Abstract
Hypochlorous acid (HOCl) is a potent cytotoxic oxidant generated by the enzyme myeloperoxidase (MPO) in the presence of hydrogen peroxide (H2O2) and chloride (Cl−). Elevated levels of HOCl play an important role in various pathological conditions through oxidative modification of several biomolecules. Recently, we have highlighted the ability of HOCl to mediate the destruction of the metal-ion derivatives of tetrapyrrole macrocyclic rings such as hemoproteins and vitamin B12 (VB12) derivatives. Destruction of cyanocobalamin, a common pharmacological form of VB12 mediated by HOCl, results in the generation of toxic molecular products such as chlorinated derivatives, corrin ring cleavage products, the toxic blood agents cyanide (CN−) and cyanogen chloride (CNCl), and redox active free cobalt. Here, we show that melatonin prevents HOCl-mediated cyanocobalamin destruction, using a combination of UV-Vis spectrophotometry, HPLC analysis, and colorimetric CNCl assay. Identification of several melatonin oxidation products suggests that the protective role of melatonin against HOCl-mediated cyanocobalamin destruction and subsequent CNCl generation is at the expense of melatonin oxidation. Collectively, this work highlights that, in addition to acting as an antioxidant and as a MPO inhibitor, melatonin can also prevent VB12 deficiency in inflammatory conditions such as cardiovascular and neurodegenerative diseases, among many others.
Keywords: vitamin B12, mammalian peroxidases, hypochlorous acid, cyanocobalamin, melatonin, circadian rhythm
INTRODUCTION
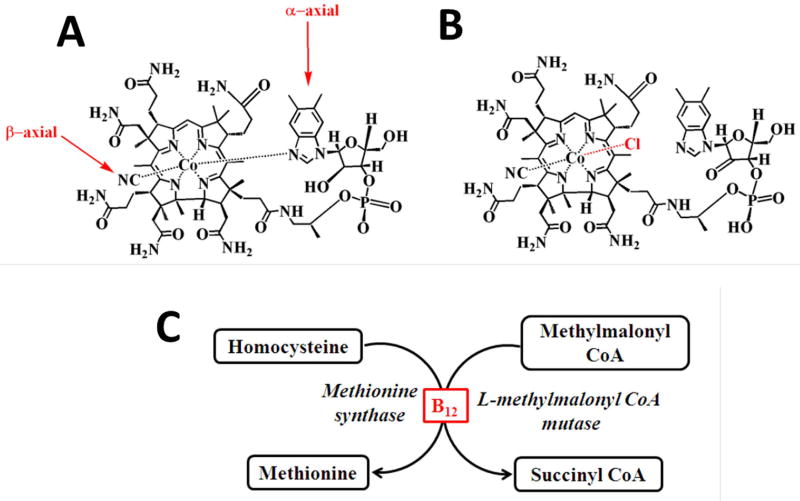
Hypochlorous acid (HOCl) is a potent microbicidal agent generated by the myeloperoxidase (MPO)-hydrogen peroxide (H2O2)-Chloride (Cl−) system in phagocytic cells including neutrophils and plays a key role in innate immunity1–3. Hypochlorous acid is a strong oxidant, modifies a wide array of biomolecules ranging from proteins, nucleic acids and lipids through its ability to react with: 1) free amine groups in amino acids, peptides and proteins to form toxic chloramines; and 2) tyrosine to form 3-chlorotyrosine and 3, 5-dichloro tyrosine, both of which are specific biomarkers of HOCl induced damage4–8. Pyrimidine nucleotides (either free or in DNA/ RNA strands) are readily chlorinated by HOCl-induced reaction and by this pathway HOCl causes extensive denaturation of double-stranded DNA and also acts as an effective mutagen, clastogen and induces sister chromatid exchange9,10. HOCl initiates alterations of the heme prosthetic group of both ferrous-dioxy and methemoglobin, ultimately leading to heme destruction11. Recently, utilizing rapid kinetic measurements, HPLC and mass spectrometric techniques we have highlighted the mechanism through which HOCl mediates the destruction of the metal-ion derivatives of tetrapyrrole macrocyclic rings such as hemoproteins and vitamin B12 (VB12) derivatives12–17. We have shown destruction of cyanocobalamin (Fig 1A), a common pharmacological form of VB12, mediated by HOCl, resulted in the generation of toxic molecular products such as chlorinated derivatives, different degradation products of corrin, the organic ring system of cobalamin, release of the toxic blood agents (CN− /cyanogen chloride (CNCl)), and redox active free cobalt (Co) through the formation of chloro-cyanocobalamin (Fig 1B)15,18.
Figure 1. Function of vitamin B12 (A) and structures of cyanocobalamin (B) and Cl- cyanocobalamin (C).
Interestingly, many of the disorders known to be associated with VB12 deficiency are also associated with increased MPO/HOCl levels19–24. For instance, deficiency of VB12 is associated with neurodegenerative disorders including but not limited to Parkinson and Alzheimer disease and megaloblastic anemia, elevated blood homocysteine level (a risk factor for cardiovascular disease and adverse pregnancy outcomes)19,20,22–25. These negative effects are primarily because the most prominent function of cyanocobalamin is to act as cofactor in two major biochemical pathways; 1) in the conversion of homocysteine to methionine by methionine synthase and 2) in the conversion of methylmalonyl CoA to succinyl CoA by methylmalonyl CoA mutase26 (Fig. 1C).
Many experiments have demonstrated that melatonin (N-acetyl-5-hydroxytryptamine) can protect against HOCl mediated damage16,27–30. This ubiquitous molecule is found in a large cross-section of organisms, ranging from unicellular organism, fungi, plants to higher invertebrate and vertebrate animals31. In humans, it is most commonly known to be a modulator of circadian rhythm and is produced in the pineal gland, from the amino acid tryptophan32–34. In addition, melatonin can be found in the gastrointestinal tract, cerebellum, retina, skin, ovary, liver, pancreas, kidneys, and the immune competent cells33,35–41. Additionally, melatonin is also involved in immune enhancing35, anti-inflammatory pathway42,43, and can inhibit cancer progression41,44,45. Several of melatonin’s functions are mediated by the membrane bound G-protein coupled receptors, MT1 and MT246,47. In addition to the receptor-mediated functions, melatonin performs various biochemical functions, for example acts as an antioxidant. Previously, melatonin has also been shown to be a major scavenger of HOCl48. In addition, melatonin has the ability to scavenge a variety of reactive oxygen species (ROS)/free radicals, such as, H2O2, hydroxyl radical (•OH), superoxide (O2•−), alkoxyl radical, peroxyl radical, singlet oxygen, and nitric oxide40,49–51. Importantly, melatonin also serves as an inhibitor for enzymes that generate reactive oxygen species, such as MPO and eosinophil peroxidase, the major sources for hypohalous acids29,30.
In this work, we examined the ability of melatonin to prevent HOCl-mediated cyanocobalamin corrin destruction and subsequent release of the blood agents CN−/CNCl, and redox active free Co release. Our results showed that melatonin prevents HOCl-mediated cyanocobalamin destruction and subsequent release of toxic agents. The conversion of several melatonin oxidation products suggests that the protective role of melatonin against HOCl-mediated cyanocobalamin destruction is at the expense of melatonin oxidation. Collectively, melatonin displays the ability to prevent VB12 destruction in inflammatory conditions and could benefit patients with disorders associated with deficiency of this vitamin.
Materials and Methods
Materials
All the materials used were of highest purity grade available and used without further purification. Melatonin, sodium hypochlorite (NaOCl), cyanocobalamin, pyridine, 1,3-dimethyl barbituric acid, L-methionine, and dimethylformamide (DMF) HPLC-grade were obtained from Sigma Aldrich (St Louis, MO, USA). HPLC-grade acetonitrile was obtained from EMD Chemicals Inc. (Gibbstown, NJ, USA).
Kinetic analysis
Kinetic measurements were performed using a 96-well plate reader (Spectra Max 190; Molecular Devices, Sunnyvale, FL, USA). Briefly, experiments were performed in 200 µL final volume containing 200 mM phosphate buffer, pH 7.4, at 25 °C. A fixed concentration of cyanocobalamin (20 µM) was incubated with increasing concentrations of melatonin (0 – 450 µM) prior to addition of 450 µM of HOCl. Immediately after addition of HOCl, the increase in absorbance at 590 nm was monitored as a function of time. Similarly, for studying the rate of HOCl-mediated cyanocobalamin destruction in presence of melatonin, cyanocobalamin (20 µM) was incubated with increasing concentrations of melatonin (0 – 4000 µM) prior to addition of 4000 µM of HOCl, and the decreases in absorbance at 590 nm was monitored as a function of time. The rate of the reaction was calculated by the slope of the tangent drawn at the initial linear part of the curve. All experiments were performed in triplicates.
High-performance liquid chromatography (HPLC) analysis
HPLC analyses were carried out using a Shimadzu HPLC system equipped with an SCL-10A system controller, with a binary pump solvent delivery (LC-10 AD) module and an SIL-10 AD autoinjector connected to an SPD-M10A diode array detector and an RF-10A XL fluorescence detector. An Alltech 5-µm particle size, 4.6 × 150-mm reverse-phase octadecylsilica (C18) HPLC column was used.
Detection of Cl-cyanocobalamin
50 µL of cyanocobalamin-HOCl reaction mixture was injected to analyze HOCl-mediated formation of Cl-cyanocobalamin and its modulation by melatonin. The photodiode array detector was set at 360 nm to monitor the chromatogram. The column was eluted isocratically at a flow rate of 2.0 ml/min with 20 % acetonitrile and 80 % water. Each sample was analyzed in triplicate.
Cyanocobalamin destruction and analysis of melatonin oxidation products
50 µL of cyanocobalamin-HOCl reaction mixture was injected to analyze HOCl-mediated destruction of cyanocobalamin and its prevention by melatonin. The column was eluted isocratically at a flow rate of 1.0 ml/min with 40 % acetonitrile and 60 % water. Each sample was analyzed in triplicate. To monitor cyanocobalamin, the photodiode array detector was set at 360 nm to obtain the chromatogram. For analyzing melatonin consumption and formation of melatonin oxidation products, the photodiode array detector was set at 260 nm. AFMK was detected by its intrinsic fluorescence by setting the fluorescence detector at excitation 321 nm and emission 465 nm to monitor the chromatogram. Each sample was analyzed in triplicate.
Colorimetric detection of CNCl
CNCl was detected colorimetrically using the pyridine-1,3-dimethyl barbituric acid reagent as described previously15,52. In this assay, CNCl reacts with pyridine to form a dialdehyde, glutacon dialdehyde, which then reacts with 1,3-dimethyl barbituric acid and condenses to form a violet-colored polymethine dye. The composition of the pyridine-1,3-dimethyl barbituric acid (coloring reagent) was as follows: 1.2 g of 1,3-dimethyl barbituric acid was dissolved in a mixture of 12.8 ml of water and 6 ml of pyridine, and then 1.2 ml of HCl was added to the solution to bring the total volume to 20 ml. Cyanocobalamin (100 µM) was treated with 3000 µM of HOCl in presence or absence of 3000 µM melatonin at 10 °C for 2 hours. Following which the reaction was stopped by adding high molar excess of methionine. Then coloring reagent (500 µL), was added to 500 µL of cyanocobalamin −HOCl reaction mixture and was incubated for 15 min at 10°C. The absorbance of the resulting violet-colored solution was measured at 587.5 nm against the cyanocobalamin–HOCl reaction mixture (without addition of coloring reagent, but diluted with an equal volume of water) to subtract the absorbance due to residual cyanocobalamin. The amount of CNCl was determined from the extinction coefficient of 1.03 × 105 M− 1cm− 1 for the violet-colored complex53.
Solution preparation
HOCl preparation
HOCl was prepared following a slight modification of a published method54. Briefly, a stock solution of HOCl was prepared by adding 1 ml of sodium hypochlorite (NaOCl) solution to 40 ml of 154 mM and the pH was adjusted to around 3 by adding HCl. The concentration of active total chlorine species in solution, determined as [HOCl]T (where [HOCl]T = [HOCl] + [Cl2] + [Cl3−] + [OCl−]), in 154 mM NaCl was based on converting all the active chlorine species to OCl− by adding a single bolus of 40 µl of 5 M NaOH and then measuring the concentration of OCl−. The concentration of OCl− was determined spectrophotometrically at 292 nm (ε = 362 M− 1 cm− 1). As HOCl is unstable, the stock solution was freshly prepared on a daily basis, stored on ice, and used within 1 h of preparation. For further experimentations, dilutions were made from the stock solution using 200 mM phosphate buffer, pH 7.4, to give working solutions of lower HOCl concentration.
Cyanocobalamin preparation
Cyanocobalamin stock solution was prepared by dissolving cyanocobalamin in distilled water. The concentration of the stock solution was determined spectrophotometrically at 361 nm (ε = 27.5 mM− 1 cm− 1).
Melatonin preparation
Melatonin was prepared by dissolving 92 mg of M melatonin elt in 1 mL of DMF, to give a solution of concentration 396 mM. Melatonin solution prepared in this manner was shielded from light and kept on ice and used within 1 hr of preparation.
Results
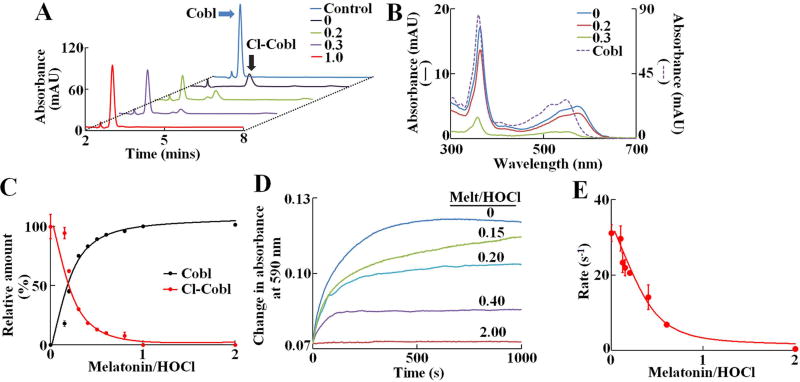
We first utilized HPLC techniques to investigate the effect of melatonin in preventing the HOCl-mediated cyanocobalamin destruction by pre-incubating cyanocobalamin with increasing molar ratios of melatonin:HOCl (0:1 up to 2:1) prior to HOCl addition and HPLC. Under our experimental conditions, cyanocobalamin eluted around 3 min and was identified based on its characteristic UV-Vis spectra (360, 540 and 550 nm) obtained from the diode array detector (Fig. 2A & 2B). Treatment of cyanocobalamin (10 µM) with 100 µM of HOCl led to a complete disappearance of the cyanocobalamin peak and instead a new peak appeared with an elution time of 4.25 mins. The spectral feature of this new peak was distinctly red-shifted, from 360 to 363 nm with an additional shift in the visible absorption region (from 550 to 590 nm), and was identified to be as Cl-cyanocobalamin, in accordance with our earlier observation15 (Figure 2B). Adding 20 µM of melatonin to the reaction mixture (melatonin −HOCl) decreased in Cl-cyanocobalamin peak and the restoration of the cyanocobalamin peak. Further increasing the melatonin concentration leads to complete restoration of the cyanocobalamin peak in a concentration dependent manner. The area under the curve for the cyanocobalamin peak and Cl-cyanocobalamin peak was plotted as a function of melatonin:HOCl ratio (Fig. 2C). The relative amount of Cl-cyanocobalamin decreased linearly until melatonin:HOCl ratio 0.4:1, after which the Cl- cyanocobalamin level gradually plateaued off, and saturating at melatonin:HOCl ratio 1:1 (Fig. 2C, red curve). A parallel trend was observed for the amount of cyanocobalamin (Fig. 2C, black curve). Next to study the effect of melatonin on the kinetics of the process of HOCl-mediated Cl-cyanocobalamin formation UV-Vis spectrophotometry was used. To phase out the effect of melatonin on the spectra of cyanocobalamin, the increase in absorbance was monitored at 590 nm, where melatonin (at the concentrations used), had no interference. Cyanocobalamin (20 µM) was preincubated with different melatonin:HOCl molar ratios (0 to 2:1) and then 450 µM HOCl was added to the reaction mixture and increase in absorbance at 590 nm was recorded. Figure 2D, shows the profile of the absorbance increase at 590 nm, as a function of time for different melatonin:HOCl ratios. In absence of melatonin, HOCl caused the absorbance to increase exponentially, and signal saturated at approximately 500s. When cyanocobalamin, was pretreated with melatonin, we observed a decrease in the amplitude of the absorbance change, as well as a decrease in the slope of the curve, signifying the rate. Indeed, when the pseudo first order rate of Cl- cyanocobalamin formation was plotted as a function of melatonin:HOCl ratio, we observed that the rate of Cl- cyanocobalamin formation decreased linearly until 0.6:1, after which the rate plateaued off and saturated around 2:1 melatonin:HOCl ratio. Thus, we conclude that melatonin can effectively prevent HOCl-mediated Cl- cyanocobalamin formation.
Figure 2. Melatonin prevents HOCl mediated α-axial ligand replacement and subsequent formation of Cl-cyanocobalamin.
(A) HPLC analysis of cyanocobalamin-HOCl reaction mixtures in presence different molar ratios of melatonin:HOCl (ratios shown in inset). Chromatograms were monitored at 360 nm. The position of the cyanocobalamin and Cl-cyanocobalamin peaks are marked by blue and black arrows, respectively. The data shown is a representative of three independent experiments. (B) UV-Vis spectra of the cyanocobalamin (dotted line) and Cl- cyanocobalamin (solid lines) peaks, as obtained from the diode array detector. The Cl-cyanocobalamin spectra was plotted as a function of melatonin:HOCl molar ratios (ratios shown in inset). (C) Relative amount of cyanocobalamin and Cl- cyanocobalamin as calculated from the area under the curve for each peaks, were plotted as a function of melatonin:HOCl molar ratios. For cyanocobalamin, the area under the curve for cyanocobalamin alone was defined as 100 %, while for Cl- cyanocobalamin, the area under the curve at 0 melatonin was defined as 100 % and subsequent areas were normalized to those values. The data points are the average of three independent experiments, and the error bars represent the standard error of measurements. (D) Kinetic traces for the cyanocobalamin-HOCl reaction monitored at 590 nm, showing the formation of Cl-cyanocobalamin, as a function of different molar ratios of melatonin:HOCl. These data are average of three independent experiments. (E) Pseudo-first order rate constants of formation of Cl-cyanocobalamin, plotted as a function of melatonin:HOCl molar ratios. The data points are the average of three independent experiments, and the error bars represent the standard error of measurements.
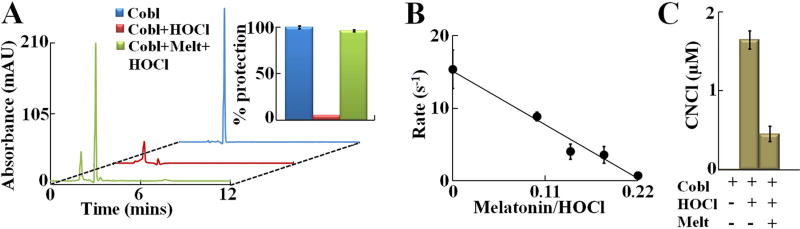
As reported earlier, the process of HOCl-mediated cyanocobalamin destruction involves two distinct steps, first conversion of cyanocobalamin to Cl- cyanocobalamin and next oxidative destruction of Cl- cyanocobalamin15. Since our current results indicated that melatonin prevents HOCl-mediated generation of Cl- cyanocobalamin we hypothesized that melatonin will prevent HOCl-mediated cyanocobalamin destruction and subsequent generation of CNCl. To test our hypothesis, cyanocobalamin (20 µM) was incubated with HOCl (1000 µM) in presence/absence of melatonin (1000 µM). HPLC analyses revealed that in absence of melatonin, HOCl treatment led to complete destruction of cyanocobalamin, while pretreatment with 1:1 melatonin:HOCl prevented cyanocobalamin destruction by 96 % (see Fig. 3A and Fig. 3A inset). Next, to test how melatonin modulates the kinetic of the process of HOCl-mediated cyanocobalamin destruction UV-Vis spectrophotometry was utilized. Experiments were performed as mentioned earlier. Briefly cyanocobalamin (20 µM) was preincubated with different melatonin:HOCl molar ratios (0 to 1:1) and then 4000 µM HOCl was added to the reaction mixture and decrease in absorbance at 590 nm was recorded. Figure 3B, shows the pseudo-first order rate of HOCl-mediated cyanocobalamin corrin ring destruction plotted as a function of melatonin:HOCl ratio. As the melatonin:HOCl ratio increased we observed a linear decrease in the rate of cyanocobalamin destruction, which ultimately approached ~0 at around 0.22:1 melatonin:HOCl ratio. Since our results showed that melatonin effectively prevents HOCl-mediated cyanocobalamin corrin destruction, we wanted to test the role of melatonin in inhibiting HOCl-mediated CNCl generation from cyanocobalamin. As shown in Fig. 3C, treatment of cyanocobalamin with HOCl in absence of melatonin, led to generation of 1.65 µM of CNCl while no CNCl was detected in control cyanocobalamin without HOCl treatment. But when cyanocobalamin was preincubated with 1:1 molar ratio of melatonin:HOCl and then HOCl was added, the amount of CNCl generated decreased by ~72 % to 0.45 µM.
Figure 3. Melatonin prevents HOCl mediated corrin destruction and CNCl generation from cyanocobalamin.
(A) HPLC analysis of cyanocobalamin-HOCl reaction mixtures in presence/absence of 1:1 melatonin:HOCl molar ratio. Chromatograms were obtained at 360 nm. Relative amount of cyanocobalamin remaining was calculated from the area under the curve for the cyanocobalamin peak, and is shown in inset. The data shown is a representative of three independent experiments and the error bars are represent the standard error of measurements. (B) Pseudo-first order rate constants of HOCl-mediated cyanocobalamin destruction, plotted as a function of melatonin:HOCl molar ratios. The data points are the average of three independent experiments, and the error bars represent the standard error of measurements. (C) cyanocobalamin (100 µM) was treated with 3000 µM of HOCl in presence/absence of melatonin (3000 µM) and CNCl generation was assayed colorimetrically as detailed under Materials and Methods section. The data are representative of three independent experiments with the error bars representing the standard error measurements.
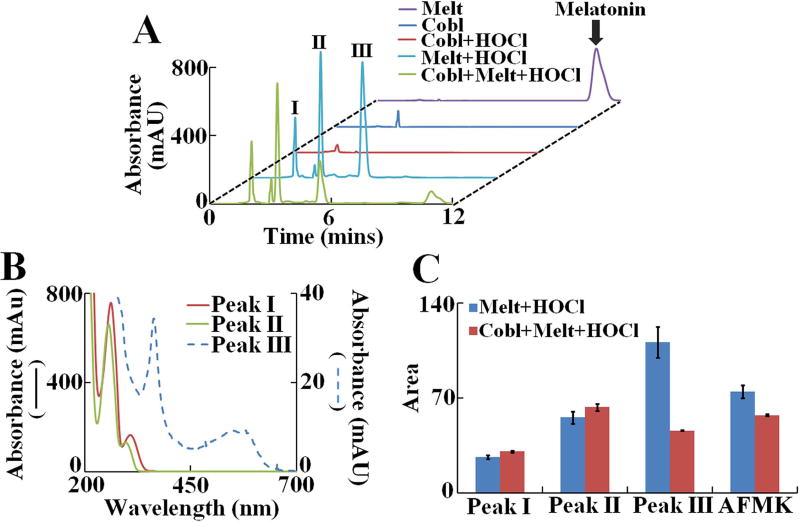
To understand the mechanism of how melatonin prevents HOCl-mediated cyanocobalamin destruction, we compared the melatonin oxidation products that were formed when HOCl oxidized melatonin in presence and absence of cyanocobalamin. The chromatograms (see Fig. 4A) were monitored at 260 nm to phase out the interference from any unreacted cyanocobalamin /Cl- cyanocobalamin derivatives. Under our chromatographic conditions, control unreacted melatonin eluted around ~ 11 min, and was identified from its characteristic spectra (purple trace in Fig. 4A). As expected, cyanocobalamin alone and cyanocobalamin treated with HOCl in absence of melatonin did not show any major peaks (Fig. 4A, traces labeled as ‘cyanocobalamin’ and ‘cyanocobalamin +HOCl’). In contrast the melatonin +HOCl and cyanocobalamin + melatonin +HOCl reaction mixtures displayed, three major UV absorbing peaks labeled as ‘Peak I’, ‘Peak II’ and ‘Peak III’. The retention time and spectral characteristics of the Peak I–III are listed in Table 1. The spectral features of the compounds eluting as ‘Peak II’ and ‘Peak III’ are very similar, while ‘Peak I’ shows a distinct red-shift (Fig. 4B). We also detected the formation of the fluorescent melatonin oxidation product N-[3-[2-(formylamino)-5-methoxyphenyl]-3-oxypropyl]-acetamide (AFMK) (chromatogram not shown) as reported earlier by Dellegar et. al.51 and Ximenes et al who also described the formation of a melatonin dimer55. Although previous reports suggested formation of 2-hydroxymelatonin as a product of HOCl-induced melatonin oxidation51, we failed to identify 2-hydroxymelatonin, from the spectral features of the three peaks. 2-hydroxymelatonin is relatively unstable and could easily convert to its keto tautomer, an indolinone, a rather lipophilic molecule56. Detailed mass-spectrometric and NMR studies are currently underway in our laboratory to characterize these novel melatonin oxidation products. The relative amount of the melatonin oxidation product (as judged by the area under the curve for each peak), formed when melatonin reacted with HOCl in presence or absence of cyanocobalamin is shown in Fig. 4C. We observed similar trend for the melatonin +HOCl and cyanocobalamin + melatonin +HOCl reaction mixtures respectively, indicating that melatonin protects HOCl-mediated cyanocobalamin corrin ring destruction by virtue of its ability to compete and scavenge HOCl.
Figure 4. Analysis of melatonin oxidation products generated when melatonin was oxidized by HOCl in presence or absence of cyanocobalamin.
(A) HPLC analysis of melatonin oxidation products generated by HOCl in presence and absence of cyanocobalamin. Final concentration of reactants cyanocobalamin (10 µM), melatonin (1000 µM) and HOCl (1000 µM). Chromatograms were monitored at 260 nm. The peak corresponding to unreacted melatonin is labeled as “Melatonin”. Three other, major UV absorbing peaks are labeled as I, II and III respectively. The data shown is a representative of three independent experiments. (B) UV-Vis spectrum of peak I, II and III as obtained from the diode array detector. (C) Relative amount of each product generated as calculated from the area under the curve for each peak. AFMK was detected via fluorescence detector (chromatogram not shown) as mentioned in the Materials and Methods section. The data shown is a representative of three independent experiments and the error bars are represent the standard error of measurements.
Table 1.
Retention time and spectral features of UV absorbing melatonin oxidation products.
| Retention time (mins) |
Absorbance maxima (nm) |
|
|---|---|---|
| Peak I | 2.06 | 362, 540, 576 |
| Peak II | 3.30 | 258, 302 |
| Peak III | 5.40 | 256, 290 |
Discussion
Here, we showed that melatonin is capable in preventing HOCl-mediated VB12 destruction and the generation of toxic molecular products such as chlorinated derivatives, corrin ring cleavage products, the toxic blood agents CN− and CNCl, and redox active free cobalt. The identification of several melatonin oxidation products clearly suggests that corrin ring protection was at the expense of melatonin. Our work may establish a direct mechanistic link by which melatonin exerts its antioxidant protective effect of VB12 under chronic inflammatory conditions where MPO chlorinating activity is elevated.
In human, melatonin and VB12 complement each other as antioxidants and in the regulation of circadian rhythm. Vitamin B12 exerts a direct influence on melatonin causing an earlier release of melatonin at night, which resets the sleep-wake cycle, by acting directly on the pineal gland to provoke a faster release of melatonin57. Vitamin B12 also causes melatonin to drop off faster by sensitizing the body to morning light58–60. Very serious sleep-wake disorders have been successfully treated with vitamin B12 in the methylcobalamin form58–61. Here we show a novel mechanistic role of melatonin, in which it can act to prevent the corrin ring destruction under inflammatory diseases where HOCl is elevated therefore preventing VB12 deficiency under such conditions.
Vitamin B12 is not only used for treating and preventing vitamin B12 deficiency, but also used for treating several conditions related to memory loss, immune system, aging, heart disease, male infertility, diabetes, sleep disorders, depression, mental disorders, and inflammation62,63. The safety in the64 use of cyanocobalamin supplementation, versus other VB12 derivatives, is a concern because: 1) biosynthesis of the B12 coenzyme requires the release of CN− that can be used by the body’s cells manifesting acute cyanide poisoning65,66 unwanted inflammation, reactions associated with the generation of higher levels of HOCl, can also be a major destructive force of the vitamin corrin ring generating CNCl, free active Co, and corrin degradation products15. Indeed, our current and previously published results suggest that the degradation of cyanocobalamin mediated by HOCl is largely modulated by the concentration of HOCl in the reaction milieu which could be partially or completely prevented by the presence of melatonin. Thus, any dysregulation of neutrophils/macrophages derived-HOCl contributes to inflammation, or reduction in HOCl consumption might get manifested as increased cyanocobalamin destruction and CNCl generation15. Therefore, it is of enormous therapeutic and pharmacologic importance, to prevent HOCl-mediated damage, especially in chronic inflammation, where a higher rate of infiltration of monocytes/macrophages over a longer period leads to pathologic alterations.
Besides HOCl mediated corrin ring destruction, there are several other causes that can lead to VB12 deficiency. For example, two widely studied causes of VB12 deficiency are: Pernicious anemia, an autoimmune condition, and food-bound VB12 malabsorption67–70. Both diseases have been associated with atrophic gastritis, a condition characterized by chronic inflammation of the gastric mucosa71. It has been generally perceived that the development of atrophic gastritis is due to chronic Helicobacter pylori (H. pylori) infection, which leads to extensive infiltration of the gastric mucosa by neutrophils72. MPO in neutrophils/macrophages amplifies the oxidative potential of H2O2 that induces gastric mucosal damage, and thus MPO is suspected to play a role in H. pylori-induced gastric injury73. The development of atrophic gastritis subsequent to H. pylori infection is determined by host-bacterial interaction with studies favoring host-related factors74. It has been shown that host MPO genetic polymorphism may play an important role in the pathogenesis of atrophic gastritis upon H. pylori infection75. In addition to lack of intrinsic factor, VB12 deficiency in pernicious anemia and atrophic gastritis, could be explained in part by enhancement of MPO activity that leads to distortion of the corrin ring of cyanocobalamin by HOCl15. Destruction of cyanocobalamin tetrapyrrole macrocycle by HOCl is also believed to be a leading event in cell injury, because it is tightly coupled with the generation of several undesired toxic molecules such as redox-active transition metals (e.g. cobalt and iron), which, through Fenton reaction forms highly reactive free radicals such as •OH76–81. VB12 destruction will also lead to the elevation of plasma homocysteine and methylmalonyl CoA levels by inhibiting methionine synthase and L-methyl malonyl CoA mutase82.
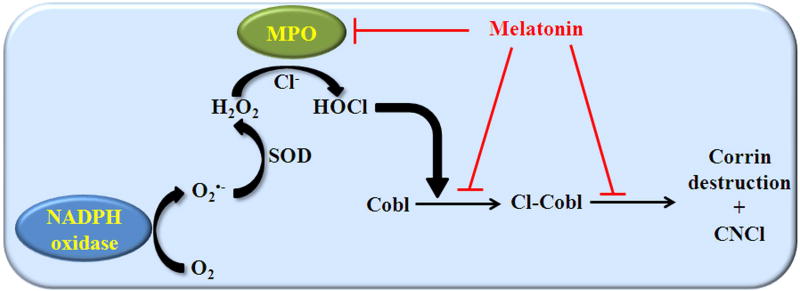
A scheme of MPO catalysis that incorporates our current findings is presented in Fig. 5. In this working model, stimulation of neutrophils activates the membrane bound NADPH oxidase, which converts molecular oxygen to O2•− 1. Superoxide either spontaneously or by an enzyme-catalyzed process (through SOD, superoxide dismutase) is converted to H2O2. Concurrent to NADH oxidase activation there is also release of MPO83. MPO utilizes H2O2 in the presence of Cl−, through the formation of a resonance-stabilized porphyrin π cation radical (Compound I), to generate HOCl54. HOCl plays an important role in the innate immune response, but sustained high levels of HOCl are implicated in several pathological conditions1. Recently we have demonstrated that HOCl can oxidatively destroy tetrapyrrole macrocycles such as free heme13, heme moiety in hemoproteins (hemoglobin and lactoperoxidase)12,14 and cyanocobalamin15. Melatonin modulates HOCl-mediated cyanocobalamin destruction in a concentration-dependent fashion by at least through two distinct pathways (Fig. 5). Firstly by serving as an inhibitor of MPO by its ability to act as a 1e− substrate for MPO Compounds I and II (MPO-Fe(IV)=O complex), or binding directly to MPO above the heme iron forming melatonin -MPO-Fe(III) complex and secondly, by direct scavenging of HOCl30,48,84. In the presence of melatonin, rapid kinetic studies suggested that the Compound I oxidizes melatonin through two sequential 1-electron steps, forming Compound II and MPO-Fe(III), respectively85. Through this mechanism, melatonin inhibits MPO chlorinating activity but not the peroxidase activity85. Alternatively, melatonin binds directly to MPO-Fe(III) presumably above the heme moiety, to readily promote reversible inhibition of peroxidase activity, to form a dead-end melatonin -MPO-Fe(III) complex85, and secondly, by its ability to directly scavenge HOCl may protect cyanocobalamin from HOCl-mediated degradation40,51. Thus, one mechanism through which melatonin might prevent VB12 mediated HOCl-damage in vivo is by minimizing the accumulation of HOCl during steady-state catalysis. As depicted in Fig. 4, the effect of melatonin on MPO-Fe(III) peroxidase activity will depend on multiple factors, including the bioavailability of melatonin versus H2O2, the affinity of MPO-Fe(III) for melatonin versus H2O2, and the rate of melatonin -MPO-Fe(III) breakdown.
Figure 5. Schematic model describing the role of melatonin in preventing HOCl-mediated cyanocobalamin destruction.
Melatonin functions as an antioxidant has been proven not only through its capacity to scavenge a variety of free radical and ROS, but also through its ability to chelate transition metals (e.g. iron (III), copper and zinc), a major source of ROS86–88. Thereby resulting in the lowering of ROS harmful effects such as lipid peroxidation, protein oxidation, and DNA damage27,35,49,87–89. Importantly, melatonin oxidation products have no biologically harmful consequences when it reacts with HOCl, therefore, its use as anti-oxidant is exceptional among other HOCl scavengers such as taurine, cystine, cysteine and uric acid),29,90. For example, 2-hydroxymelatonin is produced54 at a sufficient rate by the reaction of melatonin with HOCl to protect catalase against HOCl-mediated inactivation48. Collectively, inhibiting the MPO chlorinating activity or scavenging HOCl by melatonin might be a useful therapeutic approach in reducing VB12 deficiency, and eliminating the subsequent sequelae of the toxic cyanocobalamin degradation products generated by HOCl in a wide variety of inflammatory conditions.
Acknowledgments
Supported by The National Institutes of Health grant # RO1 HL066367, and the Department of Obstetrics and Gynecology, Wayne State University, Detroit, MI, USA.
Abbreviations
- MPO
myeloperoxidase
- HOCl
hypochlorous acid
- VB12
vitamin B12
- H2O2
hydrogen peroxide
- Cl−
chloride
- CN−
cyanide
- CNCl
cyanogen chloride
Footnotes
Author Contributions
H.M.A-S., D.M. and R.J. conceived and designed the experiments; D.M., R.J., C.C. and S.N. conducted the experiments. D.M., R.J., C.C., S.N. and R.M. analyzed the data; and R.J., D.M. and H.M.A-S wrote the manuscript. All authors reviewed the manuscript.
References
- 1.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(7):1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 2.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 3.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28(12):1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 4.Curtis MP, Neidigh JW. Kinetics of 3-nitrotyrosine modification on exposure to hypochlorous acid. Free Radic Res. 2014;48(11):1355–1362. doi: 10.3109/10715762.2014.954110. [DOI] [PubMed] [Google Scholar]
- 5.Green JN, Kettle AJ, Winterbourn CC. Protein chlorination in neutrophil phagosomes and correlation with bacterial killing. Free Radic Biol Med. 2014;77:49–56. doi: 10.1016/j.freeradbiomed.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Senthilmohan R, Kettle AJ. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch Biochem Biophys. 2006;445(2):235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Cook NL, Moeke CH, Fantoni LI, Pattison DI, Davies MJ. The myeloperoxidase-derived oxidant hypothiocyanous acid inhibits protein tyrosine phosphatases via oxidation of key cysteine residues. Free Radic Biol Med. 2016;90:195–205. doi: 10.1016/j.freeradbiomed.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Peskin AV, Winterbourn CC. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic Biol Med. 2001;30(5):572–579. doi: 10.1016/s0891-5849(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 9.Prutz WA. Interactions of hypochlorous acid with pyrimidine nucleotides, and secondary reactions of chlorinated pyrimidines with GSH, NADH, and other substrates. Arch Biochem Biophys. 1998;349(1):183–191. doi: 10.1006/abbi.1997.0440. [DOI] [PubMed] [Google Scholar]
- 10.Shishido N, Nakamura S, Nakamura M. Dissociation of DNA double strand by hypohalous acids. Redox Rep. 2000;5(4):243–247. doi: 10.1179/135100000101535690. [DOI] [PubMed] [Google Scholar]
- 11.Gebicka L, Banasiak E. Hypochlorous acid-induced heme damage of hemoglobin and its inhibition by flavonoids. Toxicol In Vitro. 2012;26(6):924–929. doi: 10.1016/j.tiv.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Souza CE, Maitra D, Saed GM, et al. Hypochlorous acid-induced heme degradation from lactoperoxidase as a novel mechanism of free iron release and tissue injury in inflammatory diseases. PLoS One. 2011;6(11):e27641. doi: 10.1371/journal.pone.0027641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maitra D, Byun J, Andreana PR, et al. Mechanism of hypochlorous acid-mediated heme destruction and free iron release. Free Radic Biol Med. 2011;51(2):364–373. doi: 10.1016/j.freeradbiomed.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maitra D, Byun J, Andreana PR, et al. Reaction of hemoglobin with HOCl: mechanism of heme destruction and free iron release. Free Radic Biol Med. 2011;51(2):374–386. doi: 10.1016/j.freeradbiomed.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Soud HM, Maitra D, Byun J, et al. The reaction of HOCl and cyanocobalamin: corrin destruction and the liberation of cyanogen chloride. Free Radic Biol Med. 2012;52(3):616–625. doi: 10.1016/j.freeradbiomed.2011.10.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maitra D, Abdulhamid I, Diamond MP, Saed GM, Abu-Soud HM. Melatonin attenuates hypochlorous acid-mediated heme destruction, free iron release, and protein aggregation in hemoglobin. J Pineal Res. 2012;53(2):198–205. doi: 10.1111/j.1600-079X.2012.00988.x. [DOI] [PubMed] [Google Scholar]
- 17.Maitra D, Shaeib F, Abdulhamid I, et al. Myeloperoxidase acts as a source of free iron during steady-state catalysis by a feedback inhibitory pathway. Free Radic Biol Med. 2013;63:90–98. doi: 10.1016/j.freeradbiomed.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maitra D, Ali I, Abdulridha RM, et al. Kinetic studies on the reaction between dicyanocobinamide and hypochlorous acid. PLoS One. 2014;9(11):e110595. doi: 10.1371/journal.pone.0110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qureshi GA, Qureshi AA, Devrajani BR, Chippa MA, Syed SA. Is the deficiency of vitamin B12 related to oxidative stress and neurotoxicity in Parkinson's patients? CNS Neurol Disord Drug Targets. 2008;7(1):20–27. doi: 10.2174/187152708783885101. [DOI] [PubMed] [Google Scholar]
- 20.Teismann P. Myeloperoxidase in the neurodegenerative process of Parkinson's disease. Dtsch Med Wochenschr. 2014;139(3):99–102. doi: 10.1055/s-0033-1359907. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo G, Lagana AS, Rapisarda AM, et al. Vitamin B12 among Vegetarians: Status, Assessment and Supplementation. Nutrients. 2016;8(12) doi: 10.3390/nu8120767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25(6):1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 23.Ohyama W, Yamaoka M, Yokoi K, et al. Maternal Crohn's disease-related vitamin B12 deficient megaloblastic anemia in an infant. Rinsho Ketsueki. 2016;57(1):15–19. doi: 10.11406/rinketsu.57.15. [DOI] [PubMed] [Google Scholar]
- 24.Gulley ML, Bentley SA, Ross DW. Neutrophil myeloperoxidase measurement uncovers masked megaloblastic anemia. Blood. 1990;76(5):1004–1007. [PubMed] [Google Scholar]
- 25.Varela-Moreiras G, Murphy MM, Scott JM. Cobalamin, folic acid, and homocysteine. Nutr Rev. 2009;67(Suppl 1):S69–72. doi: 10.1111/j.1753-4887.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- 26.Banerjee RV, Matthews RG. Cobalamin-dependent methionine synthase. FASEB J. 1990;4(5):1450–1459. doi: 10.1096/fasebj.4.5.2407589. [DOI] [PubMed] [Google Scholar]
- 27.Shaeib F, Khan SN, Ali I, et al. Melatonin prevents myeloperoxidase heme destruction and the generation of free iron mediated by self-generated hypochlorous acid. PLoS One. 2015;10(3):e0120737. doi: 10.1371/journal.pone.0120737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maitra D, Banerjee J, Shaeib F, Souza CE, Abu-Soud HM. Melatonin can mediate its vascular protective effect by modulating free iron level by inhibiting hypochlorous acid-mediated hemoprotein heme destruction. Hypertension. 2011;57(5):e22. doi: 10.1161/HYPERTENSIONAHA.111.172197. author reply e23. [DOI] [PubMed] [Google Scholar]
- 29.Lu T, Galijasevic S, Abdulhamid I, Abu-Soud HM. Analysis of the mechanism by which melatonin inhibits human eosinophil peroxidase. Br J Pharmacol. 2008;154(6):1308–1317. doi: 10.1038/bjp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galijasevic S, Abdulhamid I, Abu-Soud HM. Melatonin is a potent inhibitor for myeloperoxidase. Biochemistry. 2008;47(8):2668–2677. doi: 10.1021/bi702016q. [DOI] [PubMed] [Google Scholar]
- 31.Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: Nature's most versatile biological signal? FEBS J. 2006;273(13):2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 32.Stehle JH, Saade A, Rawashdeh O, et al. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J Pineal Res. 2011;51(1):17–43. doi: 10.1111/j.1600-079X.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- 33.Hardeland R. Melatonin: signaling mechanisms of a pleiotropic agent. Biofactors. 2009;35(2):183–192. doi: 10.1002/biof.23. [DOI] [PubMed] [Google Scholar]
- 34.Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79(1–3):C153–158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 35.Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27(2):189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- 36.Skinner DC, Malpaux B. High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology. 1999;140(10):4399–4405. doi: 10.1210/endo.140.10.7074. [DOI] [PubMed] [Google Scholar]
- 37.Yu HS, Yee RW, Howes KA, Reiter RJ. Diurnal rhythms of immunoreactive melatonin in the aqueous humor and serum of male pigmented rabbits. Neurosci Lett. 1990;116(3):309–314. doi: 10.1016/0304-3940(90)90092-n. [DOI] [PubMed] [Google Scholar]
- 38.Tan D, Manchester LC, Reiter RJ, Qi W, Hanes MA, Farley NJ. High physiological levels of melatonin in the bile of mammals. Life Sci. 1999;65(23):2523–2529. doi: 10.1016/s0024-3205(99)00519-6. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003;80(4):1012–1016. doi: 10.1016/s0015-0282(03)01008-2. [DOI] [PubMed] [Google Scholar]
- 40.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51(1):1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 41.Markus RP, Ferreira ZS, Fernandes PA, Cecon E. The immune-pineal axis: a shuttle between endocrine and paracrine melatonin sources. Neuroimmunomodulation. 2007;14(3–4):126–133. doi: 10.1159/000110635. [DOI] [PubMed] [Google Scholar]
- 42.Jung KH, Hong SW, Zheng HM, et al. Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. J Pineal Res. 2010;48(3):239–250. doi: 10.1111/j.1600-079X.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 43.Chahbouni M, Escames G, Venegas C, et al. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J Pineal Res. 2010;48(3):282–289. doi: 10.1111/j.1600-079X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 44.Jung-Hynes B, Reiter RJ, Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res. 2010;48(1):9–19. doi: 10.1111/j.1600-079X.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SY, Jang WJ, Yi EY, et al. Melatonin suppresses tumor angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res. 2010;48(2):178–184. doi: 10.1111/j.1600-079x.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- 46.Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13(5):1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 47.Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995;92(19):8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B. Evaluation of the antioxidant activity of melatonin in vitro. Free Radic Biol Med. 1996;21(3):307–315. doi: 10.1016/0891-5849(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee J, Maitra D, Diamond MP, Abu-Soud HM. Melatonin prevents hypochlorous acid-induced alterations in microtubule and chromosomal structure in metaphase-II mouse oocytes. J Pineal Res. 2012;53(2):122–128. doi: 10.1111/j.1600-079X.2012.00977.x. [DOI] [PubMed] [Google Scholar]
- 50.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444–458. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 51.Dellegar SM, Murphy SA, Bourne AE, DiCesare JC, Purser GH. Identification of the factors affecting the rate of deactivation of hypochlorous acid by melatonin. Biochem Biophys Res Commun. 1999;257(2):431–439. doi: 10.1006/bbrc.1999.0438. [DOI] [PubMed] [Google Scholar]
- 52.Lundquist P, Rosling H, Sorbo B. Determination of cyanide in whole blood, erythrocytes, and plasma. Clin Chem. 1985;31(4):591–595. [PubMed] [Google Scholar]
- 53.Gumus G, Demirata B, Apak R. Simultaneous spectrophotometric determination of cyanide and thiocyanate after separation on a melamine-formaldehyde resin. Talanta. 2000;53(2):305–315. [PubMed] [Google Scholar]
- 54.Wang L, Bassiri M, Najafi R, et al. Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity. J Burns Wounds. 2007;6:e5. [PMC free article] [PubMed] [Google Scholar]
- 55.Ximenes VF, Silva SO, Rodrigues MR, et al. Superoxide-dependent oxidation of melatonin by myeloperoxidase. J Biol Chem. 2005;280(46):38160–38169. doi: 10.1074/jbc.M506384200. [DOI] [PubMed] [Google Scholar]
- 56.Hardeland R. Taxon- and Site-Specific Melatonin Catabolism. Molecules. 2017;22(11) doi: 10.3390/molecules22112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikeda M, Asai M, Moriya T, Sagara M, Inoue S, Shibata S. Methylcobalamin amplifies melatonin-induced circadian phase shifts by facilitation of melatonin synthesis in the rat pineal gland. Brain Res. 1998;795(1–2):98–104. doi: 10.1016/s0006-8993(98)00262-5. [DOI] [PubMed] [Google Scholar]
- 58.Yamadera H, Takahashi K, Okawa M. A multicenter study of sleep-wake rhythm disorders: therapeutic effects of vitamin B12, bright light therapy, chronotherapy and hypnotics. Psychiatry Clin Neurosci. 1996;50(4):203–209. doi: 10.1111/j.1440-1819.1996.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 59.Okawa M, Takahashi K, Egashira K, et al. Vitamin B12 treatment for delayed sleep phase syndrome: a multi-center double-blind study. Psychiatry Clin Neurosci. 1997;51(5):275–279. doi: 10.1111/j.1440-1819.1997.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto S, Kohsaka M, Morita N, Fukuda N, Honma S, Honma K. Vitamin B12 enhances the phase-response of circadian melatonin rhythm to a single bright light exposure in humans. Neurosci Lett. 1996;220(2):129–132. doi: 10.1016/s0304-3940(96)13247-x. [DOI] [PubMed] [Google Scholar]
- 61.Dodson ER, Zee PC. Therapeutics for Circadian Rhythm Sleep Disorders. Sleep Med Clin. 2010;5(4):701–715. doi: 10.1016/j.jsmc.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mikkelsen K, Stojanovska L, Apostolopoulos V. The Effects of Vitamin B in Depression. Curr Med Chem. 2016;23(38):4317–4337. doi: 10.2174/0929867323666160920110810. [DOI] [PubMed] [Google Scholar]
- 63.Smith AD, Refsum H. Vitamin B-12 and cognition in the elderly. Am J Clin Nutr. 2009;89(2):707S–711S. doi: 10.3945/ajcn.2008.26947D. [DOI] [PubMed] [Google Scholar]
- 64.Bourre JM. The role of nutritional factors on the structure and function of the brain: an update on dietary requirements. Rev Neurol (Paris) 2004;160(8–9):767–792. doi: 10.1016/s0035-3787(04)71032-2. [DOI] [PubMed] [Google Scholar]
- 65.Weissbach H, Redfield BG, Peterkofsky A. Biosynthesis of the B12 coenzyme: requirements for release of cyanide and spectral changes. J Biol Chem. 1962;237:3217–3222. [PubMed] [Google Scholar]
- 66.Nelson L. Acute cyanide toxicity: mechanisms and manifestations. J Emerg Nurs. 2006;32(4 Suppl):S8–11. doi: 10.1016/j.jen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 67.Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149–160. doi: 10.1056/NEJMcp1113996. [DOI] [PubMed] [Google Scholar]
- 68.Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and Perinatal Health. Adv Nutr. 2015;6(5):552–563. doi: 10.3945/an.115.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lanska DJ. Chapter 30: historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins. Handb Clin Neurol. 2010;95:445–476. doi: 10.1016/S0072-9752(08)02130-1. [DOI] [PubMed] [Google Scholar]
- 70.Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 71.Kulnigg-Dabsch S. Autoimmune gastritis. Wien Med Wochenschr. 2016;166(13–14):424–430. doi: 10.1007/s10354-016-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valenzuela MA, Canales J, Corvalan AH, Quest AF. Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis. World J Gastroenterol. 2015;21(45):12742–12756. doi: 10.3748/wjg.v21.i45.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki H, Miura S, Imaeda H, et al. Enhanced levels of chemiluminescence and platelet activating factor in urease-positive gastric ulcers. Free Radic Biol Med. 1996;20(3):449–454. doi: 10.1016/0891-5849(96)02048-5. [DOI] [PubMed] [Google Scholar]
- 74.Cadamuro AC, Rossi AF, Maniezzo NM, Silva AE. Helicobacter pylori infection: host immune response, implications on gene expression and microRNAs. World J Gastroenterol. 2014;20(6):1424–1437. doi: 10.3748/wjg.v20.i6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roe I, Nam S, Kim J, Shin J, Bang W, Yang M. Association of the myeloperoxidase-463G-->A polymorphism with development of atrophy in Helicobacter pylori-infected gastritis. Am J Gastroenterol. 2002;97(7):1629–1634. doi: 10.1111/j.1572-0241.2002.05899.x. [DOI] [PubMed] [Google Scholar]
- 76.Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157(3):175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Clark RA. Oxidative stress and "senescent" fibroblasts in non-healing wounds as potential therapeutic targets. J Invest Dermatol. 2008;128(10):2361–2364. doi: 10.1038/jid.2008.257. [DOI] [PubMed] [Google Scholar]
- 78.Crichton RR, Wilmet S, Legssyer R, Ward RJ. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J Inorg Biochem. 2002;91(1):9–18. doi: 10.1016/s0162-0134(02)00461-0. [DOI] [PubMed] [Google Scholar]
- 79.Ong WY, Halliwell B. Iron, atherosclerosis, and neurodegeneration: a key role for cholesterol in promoting iron-dependent oxidative damage? Ann N Y Acad Sci. 2004;1012:51–64. doi: 10.1196/annals.1306.005. [DOI] [PubMed] [Google Scholar]
- 80.Trinder D, Fox C, Vautier G, Olynyk JK. Molecular pathogenesis of iron overload. Gut. 2002;51(2):290–295. doi: 10.1136/gut.51.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mates JM, Segura JA, Alonso FJ, Marquez J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med. 2010;49(9):1328–1341. doi: 10.1016/j.freeradbiomed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi-Iniguez T, Garcia-Hernandez E, Arreguin-Espinosa R, Flores ME. Role of vitamin B12 on methylmalonyl-CoA mutase activity. J Zhejiang Univ Sci B. 2012;13(6):423–437. doi: 10.1631/jzus.B1100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ximenes VF, Paino IM, Faria-Oliveira OM, Fonseca LM, Brunetti IL. Indole ring oxidation by activated leukocytes prevents the production of hypochlorous acid. Braz J Med Biol Res. 2005;38(11):1575–1583. doi: 10.1590/s0100-879x2005001100003. [DOI] [PubMed] [Google Scholar]
- 85.Morris SM. The genetic toxicology of 5-fluoropyrimidines and 5-chlorouracil. Mutat Res. 1993;297(1):39–51. doi: 10.1016/0165-1110(93)90006-9. [DOI] [PubMed] [Google Scholar]
- 86.Tekbas OF, Ogur R, Korkmaz A, Kilic A, Reiter RJ. Melatonin as an antibiotic: new insights into the actions of this ubiquitous molecule. J Pineal Res. 2008;44(2):222–226. doi: 10.1111/j.1600-079X.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 87.Gomez FJ, Raba J, Cerutti S, Silva MF. Monitoring melatonin and its isomer in Vitis vinifera cv. Malbec by UHPLC-MS/MS from grape to bottle. J Pineal Res. 2012;52(3):349–355. doi: 10.1111/j.1600-079X.2011.00949.x. [DOI] [PubMed] [Google Scholar]
- 88.Gulcin I, Buyukokuroglu ME, Kufrevioglu OI. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J Pineal Res. 2003;34(4):278–281. doi: 10.1034/j.1600-079x.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- 89.Reiter RJ, Korkmaz A, Ma S, Rosales-Corral S, Tan DX. Melatonin protection from chronic, low-level ionizing radiation. Mutat Res. 2012;751(1):7–14. doi: 10.1016/j.mrrev.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Ogino T, Than TA, Hosako M, Ozaki M, Omori M, Okada S. Taurine chloramine: a possible oxidant reservoir. Adv Exp Med Biol. 2009;643:451–461. doi: 10.1007/978-0-387-75681-3_47. [DOI] [PubMed] [Google Scholar]