Auditory and Vestibular Systems. Academic Press; 2014. pp. 449–487. [Google Scholar]
- 26.Rothwell J. Control of Human Voluntary Movement. London: Chapman & Hall; 1994. [Google Scholar]
- 27.Konner M. Universals of behavioral development in relation to brain myelination. In: Gibson KR, Petersen AC, editors. Brain Maturation and Cognitive Development. New York: Aidine De Gruyter; 1991. pp. 181–223. [Google Scholar]
- 28.Pollitt E, Gorman K. Activity, energy expenditure and energy requirements of infants and children. Vol. 1990. Lausanne, Switzerland: International Dietary Energy Consultancy Group; 1990. Long-term developmental implications of motor maturation and physical activity in infancy in a nutritionally at risk population; pp. 279–296. [Google Scholar]
- 29.Adolph KE, Eppler MA, Gibson EJ. Development of perception of affordances. Adv Infant Res. 1993;8:51–98. [PubMed] [Google Scholar]
- 30.Campos JJ, Kermoian R, Zumbahlen MR. Socioemotional transformation in the family system following infant crawling onset. In: Eisenberg N, Fabes R, editors. Emotion and Its Regulation in Early Development. San Francisco, CA: Josey Bass; 1992. p. 110. [DOI] [PubMed] [Google Scholar]
- 31.Lozoff B, Klein NK, Nelson EC, et al. Behavior of infants with iron deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 32.Perez EM, Hendricks MK, Beard JL, Murray-Kolb LE, Berg A, Tomlinson M, et al. Mother-infant interaction and infant development are altered by maternal iron deficiency anemia. J Nutr. 2005;135:850–855. doi: 10.1093/jn/135.4.850. [DOI] [PubMed] [Google Scholar]
- 33.Armony-Sivan R, Kaplan-Estrin M, Jacobson SW, Lozoff B. Iron-deficiency anemia in infancy and mother-infant interaction during feeding. J Dev Behav Pediatr. 2010;31:526–532. doi: 10.1097/DBP.0b013e3181dc525d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corapci F, Radan AE, Lozoff B. Iron deficiency in infancy and mother-child interaction at 5 years. J Behav Dev Pediatr. 2006;27:371–378. doi: 10.1097/00004703-200610000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]