Sodium (Na+) balance is flow dependent and occurs along the nephron due to basic communication between nephron segments in response to Na+ levels and to maintain Na+ delivery to the distal convoluted tubule (DCT).1 Decreased distal Na+ delivery increases Na+ reabsorption by facilitating epithelial Na+ channel (ENaC) activity in the distal nephron at the expense of K+ excretion to maintain the ionic gradient. This process is very dynamic and is affected by multiple factors. Angiotensin-II (ang-II) and its type 1 receptor (AT1R) constitute a well-known anti-natriuretic hormone/receptor system that promotes Na+ reabsorption and decreases distal Na+ delivery. Now, there is ample evidence demonstrating the natriuretic role of the angiotensin type 2 receptor (AT2R) in normal as well as pathological conditions. Along the nephron, the proximal and distal tubules are the primary sites where AT2R is expressed, albeit at a very low receptor density. However, the expression of renal AT2R increases in pathological conditions, such as obesity and diabetes, including in the diabetic human kidney. AT2R expressed in the proximal tubule seems to play a significant role in natriuresis potentially via inhibition of sodium transporters, such as the Na+-K+-ATPase (NKA). According to at least one study, AT2R-mediated natriuresis is not impacted by inhibitors of the distal tubule transporter Na+-Cl− co-transporter (NCC) and ENaC activity.2
Compared to that discussing the role of AT2R in Na+ excretion, literature describing the effect of AT2R on the K+ channel is limited only to neuronal cells3 and the renal outer medullary K+ channel4 in the cortical collecting duct. Wu et al.5 reported important and relevant information in the field of AT2R and K+ excretion through a patch-clamp and renal clearance study published in the current issue of Hypertension. They showed that AT2R activation by the AT2R agonist, CGP42112a, inhibited the activity of the basolateral 40-pS K+ channel (a Kir4.1/Kir5.1 heteromer) in the DCT (Fig.1), and the AT2R antagonist, PD123319, stimulated the activity of this K+ channel. The inhibitory effect of AT2R on the K+ channel activity would be definite if the CGP42112a response was blocked by PD123319 or by an inhibitor of Src homology region 2 domain-containing phosphatase-1 (SHP-1), which is a downstream signaling component of AT2R. Furthermore, ang-II inhibited the K+ channel only when AT1R was blocked,3 although it is surprising that ang-II alone had no effect on the channel activity. Another study6 performed on the thick ascending limb revealed that there was no effect of ang-II on the 50-pS K+ channel activity while AT1R was blocked with losartan. It is likely that a differential region-specific outcome exists for the K+ channel in response to ang-II. In light of the numerous studies showing that AT2R expression and function in the renal/cardiovascular system increases in pathological conditions, it would be relevant to further explore the effect of pharmacological activation of AT2R on the K+ channel in the DCT and other nephron segments in the conditions of obesity, diabetes, and high dietary K+ intake. Increased AT2R expression, by virtue of higher receptor numbers, can not only produce a higher response but can also form more heterodimers with AT1R, thereby, reducing AT1R function.
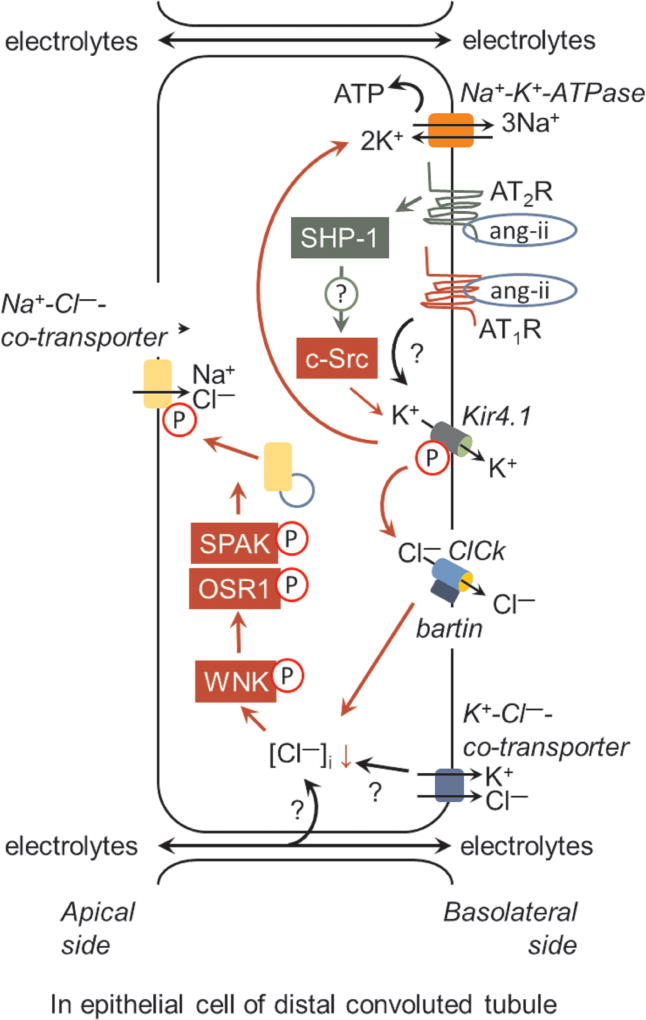
Figure 1.
Role of the angiotensin AT2 receptor (AT2R) in regulation of the apical Na+-Cl− co-transporter (NCC) activity via inhibition of the basolateral 40-pS K+ channel (Kir4.1) activity. The Src family protein kinase, c-Src-, mediated phosphorylation of Kir4.1 is critically required for K+ retrieval. This process facilitates the activity of Na+-K+-ATPase (NKA) for maintaing the cell membrane potential and provides the driving force for Cl− efflux via basolateral Cl− channel (ClCk)-bartin. Cl− efflux results in the decrease of [Cl−]i and hyperpolarization of the basolateral membrane, which is rapidly sensed by with-no-lysine kinase (WNK). This results in the phosphorylation and activation of WNK and phosphorylation of Sterile 20/SPS-1-44-related proline-alanine-rich protein kinase (SPAK)/oxidative stress-related kinase (OSR1). These events are required for vesicular trafficking, phosphorylation, and efficient activity of NCC. However, the following several mechanisms require further elucidation: (a) whether the Src homology region 2 domain-containing phosphatase-1 (SHP-1) is involved in AT2R mediated inhibition of c-Src and decrease in Kir4.1 activity; (b) elucidation of the molecular targets involved in WNK phosphorylation in response to the decrease in [Cl−]i, (c) the role of the K+-Cl−-co-transporter and paracellular transport of anions in the maintenance of the membrane potential and [Cl−]i, and (d) the lack of responsiveness of Kir4.1 to ang-II/AT1R.
Previously, Zhang et al.7 reported that Src family protein tyrosine kinase, c-Src, phosphorylated KCNJ10 (Kir4.1), a key component of the 40-pS K+ channel at Tyr8 and Tyr9 and stimulated the basolateral K+ channel in the DCT. Activation of AT2R induces SHP-1. Both c-Src and SHP-1 can bind to membrane lipids and may act upon complementary substrates. Upon stimulation of AT2R, AT2R associates with c-Src and SHP-1. Moreover, the ang-II/NOX/c-Src axis has been known to induce oxidative stress, and AT2R stimulation by C21 reduces oxidative stress.8 Hence, further studies are needed to delineate whether AT2R-mediated inhibition of Kir4.1 involves interactions between SHP-1 and c-Src.
There seems to be a disconnect between the acute in vitro patch-clamp experiment and the 4-day in vivo renal clearance experiment. In the in vitro patch-clamp experiment, acute application of ang-II, PD123319, or PD123319+ang-II did not affect Kir4.1 activity, and a blockade of AT1R allowed analysis of ang-II-mediated inhibition of the Kir4.1 channel activity. Acute application of PD123319 only reversed ang-II inhibition of Kir4.1 in the presence of losartan. In contrast, the authors were able to observe the in vivo effects of PD123319 infusion in the absence of an AT1R blockade in the renal clearance experiment. They suggested that 4-day long inhibition of AT2R stimulated AT1R, which indirectly increased Kir4.1 activity. This short-term vs. long-term inhibition controversy of the angiotensin receptors in K+ regulation requires detailed investigation.
KS-Kir4.1-KO mice showed salt (Na+, K+) wasting. The KS-Kir4.1-KO mice may exhibit mistrafficking of renal transporters and reduced basolateral infoldings and surface area that may have resulted in reduced availability of Na+ and K+ transporters and their activity. This might have altered the renal response to hydrochlorothiazide (HCTZ), an NCC inhibitor, and PD123319 in KS-Kir4.1-KO mice. Perhaps, due to the same reason, the change in CGP42112a-induced Na+-K+ excretion was less in the KS-Kir4.1-KO mice than that in WT mice. However, the effect of AT2R on Na+-K+ excretion observed in the present study is corroborated by results of studies involving patients with Gitelman/Bartter syndrome showing reduced renal transporter expression and endogenous AT2R stimulation.
Additionally, PD123319 infusion enhanced HCTZ-induced Na+ excretion but decreased K+ excretion only in WT mice, resulting in hyperkalemia. In perspective, this outcome is of significant consideration, as hyperkalemia is an associated condition induced by inhibitors of the renin-angiotensin-aldosterone system, and severe hyperkalemia may lead to respiratory paralysis, cardiac arrest, chronic kidney disease, or even death. However, direct pharmacological activation of AT2R increased excretion of Na+ and K+. From this finding, it may be inferred that selective activation of AT2R would be helpful in maintaining K+ homeostasis in patients with pseudohypoaldosteronism type-II and other genetic syndromes that show high NCC activity and hyperkalemia. The CGP42112a-induced increase in Na+-K+ excretion is also of great importance in patients resistant to loop diuretics. Loop diuretics inhibit Na+-K+-2Cl− cotransporter (NKCC2) and sustain Na+ delivery and reabsorption in the DCT, causing Na+-related hypertrophic changes in the DCT. This dramatically affects the expression of NCC in the DCT and alters the electrolyte balance.9 Hence, it will be interesting to determine the impact of direct AT2R stimulation on the K+ balance in patients receiving diuretics.
There are additional players involved in the renal electrolyte balance. First, even HCTZ inhibited NCC and Cl− reabsorption; passive paracellular electrolyte movement (Fig. 1) may occur due to electrogenic ENaC and NKA, which may impact luminal polarity and tonicity, affecting electrolyte transport. Second, luminal hypotonicity of the DCT relative to the plasma favors electroneutral K+-Cl− cotransporter (KCC) activity, which may affect the electrolyte gradient and, therefore, with-no-lysine kinase (WNK) activity (Fig. 1). Third, the authors suggested that the Kir4.1/Kir5.1 heteromer is the only active K+ channel in the DCT, but there are other types of inwardly rectifying K+ channels present in the basolateral infoldings of the DCT, which may have affected the renal response to HCTZ and PD123319. Therefore, additional studies are required to delineate the precise role of AT2R activation in the K+ balance.
Overall, the findings of Wu et al. fill in gaps regarding the role of AT2R in Na+-K+ excretion and advance our understanding of the role that it plays in renal physiology. Renal Na+-K+ handling is under the fine tuning of distal Na+ delivery, a major determinant of pressure-natriuresis. Regulation of distal Na+ delivery and the final Na+-K+ balance is secondary to the electrolyte balance in the proximal tubule. For instance, in response to salt-sensitive hypertension, proximal Na+-transporters redistribute between the apical and basolateral membrane to regulate Na+ delivery in the distal nephron. AT2R activation inhibits NKA in the proximal tubule, increases the natriuretic effects of inhibitors of NKCC2 and ENaC,2 and limits Na+-mediated inflammatory and hypertensive injury in case of chronic kidney diseases in obesity. The expression of Na+ transporters is increased after intake of a high fat diet and in obesity, which is important in hypertensive kidney injury. Likewise, hyperkalemia is prevalent in patients with chronic kidney disease. This previously undocumented role of ang-II in regulation of NCC via the Kir4.1 channel showing the occurrence of hyperkalemia during a blockade of AT2R suggests activation of natriuretic AT2R as a strategy to mitigate salt-sensitive hypertension and hyperkalemia.
Acknowledgments
Sources of Funding: This study was supported by NIH R01 grant DK61578.
Footnotes
Disclosures: none
References
- 1.Kamel KS, Halperin ML. Potassium Physiology. 2017:359–392. [Google Scholar]
- 2.Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens Res. 2012;35:654–660. doi: 10.1038/hr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao L, Zucker IH. Protective Arm of the Renin Angiotensin System (RAS): Functional Aspects and Therapeutic Implications. Ch. 15. Elsevier Inc.; 2015. pp. 109–118. [Google Scholar]
- 4.Wei Y, Liao Y, Zavilowitz B, Ren J, Liu W, Chan P, Rohatgi R, Estilo G, Jackson EK, Wang WH, Satlin LM. Angiotensin II type 2 receptor regulates ROMK-like K(+) channel activity in the renal cortical collecting duct during high dietary K(+) adaptation. Am J Physiol Renal Physiol. 2014;307:F833–843. doi: 10.1152/ajprenal.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu P, Gao Z-X, Duan X-P, Su X-T, Wang M-X, Lin D-H, Gu R, Wang W-H. AT2R-mediated regulation of Na-Cl cotransporter (NCC) and renal K excretion depends on the K channel, Kir4.1. Hypertension. 2018 doi: 10.1161/HYPERTENSIONAHA.117.10471. published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Luan H, Wu P, Fan L, Wang L, Duan X, Zhang D, Wang WH, Gu R. Angiotensin II stimulates basolateral 50-pS K channels in the thick ascending limb. Am J Physiol Renal Physiol. 2014;306:F509–516. doi: 10.1152/ajprenal.00476.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH. Src family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10 protein. J Biol Chem. 2013;288:26135–26146. doi: 10.1074/jbc.M113.478453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel SN, Ali Q, Hussain T. Angiotensin II type 2-receptor agonist C21 reduces proteinuria and oxidative stress in kidney of high-salt-fed obese Zucker rats. Hypertension. 2016;67:906–915. doi: 10.1161/HYPERTENSIONAHA.115.06881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanya AR, Ellison DH. Distal convoluted tubule. Clin J Am Soc Nephrol. 2014;9:2147–2163. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]