Abstract
Background
Biomarkers of cardiac damages, such as troponin T (TnT) and the amino-terminal fragment of brain natriuretic peptide (NT-proBNP), may be useful as early predictors of cardiac dysfunction. The role of these biomarkers in patients receiving lapatinib and/or trastuzumab before anthracyclines is unknown. This study explores TnT and NT-proBNP as predictors of early cardiac toxicity in neoadjuvant breast cancer patients.
Methods
This sub-study of the NEOALTTO trial tested if changes in the levels of TnT and NT-proBNP occurred after 2 weeks of anti-HER2 therapy (lapatinib, trastuzumab or their combination) alone and/or after 18 weeks of anti-HER2 therapy plus weekly paclitaxel.
Results
173 and 172 were tested at all three timepoints for NT-proBNP and TnT, respectively. The incidence of biomarker elevation was overall low at all timepoints for all the three treatment arms. A total of 13 CEs in 11 patients occurred. Biomarker elevations in patients with CEs were very rare; only one patient with subsequent CE had a NT-proBNP elevation at baseline and at week 2.
Conclusion
These results suggest that TnT and proBNP may not be useful as early predictors of cardiac toxicity in anthracycline-naïve patients receiving trastuzumab and/or lapatinib.
Keywords: HER2 positive breast cancer, Lapatinib, Cardiotoxicity, Troponin T, Brain natriuretic peptide
Introduction
Anti-HER2 therapy is the cornerstone of the management of HER2-positive breast cancer in both the early and advanced settings [1, 2]. Cardiac toxicity remains the main adverse event associated with anti-HER2 therapy especially when used concomitantly with or sequentially to anthracyclinebased chemotherapy [3]. This finding is consistent with the important role of HER2 signalling in cardiac muscle development and function [3]. Several studies have shown that, when added to chemotherapy, trastuzumab is associated with a low but nevertheless increased rate of cardiac toxicity compared to cytotoxic chemotherapy alone [4]. Comparatively, few data are available about the potential cardiac toxicity of lapatinib alone or combined with trastuzumab [3, 5, 6]. This combination, despite initial promising results, did not improve patient’s outcome (survival) in the early setting [7–9]. Nevertheless, it remains an important therapeutic alternative in patients with advanced disease [6, 10]. In fact, trastuzumab and lapatinib, individually or in combination with chemotherapy or endocrine therapy, are commonly used in advanced disease [1, 10–15]. Therefore, a better understanding of the cardiac toxicity of these agents remains a relevant area of research.
Currently, the main methods of cardiac evaluation prior and during the use of anti-HER2 therapy are serial echocardiography or multigated acquisition scan (MUGA) [16, 17]. These exams have the serious limitation of detecting cardiac toxicity only when myocyte dysfunction has occurred. Developing tools that would be adequately able to predict cardiac toxicity at an early stage remains an important area where further research efforts are needed. To improve upon current standards, cardiac biomarkers for myocyte damages, including troponin T (TnT) and the amino-terminal fragment of brain natriuretic peptide (NT-proBNP), have been studied [3]. Both cardiac markers are currently in use for a variety of heart conditions in non-oncologic patients [18].
With the present sub-study of the neoadjuvant Neo-ALTTO trial [8], we investigated the potential utility values of TnT and NT-proBNP as early predictors of cardiac toxicity in patients receiving neoadjuvant trastuzumab, lapatinib, or their combination as single agents and then combined with weekly paclitaxel.
Patients and methods
Study design
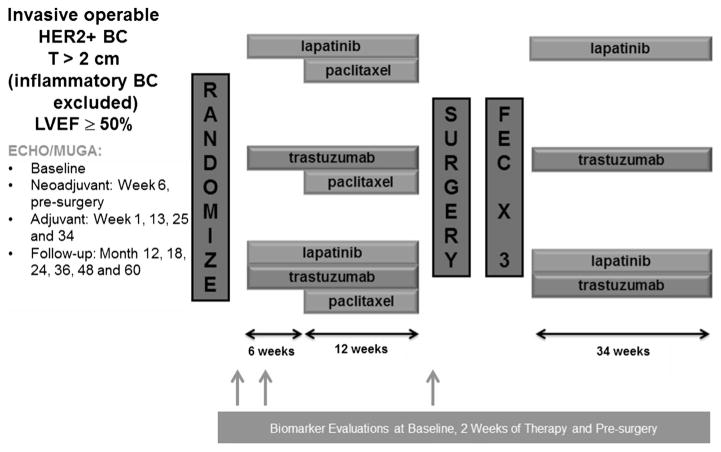
Details of the NeoALTTO trial (Breast International Group 1-06) have been previously reported [7, 8]. Briefly, the study was a randomized, multicentre, open-label, phase III trial that evaluated lapatinib, trastuzumab, or their combination as neoadjuvant therapy in patients with HER2-positive primary breast cancer. Adequate baseline left ventricular ejection fraction (LVEF) ≥ 50% was required at study entry. Eligible patients were randomly assigned to oral lapatinib, intravenous trastuzumab, or lapatinib plus trastuzumab, given alone for the first 6 weeks and then with weekly paclitaxel for further 12 weeks before definitive surgery was performed. After surgery, patients received three cycles of adjuvant 3-weekly FEC (fluorouracil, epirubicin and cyclophosphamide) chemotherapy followed by the same targeted therapy as in the neoadjuvant phase to complete 1 year of anti-HER2 treatment. Figure 1 summarizes the NeoALTTO trial design.
Fig. 1.
NeoALTTO design legend, T tumour, BC breast cancer, LVEF left ventricular ejection fraction, Echo echocardiogram, MUGA multigated acquisition scan
All NeoALTTO sites with facilities for management of additional blood samples could participate in this study. Patients’ participation in this sub-study was allowed after signing the main study consent form, which included a non-specific clause for use of blood samples for biomarker research.
Cardiac biomarker sub-study: objectives and procedures
The present sub-study aimed to evaluate the utility values of TnT and NT-proBNP as predictive biomarkers of cardiac dysfunction with anti-HER2-targeted treatments in an anthracycline-naïve breast cancer population. Specifically, the study tested whether the level of TnT or NT-proBNP changed after 2 and/or 18 weeks (pre-surgery) after anti-HER2 therapy compared to baseline values, and if elevated levels of TnT and/or NT-proBNP observed at any timepoint (baseline, week 2 or pre-surgery) correlated with the occurrence of cardiac events. Finally, the study aimed to compare the incidences of cardiac events with lapatinib, trastuzumab or their combination.
The hypothesis was that the use of trastuzumab, lapatinib or their combination as single agents and added to weekly paclitaxel would not increase the levels of these biomarkers, and that if an elevation of TnT and/or NT-proBNP was to occur, it would precede LVEF decline.
Blood samples were collected and centrally tested (Inselspital, Bern University Hospital, Bern, Switzerland) at 3 timepoints: baseline, after 2 weeks of anti-HER2 treatment alone and immediately before surgery (week 18). Cardiac function was closely monitored with either echocardiography or MUGA throughout the trial across all three arms at the following pre-specified timepoints: Baseline, week 6 of neoadjuvant phase, pre-surgery, weeks 1, 13, 25 and 34 of adjuvant phase, months 12, 18, 24, 36, 48 and 60 of follow-up [8]. Study protocol required that only one method of cardiac evaluation was used per patient, and all possible efforts were made to ensure the exam was performed by the same physician. Physicians performing the cardiac function test were not blinded to study treatment arm (as this was an open label study) and results of cardiac function tests were not centrally reviewed (as is the case for most studies in the oncology field that don’t specifically focus on cardiac function). The thresholds for positivity (i.e. elevated biomarker) were > 0.015 μg/for TnT and > 125 pg/mL for NT-proBNP [19]. For both TnT and NT-proBNP, tests manufactured by Cobas ® were employed. The antibodies use were, respectively: Anti-Troponin T-Ak ~ Biotin/Anti-Troponin T-Ak ~ Ru(bpy) and Anti-NT-proBNP-Ak ~ Biotin/Anti-NT-proBNP-Ak ~ Ru(bpy).
Cardiac endpoints (CEs) were defined as in the main trial: (a) primary cardiac endpoint was defined as symptomatic congestive heart failure (CHF) according to the New York Heart Association (NYHA) class III or IV, or as cardiac death; (b) secondary cardiac endpoint was defined as asymptomatic (NYHA class I) or mildly symptomatic (NYHA class II) significant drop in LVEF (i.e. to below 50% and > 10 points) confirmed by a second LVEF assessment within approximately 3 weeks.
Statistical analysis
All the analyses performed were exploratory with no prespecified statistical hypothesis to be tested. Due to the small number of cardiac events and the rarity of marker elevation, only descriptive analyses were conducted.
Besides type of anti-HER2 treatment administered, the impact of the following cardiac risk factors at study entry was evaluated: diabetes, hypertension, body mass index (BMI), age and blood pressure (systolic and diastolic).
Biomarker levels (elevated/not elevated) across time were tabulated by treatment arm, post-surgery LVEF and incidence of cardiac event (secondary or primary).
Results
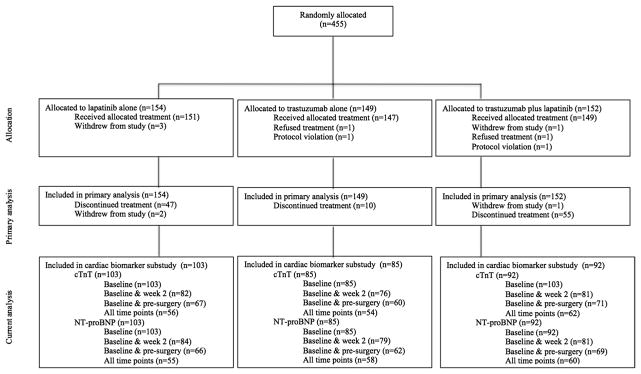
Of the 455 patients randomized into the NeoALTTO trial, 280 (62%) were evaluated at baseline for both TnT and NT pro-BNP; 239 (52.5%) and 244 (53.6%) at 2 weeks; and 198 (43.5%) and 197 (43.2%) at week 18, respectively (Fig. 2). NT-proBNP and TnT were tested at all three timepoints in 173 (38%) and 172 (38%) patients, respectively. Of the 280 patients tested at baseline, 29 (10.36%) were under an angiotensin-converting-enzyme (ACE) inhibitor, 25 (8.93% a diuretic and 17 (6.07% a betablocker). Cardiac safety follow-up for this population was mostly performed with echocardiogram—211 (75.36%), with a minority being followed with MUGA 16.43%) or both (8.21%). As this project is not a pre-planned sub-study, participation was limited by the availability of samples at each of the timepoints for testing.
Fig. 2.
Study flow-chart
Table 1 summarizes baseline characteristics of all the patients included in the present cardiac biomarker substudy. Tables 2 and 3 report the alterations in NT-proBNP and TnT at each timepoint for all patients tested at least one time, and for those tested at all three timepoints, respectively.
Table 1.
Baseline patients’ characteristics
| All patientsa (n = 280) no. (%) | No cardiac biomarkers elevation (n = 221) no. (%) | Any cardiac biomarkers elevationb (n = 59) no. (%) | P value | |
|---|---|---|---|---|
| Age at diagnosis, median (range) | 50 (25–75) | 49 (25–75) | 58 (28–73) | < 0.001 |
| BMI (kg/m2), median (range) | 24.9 (16.6–47.6) | 24.7 (16.6–47.6) | 25.9 (18.2–42.6) | 0.185 |
| Diabetes | ||||
| Yes | 9 (3) | 8 (4) | 1 (2) | 0.456 |
| No | 271 (97) | 213 (96) | 58 (98) | |
| Hypertension | ||||
| Yes | 57 (20) | 34 (15) | 23 (39) | < 0.001 |
| No | 223 (80) | 187 (85) | 36 (61) | |
| Ethnicity | ||||
| White | 177 (63) | 127 (57) | 50 (85) | 0.002 |
| Black | 4 (1) | 4 (2) | 0 (0) | |
| Asian | 75 (27) | 69 (31) | 6 (10) | |
| Other | 24 (9) | 21 (10) | 3 (5) | |
| Radiotherapy | 0.763 | |||
| Yes | 199 (71) | 158 (71) | 41 (69) | |
| No | 81 (29) | 63 (29) | 18 (31) | |
| Histology | 0.318 | |||
| Ductal carcinoma | 143 (51) | 112 (51) | 31 (53) | |
| lobular carcinoma | 7 (3) | 4 (2) | 3 (5) | |
| Others/missing | 130 (46) | 105 (47) | 25 (42) | |
| Tumour size (cm): | 0.158 | |||
| ≤ 2 | 0 (0) | 0 (0) | (0) | |
| > 2/≤ 5 | 148 (53) | 112 (51) | 36 (61) | |
| > 5 | 132 (47) | 109 (49) | 23 (39) | |
| Nodal status | 0.718 | |||
| N0/N1 | 228 (81) | 179 (81) | 49 (83) | |
| N2/N3 or missing | 52 (19) | 42 (19) | 10 (17) | |
| Hormone receptor status | 0.088 | |||
| ER and/or PR positive | 148 (53) | 111 (50) | 37 (63) | |
| ER and PR negative | 132 (47) | 110 (50) | 22 (37) | |
| Cardiac events | 10 | 9 | 1 | – |
All patients with any troponin T and NT-proBNP measurement at baseline
At baseline, week 2, and/or pre-surgery
BMI bodymass index, NT-proBNP amino-terminal fragment of brain natriuretic peptide, ER estrogene receptor, PR progesterone receptor
Table 2.
Number of patients with elevated serum troponin T and/or NT-proBNP for all patients who were tested at least one time
| Baseline | Week 2 | Pre-surgery | |
|---|---|---|---|
| Troponin T | (N = 280) | (N = 239) | (N = 198) |
| Elevated TnT, n (%) | 1 (0.4) | 1 (0.4) | 5 (2.5) |
| NT-proBNP | (N = 280) | (N = 244) | (N = 197) |
| Elevated NT-proBNP, n (%) | 39 (13.9) | 17 (7.0) | 23 (11.7) |
TnT troponin T, NT-proBNP amino-terminal fragment of brain natriuretic peptide
Table 3.
Number of patients with elevated serum troponin T and/or NT-proBNP for patients who were tested at all timepoints
| Baseline | Week 2 | Pre-surgery | |
|---|---|---|---|
| Troponin T | (n = 172) | (n = 172) | (n = 172) |
| Elevated TnT, n (%) | 1 (0.6) | 1 (0.6) | 5 (2.9) |
| NT-proBNP | (n = 173) | (n = 173) | (n = 173) |
| Elevated NT-proBNP, n (%) | 25 (14.5) | 10 (5.8) | 21 (12.1) |
TnT troponin T, NT-proBNP amino-terminal fragment of brain natriuretic peptide
In the whole NeoALLTO population, 13 cardiac events were recorded in 11 patients, with 4 being primary CE and 9 secondary CE. Overall, the incidence of biomarker alterations in the entire sample and in patients who experienced cardiac events was very low. Among patients with cardiac events, five had a baseline measurement of biomarkers, with one having a NT-proBNP increase and none a TnT increase. At week 2, results were similar, with nine patients evaluated and only one having a NT-proBNP elevation. Of note, at both timepoints, it was the same patient who had an elevation. At week 18, 3 patients had a CE and none had biomarker alterations. The overall low number of cardiac events and of elevations precluded the performance of any statistical analysis for correlation between cardiac event and biomarker elevation in this sample as previously planned in the statistical analyses plan.
Known risk factors for anti-HER2 therapy-related cardiac toxicity were evaluated in this population. A total of 9 patients had diabetes and 57 patients had hypertension. One diabetic patient and 23 patients with hypertension had biomarker alterations, respectively. Patients with biomarker alteration were significantly older (58 vs. 49 years, respectively, p < 0.001) and had more history of hypertension (39% vs. 15%, respectively, p < 0.001) compared to those without. No differences were found in BMI between patients with biomarker alterations and those without (25.9 and 24.7, respectively) (Table 1). The low number of events precluded statistical determination of any correlations between risk factors and biomarker alterations.
Discussion
To our knowledge, this is the first study investigating the potential role of cardiac biomarkers as early predictors of cardiac toxicity in anthracycline-naïve patients receiving anti-HER2 targeted therapy. The results of this Neo-ALTTO sub-study highlights the low risk of cardiac events with trastuzumab, lapatinib or their combination given alone or in combination with weekly paclitaxel followed by FEC chemotherapy.
The potential risk of cardiac toxicity in patients receiving anti-HER2 therapy has led to the need of cardiac surveillance during and post-treatment. Currently, the National Comprehensive Cancer Network (NCCN), European Society of Medical Oncology (ESMO) and the European Society of Cardiology (ESC) recommend evaluations of heart function with cardiac imaging during therapy at 3 month intervals (4 cycles) [16, 20, 21]. However, this approach has the significant drawback of detecting cardiotoxicity only once it becomes functionally significant. Despite data showing high rates of recovery following therapy interruption and proper cardiac medications, it is not possible at this point to be sure that on the very long term (a decade or more) these patients will not be at a higher risk of CHF [5]. Therefore, the use of cardiac biomarkers as means of early predictor of cardiac toxicity would be, if validated, of critical importance making possible both early interruption of therapy and more personalized follow-up schedules [22].
Troponins I (TnI) and TnT, and NT-proBNP are markers of tissue damage (death of heart muscle cells) and of left ventricle distension (an early marker of dysfunction), respectively [22]. In the present study, the numbers of TnT and NT-proBNP elevations were overall low, thus precluding the possibility to test for correlation with cardiac events. Of note, this is the largest study dealing with cardiac biomarkers in patients receiving lapatinib and the only one evaluating both TnT and NT-proBNP in an anthracycline-naïve early breast cancer population (as all biomarker measurements were taken before patients received adjuvant FEC).
Other available studies investigating the role of cardiac biomarkers and trastuzumab use have shown mixed results [22]. The largest study conducted in 251 women receiving trastuzumab-based therapy suggested that TnI positively correlates with cardiac events [23]. Moreover, the study showed that TnI-positive patients recovered less often from cardiac events when compared to TnI-negative patients [23]. Most elevations were detected at baseline or post-first cycle with rapid normalization within 3 months. It is important to note that most patients in this sample who experienced CEs had been previously exposed to anthracyclines. Another study investigated the role of TnI and c-reactive protein in a population of patients that received 4 cycles of dose-dense AC (anthracycline and cyclophosphamide) followed by paclitaxel plus trastuzumab and lapatinib followed by a year of trastuzumab plus lapatinib [24]. In this study, 99 patients were tested for biomarkers every 2 weeks while on treatment and then at every 3-month interval. A total of 67% of patients had at least one elevated TnI measurement, with a peak after 3 months of therapy. Although elevation in TnI often occurred before maximum LVEF reduction, no correlation was found between maximum TnI elevation and LVEF decline [25]. Once again most alterations were detected early during anti-HER2 treatment and resolved rapidly. In addition, another study with 214 patients found TnI elevations in patients who received trastuzumab after epirubicin, but no elevations in patients who received trastuzumab only [26].
Taking into account all these findings together with the results from our NeoALTTO sub-study, it is possible to hypothesize that the elevation in biomarkers is fundamentally determined by anthracyclines, in most of the cases. This is congruent with currently held hypothesis on the mechanisms of anthracycline vs anti-HER2 therapy cardiac toxicity. Anthracyclines cause direct damage to the cell membrane of cardiomyocytes by promoting oxidative stress [27] that ultimately leads to cell death, and thus to marker elevation. HER2 signalling may be a key component of cardiomyocyte resistance to anthracycline toxicity, explaining the higher risk of the combination. Trastuzumab, on the other hand, impairs cardiomyocyte function by interfering with contractility by decreasing the expression of anti-apoptotic Bcl-xL. Lapatinib may indeed antagonize this particular effect of trastuzumab and therefore be a cardioprotective factor [28].
A recently published sub-study of the HERA trial investigating adjuvant trastuzumab and chemotherapy versus chemotherapy alone (nearly all patients in HERA received anthracyclines before trastuzumab) evaluated the potential role of cardiac biomarkers in identifying patients at risk of developing cardiac events. In this HERA sub-study, both baseline TnT and TnI levels correlated with cardiac events, even if the risk was still overall low [29]. Interestingly, the study showed that the risk of cardiac events increased by approximately 30% for each 10 ng/dl raise in NT-proBNP values from baseline levels. Few other studies have explored the usefulness of NT-proBNP in patients receiving anti-HER2 therapy. Overall, only a small number of elevations and no correlation with events were observed, as was the case in our study, although the detected NT-proBNP alterations were higher in our study [30–32].
Several limitations should be taken into account in interpreting the results from our study. The low rates of both biomarker alterations and incidence of cardiac events reduce the reliability of our findings. Lack of prior exposure to anthracyclines may be a possible explanation for these findings. The different physiopathology of pure anti-HER2 therapy-induced cardiac toxicity, causing more dysfunction than direct necrosis of heart tissue can be another explanation [3]. Finally, the half-life of troponin is short; therefore, it is possible that the timing of measurements might have missed them [22]. Furthermore, evidence posterior to the design of our study suggests TnI to be a better target for investigation than the two biomarkers chosen for this study.
To conclude, in the present NeoALTTO sub-study, trastuzumab, lapatinib or their combination alone and combined with taxanes seemed to be a safe treatment option from a cardiac perspective with a low rate of cardiac events. TnT and NT-proBNP in an anthracycline-naïve population were detected in a few patients; hence, these biomarkers seem not to be useful in this population. Further studies investigating the role of cardiac biomarkers are warranted in patients receiving anti-HER2 therapy, specifically in those previously exposed to anthracyclines where prophylactic treatment may have a role.
Acknowledgments
Dr. Matteo Lambertini acknowledges the support from the European Society for Medical Oncology (ESMO) for a Translational Research Fellowship at the Institut Jules Bordet in Brussels (Belgium).
Funding This research has received research grant from “Les Amis de L’Institut Bordet” (Grant 2016-02).
Footnotes
This work was partially presented at the San Antonio Breast Cancer 2016 (abstract # P4-21-09).
Compliance with ethical standards
Conflicts of interest The authors of this manuscript hereby declare the following conflicts of interest regarding this manuscript: Dimitrios Zardavas—Research Grants to his institution from Roche, Genentech, Novartis, Pfizer, AstraZeneca, Tesaro, Puma Biotechnology; Jens Huober—Roche honoraria and advisory role, Novartis: honoraria and advisory role, GSK: research funding; Evandro de Azambuja—received honoraria from Roche and travel grants from Roche and Glaxo-SmithKline outside the submitted work; Martine Piccart—is a board member of Radius, is a consultant (honoraria): AstraZeneca, Lilly, MSD, Novartis, Odonate, Pfizer, Roche-Genentech, Crescendo Biologics, Periphagen, Huya, Debiopharm, PharmaMar and has received research grants to her Institute: AstraZeneca, Lilly, MSD, Novartis, Pfizer, Roche-Genentech, Synthon, Radius, Servier. All remaining authors have declared no conflicts of interest.
References
- 1.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–v30. doi: 10.1093/annonc/mdv298. https://doi.org/10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Costa A, Senkus E, et al. 3rd ESO–ESMO international consensus guidelines for advanced breast cancer (ABC 3) Ann Oncol. 2016;28:16–33. doi: 10.1093/annonc/mdw544. https://doi.org/10.1093/annonc/mdw544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pondé N, Lambertini M, De Azambuja E. Twenty years of anti-HER2 therapy-associated cardiotoxicity. ESMO Open. 2016;1:e000073. doi: 10.1136/esmoopen-2016-000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ades F, Zardavas D, Pinto AC, et al. Cardiotoxicity of systemic agents used in breast cancer. Breast Edinb Scotl. 2014;23:317–328. doi: 10.1016/j.breast.2014.04.002. https://doi.org/10.1016/j.breast.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 5.de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the herceptin Adjuvant trial (BIG 1-01) J Clin Oncol Off J Am Soc Clin Oncol. 2014;32:2159–2165. doi: 10.1200/JCO.2013.53.9288. https://doi.org/10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. https://doi.org/10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (Neo-ALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet Lond Engl. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. https://doi.org/10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137–1146. doi: 10.1016/S1470-2045(14)70320-1. https://doi.org/10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- 9.Piccart-Gebhart M, Holmes E, Baselga J, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. https://doi.org/10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso F, Costa A, Norton L, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Ann Oncol. 2014;25:1871–1888. doi: 10.1093/annonc/mdu385. https://doi.org/10.1093/annonc/mdu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. https://doi.org/10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 12.Johnston S, Pippen J, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. https://doi.org/10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. https://doi.org/10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that over expresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. https://doi.org/10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 15.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. https://doi.org/10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 16.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. 2012;23:vii155–vii166. doi: 10.1093/annonc/mds293. https://doi.org/10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 17.Eschenhagen T, Force T, Ewer MS, et al. Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. doi: 10.1093/eurjhf/hfq213. https://doi.org/10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
- 18.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. https://doi.org/10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 19.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. https://doi.org/10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed 3 Nov 2016];Breast.pdf. 2016 https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 21.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. https://doi.org/10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 22.Witteles R. Biomarkers as predictors of cardiac toxicity from targeted cancer therapies. J Card Fail. 2016 doi: 10.1016/j.cardfail.2016.03.016. https://doi.org/10.1016/j.cardfail.2016.03.016. [DOI] [PubMed]
- 23.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. https://doi.org/10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 24.Dang C, Lin N, Moy B, et al. Dose-dense doxorubicin and cyclophosphamide followed by weekly paclitaxel with trastuzumab and lapatinib in HER2/neu-overexpressed/amplified breast cancer is not feasible because of excessive diarrhea. J Clin Oncol. 2010;28:2982–2988. doi: 10.1200/JCO.2009.26.5900. https://doi.org/10.1200/JCO.2009.26.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris PG, Chen C, Steingart R, et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin Cancer Res Off J Am Assoc Cancer Res. 2011;17:3490–3499. doi: 10.1158/1078-0432.CCR-10-1359. https://doi.org/10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 26.Mokuyasu S, Suzuki Y, Kawahara E, et al. High-sensitivity cardiac troponin I detection for 2 types of drug-induced cardiotoxicity in patients with breast cancer. Breast Cancer. 2015;22:563–569. doi: 10.1007/s12282-014-0520-8. https://doi.org/10.1007/s12282-014-0520-8. [DOI] [PubMed] [Google Scholar]
- 27.Sawyer DB, Peng X, Chen B, et al. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53:105–113. doi: 10.1016/j.pcad.2010.06.007. https://doi.org/10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hervent A-S, De Keulenaer GW. Molecular mechanisms of cardiotoxicity induced by ErbB receptor inhibitor cancer therapeutics. Int J Mol Sci. 2012;13:12268–12286. doi: 10.3390/ijms131012268. https://doi.org/10.3390/ijms131012268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zardavas D, Suter TM, Van Veldhuisen DJ, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast cancer receiving trastuzumab: a herceptin adjuvant study cardiac marker substudy. J Clin Oncol. 2016;35(8):878–884. doi: 10.1200/JCO.2015.65.7916. https://doi.org/10.1200/jco.2015.65.7916. [DOI] [PubMed] [Google Scholar]
- 30.Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. https://doi.org/10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu AF, Manrique C, Pun S, et al. Cardiac safety of paclitaxel plus trastuzumab and pertuzumab in patients with HER2-positive metastatic breast cancer. Oncologist. 2016;21:418–424. doi: 10.1634/theoncologist.2015-0321. https://doi.org/10.1634/theoncologist.2015-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–2270. doi: 10.1016/j.jacc.2010.11.063. https://doi.org/10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]