Abstract
Spinocerebellar ataxias are a genetically heterogeneous group of degenerative diseases typically characterized by progressive ataxia and to various degrees, neuropathy, amyotrophy, and ocular abnormalities. There is increasing evidence for non-motor manifestations associated with cerebellar syndromes including cognitive and psychiatric features. We studied a retrospective clinical case series of eight subjects with spinocerebellar ataxias (SCAs) 2, 3, 7, and 17, all displaying features of psychosis, and also measured tyrosine hydroxylase (TH) staining of the substantia nigra (SN) at autopsy, among four of the subjects. We hypothesized that increased dopamine production in the SN may underlie the pathophysiology of psychosis in SCAs, given evidence of increased dopamine production in the SN in schizophrenia, as measured by TH staining. We analyzed differences in TH staining between the SCA psychosis cohort (n = 4), a heterogeneous ataxic cohort without psychosis (n = 22), and non-diseased age- and sex-matched control group (n = 12). SCA subjects with psychosis did not differ significantly in TH staining versus ataxic cases without psychosis. There was, however, increased TH staining in the ataxic cohort with and without psychosis (n = 26), compared to non-diseased controls (n = 12). Psychotic features were similar across subjects, with the presence of delusions, paranoia, and auditory hallucinations. Our findings are preliminary because of small numbers of subjects and variable neuropathology; however, they suggest that psychosis is a clinical feature of SCAs and may be under-recognized. While the underlying pathophysiology remains to be fully established, it may be related to extra-cerebellar pathology, including a possible propensity for increased dopamine activity in the SN.
Keywords: spinocerebellar ataxia, psychosis, neuropathology, dopamine
INTRODUCTION
The cerebellum was previously conceptualized as having pure motor function, assisting in the fine tuning of motor control. More recently, this important brain region has been recognized for a perhaps equally vital, non-motor role in cognitive processing. Evidence from numerous functional imaging studies note activation of the cerebellum of human subjects undergoing various cognitive tasks including those involving working-memory, executive function, visuospatial function, and during tasks involving language and emotion [1, 2, 3, 4, 5, 6]. Other anatomical studies have elucidated connections between the ventral dentate nucleus of the cerebellum and the frontal and parietal cortices [7, 8, 9], which are distinct from other motor networks between the cerebellum and the primary motor cortices. Additionally, connections between the cerebellum and brainstem have also been implicated in non-motor cerebellar functions. Lesion studies of patients with cerebellar damage have demonstrated similar findings to functional imaging studies in healthy adults and have found that the cerebellum likely modulates many cognitive processes including working [10, 11, 12], visuospatial [11, 13, 14], and episodic memory [11, 15].
The constellation of evidence from lesion, imaging, and neuropsychologic studies of patients with cerebellar lesions and cerebellar degeneration has led Schmahmann and others to define a cerebellar cognitive affective syndrome (CCAS) [16]. This syndrome includes a wide-ranging set of cognitive deficits including executive dysfunction, impaired visuospatial memory and functions, and changes in personality and affective state, which can manifest in mood disorders and flattened affect, as well as impairment of language. Of note, CCAS is not usually described in association with psychosis, rather mainly in association with depression, mood alterations, and other changes in neuropsychological measures of executive function and memory.
Spinocerebellar ataxias (SCAs) are a group of heterogeneous genetic disorders involving neurodegeneration of the cerebellum. SCAs 1, 2, 3, 6, 7, 8, and 17 are trinucleotide repeat disorders, inherited in an autosomal dominant fashion. The hallmark feature of SCAs is a typically late-onset, progressive cerebellar ataxia thought to be due primarily to degeneration of Purkinje cells of the cerebellum. However, depending on the SCA type, there is often more widespread neuropathologic involvement of the brainstem, basal ganglia, spinal cord, and even peripheral nervous system as noted in SCAs 1, 2,3, and 7 [17, 18, 19].
Though less recognized than the motor manifestations of the disease, significant cognitive impairment among SCA patients has been noted in 25–50% of cases and psychiatric symptoms (usually depression) in up to 30% [20, 21, 22, 23, 24, 25, 26]. Others have studied the longitudinal progression of cognitive impairment versus motor progression in SCAs 1 and 2 and found that there is dissociation between cognitive and motor progression [27]. Despite the fact that a wide range of neuropsychologic impairments has been noted in the SCA literature, cognitive disorders in SCA patients are often clinically under-recognized and under-reported [20], making accurate estimates of prevalence difficult.
Psychiatric symptoms among SCA patients are not unusual and are also likely under-recognized [21, 22]. These psychiatric symptoms usually refer to depression and personality changes. In the study by Leroi et al. of 31 subjects with ataxia (15 with SCA) psychotic symptoms were found in 10 cases [10]. In all 10 subjects, there was also basal ganglia involvement as evidenced by MRI atrophy of the region or clinical extra-pyramidal signs. Case reports describe a variety of SCA types accompanied by psychosis [28], including SCA 1 [29], SCA 2 [30, 31], SCA 6 [32], SCA 7 [33], and SCA 17 [34]. However, little is known about the prevalence, clinical features and pathophysiology of psychosis in the broad SCA population or in particular SCAs. SCA 17 stands out as a hereditary ataxia which typically involves the basal ganglia, is often associated with psychosis, and is often considered a mimic of Huntington’s disease [21, 22, 35].
The extra-cerebellar nature of SCA cases with psychosis described by Liszewksi et al. and Leroi et al. [20, 21] leads to the hypothesis that the pathophysiology of psychosis in SCA may include dysfunction of brain regions outside the cerebellum. Furthermore, the dopamine hypothesis of schizophrenia supposes that dysregulation of pre-synaptic dopamine in the striatum underlies the illness and susceptibility to psychosis [23, 24, 25, 26]. Schizophrenia has been associated with increased striatal dopamine synthesis as seen by PET radiotracer studies using 18 fluoro-dihydroxyphenyl-l-alanine (DOPA), an abnormality found to be specific to psychotic disorders compared to non-psychotic depression and other psychiatric disorders lacking psychosis [24, 35, 36]. Dopamine is synthesized from tyrosine through the action of tyrosine hydroxylase (TH), converting tyrosine to DOPA, which then is converted into dopamine [37]. Autopsy studies of schizophrenia patients have noted increased tyrosine hydroxylase activity in the substantia nigra versus that of controls [28, 38]. Howes et al. also found that tyrosine hydroxylase staining in substantia nigra was significantly increased in a post-mortem schizophrenia group compared with controls and subjects with depression [29]. Thus, we hypothesized that tyrosine hydroxylase levels in the substantia nigra would also be increased post-mortem in an SCA population versus controls and that SCA cases with psychosis may show even further increases in tyrosine hydroxylase levels than SCA cases without psychosis. We had the unique opportunity to study eight cases of SCA, all of whom displayed psychosis and four of whom ultimately went to autopsy. This allowed us to initiate a pilot study evaluating both their unusual clinical phenotype and TH staining levels in the midbrain.
METHODS
This study was approved by the University of Washington Institutional Review Board. Informed consent was obtained from all individual participants included in the study. We present a clinical cohort of eight SCA patients (SCAs 2, 3, 7, and 17), all of whom were seen in the University of Washington Neurogenetics Clinic and displayed prominent clinical features of psychosis (Table 1). The clinical characteristics of this cohort were recorded following retrospective chart review in addition to live examination and interview of each case.
Table 1.
SCA psychosis clinical cohort
| Subject | Mutation | CAG repeat length | Age of motor onset (years) | Sex | Age at psychosis onset (years) | Type of psychosis | Antipsychotic | Age at death (if died) (years) |
|---|---|---|---|---|---|---|---|---|
| 1 | SCA 7a | 48 | 22 | F | 38 | Paranoia/delusions | Olanzapine | 49 |
| 2 | SCA 7a | 50 | 22 | M | 38 | Delusions | Risperdal | 48 |
| 3 | SCA 7 | 41 | 48 | F | 71 | Paranoia/delusions | Olanzapine | Living |
| 4 | SCA 3 | 73 | 30 | F | 51 | Paranoia/delusions and auditory hallucinations | Olanzapine | Living |
| 5 | SCA 3 | 72 | 46 | M | 50 | Hallucinations | Risperdal | Living |
| 6 | SCA 3 | 81 | 24 | F | 30 | Delusions/paranoia/ | Trazodone, citalopram | Living |
| 7 | SCA 17a | 53 | 59 | M | 20 | Delusions/paranoia/hallucinations (schizophrenia DX age 20) | Olanzapine, buspirone | 68 |
| 8 | SCA 2a (AD pathology present) | 37 | 20 | F | 57 | Depression, paranoia, delusions, auditory hallucinations | Olanzapine trazodone risperdal |
58 |
Subject deceased at time of study and included in autopsy study results as SCA psychotic subject
Four of the cases with psychosis came to autopsy, and brain tissue was compared to two control groups. One non-disease control group included 12 age- and sex-matched subjects (all with no history of SCA or other neurodegenerative disorder). The second control group consisted of a heterogeneous group of 22 patients with various forms of ataxia who did not display psychosis clinically (see Table 4). Paraffin sections of the substantia nigra at the level of the superior colliculus were obtained from archived tissue blocks and were immunostained with TH antibodies (1: Pel-Freez, Rogers, AR) using standard techniques.
Table 4.
Clinical characteristics of ataxic non-psychotic autopsy study cohort (n = 22)
| SCA type | No. for ataxia type |
|---|---|
| Familial ataxia, gene unknown | 8 |
| Episodic ataxia type II (genetically confirmed) | 1 |
| Friedrich’s ataxia (genetically confirmed) | 2 |
| SCA 2 (genetically confirmed) | 2 |
| SCA 3 (genetically confirmed) | 5 |
| SCA 5 (genetically confirmed) | 1 |
| Fragile X tremor ataxia syndrome (genetically confirmed) with Alzheimer’s pathology | 1 |
| SCA 17 (genetically confirmed) | 1 |
| Ataxia telangiectasia (genetically confirmed) | 1 |
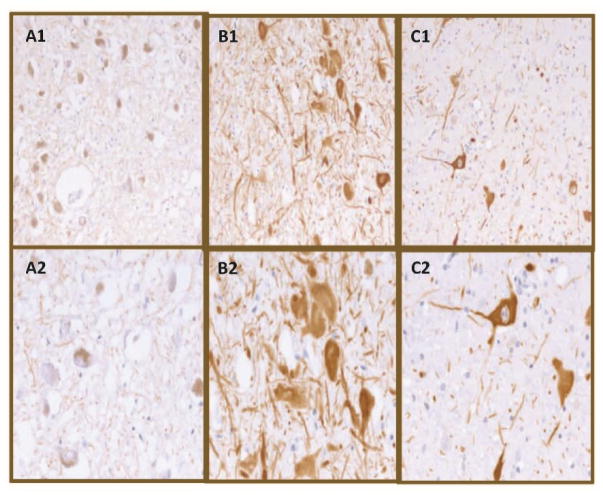
Two blinded, independent neuropathologists performed TH scoring/analysis limited to substantia nigra dopaminergic neurons at × 400 magnification following published protocol from studies of schizophrenia and depression [29], where 0 was rated as no staining, 1 was equivalent to moderate cytoplasmic staining, and 2 was rated as heavy cytoplasmic staining with a clear perinuclear halo, as previously described in the literature [29]. Representative TH staining patterns are shown in Fig. 1.
Figure 1.
Representative tyrosine hydroxylase staining. A1 and A2: No visible cytoplasmic staining (0) at × 200 (A1) and × 400 (A2) magnifications. B1 and B2: Moderate staining visible in the cytoplasm distinct from neuromelanin (1) at × 200 (B1) and × 400 (B2) magnifications. C1 and C2: Heavy staining forming a clear halo around the nucleus and intense staining observed elsewhere in the cytoplasm (2) at × 200 (C1) and × 400 (C2) magnifications.
Statistical Analysis
Two neuropathologists independently and blindly scored cases and controls without image capture. Quantification used the described scale, 0–2, and gave an overall score for each, which was then averaged for each sample for statistical analysis.
Data analysis compared non-diseased controls (n = 12), ataxia cases without psychosis (n = 22), and SCA cases with psychosis (n = 4). All statistical analyses were performed using SPSS 18.0. Analysis of variance was used to determine if there was an effect of group, age, sex, or postmortem interval. Primary analysis was a Kruskal-Wallis test to determine if there was an effect of diagnostic group (control, SCA non-psychotic, SCA psychotic) on average staining score.
RESULTS
Clinical SCA Psychosis Case Series
Retrospective chart review, clinical examination, and history contributed to the case series for eight SCA subjects with prominent psychosis. The cohort contained subjects with SCAs 2, 3, 7, and 17 (Table 4).
Detailed Clinical Description of Cognitive/Psychotic Features of Three Representative Cases from Case Series
Subject 1 had onset of her motor symptoms at age 22 and onset of her first symptoms of psychosis at age 38 years, for which she was started on olanzapine. Genetic testing for SCA 7 revealed 48 trinucleotide CAG repeats in the ataxin-7 gene. She developed a progressive psychotic disorder with hallucinations and delusions and died at age 49 years. A Neuropsychiatric Inventory battery (NPI) was completed by two of the authors (SJ and KT), interviewing the patient’s caregiver/mother post-mortem. The subject displayed delusions and paranoia, believing that others were attempting to hurt her and steal from her. She believed that radio waves were communicating with her telepathically. These delusional symptoms were present several times a week and were moderately distressing to the patient and severely distressing to the caregiver. Hallucinations were also present, including seeing things that were not seen by others and at times feeling insects crawling on her or touching her, though these symptoms were rarely present and moderately disruptive to the patient. The patient was noted to have episodic agitation and anxiety as well as persistent depression and dysphoria. Total score on the NPI was 21, where 144 points is the maximum possible score. A score of 20–50 points is considered to be in the moderate range, signifying behavioral disturbances which are significant functionally, but not severe.
Subject 3 had onset of her motor symptoms at age 48 years and onset of her first symptoms of psychosis at 71 years, for which she was started on olanzapine. Genetic testing for SCA 7 revealed 41 CAG repeats. NPI interview with her caregiver/daughter was conducted when the subject was age 74 years. She displayed frequent delusions and paranoia as well as a belief that others were trying to steal from her and that family members were planning to abandon her. She thought others were coming into her room through her window and that people were stealing her underwear from the bathroom. These thoughts were severely distressful to the patient and the caregiver. She displayed frequent and severe agitation and aggression, including being uncooperative and shouting angrily. She displayed depression and dysphoria and had periods of tearfulness with low spirits about once a week. She also displayed anxiety and was often worried about planned events, felt tense, avoiding certain places or situations, and became nervous or upset when separated from the caregiver. There was endorsement of irritability including a bad temper, rapid mood changes, and sudden flashes of anger. Her total score on the NPI was 42, signifying behavioral disturbances which were significant functionally, but not severe. Subject 8 had onset of motor symptoms at age 20 years and onset of her psychosis at age 57 years. Genetic testing for SCA 2 revealed 37 CAG repeats. She had paranoid delusions and auditory hallucinations. She thought that her step-father was watching her and coming into her room to injure her. Her psychotic symptoms required an inpatient psychiatric hospitalization. Throughout this admission, she was noted to be screaming and swearing at the man in her delusional system. She was afraid to go to sleep because she thought she was going to be attacked. Her auditory hallucinations manifested as hearing her step-father threatening her. The psychiatrist had difficulty interviewing her because she thought her step-father was listening to the conversation. She was treated with olanzapine, trazadone and risperdal.
Pathologic Results
Autopsy results from the SCA psychosis cohort (n = 4) are described in Table 3. All cases in this cohort had a loss of Purkinje cells in the cerebellum as well as neuropathologic findings beyond the cerebellum, including involvement of the substantia nigra, or striatum in the case of the SCA 17 patient.
Table 3.
Autopsy findings from SCA psychosis study cohort (n=4)
| Subject | SCA type | CAG repeat size | Age at death (years) | Cerebellar findings | Extra-cerebellar: cortical, brainstem, basal ganglia, and nigral findings |
|---|---|---|---|---|---|
| 1 | SCA 7 | 48 | 49 | Cerebellum: grossly atrophic, marked patchy loss of Purkinje cells posterior-superior greater than anterior-inferior. Mild gliosis of deep cerebellar white matter tracts. Ubiquitin-positive intranuclear inclusions. Neuronal loss in dentate nuclei. | Substantia nigra: moderate pallor grossly and loss of pigmented neurons microscopically, without Lewy bodies. Normal locus ceruleus Note of grossly atrophy brainstem as well as gliosis and neuronal loss in thalamus, pons, and medulla. Normal striatum Cortex: moderate frontal, parietal and temporal lobe atrophy. Braak stage 1 |
| 2 | SCA 7 | 50 | 48 | Cerebellum: Moderate vermian atrophy grossly with marked atrophy and astrocytic gliosis of white matter tracts of cerebellar peduncles and basis pontis crossing fibers. Dentate nuclei showed severe neuronal loss and pronounced gliosis. Moderate Purkinje cell loss with anterior vermis most affected. Numerous empty basket cells. |
Substantia nigra: moderate pallor grossly and neuronal loss microscopically, without Lewy bodies. Normal locus ceruleus. Inferior olivary nuclei: small grossly with severe neuronal loss. Normal striatum |
| 7 | SCA 17 | 53 | 68 | Cerebellum: grossly small medially more so than laterally. Severe Purkinje cell loss. | Cortex: Moderate atrophy involving frontal, temporal, and parietal lobes. Basal ganglia: Severe atrophy of caudate nuclei and less severe putamen atrophy Inferior olivary nuclei: normal appearing |
| 8 | SCA 2 | 37 | 58 | Cerebellum: atrophy grossly and neuronal loss microscopically, diffuse Purkinje cell loss with empty baskets and reactive proliferation of Bergmann astrocytes. Mild granule cell loss. Dentate nucleus with areas of gliosis and ischemic neurons without obvious cell loss. | Substantia nigra: normal appearing Substantia nigra: neuronal loss, gliosis, and macrophage infiltration without Lewy bodies. Normal striatum and thalamus Inferior olivary nuclei: atrophy grossly and marked neuronal loss Cortex: Some Alzheimer changes with Braak stage 3 |
Demographic Results
There was no significant differences between ataxia cases without psychosis n = 22, SCA cases with psychosis n = 4, and non-diseased controls n = 12 in age [F (2, 37) = 1.9; P =.157], sex [F (2, 37) = 1.7; P =.85], or post-mortem interval [F (2, 23) = 0.31; P = .74] (see Table 2 for details of demographics between groups). Furthermore, there was no significant difference in age between non-diseased controls (n = 12) and all ataxia cases with and without psychosis (n = 26) by two-tailed independent t test. Mean age for controls was 75.0 years ± 3.5, and mean age of all ataxia cases was 64.7 ± 18, t (36) = 2.0, P =.058. Tables 2 and 3 show descriptive clinical characteristics of subject groups by SCA or ataxia type.
Table 2.
Demographic characteristics of cohorts: non-diseased controls, ataxia cases without psychosis, and SCA cases with psychosis
| Group | Total n | Mean age, years (stdev) | Sex ratio (F/M) | Mean PMI in hours (stdev) |
|---|---|---|---|---|
| Ataxia cases without psychosis | 22 | 62.2 (18.6) | 8/22 | 24.8 (34) |
| SCA psychosis cases | 4 | 61.8 (16.3) | 2/4 | 7.5 (2.7) |
| Non-diseased controls | 12 | 75 (3.5) | 4/12 | 21.2 (21.2) |
None of the differences in age, sex, and pmi were found to be significantly different between groups. See the “Results” section for details
General Neuropathologic Findings
All four cases with psychosis had cerebellar atrophy with moderate to marked loss of Purkinje cells as well as frequent neuronal loss in dentate and inferior olives. Three cases had pallor and mild loss of pigmented neurons in the substantia nigra without Lewy bodies. Case 7 (SCA 17) showed severe caudate atrophy and moderate putamen atrophy. Cases 1 and 7 had mild frontal, temporal, and parietal cortex atrophy, and case 8 had early signs of Alzheimer’s disease with Braak stage 3.
The non-psychotic ataxia control group was composed of 22 subjects (12 male, 10 female) with a mean age of 66.0 years (range 27–88 years). The general neuropathologic findings in the majority of these cases consisted of moderate to severe loss of Purkinje cells and mild to moderate loss of neurons in the dentate nucleus and inferior olives. Mild loss of pigmented neurons in the substantia nigra was also relatively common with no evidence of Lewy bodies. Caudate, putamen, and globus pallidus were unremarkable except for a single case that had mild gliosis of the globus pallidus. Four subjects were members of a family with SCA 3 showing predominant pontine atrophy as previously reported [30]. Two of the elderly subjects also met criteria for Alzheimer’s disease.
TH Staining Results
There was a significant main effect of group on average tyrosine hydroxylase staining score when non-diseased controls (n = 12) were compared to all ataxia cases, with and without psychosis (n = 26) (X2 = 3.95, df = 1, P =.047, Fig. 2, error bars indicate standard deviation), but no significant effect of group was found for average TH staining between ataxia cases without psychosis (n = 22) and SCA cases with psychosis (n = 4), (X2 = 0.001, df = 1, P =.97). No significant difference was identified between non-disease control and ataxia without psychosis (P =.053).
Figure 2.
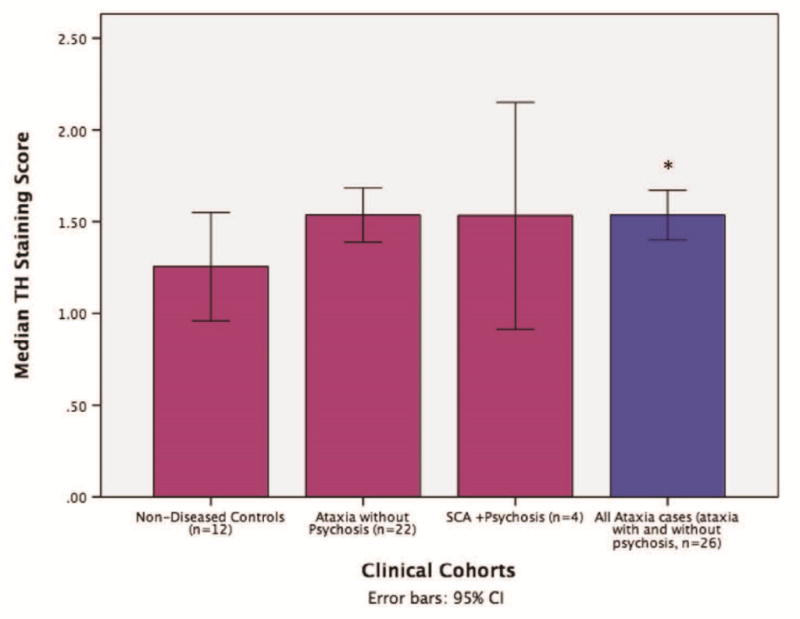
Median midbrain TH staining scores, averaged between two blinded independent raters with 95% CIs (error bars represent standard deviation) for non-diseased control subjects (n = 12), ataxia without psychosis cohort (n = 33), and SCA with psychosis cohort (n = 4). *All SCA cases with and without psychosis (combination of cohorts 2 and 3) had higher TH staining scores versus non-diseased controls, (n = 26), P = .047.
DISCUSSION
Our study has two major findings: one clinical and the other neuropathologic. The clinical findings include a description of eight cases with SCAs (2, 3, 7, 17), all of whom displayed psychosis. These clinical findings confirm and enlarge the similar previous mostly anecdotal case reports. It should be noted that onset for symptoms of schizophrenia is typically in the second or third decades. Six of the eight SCA cases with psychosis we describe had onset of psychosis after age 35 years arguing against a coincidental occurrence of schizophrenia. Rather the cases described here are more likely to demonstrate a shared underlying pathophysiologic mechanism between genetic ataxia and psychosis. In all but one of the subjects described here, the age of psychosis onset was typically years or decades after the age of motor onset.
Furthermore, the clinical psychotic features were relatively stereotyped across all eight subjects, with the presence of delusions and paranoia being most common as seen in six out of eight cases, followed by auditory hallucinations in four of the eight cases. Intriguingly, others have noted a potential association between schizophrenia and cerebellar dysfunction [31], reporting both MRI and functional imaging abnormalities of the cerebellum in schizophrenia [32, 33].
Our second finding was neuropathologic. Four of the SCA psychosis clinical cohort went on to autopsy and we reported their cerebellar and extra-cerebellar neuropathologic findings as well as measurement of nigral TH staining levels. This study is the first of its kind to evaluate tyrosine hydroxylase (TH) levels in cerebellar ataxia, specifically SCA cases displaying psychosis. Although, SCA subjects with ataxia were not found to have significantly increased TH staining in the midbrain versus ataxia cases without psychosis, as we had hypothesized, we did find increased TH staining in a diverse ataxia cohort (with and without psychosis), compared to non-diseased age-matched controls.
Our finding of increased TH staining in the ataxia cohort is similar to what others have found in primary schizophrenia related psychosis subjects [29]. Increased TH staining in the midbrain of our ataxia cohort further implicates extra-cerebellar structures, including the substantia nigra, more clearly in the pathophysiology of neurodegenerative ataxias, a set of disorders previously considered to be primarily cerebellar.
Due to the heterogeneity of the ataxia cohort with several cases of non-SCA ataxias, it is not possible to conclude that SCAs themselves, as opposed to all ataxias examined here more generally, result in higher tyrosine hydroxylase activity in the midbrain. Nor can we draw conclusions about midbrain tyrosine hydroxylase activity in SCA cases displaying psychosis versus those without psychosis, though this is certainly of interest and should be re-visited when a greater number of subjects displaying SCA and psychosis are able to be studied. However, the full ataxia cohort in this study all had a genetic background potentially indicating that increased TH production, as measured by TH staining, may act as a biomarker of increased psychosis risk among patients with genetic ataxia. This finding is preliminary because of the small number of subjects and variable neuropathologic findings.
Increased TH activity in the brainstem of cerebellar ataxia subjects may point to the cerebellum’s interaction with other extra-cerebellar, particularly brainstem structures in the pathophysiology of SCA psychosis. Intriguingly, our results underscore the potential importance of the midbrain in ataxic psychosis, similar to Leroi et al. who found that among SCA patients, psychotic disorders and hallucinations were found to be present exclusively in cases where there was also basal ganglia involvement as seen by MRI atrophy of the region or clinical extra-pyramidal signs [20, 34]. Similarly, in our pathologic case series, we found that three out of four of our psychotic SCA patients had brainstem involvement at time of autopsy (Tables 3 and 4). While Schmahmann et al. have reported that cerebellar dysregulation impacts behavior and mood, our results implicate psychosis as an additional feature of the non-motor syndrome associated with the cerebellum and its connections to the brainstem in ataxia patients. [16] Although it is becoming increasingly clear that the SCAs should not be thought of as purely cerebellar disorders, the relative contribution of cerebellar and extra-cerebellar structures, including the brainstem, to the development of psychosis in SCA patients remains unclear.
Notably, neuropsychologic test performance in SCA 2 has been found to be related to disease duration rather than to expansion size of the CAG repeat allele [39]. It may be that extra-cerebellar sites of pathology accumulate over time consistent with psychotic symptoms following motor symptoms in seven of eight of our subjects. SCA 17 may be an exception with early involvement of the basal ganglia consistent with our subject no. 7. Future studies comparing SCA subjects with and without psychosis should seek to account for disease duration. Future studies should also screen all SCA subjects using a neuropsychologic battery such as the neuropsychologic inventory (NPI) to better assess the nature and the prevalence of psychotic symptoms in this population.
In summary, we report additional evidence of an association of hereditary cerebellar ataxias with psychotic symptoms and suggestive evidence that increased tyrosine hydroxylase activity in the substantia nigra could be a contributing factor to this phenomenon.
Footnotes
Compliance with Ethical Standards. This study was approved by the University of Washington Institutional Review Board. Informed consent was obtained from all individual participants included in the study.
Ethical Approval. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments.
References
- 1.Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. NeuroImage. 2006;32:821–41. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- 2.Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–37. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Ciesielski KT, Lesnik PG, Savoy RL, Grant EP, Ahlfors SP. Developmental neural networks in children performing a categorical N-back task. NeuroImage. 2006;33:980–90. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Collette F, Van der Linden M, Laureys S, et al. Mapping the updating process: common and specific brain activations across different versions of the running span task. Cortex. 2007;43:146–58. doi: 10.1016/s0010-9452(08)70452-0. [DOI] [PubMed] [Google Scholar]
- 5.Geier CF, Garver KE, Luna B. Circuitry underlying temporally extended spatial working memory. NeuroImage. 2007;35:904–15. doi: 10.1016/j.neuroimage.2006.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. NeuroImage. 2006;30:1038–49. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Dum RP, Li C, Strick PL. Motor and nonmotor domains in the monkey dentate. Ann N Y Acad Sci. 2002;978:289–301. doi: 10.1111/j.1749-6632.2002.tb07575.x. [DOI] [PubMed] [Google Scholar]
- 8.Leiner HC, Leiner AL, Dow RS. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100:443–54. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- 9.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–12. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ribaupierre S, Ryser C, Villemure JG, Clarke S. Cerebellar lesions: is there a lateralisation effect on memory deficits? Acta Neurochir. 2008;150:545–50. doi: 10.1007/s00701-008-1562-5. [DOI] [PubMed] [Google Scholar]
- 11.Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry. 2004;75:1524–31. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirschen MP, Davis-Ratner MS, Milner MW, et al. Verbal memory impairments in children after cerebellar tumor resection. Behav Neurol. 2008;20:39–53. doi: 10.3233/BEN-2008-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbro F, Tavano A, Corti S, Bresolin N, De Fabritiis P, Borgatti R. Long-term neuropsychological deficits after cerebellar infarctions in two young adult twins. Neuropsychologia. 2004;42:536–45. doi: 10.1016/j.neuropsychologia.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Gottwald B, Mihajlovic Z, Wilde B, Mehdorn HM. Does the cerebellum contribute to specific aspects of attention? Neuropsychologia. 2003;41:1452–60. doi: 10.1016/s0028-3932(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 15.Lilja A, Hamalainen P, Kaitaranta E, Rinne R. Cognitive impairment in spinocerebellar ataxia type 8. J Neurol Sci. 2005;237:31–8. doi: 10.1016/j.jns.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 17.Burk K, Globas C, Bosch S, et al. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol. 2003;250:207–11. doi: 10.1007/s00415-003-0976-5. [DOI] [PubMed] [Google Scholar]
- 18.Rub U, Burk K, Timmann D, et al. Spinocerebellar ataxia type 1 (SCA1): new pathoanatomical and clinico-pathological insights. Neuropathol Appl Neurobiol. 2012;38:665–80. doi: 10.1111/j.1365-2990.2012.01259.x. [DOI] [PubMed] [Google Scholar]
- 19.Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21. doi: 10.1007/s00401-012-1000-x. [DOI] [PubMed] [Google Scholar]
- 20.Liszewski CM, O'Hearn E, Leroi I, Gourley L, Ross CA, Margolis RL. Cognitive impairment and psychiatric symptoms in 133 patients with diseases associated with cerebellar degeneration. J Neuropsychiatry Clin Neurosci. 2004;16:109–12. doi: 10.1176/jnp.16.1.109. [DOI] [PubMed] [Google Scholar]
- 21.Salvatore E, Varrone A, Sansone V, et al. Characterization of nigrostriatal dysfunction in spinocerebellar ataxia 17. Mov Disord. 2006;21:872–5. doi: 10.1002/mds.20827. [DOI] [PubMed] [Google Scholar]
- 22.Stevanin G, Brice A. Spinocerebellar ataxia 17 (SCA17) and Huntington’s disease-like 4 (HDL4) Cerebellum. 2008;7:170–8. doi: 10.1007/s12311-008-0016-1. [DOI] [PubMed] [Google Scholar]
- 23.Lyon GJ, Abi-Dargham A, Moore H, Lieberman JA, Javitch JA, Sulzer D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr Bull. 2011;37:108–17. doi: 10.1093/schbul/sbp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Grasby PM, McGuire PK. Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry Suppl. 2007;51:s13–8. doi: 10.1192/bjp.191.51.s13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15:2550–9. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–86. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 27.Fancellu R, Paridi D, Tomasello C, et al. Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J Neurol. 2013;260:3134–43. doi: 10.1007/s00415-013-7138-1. [DOI] [PubMed] [Google Scholar]
- 28.Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Brain Res Mol Brain Res. 2004;121:60–9. doi: 10.1016/j.molbrainres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Howes OD, Williams M, Ibrahim K, et al. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain. 2013;136:3242–51. doi: 10.1093/brain/awt264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eto K, Sumi SM, Bird TD, McEvoy-Bush T, Boehnke M, Schellenberg G. Family with dominantly inherited ataxia, amyotrophy, and peripheral sensory loss. Spinopontine atrophy or Machado-Joseph Azorean disease in another non-Portuguese family? Arch Neurol. 1990;47:968–74. doi: 10.1001/archneur.1990.00530090038011. [DOI] [PubMed] [Google Scholar]
- 31.Picard H, Amado I, Mouchet-Mages S, Olie JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34:155–72. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard JA, Seidler RD. Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev. 2014;42:193–207. doi: 10.1016/j.neubiorev.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker KL, Narayanan NS, Andreasen NC. The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci. 2014;8:163. doi: 10.3389/fnsys.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leroi I, O'Hearn E, Marsh L, et al. Psychopathology in patients with degenerative cerebellar diseases: a comparison to Huntington’s disease. Am J Psychiatry. 2002;159:1306–14. doi: 10.1176/appi.ajp.159.8.1306. [DOI] [PubMed] [Google Scholar]
- 35.Howes OD, Shotbolt P, Bloomfield M, et al. Dopaminergic function in the psychosis spectrum: an [18F]-DOPA imaging study in healthy individuals with auditory hallucinations. Schizophr Bull. 2013;39:807–14. doi: 10.1093/schbul/sbr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–7. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr. 2007;137:1539S–47S. doi: 10.1093/jn/137.6.1539S. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Costas E, Melendez-Ferro M, Rice MW, Conley RR, Roberts RC. Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Front Psychiatry. 2012;3:31. doi: 10.3389/fpsyt.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burk K, Globas C, Bosch S, et al. Cognitive deficits in spinocerebellar ataxia 2. Brain. 1999;122(Pt 4):769–77. doi: 10.1093/brain/122.4.769. [DOI] [PubMed] [Google Scholar]