Abstract
Background
The impact of blood flow regulation and oxidative stress during exercise in cystic fibrosis (CF) has yet to be investigated.
Methods
A maximal graded exercise test was conducted to determine exercise capacity (VO2 peak) and peak workload in 14 pediatric patients with mild CF (age 14±3 y, FEV1 93±16 %predicted) and 14 demographically-matched controls. On a separate visit, participants performed submaximal cycling up to 60% of peak workload where brachial artery blood velocity was determined using Doppler ultrasound. Retrograde and antegrade components were further analyzed as indices of blood flow regulation.
Results
The cumulative AUC for retrograde velocity was lower in patients versus controls (1770±554 vs. 3440±522 cm, P=0.038). In addition, an exaggerated oxidative stress response during exercise occurred in patients only (P=0.004).
Conclusion
These data suggest that patients with mild CF exhibit impaired blood flow regulation and an exaggerated oxidative stress response to submaximal exercise.
Keywords: exercise intolerance, reactive oxygen species, retrograde velocity, electron paramagnetic resonance spectroscopy
1. Introduction
Cystic Fibrosis (CF) is an autosomal recessive genetic disorder that is the result of a mutation in the cAMP-dependent CFTR (cystic fibrosis transmembrane conductance regulator) chloride channel protein. There are many systemic complications in CF which include dysfunction of pulmonary, gastrointestinal, immune, endocrine, and musculoskeletal systems [1,2]. While the primary cause of morbidity and mortality in CF is pulmonary dysfunction and lung infection [3], exercise intolerance is a hallmark of CF [4,5] and a reduction in maximal aerobic capacity (VO2 peak) is a significant predictor of mortality in this patient population, independent of lung function [6,7]. However, the mechanisms which contribute to exercise intolerance in CF remain unclear.
The ability to maintain adequate oxygen delivery to the working musculature is of critical importance, particularly under conditions of increased demand, such as during exercise. Both macro- and micro-vascular endothelial dysfunction is present in patients with CF [8,9]. Accordingly, it is plausible that impaired nutritive flow to the working musculature during exercise may contribute to exercise intolerance [10,11]. In fact, the pattern of brachial artery flow during lower limb cycling can provide valuable information regarding appropriate exercise induced vasodilatory/vasoconstrictor responses [12]. While a previous study demonstrated similar forearm blood flow responses during handgrip exercise in patients with CF versus healthy counterparts [13], whether or not 1) patients with CF exhibit abnormal blood flow regulation during exercise of large muscle-mass or 2) dysfunctional blood flow regulation affects exercise capacity in this patient population remains unknown.
There is convincing evidence to indicate the presence of chronic oxidative stress in patients with CF [14,15]. Oxidative stress in its basic form represents an imbalance between free radical production and neutralization of radicals by antioxidants and has been suggested to contribute to the multi-organ pathophysiology [14] and decline in pulmonary function over time in CF [16]. Oxidative stress is related to exercise capacity in several patient populations [17,18] and may partly be linked to pancreatic insufficiency and the intestinal malabsorption of endogenous fat-soluble vitamins and antioxidants in CF [19]. However, whether or not oxidative stress impacts exercise capacity in patients with CF is unknown.
While exercise intolerance in CF is related to a number of factors, the degree to which blood flow regulation and oxidative stress influences maximal exercise capacity in CF is unknown. Therefore, this study sought to test the hypotheses that 1) blood flow regulation during submaximal cycling exercise is compromised in patients with CF compared to controls, and 2) patients with CF exhibit an exaggerated exercise induced increase in oxidative stress compared to controls.
2. Materials and Methods
2.1 Participants
A total of 28 volunteers (14 patients with CF and 14 healthy controls) ages 8 – 20 years old participated in this study. Patients were enrolled if they had a clinical diagnosis of CF based on positive sweat test metrics and genotype analysis. Based on the patients’ characteristics, demographically-matched apparently healthy controls were recruited. Detailed exclusion criteria can be found in the online supplement. All study protocols were approved by the Institutional Review Board at Augusta University and written and verbal assent/consent was obtained by all participants and parents prior to participation.
2.2 Experimental Design
All participants reported for two days of testing; a preliminary and an experimental visit. The preliminary visit consisted of the informed consent process, body composition assessments, a baseline pulmonary function test (PFT), and a maximal exercise capacity test. On the experimental visit, a second PFT was performed prior to beginning the submaximal exercise protocol. Brachial artery diameter and blood velocity were measured while sitting on a cycle ergometer at baseline and during submaximal cycling exercise at 20, 40 and 60% of peak workload. Blood samples were taken at baseline and at 60% intensity for assessment of oxidative stress biomarkers. Patients were instructed to adhere to the timing of their daily treatments and report to the laboratory following their morning airway clearance technique and inhaled medicine regimen.
2.3 Participant Characteristics and Clinical Laboratory Values
Participant testing included assessments of height, weight, calculated body mass index (BMI), and body composition via dual-energy X-ray absorptiometry (DXA; QDR-4500W; Hologic, Waltham, MA). An estimate of daily physical activity levels was determined from a self-reported health-history questionnaire. Blood pressure was measured in triplicate according to American Heart Association recommendations. Concentrations of total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TG), glucose, and high sensitivity C-reactive protein (hsCRP) were obtained using a Cholestech LDX point of care analyzer (Alere Inc., Scarborough, ME). Hemoglobin and hematocrit were obtained using a HemoPoint H2 analyzer (Stanbio Laboratory, Boerne, TX).
2.4 Pulmonary Function Testing
Pulmonary function testing (PFT) was performed in all participants using closed circuit spirometry (ParvoMedics, Sandy, UT) according to the American Thoracic Society standards [20] to determine FEV1, forced vital capacity (FVC), FEV1/FVC ratio, and forced expiratory flow at 25–75% (FEF25–75). The national health and nutrition examination survey (NHANES) III spirometric reference standards were used to determine the percent predicted data outcomes.
2.5 Maximal Exercise Capacity
On the preliminary visit, maximal exercise capacity (VO2 peak) was determined on a cycle ergometer using the Godfrey Protocol [21]. Briefly, participants pedaled on an appropriate sized electronically braked cycle ergometer (Lode Corival or Lode Corival Pediatric, Groningen, Netherlands) with work intensities increasing every minute until volitional fatigue, breathlessness, chest discomfort, and/or any other signs observed by the investigators that the test should be terminated. Throughout maximal exercise testing, breath by breath expired gases were analyzed by a TruOne® 2400 metabolic cart (ParvoMedics, Sandy, UT) and reported as 30 second averages to obtain VO2 peak. Heart rate, blood pressure, and SpO2 were monitored throughout the test.
2.6 Submaximal Exercise
On the experimental visit (at least one week following preliminary testing), all participants performed submaximal exercise on a cycle ergometer at 20, 40, and 60% of the maximum workload (Watts) that was obtained from the maximal exercise test. Following a 2 minute unloaded warm-up, each workload intensity was performed for 5 minutes, equating to a total of 15 minutes of continuous submaximal exercise.
2.7 Brachial Artery Diameter, Blood Velocity, and Shear Rate
Blood flow regulation was determined by assessing changes in brachial artery diameter, blood flow, and blood velocity during increasing exercise intensity. At baseline and during submaximal exercise at 20, 40, and 60% intensity, assessment of brachial artery diameter and velocity was determined using Doppler ultrasound (LOGIQ 7, General Electric Company, Chicago, IL). Further details on the ultrasound methodology used can be found in the online supplement.
2.8 Oxidative Stress and Free Radical Biomarkers
Antioxidant capacity (AOC) and 8-isoprostane (8-ISO) were determined from plasma via colorimetric assay following the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI). Details on methods used to determine plasma trolox-equivalent antioxidant capacity (TEAC), ferric reducing antioxidant potential (FRAP), plasma lipid hydroperoxides (LPO), and protein carbonyls (PC) can be found in the online supplement.
Electron Paramagnetic Resonance (EPR) spectroscopy was used to determine alkoxyl and ascorbyl free radicals as previously described [22]. Comprehensive details on the EPR methodology used can be found in the online supplement. Due to technical difficulties, not all participants’ samples were analyzed. Data comparing oxidative stress and free radical biomarkers are presented with a minimum sample of nine participants per group.
2.9 Statistical Analyses
All analyses were performed using SPSS version 23 (IBM Corporation, Somers, NY). Descriptive statistics were generated and range as well as normality checks performed. Two-way repeated measures ANOVA (group × time) were used to test for group differences in blood flow regulation and changes in oxidative stress markers during exercise. The Greenhouse-Geisser correction was applied when the assumption of sphericity was violated and follow up pairwise comparisons were performed and adjusted for multiple comparisons using a Bonferroni correction. Effect sizes (partial eta squared ) are reported for the interaction terms of the ANOVA, where values of 0.01, 0.06, and 0.14 correspond to small, medium, and large effects, respectively [23]. Comparisons of participant characteristics, baseline values of oxidative stress markers, and AUC values between groups were performed using independent t-tests or Mann-Whitney U tests if data were not normally distributed. Pearson product-moment correlations were used to examine relationships between blood flow regulation, exercise capacity, and oxidative stress. An alpha <0.05 was considered statistically significant for all analyses. Data are presented as mean ± standard deviation (SD) unless stated otherwise.
3. Results
3.1 Participant Characteristics and Clinical Laboratory Values
Estimated habitual daily physical activity was not different between groups (P>0.05). Participant characteristics and clinical laboratory values are presented in Table 1. There were no differences in age, height, weight, BMI, LDL, TG, TC:HDL ratio, fasting glucose, hematocrit, hemoglobin, or blood pressure between patients and controls. Resting SpO2 and lung function values (FEV1 % predicted, FEV1/FVC, and FEF25–75) were all lower in patients versus controls. Importantly, the pulmonary function values are representative of a relatively healthy and mild disease patient cohort [24]. All pulmonary function values during the preliminary and experimental visits were similar (P>0.05; intra-class correlations [ICC] >0.903; P<0.001). Although exercise capacity expressed relative to body mass was similar between groups, it was significantly lower in patients when expressed relative to fat-free mass. Differences in exercise capacity (expressed as % predicted) between groups was approaching significance (P=0.054). No difference in peak work during maximal exercise was observed between groups.
Table 1.
Participant characteristics and clinical laboratory values.
| Variable | Controls | CF | P value |
|---|---|---|---|
| Demographics | |||
|
| |||
| N | 14 | 14 | |
| Sex (M/F) | 6/8 | 6/8 | |
| Age (y) | 14 ± 3 | 14 ± 3 | 0.905 |
| Height (cm) | 158 ± 18 | 156 ± 17 | 0.784 |
| Weight (kg) | 49.7 ± 13.8 | 46.9 ± 14.3 | 0.597 |
| BMI (kg/m2) | 19.7 ± 2.9 | 18.7 ± 2.0 | 0.333 |
| Body Fat (%) | 23.7 ± 8.4 | 19.8 ± 6.0 | 0.177 |
| SBP (mm Hg) | 107 ± 16 | 104 ± 12 | 0.553 |
| DBP (mm Hg) | 63 ± 9 | 61 ± 6 | 0.452 |
| Resting O2 Saturation (%) | 98.8 ± 0.4 | 98.2 ± 0.7 | 0.035 |
|
| |||
| Pulmonary Function | |||
|
| |||
| FVC (L) | 3.80 ± 1.27 | 3.58 ± 1.42 | 0.668 |
| FEV1 (L) | 3.36 ± 1.08 | 2.91 ± 1.29 | 0.339 |
| FEV1 (% predicted) | 104 ± 12 | 93 ± 16 | 0.038 |
| FEV1/FVC (%) | 89 ± 7 | 80 ± 7 | 0.004 |
| FEF25–75 (L/s) | 4.0 ± 1.5 | 2.9 ± 1.4 | 0.044 |
|
| |||
| Exercise Capacity | |||
|
| |||
| VO2 peak (ml/kg/min) | 36.4 ± 8.9 | 33.0 ± 6.2 | 0.252 |
| VO2 peak (ml/kg−FFM/min) | 50.4 ± 7.6 | 43.7 ± 6.8 | 0.022 |
| VO2 peak (% predicted) | 85 ± 16 | 74 ± 12 | 0.054 |
| Peak Work (W) | 149 ± 56 | 122 ± 44 | 0.165 |
|
| |||
| Clinical Laboratory Markers | |||
|
| |||
| TC (mg/dL) | 149 ± 27 | 122 ± 25 | 0.013 |
| HDL (mg/dL) | 54 ±11 | 36 ± 13 | 0.001 |
| LDL (mg/dL) | 83 ± 20 | 69 ± 20 | 0.119 |
| Triglycerides (mg/dL) | 71 ± 27 | 82 ± 31 | 0.351 |
| Glucose (mg/dL) | 85 ± 8 | 91 ± 11 | 0.149 |
| TC:HDL | 2.8 ± 0.6 | 3.8 ± 1.8 | 0.072 |
| hsCRP (mg/L) | 0.4 ± 0.2 | 1.6 ± 1.8 | 0.032 |
| Hemoglobin (g/dL) | 13.9 ± 1.4 | 13.5 ± 1.3 | 0.372 |
| Hematocrit (%) | 41.0 ± 4.1 | 40.5 ± 3.1 | 0.766 |
Values are mean ± SD.
Bold face indicates significance.
BMI = body mass index; SBP = systolic blood pressure; DBP = diastolic blood pressure; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; FEF = forced expiratory flow; VO2 = volume of oxygen consumed; TC = total cholesterol; HDL = high density lipoprotein; LDL = low density lipoprotein; hsCRP = high sensitivity C-reactive protein.
Both TC and HDL were higher in controls when compared to patients. A 3-fold higher concentration of hsCRP was also observed in patients with CF compared to controls.
3.2 Brachial Artery Hemodynamics
Brachial artery hemodynamics at rest and during submaximal exercise are presented in Table 2.
Table 2.
Blood flow variables at rest and during exercise at 20, 40, and 60% intensity.
| Baseline | 20% | 40% | 60% | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Variable | Controls | CF | Controls | CF | Controls | CF | Controls | CF |
|
|
|
|
|
|||||
| Diameter (cm) | 0.28 ± 0.05 | 0.28 ± 0.05 | 0.28 ± 0.05 | 0.27 ± 0.05 | 0.28 ± 0.05 | 0.27 ± 0.05 | 0.28 ± 0.05 | 0.27 ± 0.05 |
| Mean velocity (cm/s) | 9.3 ± 4.4 | 5.8 ± 1.4 | 8.6 ± 4.6 | 7.8 ± 2.2 | 15.3 ± 9.6* | 10.2 ± 3.7* | 18.9 ± 8.4* | 13.7 ± 4.4* |
| Blood flow (ml/min) | 34.4 ± 19.1 | 21.8 ± 9.3 | 29.0 ± 13.4 | 28.0 ± 13.2 | 54.5 ± 35.4* | 36.1 ± 15.8* | 71.4 ± 38.1† | 49.4 ± 19.2† |
| Retrograde blood flow (ml/min) | 2.8 ± 4.1 | 4.4 ± 6.8 | 10.3 ± 12.6‡ | 11.0 ± 9.4‡ | 12.5 ± 15.4* | 19.8 ± 15.8* | 14.3 ± 17.7* | 23.6 ± 17.1* |
| Antegrade blood flow (ml/min) | 37.0 ± 20.5 | 26.3 ± 12.1 | 38.7 ± 17.6‡ | 39.8 ± 17.3‡ | 63.9 ± 32.8* | 56.0 ± 25.2* | 80.2 ± 37.7† | 73.1 ± 29.8† |
| Mean shear rate (s−1) | 171 ± 54 | 277 ± 141 | 231 ± 62 | 266 ± 166 | 305 ± 131* | 458 ± 297* | 412 ± 166† | 554 ± 254† |
| Heart rate (bpm) | 82 ± 10 | 76 ± 11 | 100 ± 9 | 101 ± 11 | 118 ± 16 | 127 ± 16 | 139 ± 19 | 157 ± 17# |
Values are mean ± SD.
significantly higher versus baseline and 20% intensity, independent of group (P<0.05);
significantly higher versus baseline, 20%, and 40% intensity, independent of group (P<0.05);
significantly higher versus baseline, independent of group (P<0.01);
significantly higher versus controls within same level of intensity (P=0.017).
3.2.1. Brachial Artery Diameter, Mean Blood Velocity, and Blood Flow
Brachial artery diameter (cm) did not change over time (P=0.471), nor was there a main effect of group (P=0.897) or a significant group × time interaction (P=0.293, ). Mean velocity (cm/s) increased over time (P<0.001); however, the group × time interaction was not significant (P=0.119, ). Overall, mean velocity at 40% and 60% intensity was higher (P<0.001) compared to both 20% intensity and baseline values. In addition, there was a main effect of group, such that mean velocity was lower in patients (9.4 ± 1.2 cm/s) compared to controls (13.0 ± 1.2 cm/s, P=0.043).
Blood flow (ml/min) increased over time (P<0.001) but the group × time interaction was not significant (P=0.089, ). Across groups, mean blood flow at 20% intensity was not different versus baseline (P=0.881) but progressively increased during exercise. Specifically, mean blood flow at 60% intensity was higher versus all other time points (P<0.01) and 40% intensity was higher compared to both 20% intensity and baseline values (P<0.01).
3.2.2. Retrograde and Antegrade Blood Velocity and Blood Flow
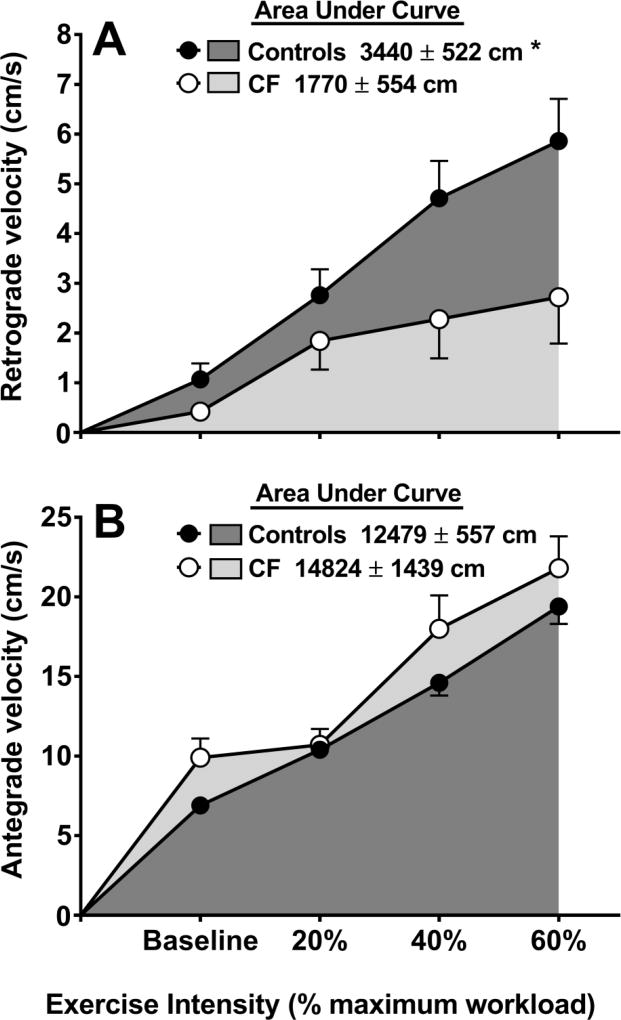
Figure 1A illustrates the significant (P=0.030, ) group × time interaction for retrograde velocity. Specifically, the total AUC for retrograde velocity was lower in patients versus controls (P=0.038). Retrograde blood flow (ml/min) increased over time (P<0.001) but the group × time interaction was not significant (P=0.130, ; Table 2). Overall, retrograde blood flow increased progressively such that it was higher at each successive exercise intensity (all P<0.01) with the exception of a non-significant difference between 40% and 60% intensity (P=0.242).
Figure 1.
Retrograde (Panel A) and antegrade (Panel B) blood velocity at baseline, and during sub-maximal exercise at 20, 40, and 60% of VO2 peak in controls (n=14) and patients with CF (n=14). *significant difference in total AUC between groups (P<0.05).
Antegrade velocity (cm/s) increased over time (P<0.001) but the group × time interaction was not significant (P=0.225, ; Figure 1B). Moreover, the total AUC for antegrade velocity was similar between groups (P=0.147). Antegrade blood flow (ml/min) increased over time (P<0.001) but the group × time interaction was not significant (P=0.322, ). Overall, antegrade blood flow increased progressively such that it was significantly higher at each successive exercise intensity (all P<0.01).
3.2.3. Shear Rate and Heart Rate
Mean shear rate (s−1) increased over time (P<0.001) but the group × time interaction was not significant (P=0.183, ; Table 2). Overall, mean shear rate at 20% intensity was not different versus baseline (P=0.263); however, it was higher at each successive exercise intensity (all P<0.01).
A significant group × time interaction (P=0.001, ) was present for changes in heart rate. Specifically, heart rate was higher in patients at 60% intensity versus controls (P=0.017). No differences between groups were observed at baseline (P=0.133), 20% (P=0.756), or 40% intensity (P=0.180).
3.3. Oxidative Stress Biomarkers
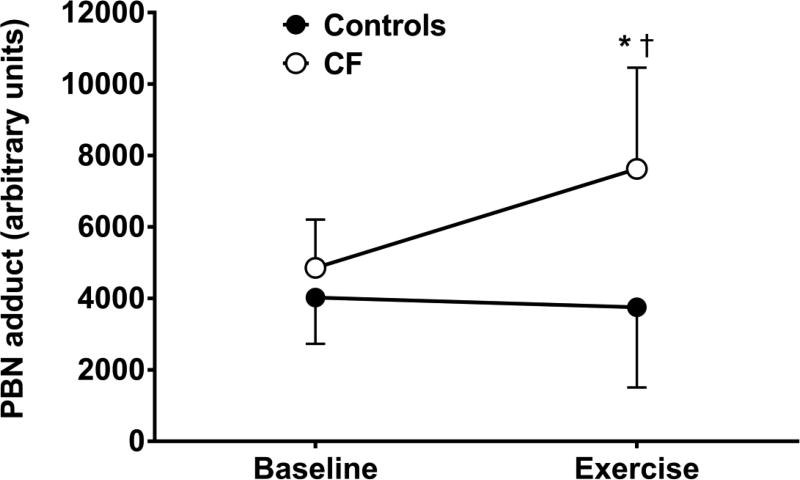
Oxidative stress biomarkers at rest and during exercise are presented in Table 3. A significant group × time interaction was present for alkoxyl free radical (P=0.004, ). Specifically, alkoxyl free radical was higher during exercise in patients versus controls (P=0.005; Figure 2). No differences in baseline concentrations of ascorbyl free radical, AOC, 8-ISO, FRAP, TEAC, PC, or LPO were present between groups (all P>0.05). Changes in additional markers of oxidative stress from baseline to exercise were not dependent on group (i.e., no significant interactions [all P>0.05]). Independent of group, there was a main effect of time for AOC as it decreased following exercise (P=0.008). No significant main effect of time was present for ascorbyl free radical (P=0.083), 8-ISO (P=0.085), or FRAP (P=0.079).
Table 3.
Oxidative stress biomarkers at rest and during exercise at 60% intensity.
| Baseline | Exercise | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | Controls | CF | Controls | CF |
|
|
|
|||
| Ascorbyl FR × 103 (a.u.)a | 650 ± 205 | 669 ± 501 | 874 ± 301 | 2,198 ± 2,811 |
| AOC (mM)b | 1.34 ± 0.34 | 1.43 ± 0.50 | 1.13 ± 0.29* | 1.12 ± 0.49* |
| 8-ISO (ng/ml)c | 16.2 ± 10.7 | 16.0 ± 7.8 | 17.1 ± 9.6 | 24.5 ± 20.1 |
| FRAP (µmol/L)b | 0.64 ± 0.21 | 0.69 ± 0.24 | 0.72 ± 0.09 | 0.77 ± 0.11 |
| TEAC (µmol/L)b | 0.43 ± 0.14 | 0.41 ± 0.14 | 0.46 ± 0.03 | 0.46 ± 0.08 |
| PC (µmol/mg protein)d | 0.45 ± 0.04 | 0.39 ± 0.13 | 0.45 ± 0.05 | 0.44 ± 0.11 |
| LPO (µmol/L)e | 0.55 ± 0.55 | 0.78 ± 0.54 | 0.79 ± 0.75 | 0.69 ± 0.55 |
Values are mean ± SD.
FR = free radicals; AOC = antioxidant capacity; 8-ISO = 8-isoprostane; FRAP = ferric reducing antioxidant power; TEAC = trolox equivalent antioxidant capacity; PC = protein carbonyls; LPO = lipid hydroperoxide.
main effect of time, independent of group (P=0.008).
Controls (n=9), CF (n=9);
Controls (n=12), CF (n=14);
Controls (n=12), CF (n=11);
Controls (n=10), CF (n=10);
Controls (n=11), CF (n=13).
Figure 2.
PBN-adduct at baseline and during sub-maximal exercise at 60% VO2 peak in controls (n=9) and patients with CF (n=11). *significantly different from controls at 60% (P=0.005); †significant change from baseline in patients with CF (P=0.001).
3.4 Relationships between Blood flow regulation, Exercise Capacity, and Oxidative Stress
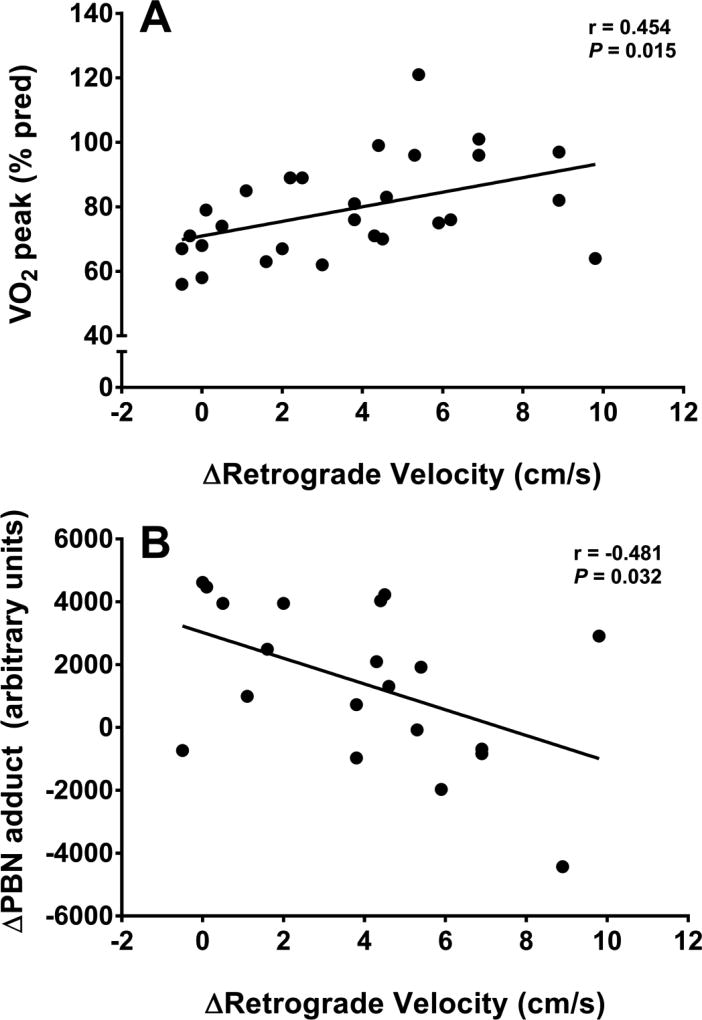
Figure 3A illustrates the significant relationship (r=0.454, P=0.015) between the change (Δ) in retrograde velocity during exercise and exercise capacity (VO2 peak, % predicted). Similarly, the relationship between Δretrograde velocity and peak workload (Watts) was significant, both overall (r=0.551, P=0.024) and when only examining patients with CF (r=0.604, P=0.011, n=14). In addition, there was an overall significant negative relationship (r=−0.481, P=0.032) between Δretrograde velocity and the change in oxidative stress during exercise (ΔPBN adduct; Figure 3B).
Figure 3.
Significant relationships between A) basal exercise capacity (VO2 peak, % predicted) and the change in retrograde velocity, and B) the change in oxidative stress during exercise and the change in retrograde velocity.
4. Discussion
A number of factors contribute to exercise intolerance in patients with CF including pulmonary dysfunction [5], impaired skeletal muscle function [25], and impaired oxygen uptake kinetics [26]. However, whether or not alterations in blood flow and oxidative stress contribute to exercise intolerance is unclear, particularly in patients with mild disease severity (such as the pediatric patients tested in the present study). For the first time, this investigation provides compelling evidence to indicate that, compared to controls, younger patients with CF have impaired blood flow regulation during submaximal exercise that is accompanied by an exaggerated exercise-induced oxidative stress. In addition, our data suggest a potential link between impaired blood flow regulation, exercise-induced oxidative stress, and exercise capacity.
4.1 Impaired Blood Flow Regulation during Exercise in CF
During exercise that involves a large muscle mass (i.e., cycling), the demand for adequate nutrient supply to the working musculature results in a peripheral (i.e. upper body) vasoconstrictor response [27,28]. In the present study, we identified a substantial difference in brachial artery retrograde velocity during submaximal exercise between patients and controls (Figure 1A), suggestive of an inadequate upper body vasoconstrictor response in patients. While there were no differences in retrograde velocity at baseline or the lightest exercise intensity (20%) between groups, as exercise intensity increased, retrograde velocity appeared to plateau in patients with CF, whereas an increased trajectory was observed in controls. Interestingly, our findings are in contrast to a previous investigation investigating forearm blood flow responses to handgrip exercise in patients with CF [13], although differences between studies may be partly due to exercise modality and intensity. No group differences up to 15% of maximal grip strength were observed previously [13] and data from the present study show a sizeable separation between groups beginning at 40% intensity (Figure 1A). Taken together, data from both studies seem to indicate that differences in exercise blood flow responses between patients with CF and controls may only be evident at higher workloads.
Given that retrograde velocity of the brachial artery during cycling is representative of the peripheral vasoconstrictor response in a non-exercising limb, the appropriate response (as observed in controls) should be a stepwise escalation in retrograde velocity as lower leg exercise intensity increases [29]. The lack of increase in retrograde velocity in young patients with CF suggests an abnormal pattern of blood flow during exercise, likely resulting in reduced blood flow to the working muscle and contributing to a lower exercise capacity. Impaired blood flow regulation can induce additional physiological strain which is evidenced by the higher heart rate in patients versus controls at 60% exercise intensity (Table 2), likely in an effort to increase cardiac output and maintain sufficient blood flow to the working muscle. While we did not measure femoral artery blood flow in the present study, previous studies have shown that reduced blood flow to the working musculature limits exercise capacity in healthy [30] and clinical populations [10,11]. Moreover, our data suggest that a greater increase in retrograde velocity is significantly related to a higher exercise capacity (Figure 3A). Thus, these data collectively suggest that impaired blood flow regulation may contribute to exercise intolerance in CF. Future studies should measure blood flow to the working musculature during exercise to confirm the findings of the present study that suggest this may be reduced in patients with CF.
4.2 Oxidative Stress during Exercise Impacts Blood Flow Regulation in CF
Interpretation of exercise-induced oxidative stress responses are challenging due to multiple ROS sources, endogenous radical quenching compounds, and confounding effects of chronic disease conditions [31], including CF. Unequivocal evidence indicates that patients with CF exhibit an elevated basal oxidative stress profile [14,15]; however, the oxidative stress response to exercise and its potential role in augmenting blood flow regulation and limiting exercise capacity in this population is unknown. Given the preserved clinical presentation of the pediatric patients with CF recruited for this investigation, it is not surprising that basal markers of oxidative stress were similar between patients and controls (Table 3). Perhaps more importantly, the free radical response to sub-maximal exercise was markedly increased in patients with CF compared to controls (Figure 2). The disparity in the free radical response between groups could be due to different endogenous antioxidant buffering capacities during exercise. Interestingly, the changes in different markers of antioxidant activity (i.e., AOC, FRAP, TEAC) during exercise were similar between groups, which may suggest that the antioxidant defence system per se in patients with CF is preserved, yet is overloaded by the excessive ROS production during exercise. Patients with CF may benefit from exogenous antioxidant supplementation as a means to enhance free radical buffering and improve exercise capacity, particularly as benefits of this therapy have been reported in other clinical populations with a similar phenotype as CF [32,33].
Unique to the present investigation, there appeared to be a negative association between changes in oxidative stress and retrograde velocity (Figure 3B). These data suggest the possibility that exercise-induced oxidative stress, in part, may contribute to impaired blood flow regulation. Conceptually, there are many ways to link oxidative stress to impaired blood flow regulation; however, one of the key mediators of blood flow is vascular tone and NO production [34]. Moreover, while there are disparate findings regarding the role of NO in exercise hyperemia [35,36], previous work has shown that NO contributes significantly to blood flow in a non-exercising limb during lower limb cycling exercise [12]. It is well established that the superoxide anion can limit the bioavailability of NO and our group has described both macro- [8] and micro-vascular [9] endothelial dysfunction in patients with CF. Although the present study was not designed to specifically examine the role of endothelial function on blood flow regulation, it is plausible that the increased oxidative stress contributed to vascular dysfunction and subsequent impairment in blood flow regulation during exercise. Nonetheless, future studies are warranted to evaluate changes in blood flow regulation following interventions that improve endothelial function, and further investigate the interactions between endothelial function, oxidative stress, and blood flow regulation during exercise in patients with CF.
4.3 Clinical Perspectives
The observation of exercise intolerance in patients with CF is due to a variety of complications associated with the disease. Data from the present study highlight the importance of appropriate regulation of blood flow during exercise in this patient population and provide a link between exercise-induced oxidative stress and exercise capacity in CF. Accordingly, therapies that target blood flow regulation and oxidative stress in CF may yield an improvement in exercise capacity, subsequently increasing quality of life and survival in this patient group.
Interestingly, these findings were in a young cohort of patients with well-preserved lung function. While not statistically significant, disease severity (FEV1, % predicted) appears to be negatively associated with the change in oxidative stress (PBN adduct) during exercise in patients with CF (r=−0.501, P=0.116). These data provide insight into the role that disease severity may play in mediating the oxidative stress response to exercise and, subsequently, exercise capacity in patients with moderate to severe disease severity. Future studies should seek to examine blood flow and oxidative stress responses to exercise in patients with CF at more advanced stages of the disease.
4.4 Conclusions
The present investigation provides unique evidence of impaired blood flow regulation during sub-maximal exercise in pediatric patients with CF, which may be partially mediated by an exaggerated, exercise-induced oxidative stress response. Additionally, dysfunctional blood flow regulation during exercise may be associated with exercise capacity and oxidative stress in CF, although further investigation is needed to confirm these observations. Impaired blood flow regulation and the exaggerated oxidative stress response to exercise provides two potential mechanisms that may be contributing to exercise intolerance in patients with CF. Since exercise capacity predicts mortality in patients with CF, future studies are warranted to identify interventions that may increase survival rates by improving blood flow regulation and reducing oxidative stress during exercise.
Supplementary Material
Acknowledgments
Funding
This study was supported in part by an NIH/NIDDK R21 grant (DK100783) awarded to R.A.H.
Abbreviations
- 8-ISO
8-isoprostane
- AOC
antioxidant capacity
- CRP
C-reactive protein
- DXA
dual-energy X-ray absorptiometry
- EPR
electron paramagnetic resonance
- FRAP
ferric reducing antioxidant potential
- LPO
lipid hydroperoxides
- PBN
α-phenyl-tert-butylnitrone
- PC
protein carbonyls
- PFT
pulmonary function test
- TEAC
trolox-equivalent antioxidant capacity
- V̇O2
volume of oxygen uptake
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
None to declare.
References
- 1.Gruet M, Troosters T, Verges S. Peripheral muscle abnormalities in cystic fibrosis: Etiology, clinical implications and response to therapeutic interventions. J Cyst Fibros. 2017 doi: 10.1016/j.jcf.2017.02.007. (In Press). doi.org/10.1016/j.jcf.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Plant BJ, Goss CH, Plant WD, Bell SC. Management of comorbidities in older patients with cystic fibrosis. Lancet Respir Med. 2013;1(2):164–74. doi: 10.1016/S2213-2600(13)70025-0. [DOI] [PubMed] [Google Scholar]
- 3.Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. Am J Respir Crit Care Med. 1995;151(4):939–41. doi: 10.1164/ajrccm.151.4.7697269. [DOI] [PubMed] [Google Scholar]
- 4.Troosters T, Langer D, Vrijsen B, Segers J, Wouters K, Janssens W, et al. Skeletal muscle weakness, exercise tolerance and physical activity in adults with cystic fibrosis. Eur Respir J. 2009;33(1):99–106. doi: 10.1183/09031936.00091607. [DOI] [PubMed] [Google Scholar]
- 5.Lands LC, Heigenhauser GJ, Jones NL. Analysis of factors limiting maximal exercise performance in cystic fibrosis. Clin Sci. 1992;83(4):391–7. doi: 10.1042/cs0830391. [DOI] [PubMed] [Google Scholar]
- 6.Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med. 1992;327(25):1785–8. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 7.Pianosi P, Leblanc J, Almudevar A. Peak oxygen uptake and mortality in children with cystic fibrosis. Thorax. 2005;60(1):50–4. doi: 10.1136/thx.2003.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poore S, Berry B, Eidson D, McKie KT, Harris RA. Evidence of vascular endothelial dysfunction in young patients with cystic fibrosis. Chest. 2013;143(4):939–45. doi: 10.1378/chest.12-1934. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Miguelez P, Thomas J, Seigler N, Crandall R, McKie KT, Forseen C, et al. Evidence of microvascular dysfunction in patients with cystic fibrosis. Am J Physiol Heart Circ Physiol. 2016;310(11):H1479–H85. doi: 10.1152/ajpheart.00136.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley JR, Anderson JR, Evans DB, Cowley AJ. Impaired nutritive skeletal muscle blood flow in patients with chronic renal failure. Clin Sci. 1990;79(3):239–45. doi: 10.1042/cs0790239. [DOI] [PubMed] [Google Scholar]
- 11.Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation. 1984;69(6):1079–87. doi: 10.1161/01.cir.69.6.1079. [DOI] [PubMed] [Google Scholar]
- 12.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'driscoll G, et al. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol. 2005;562(2):617–28. doi: 10.1113/jphysiol.2004.075929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrage WG, Wilkins BW, Dean VL, Scott JP, Henry NK, Wylam ME, et al. Exercise hyperemia and vasoconstrictor responses in humans with cystic fibrosis. J Appl Physiol. 2005;99(5):1866–71. doi: 10.1152/japplphysiol.00616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Der Vliet A, Eiserich JP, Marelich GP, Halliwell B, Cross CE. Oxidative stress in cystic fibrosis: does it occur and does it matter? Adv Pharmacol. 1996;38:491–513. doi: 10.1016/s1054-3589(08)60996-5. [DOI] [PubMed] [Google Scholar]
- 15.Winklhofer-Roob BM. Oxygen free radicals and antioxidants in cystic fibrosis: the concept of an oxidant-antioxidant imbalance. Acta Paediatrica. 1994;83(s395):49–57. doi: 10.1111/j.1651-2227.1994.tb13229.x. [DOI] [PubMed] [Google Scholar]
- 16.Brown R, Wyatt H, Price J, Kelly F. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur Respir J. 1996;9(2):334–9. doi: 10.1183/09031936.96.09020334. [DOI] [PubMed] [Google Scholar]
- 17.Dekleva M, Celic V, Kostic N, Pencic B, Ivanovic AM, Caparevic Z. Left ventricular diastolic dysfunction is related to oxidative stress and exercise capacity in hypertensive patients with preserved systolic function. Cardiology. 2006;108(1):62–70. doi: 10.1159/000095883. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama Y, Ikeda H, Haramaki N, Yoshida N, Imaizumi T. Oxidative stress is related to exercise intolerance in patients with heart failure. Am Heart J. 1998;135(1):115–20. doi: 10.1016/s0002-8703(98)70351-5. [DOI] [PubMed] [Google Scholar]
- 19.Lancellotti L, D'Orazio C, Mastella G, Mazzi G, Lippi U. Deficiency of vitamins E and A in cystic fibrosis is independent of pancreatic function and current enzyme and vitamin supplementation. Eur J Pediatr. 1996;155(4):281–5. doi: 10.1007/BF02002713. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 21.Godfrey S, Davies C, Wozniak E, Barnes CA. Cardio-respiratory response to exercise in normal children. Clin Sci. 1971;40(5):419–31. doi: 10.1042/cs0400419. [DOI] [PubMed] [Google Scholar]
- 22.Davison GW, Ashton T, Davies B, Bailey DM. In vitro electron paramagnetic resonance characterization of free radicals: relevance to exercise-induced lipid peroxidation and implications of ascorbate prophylaxis. Free Rad Res. 2008;42(4):379–86. doi: 10.1080/10715760801976618. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. Hilsdale. Vol. 2 NJ: Lawrence Earlbaum Associates; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 24.Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Jr, Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176(10):957–69. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 25.Erickson ML, Seigler N, McKie KT, McCully KK, Harris RA. Skeletal muscle oxidative capacity in patients with cystic fibrosis. Exp Physiol. 2015;100(5):545–52. doi: 10.1113/EP085037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fielding J, Brantley L, Seigler N, McKie KT, Davison GW, Harris RA. Oxygen uptake kinetics and exercise capacity in children with cystic fibrosis. Pediatr Pulmonol. 2015;50(7):647–54. doi: 10.1002/ppul.23189. [DOI] [PubMed] [Google Scholar]
- 27.Blair DA, Glover WE, Roddie JC. Vasomotor responses in the human arm during leg exercise. Circ Res. 1961;9(2):264–74. [Google Scholar]
- 28.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, et al. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: role of thermoregulatory vasodilation. J Appl Physiol. 2011;110(2):389–97. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thijssen D, Dawson EA, Black MA, Hopman M, Cable NT, Green DJ. Brachial artery blood flow responses to different modalities of lower limb exercise. Med Sci Sports Exerc. 2009;41(5):1072–9. doi: 10.1249/MSS.0b013e3181923957. [DOI] [PubMed] [Google Scholar]
- 30.Wilmore JH, Horvath SM. Alterations in peripheral blood flow consequent to maximal exercise. Am Heart J. 1963;66(3):353–62. doi: 10.1016/0002-8703(63)90267-9. [DOI] [PubMed] [Google Scholar]
- 31.Reid MB. Reactive Oxygen Species as Agents of Fatigue. Med Sci Sports Exerc. 2016;48(11):2239–46. doi: 10.1249/MSS.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 32.Ives SJ, Harris RA, Witman MA, Fjeldstad AS, Garten RS, McDaniel J, et al. Vascular Dysfunction and Chronic Obstructive Pulmonary Disease The Role of Redox Balance. Hypertension. 2014;63(3):459–67. doi: 10.1161/HYPERTENSIONAHA.113.02255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, et al. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension. 2012;59(4):818–24. doi: 10.1161/HYPERTENSIONAHA.111.189456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer RM, Ferrige A, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 35.Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S-i, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation. 1994;90(6):2886–90. doi: 10.1161/01.cir.90.6.2886. [DOI] [PubMed] [Google Scholar]
- 36.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557(2):599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.