Abstract
Angiotensin II (AngII) type 2 receptor (AT2R) is expressed in the distal nephrons. The aim of the present study is to examine whether AT2R regulates Na-Cl cotransporter (NCC) and Kir4.1 of the distal convoluted tubule (DCT). AngII inhibited the basolateral 40 pS K channel (a Kir4.1/5.1 heterotetramer) in the DCT treated with losartan but not with PD123319. AT2R agonist also inhibits the K channel, indicating that AT2R was involved in tonic regulation of Kir4.1. The infusion of PD123319 stimulated the expression of total NCC (tNCC) and phosphorylated NCC (pNCC) (Thr53) by a time-dependent way with the peak at 4 days. PD123319 treatment (4 days) stimulated the basolateral 40 pS K channel activity, augmented the basolateral K conductance and increased the negativity of DCT membrane. The stimulation of Kir4.1 was essential for PD123319-induced increase in NCC since inhibiting AT2R increased the expression of tNCC and pNCC only in WT but not in the kidney-specific Kir4.1 knockout (KS-Kir4.1 KO) mice. Renal clearance study showed that thiazide-induced natriuretic effect was larger in PD123319 treated mice for 4 days than untreated mice. However, this effect was absent in KS-Kir4.1 KO mice which were also Na wasting under basal conditions. Finally, application of AT2R antagonist decreased the renal ability of K excretion and caused hyperkalemia in WT but not in KS-Kir4.1 KO mice. We conclude that AT2R dependent regulation of NCC requires Kir4.1 in the DCT and that AT2R plays a role in stimulating K excretion by inhibiting Kir4.1 and NCC.
Keywords: Angiotensin II, Na transport, hypertension, hyperkalemia and hypokalemia
Introduction
It is well established that two types of angiotensin II (AngII) receptors, AT1R and AT2R, are expressed in the kidney and involved in the regulation of a variety of epithelial transport processes (1–5). While the role of AT1R in regulating membrane transport in the kidney is extensively investigated (3–6), the role of AT2R in the regulation of Na and K transport is less well known. The deletion of AT2R has been shown to increase blood pressure, augmenting AngII-induced hypertension and causing renal Na retention (7–9). Conversely, the activation of AT2R has been shown to stimulate renal Na excretion and to lower blood pressure (10;11). For instance, stimulation of AT2R has been shown to promote natriuresis, an effect attributed to the inhibition of Na transport in the proximal tubule by NO-dependent pathway (11). Thus, the activation of AT2R is expected to antagonize the stimulatory effect of AT1R on renal Na transport (1). Not only regulating Na transport, AT2R-dependent signaling pathway may also play a role in the regulation of renal K excretion and K homeostasis (12). First, an increase in dietary K intake augmented the expression of AT2R in the renal tissue including cortical collecting duct (CCD). Second, AngII has been shown to stimulate ROMK channel (Kir1.1), a K secretory channel, only in animals on a high K (HK) diet (12). Since the inhibition of AT2R abolished the stimulatory effect of AngII on ROMK, this strongly suggests the possible role of AT2R in mediating renal K excretion in the aldosterone-sensitive distal nephron segments (ASDN) including distal convoluted tubule (DCT) and CCD. Immunostaining shows that AT2R is expressed in proximal tubules and the cortical portion of distal tubules (2). Since the DCT is a main component of the cortical portion of the distal nephrons, it raises the possibility that AT2R may regulate the membrane transport in the DCT. The DCT is responsible not only for the reabsorption of 5% filtered Na load but it also plays a key role in the regulation of renal K transport and K homeostasis since the DCT determines Na and volume delivery to the late ASDN (13;14). Previous studies have demonstrated that both apical NCC and basolateral Kir4.1 K channel play an essential role in mediating the membrane transport in the DCT (15–17). The deletion of Kir4.1 has been shown to inhibit NCC in the DCT and to increase ENaC and plasma aldosterone as compensation (16;18). Thus, the aim of the present study is to explore the role of AT2R in the regulation of NCC and Kir4.1 in the DCT.
Methods
The authors declare that all supporting data and detailed methods including animal preparation, electrophysiology, western blot and renal clearance method are available within the article and its online supplementary file.
Animals
Kcnj10flox/flox (WT) and inducible kidney-specific Kir4.1 knockout (KS-Kir4.1 KO) mice with C57BL/6 background were obtained as described previously (16). To generate KS-Kir4.1 KO mice, mice expressing Pax8-rtTA and tet-on LC-1 transgene were crossed with Kcnj10flox/flox mice (detailed information is provided in supplement). The mice were fed with normal rodent chow diet and had free access to water. For perfusion of PD123319 (4 ¼g/min/kg BW) or vehicles, the mice were implanted subcutaneously with an osmotic mini-pump (Model. 1007D, Alzet) for 1, 4 and 7 days, respectively. The animal use was approved by the independent Institutional Animal Care and Use Committee at New York Medical College.
Chemicals and statistics
Angiotensin II, hydrochlorothiazide (HCTZ), CGP42112A, losartan and PD123319 were purchased from Sigma (St Louis, MO). The NCC antibody was a gift from Robert Hoover at Emory University, whereas antibody of phosphorylated NCC at threonine 53 (Thr53) was kindly provided by David H. Ellison at Oregon Health & Science University. The data are shown as mean ± SEM. We used the paired t test or one-way ANOVA test to determine the statistical significance.
Results
AT2R regulates the basolateral K channel in the DCT
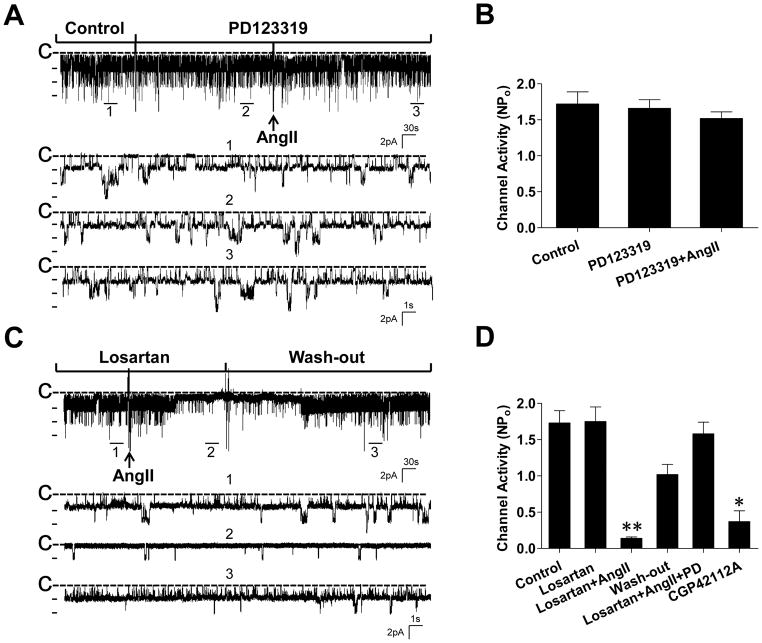
We first performed the patch-clamp experiments to examine the effect of AngII on the basolateral 40 pS K channel (a Kir4.1/5.1 heterotetramer) in the DCT. The results summarized in Figure S1 depict that AngII had no significant effect on the K channel activity (control, 1.59±0.15; AngII, 1.42±0.13). We speculate that the AT1R signaling may antagonize AT2R effect or vise versa since both AT1R and AT2R are expressed in the distal nephrons (2). Thus, we examined the effect of 100 nmol/L AngII on the basolateral K channels in the presence of either PD123319 (an AT2R antagonist) or losartan (an AT1R antagonist), respectively. Figure 1A is a typical channel recording showing that the application of neither PD123319 (10 μmol/L) nor AngII (100 nmol/L)+PD123319 had a significant effect on the 40 pS K channel activity in the DCT. Figure 1B summarizes the results from five experiments showing that the 40 pS K channel activity defined by NPo was 1.72±0.17 (control), 1.66±0.12 (PD123319) and 1.52±0.1 (AngII+PD123319), respectively. The results suggest that acute stimulation of AT1R does not affect the basolateral 40 pS K channel in the DCT. We next examined the effect of AngII on the basolateral 40 pS K channel in the DCT treated with losartan. Figure 1C is a recording demonstrating the effect of AngII on the basolateral 40 pS K channel. Application of losartan (10 μmol/L) alone had no significant effect on the 40 pS K channel since NPo with losartan treatment (1.75±0.2) was similar to the control value (1.73±0.17) (Figure 1D). However, in the presence of 10 μmol/L losartan, AngII significantly inhibited the 40 pS K channel and the inhibitory effect of AngII on the 40 pS K channel was reversible since the washout largely restored the channel activity. Also, adding AT2R antagonist abolished the inhibitory effect of AngII+losartan (NPo =1.58±0.16) (Figure 1D). Results are summarized in Figure 1D demonstrating that AngII decreased channel activity to 0.14±0.02 in the DCT treated with losartan (n=6). The notion that AT2R rather than AT1R is involved in tonic regulation of the basolateral K channel activity was also supported by the finding that application of CGP42112A (1 μmol/L), an AT2R agonist (19), inhibited the basolateral K channel in the DCT and decreased NPo to 0.37±0.15 (Figure 1D).
Figure 1. Stimulation of AT2R inhibits the basolateral Kir4.1/5.1 (40 pS) K channels in the DCT.
(A) A recording shows the effect of 100 nmol/L angiotensin II (AngII) on the 40 pS K channel in the DCT treated with PD123319 (10 μmol/L). Three parts of the trace indicated by numbers are extended to show a fast time resolution. (B) A bar graph summarizes the results from 5 similar experiments. (C) A recording shows the effect of 100 nmol/L AngII on the 40 pS K channel in the DCT treated with losartan (10 μmol/L). Three parts of the trace indicated by numbers are extended to show a fast time resolution. (D) A bar graph summarizes the results from 6 similar experiments in which the effect of losartan, AngII+losartan, PD123319+losartan+AngII and CGP42112A (1 μmol/L) on the basolateral 40 pS K channel in the DCT was examined. The experiments were performed in cell-attached patches at 0 mV and the channel closed line is indicated by “C”. “**” indicates a significant difference in comparison to other groups (P<0.01).
AT2R regulates NCC
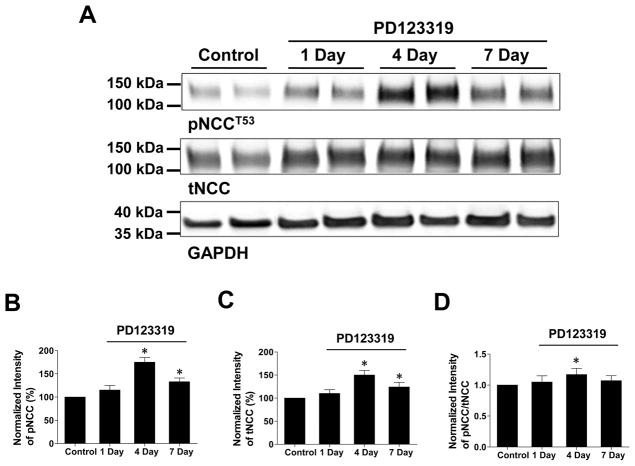
The basolateral K channels in the DCT have been shown to play a key role in the regulation of thiazide-sensitive NCC by modulating the Cl-sensitive WNK pathway (16;20). Having demonstrated that AngII inhibited the basolateral Kir4.1/5.1 by AT2R, we next examined whether inhibiting AngII-AT2R pathway in vivo should affect NCC activity. Thus, we examined the expression of tNCC and pNCC in the mice which were continuously infused with PD123319 (4 μg/min/kg BW) (a similar dose has been used to inhibit AT2R in vivo) through an osmotic mini-pump for 1, 4 and 7 days, respectively (21). Infusion of PD123319 did not affect the food and water intakes in comparison to the control mice (vehicle) (Table S1) but it affected the expression of tNCC and pNCC (Figure 2). From the inspection of Figure 2A, it is apparent that the expression of both tNCC (150±10% of the control) and pNCC (175±10% of the control) was significantly increased in the mice treated with PD123319 infusion for 4 days (Figure 2B & C). Although both tNCC (125±10% of the control) and pNCC (135±8% of the control) were slightly decreased in the mice treated with 7-day PD123319 treatment in comparison to 4 days, they were still significantly higher than that of the untreated control (Figure 2B & C). Figure 2D also shows that pNCC expression is slightly but significantly larger than tNCC in the mice treated with PD123319 for 4 days when pNCC value is normalized with tNCC (pNCC/tNCC ratios were 1.05±0.1, 1.17±0.1 and 1.07±0.1 for 1 day, 4 days and 7 days PD123319, respectively). Moreover, the effect of PD123319 on NCC expression was specific since the inhibition of AT2R had no significant effect on the expression of NKCC2 and ENaC-α subunit (Figure S2). Thus, AT2R inhibition specifically stimulates NCC activity in the DCT.
Figure 2. Inhibition of AT2R increases the abundance of pNCC and tNCC.
(A) Western blots show the abundance of phosphor-NCC (pNCCT53) and total NCC (tNCC) in mice treated with vehicle (control) and PD123319 (1 day, 4 days and 7 days), respectively. A bar graph summarizes the normalized (using GAPDH as a loading reference) band intensity of above results for pNCCT53 (B) and tNCC (C), respectively. (D) The normalized ratio between pNCC and tNCC in the mice treated with PD123319 for 1, 4 and 7 days, respectively. “*” indicates a significant difference in comparison to the control (P<0.05). PD123319 (4 μg/min/kg BW) was applied through an osmotic mini-pump.
Inhibition of AT2R increases Kir4.1 in the DCT
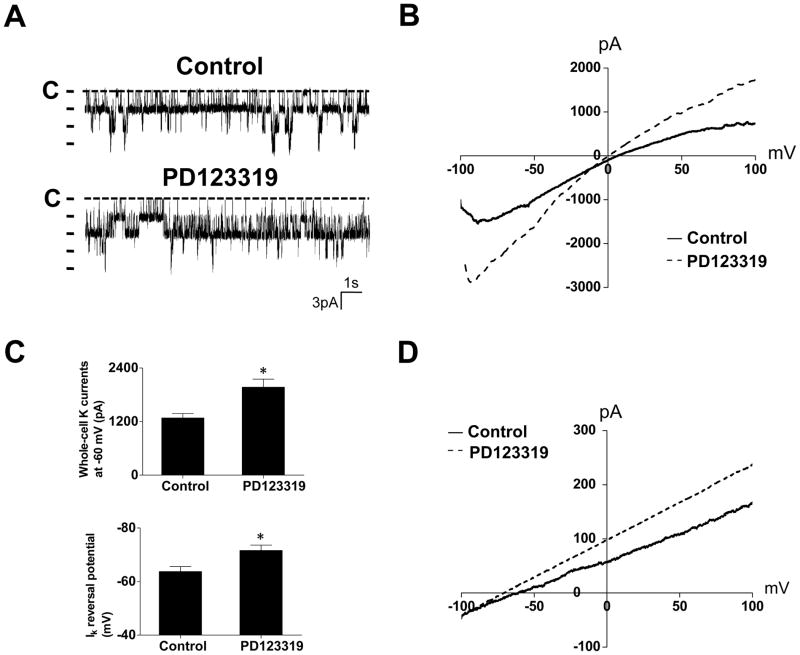
Previous studies have shown that a high basolateral K conductance was linked to a high NCC activity (16). Thus, we tested whether PD123319-induced stimulation of NCC was due to the augmentation of the basolateral K conductance in the DCT by examining the basolateral K channel activity in the mice treated with PD123319 for 4 days since it had the largest effect on NCC (Figure 2). From the inspection of Figure 3A, it is apparent that the 40 pS K channel activity in the DCT of the mice treated with PD123319 for 4 days was significantly higher (2.16±0.2) than that of untreated control animals (1.53±0.12) (Table S2). Further analysis shows that PD123319-induced increase in the 40 pS K channel activity was the result of increasing channel open probability (control, 0.47±0.04; PD123319, 0.59±0.05) and the number of functional K channels in the membrane (Table S2). We found the 40 pS K channel in 44 patches from a total of 61 experiments (72%) in mice treated with AT2R antagonist whereas the probability of finding the 40 pS K channel was only 51% (43 patches with K channel from total 84 experiments) in the untreated group.
Figure 3. Inhibition of AT2R increases the Kir4.1/5.1 activity in the DCT.
(A) Representative recordings show the activity of the basolateral 40 pS K channel in the DCT from mice treated with vehicle (control) and PD123319 for 4 days, respectively. The experiments were performed in cell-attached patches with 140 mmol/L K in the pipette and 140 mmol/L Na+5 mmol/L K in the bath solution. The closed level is indicated by a dotted line and “C”. (B) A whole-cell recording showing Ba (barium)-sensitive K currents in the DCT of the mice treated with vehicle (control) and PD123319 for 4 days, respectively. The K currents were measured with a ramp protocol from -100 to 100 mV using symmetrical 140 mmol/L KCl solution in the bath and pipette. (C) A bar graph (top panel) summarizes the results of experiments in which Ba-sensitive K currents of the DCT were measured at -60 mV from the PD123319-treated and untreated (control) mice (n=6). (D) K-current (IK) reversal potential of the DCT measured with perforated whole-cell recording in the mice treated with vehicle (control) or PD123319 for 4 days. The bath solution contains (in mmol/L) 140 NaCl and 5 KCl while the pipette solution has 140 KCl. A bar graph (low panel of Figure 3C) summarizes the results of above experiments (n=6). “*” means that the difference is significant in comparison to the control value (P<0.05). PD123319 (4 μg/min/kg BW) was applied through an osmotic mini-pump.
The possibility that the inhibition of AT2R for 4 days stimulated the basolateral K channels in the DCT was further suggested by measuring Ba (barium)-sensitive whole-cell K currents in the DCT of the mice treated with PD123319. Figure 3B is a recording showing the currents of DCT cells clamped from -100 mV to 100 mV with a ramp protocol from untreated control and PD123319-treated mice. As summarized in Figure 3C, the mean whole-cell K current at -60 mV was -1280±100 pA (n=6) in control (vehicle) mice and it was -1970±180 pA (n=6) in PD123319-treated mice. Because the basolateral K channels participate in generating the membrane potential of DCT cells, a stimulation of the basolateral K channels is expected to hyperpolarize DCT membrane. Thus, we measured the K-current (IK) reversal potential, which serves as an index of DCT membrane potential. Figure 3D is a perforated whole-cell recording showing current/voltage curve of the DCT with 140 mmol/L K in the pipette (intracellular solution) and 140 mmol/L Na+5 mmol/L K in the bath from the control and PD123319-treated mice. The results from seven experiments are summarized in Figure 3C (lower panel) showing IK reversal potential of the DCT in the control mice (-63.7±1.9 mV) and in PD123319-treated mice (-72±2 mV). Thus, patch-clamp experiments confirm that the inhibition of AT2R for 4 days increases the basolateral K channel activity in the DCT and causes hyperpolarization of the membrane.
Kir4.1 is required for inhibiting AT2R-induced stimulation of NCC
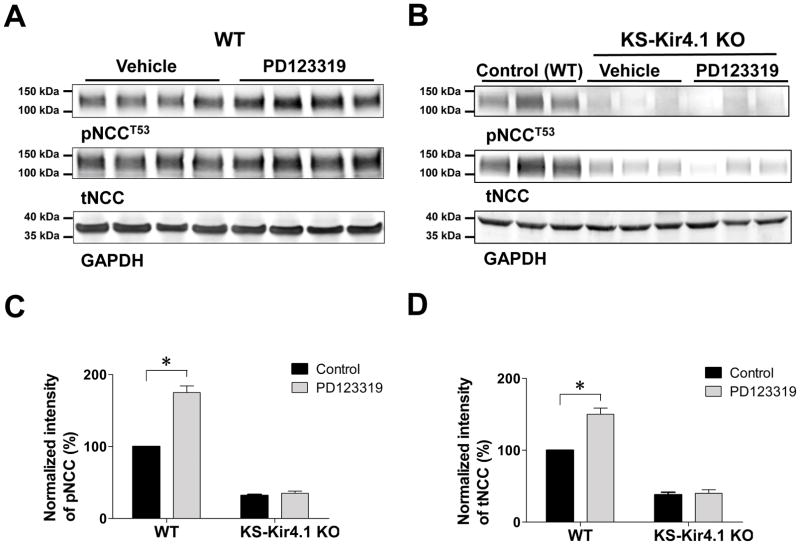
If the activation of the basolateral Kir4.1/5.1 channels in the DCT is directly responsible for PD123319 perfusion-induced stimulation of NCC activity, we expect that PD123319 treatment should have no effect on NCC in the absence of Kir4.1. Thus, we examined the effect of PD123319 infusion (4 days) on NCC expression in both WT and in KS-Kir4.1 KO mice. Figure 4 is a western blot showing the effect of PD123319 (4 days) on tNCC and pNCC in WT (Figure 4A) and in KS-Kir4.1 KO mice (Figure 4B). The normalized band intensity for pNCC and tNCC is summarized in Figures 4C and 4D, respectively. We confirm that PD123319 infusion for 4 days significantly increased the abundance of tNCC (150±10% of the control) and pNCC (175±10% of the control) in WT mice. The reason for paralleled changes in tNCC and pNCC is possibly due to unphosphorylated NCC is not stable (22). However, deletion of Kir4.1 not only decreased both tNCC (38±5% of WT) and pNCC (32±5% of WT) under control conditions (untreated) but also abolished the effect of PD123319 on tNCC (40±5% of WT) and pNCC (35±3% of WT). Also, PD123319 treatment failed to cause hyperpolarization of the DCT membrane in KS-Kir4.1 KO mice (Figure S3). Thus, AT2R inhibition failed to stimulate NCC and to induce hyperpolarization of the DCT in KS-Kir4.1 KO mice.
Figure 4. Inhibition of AT2R increases the abundance of pNCC and tNCC in WT mice but not in KS-Kir4.1 KO mice.
Western blots show the abundance of phosphor-NCC (pNCCT53) and total NCC (tNCC) in WT mice (A) and in KS-Kir4.1 KO mice (B) treated with vehicle (control) and PD123319 for 4 days, respectively. A bar graph summarizes the normalized band intensity of above results for pNCCT53 (C) and tNCC (D), respectively. “*” indicates a significant difference in comparison to the corresponding control (P<0.05). PD123319 (4 μg/min/kg BW) was applied through an osmotic mini-pump.
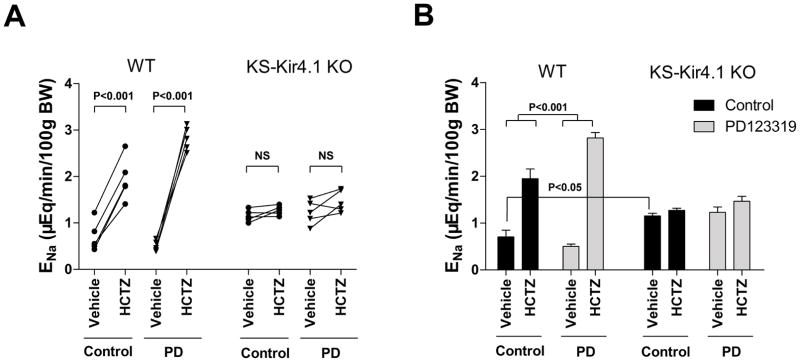
The notion that Kir4.1 is essential for the activation of NCC induced by AT2R inhibition was also supported by renal clearance experiments in which HCTZ-induced natriuretic effect was examined. For the clearance study, the mice were intravenously perfused with isotonic saline for 4 hr (0.3 ml/1 hr) and urine collections started one hr after infusion of 0.3 ml saline. Figure 5A shows results from each individual experiment in which urinary Na excretion (ENa) was measured before and after a single dose of HCTZ (25 mg/kg BW) in WT mice and KS-Kir4.1 KO mice with or without PD123319 treatment. Figure 5B summarizes all above results showing that HCTZ-induced natriuretic effect (from 0.5±0.06 to 2.82±0.14 μEq/min/100g BW) in PD123319-treated mice was significantly larger than those in the untreated group (from 0.7±0.14 to 1.95±0.21 μEq/min/100g BW). This finding is consistent with immunoblotting suggesting that PD123319 treatment for 4 days increases NCC activity. However, deletion of Kir4.1 not only increased basal level of ENa (1.15±0.06 μEq/min/100g BW) and abolished HCTZ-induced natriuretic effect (HCTZ, 1.27±0.05 μEq/min/100g BW), but also eliminated the effect of PD123319 treatment (control, 1.23±0.11; HCTZ, 1.47±0.13 μEq/min/100g BW). This strongly suggests that AT2R inhibition-induced stimulation of NCC function is the result of the activation of basolateral Kir4.1 in the DCT. We also used renal clearance method to examine the acute effect of CGP42112A (AT2R agonist) at 1.5 mg/kg BW on ENa in WT and KS-Kir4.1 KO mice (Figure S4). AT2R agonist stimulated Na excretion (control, 0.61±0.06; CGP42112A, 1.82±0.15 μEq/min/100g BW), the deletion of Kir4.1 attenuated CGP42112A-induced natriuretic effect (control, 1.24±0.11; CGP42112A, 2.03±0.17 μEq/min/100g BW) (Figure S4A) since the net ENa induced by CGP42112A is smaller in KS-Kir4.1 KO mice than WT (WT, 1.21±0.11; KS-Kir4.1 KO, 0.79±0.06 μEq/min/100g BW, Figure S4B).
Figure 5. Inhibition of AT2R upregulates NCC activity in WT mice but not in KS-Kir4.1 KO mice.
(A) A line graph shows the results of each experiment in which urinary sodium excretion (ENa) was measured before and after a single dose of HCTZ (25 mg/kg BW) in WT mice and KS-Kir4.1 KO mice with (triangle) or without (circle) PD123319 infusion for 4 days. (B) A bar graph summarizes all above experiments. HCTZ-induced natriuretic effect was significantly different between control and PD123319 (PD) treated mice. The basal level of ENa of KS-Kir4.1 KO mice is significantly different in comparison to WT mice. PD123319 (4 μg/min/kg BW) was applied through an osmotic mini-pump.
Inhibition of AT2R impairs K homeostasis
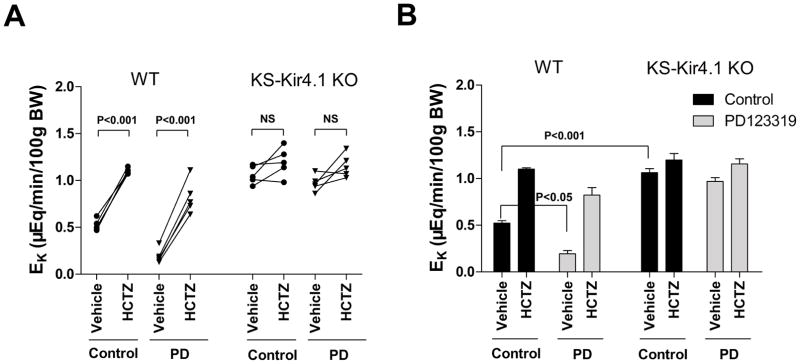
It is well established that NCC plays a key role in the regulation of K homeostasis (15). Thus, we next examined the effect of AT2R antagonist on urinary K excretion (EK) in WT mice and in KS-Kir4.1 KO mice using renal clearance method. Figure 6A shows the results from each individual experiment which are then summarized in Figure 6B demonstrating the mean value and statistical information. The application of HCTZ increased K excretion from 0.52±0.03 to 1.10±0.02 μEq/min/100g BW in untreated WT mice and from 0.19±0.05 to 0.82±0.11 μEq/min/100g BW in PD123319-treated WT mice. Moreover, from the inspection of Figure 6B it is apparent that basal level of EK in PD123319-treated mice was significantly lower than that of the untreated group (0.19±0.05 vs 0.52±0.03 μEq/min/100g BW). The notion that PD123319 treatment for 4 days impairs renal K excretion is also suggested by observations that PD123319 treatment significantly increased the plasma K concentration from 4.1±0.2 to 5.2±0.3 mmol/L (n=8) (Table S3) in WT mice. We also confirm the previous finding that the deletion of Kir4.1 caused K wasting in the mice with or without PD123319 treatment (Figure 6A). The results from 5 experiments are summarized in Figure 6B showing that HCTZ did not significantly alter EK in KS-Kir4.1 KO mice treated with PD123319 (from 0.97±0.03 to 1.16±0.05 μEq/min/100g BW) or without (1.06±0.04 to 1.20±0.07 μEq/min/100g BW). Consequently, KS-Kir4.1 KO mice were hypokalemic under control conditions (2.8±0.1 mmol/L) or during PD123319 treatment for 4 days (2.9±0.2 mmol/L) (Table S3). Thus, PD123319 treatment (4 days) caused hyperkalemia in WT mice and this effect was absent in KS-Kir4.1 KO mice. Also, CGP42112A stimulates renal K secretion (0.57±0.02 to 1.10±0.10 μEq/min/100g BW) and this effect was abolished in KS-Kir4.1 KO mice (1.10±0.1 to 1.27±0.1 μEq/min/100g BW) (Figure S4C), suggesting that Kir4.1 is required for AT2R agonist-induced stimulation of renal K excretion.
Figure 6. Inhibition of AT2R diminishes the renal ability of K excretion.
(A) A line graph shows the results of each experiment in which urinary potassium excretion (EK) was measured before and after a single dose of HCTZ (25 mg/kg BW) in WT mice and KS-Kir4.1 KO mice with (triangle) or without (circle) PD123319 treatment (PD) for 4 days. (B) A bar graph summarizes all above experiments shows the mean value and statistic information. The basal level of EK in PD123319 treated WT mice is significantly different from vehicle groups (control). PD123319 (4 μg/min/kg BW) was applied through an osmotic mini-pump.
Discussion
In the present study, we observed that AngII inhibited the basolateral 40 pS K channel in the DCT treated with losartan, suggesting that AT2R is responsible for mediating the inhibitory effect of AngII on basolateral K channels in the DCT. This notion was also suggested by the observation that the infusion of PD123319 increased the basolateral K conductance in the DCT. The reason that acute application of AT2R antagonist had no effect on K channels is possibly due to the inactivated AT2R without AngII in the isolated DCT. This view is supported by the finding that AT2R agonist inhibits the K channel in the DCT. Although the inhibition of AT2R could indirectly enhance AT1R-dependent pathway, it is unlikely that AT1R was responsible for the upregulation of the basolateral 40 pS K channel activity since AngII had no effect on the 40 pS K channel in the DCT treated with PD123319. However, it is possible that the long-term stimulation of AT1R may be able to indirectly influence the basolateral K channel activity in the DCT. The stimulation of AT1R has been shown to increase Na transport in the thick ascending limb and in distal nephrons (6;23), and to stimulate ENaC in the CCD (5;24;25). Consequently, the stimulation of Na transport not only causes volume expansion but it may also increase renal K excretion in ASDN thereby leading to hypokalemia which could also increase the basolateral K conductance. But, this possibility is largely excluded by the observation that the mice treated with AT2R inhibitor were hyperkalemic rather than hypokalemic. Thus, the results strongly suggest that AT2R rather than AT1R in the DCT is involved in mediating a tonic regulation of the basolateral 40 pS K channel.
The basolateral 40 pS K channel in the DCT is composed of Kir4.1 (encoded by Kcnj10) and Kir5.1 (encoded by Kcnj16) and Kir4.1 is a key component for forming the basolateral K conductance because the deletion of Kcnj10 completely eliminates the basolateral K conductance (16;26;27). Since this 40 pS K channel is the only type of K channel in the basolateral membrane of the DCT (16), it is conceivable that a factor which regulates the basolateral 40 pS K channel activity should have a profound effect on the basolateral K conductance and the membrane potentials. Indeed, we observed that the inhibition of AT2R not only increased the basolateral K conductance but also hyperpolarized the DCT membrane. This suggests that AT2R plays a role in tonic regulation of the membrane potential in the DCT under a variety of physiological conditions.
A large body of evidence has suggested the membrane potential in the DCT plays an important role in the regulation of NCC activity such that an increase in the membrane negativity (hyperpolarization) stimulates, whereas a decrease in membrane negativity (depolarization) inhibits NCC activity (16;20;28;29). Our present study has also demonstrated that AT2R inhibition-induced hyperpolarization is correlated with an increase in NCC activity. The mechanism by which the membrane potential is linked to NCC expression is most likely due to the modulation of Cl-sensitive with-no-lysine kinase (WNK) which is activated by low intracellular Cl and inhibited by high intracellular Cl (20;30;31). It is well established that WNK activity plays a key role in increasing NCC activity by stimulating ste20-proline-and-alanine-rich kinase and oxidative-sensitive responsive kinase (32–36). Two lines of evidence suggest that the activation of Kir4.1 in the DCT is responsible for mediating the effect of AT2R on NCC. First, the AT2R inhibition significantly hyperpolarizes the DCT membrane and stimulates NCC. Second, PD123319 infusion failed to stimulate NCC in KS-Kir4.1 KO mice in which the AT2R inhibition also did not cause hyperpolarization of the DCT. This finding also excludes the possibility that an indirect activation of AT1R, which has been shown to stimulate WNK4 (37), is responsible for PD123319 perfusion-induced stimulation of NCC expression. Thus, our results strongly suggest that the activation of the basolateral Kir4.1 in the DCT induced by AT2R inhibition is responsible for PD123319-induced increase in NCC expression and activity.
Recent developments in the field of renal K excretion have indicated that NCC activity in the DCT plays an important role in the regulation of K excretion and K homeostasis (20;38;39). For instance, an increase in dietary K intake suppresses the NCC activity thereby increasing Na and volume delivery to the late ASDN, whereas a decrease in dietary K intake stimulates the NCC activity thereby decreasing Na and volume delivery to the late ASDN. The role of NCC in regulating renal K secretion and K homeostasis is also convincingly established by human genetic and clinical studies demonstrating that an abnormal NCC activity is responsible for causing hyperkalemia or hypokalemia. For instance, pseudohypoaldosteronism type II (PHAII) or familial hyperkalemic hypertension (FHHt) is caused by high activity of NCC (34;40;41), whereas hypokalemia in patients with Gitelman syndrome is due to the loss-of-function mutations of NCC (42). Indeed, our present study has also demonstrated that AT2R inhibition induced upregulation of NCC diminished renal ability for K excretion such that the mice treated with PD123319 were hyperkalemic. Moreover, we observed that hyperkalemia was more severe in the mice treated with PD123319 for 7 days than in those treated for 4 days (Figure S5). This hyperkalemia may be responsible for a compensatory decrease in NCC expression in the mice treated with PD123319 for 7 days.
Perspectives
The physiological significance of our finding is to illustrate a possible signaling pathway which mediates the effect of HK diet on the basolateral Kir4.1and apical NCC activity. Although it is demonstrated that increased dietary K intake inhibits the basolateral Kir4.1/5.1 K channels (29), the mechanism by which HK intake inhibits the basolateral Kir4.1 is not clear. It is possible that AT2R may be involved in mediating the effect of HK intake on the basolateral K channels in the DCT since HK intake has been shown to upregulate AT2R expression (12). An upregulation of AT2R in the DCT should lead to the inhibition of the basolateral K channel activity thereby depolarizing DCT membrane. A depolarization of the DCT inhibits NCC expression and increases the Na and volume delivery to the late ASDN. Since a HK intake is also expected to stimulate the expression of ROMK and ENaC (43–45), a high ENaC/ROMK activity and a high Na delivery should stimulate K secretion in the late ASDN. In addition, the present study suggests that the stimulation of AT2R in the kidney not only inhibits type III Na-H exchanger and Na-K-ATPase in the proximal tubule but also NCC in the DCT(10). We conclude that AT2R plays a role in regulating NCC activity by modulating the basolateral Kir4.1 activity in the DCT and that AT2R plays a role in mediating renal K excretion and K homeostasis.
Supplementary Material
Novelty and Significance.
1) What is New?
Angiotensin II inhibits the basolateral Kir4.1 in the DCT by AT2R-pathway and the inhibition of AT2R hyperpolarizes DCT membrane.
Inhibition of AT2R causes hyperkalemia and high NCC activity. This effect depends on the presence of Kir4.1 in the DCT because PD123319 did not increase NCC and failed to induce hyperkalemia in KS-Kir4.1 KO mice.
2) What is relevant?
Stimulation of AT2R increases renal Na excretion and is antihypertensive. The finding that the inhibition of AT2R increases NCC activity is highly relevant for understanding the mechanism of AT2R-induced natriuretic effect.
NCC plays a key role in regulating renal K excretion and maintaining K homeostasis while Kir.4.1 plays a key role in controlling NCC activity. The finding that AT2R is involved in the regulation of Kir4.1 and NCC is relevant for understanding an integrated mechanism of regulating K homeostasis.
Summary
AT2R plays a role in regulating NCC activity by modulating the basolateral Kir4.1 activity in the DCT and that AT2R plays a role in mediating renal K excretion and K homeostasis.
Acknowledgments
Source of Funding:
The work is supported by NIH grant DK 54983. Dr. Peng Wu is supported by the National Natural Science Foundation of China # 31400993.
Authors thank Drs. R. Hoover and DH Ellison for the gift of NCC and pNCC antibodies. We also thank Dr. Tong Wang and the O’Brien Kidney Center at Yale University School of Medicine for providing the technique support in performing renal clearance and Ms. Gail Anderson for her assistance in editing.
Footnotes
Conflict(s) of Interest/Disclosure:
None.
References
- 1.Carey RM. Update on angiotensin AT2 receptors. Curr Opin Nephrol Hypertens. 2017;26:91–96. doi: 10.1097/MNH.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyata N, Park F, Li XF, Cowley AW. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. American Journal of Physiology - Renal Physiology. 1999;277(3):F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 3.Oliverio MI, Best CF, Smithies O, Coffman TM. Regulation of Sodium Balance and Blood Pressure by the AT1A Receptor for Angiotensin II. Hypertension. 2000;35(2):550–554. doi: 10.1161/01.hyp.35.2.550. [DOI] [PubMed] [Google Scholar]
- 4.Li XC, Shao Y, Zhuo JL. AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int. 2009;76(2):169–177. doi: 10.1038/ki.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II Directly Stimulates ENaC Activity in the Cortical Collecting Duct via AT1 Receptors. J Am Soc Nephrol. 2002;13(5):1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 6.Silva GB, Garvin JL. Angiotensin II Dependent Hypertension Increases Na Transport-Related Oxygen Consumption by the Thick Ascending Limb. Hypertension. 2008;52(6):1091–1098. doi: 10.1161/HYPERTENSIONAHA.108.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siragy HM, Senbonmatsu T, Ichiki T, Inagami T, Carey RM. Increased renal vasodilator prostanoids prevent hypertension in mice lacking the angiotensin subtype-2 receptor. J Clin Invest. 1999;104(2):181–188. doi: 10.1172/JCI6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proceedings of the National Academy of Sciences. 1999;96(11):6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BLM, lnagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377(6551):748–750. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- 10.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT2 receptor activation induces natriuresis and lower blood preesssure. Circ Res. 2014;115(3):388–399. doi: 10.1161/CIRCRESAHA.115.304110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertension Research. 2012;35:654–660. doi: 10.1038/hr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Y, Liao Y, Zavilowitz B, Ren J, Liu W, Chan P, Rohatgi R, Estilo G, Jackson EK, Wang WH, Satlin LM. Angiotensin II type 2 receptor regulates ROMK-like K+ channel activity in the renal cortical collecting duct during high dietary K+ adaptation. American Journal of Physiology - Renal Physiology. 2014;307(7):F833–F843. doi: 10.1152/ajprenal.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison DH, Valazquez H, Wright FS. Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol (Renal Fluid Electrolyte Physiol ) 1987;253–22:F546–F554. doi: 10.1152/ajprenal.1987.253.3.F546. [DOI] [PubMed] [Google Scholar]
- 14.Obermuller N, Bernstein P, Velazquez H, Reilly R, Moser D, Ellison DH, Bachmann S. Expression of the thiazide-sensitive Na-Cl cotransporter in rat and human kidney. Am J Physiol. 1995;269(6 Pt 2):F900–F910. doi: 10.1152/ajprenal.1995.269.6.F900. [DOI] [PubMed] [Google Scholar]
- 15.Ellison DH, Terker AS, Gamba G. Potassium and Its Discontents: New Insight, New Treatments. J Am Soc Nephrol. 2015;27:981–989. doi: 10.1681/ASN.2015070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuevas CA, Su XT, Wang MX, Terker AS, Lin DH, McCormick JA, Yang C-L, Ellison DH, Wang WH. Potassium sensing by renal distal tubules requires Kir4. 1. J Am Soc Nephrol. 2017;28:1814–1825. doi: 10.1681/ASN.2016090935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yong-Feng Y, David LL, Xiaoman Z, Chao-Ling Y, Ellison DH. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest. 2009;119(9):2601–2612. doi: 10.1172/JCI38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su XT, Zhang C, Wang L, Gu R, Lin DH, Wang WH. Disruption of KCNJ10 (Kir4. 1) stimulates the expression of ENaC in the collecting duct. American Journal of Physiology - Renal Physiology. 2016;310(10):F985–F993. doi: 10.1152/ajprenal.00584.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipeanu CM, Henning RH, De Zeeuw D, Nelemans A. Intracellular Angiotensin II and cell growth of vascular smooth muscle cells. Br J Pharmacol. 2001;132(7):1590–1596. doi: 10.1038/sj.bjp.0703984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terker A-S, Zhang C, McCormick J-A, Lazelle R-A, Zhang C, Meermeier N-P, Siler D-A, Park H-J, Fu Y, Cohen D-M, Weinstein A-M, Wang WH, Yang CL, Ellison D-H. Potassium Modulates Electrolyte Balance and Blood Pressure through Effects on Distal Cell Voltage and Chloride. Cell Metabolism. 2015;21(1):39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Te Chao F, Wann-Chu H. Angiotensin receptor blockade blunts hyperinsulinemia-induced hypertension in rats. Hypertension. 1998;32:235–242. doi: 10.1161/01.hyp.32.2.235. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaek LL, Kortenoeven MLA, Aroankins TS, Fenton RA. Phosphorylation Decreases Ubiquitylation of the Thiazide-sensitive Cotransporter NCC and Subsequent Clathrin-mediated Endocytosis. J Biol Chem. 2014;289(19):13347–13361. doi: 10.1074/jbc.M113.543710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao D, Seth DM, Navar LG. Enhanced Distal Nephron Sodium Reabsorption in Chronic Angiotensin II-Infused Mice. Hypertension. 2009;54(1):120–126. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-Term Regulation of ENaC Expression in Kidney by Angiotensin II. Hypertension. 2003;41(5):1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 25.Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. American Journal of Physiology - Renal Physiology. 2012;302(6):F679–F687. doi: 10.1152/ajprenal.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5. 1 heteromeric channels. J Physiol. 2002;538(Pt 2):391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15(12):2980–2987. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Wang L, Zhang J, Su X-T, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1) Proc Natl Acad Sci USA. 2014;111:11864–11869. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang MX, Cuevas-Gallardo C, Su XT, Wu P, Gao Z-X, Lin DH, McCormick JA, Yang CL, Wang WH, Ellison DH. Potassium (K+) intake modulates NCC activity via the K+ channel. Kir4. 1. Kid Int. 2017;00:00. doi: 10.1016/j.kint.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.piala AT, Moon TM, Akella R, He HX, Cobb MH, Goldsmith EJ. Chloride sensing by WNK1 involves inhibition of autophosphosphorylation. Sciencesignaling. 2014;7(324):ra41. doi: 10.1126/scisignal.2005050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, Gonzalez-Rodriguez X, Vazquez N, Rodriguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, Garcia-Valdes J, Hadchouel J, Gamba G. The Effect of WNK4 on the Na+Cl Cotransporter Is Modulated by Intracellular Chloride. J Am Soc Nephrol. 2014;26:1781–1786. doi: 10.1681/ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang C-L, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metabolism. 2011;14:352–364. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-Knockout Mice Manifest Gitelman Syndrome and Impaired Vasoconstriction. Journal of the American Society of nephrology. 2010;21(11):1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38(10):1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 35.Piechotta K, Lu J, Delpire E. Cation Chloride Cotransporters Interact with the Stress-related Kinases Ste20-related Proline-Alanine-rich Kinase (SPAK) and Oxidative Stress Response 1 (OSR1) J Biol Chem. 2002;277(52):50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL. Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models. Human Molecular Genetics. 2011;20(5):855–866. doi: 10.1093/hmg/ddq525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata S, Arroyo JP, Castañeda-Bueno Ma, Puthumana J, Zhang J, Uchida S, Stone KL, Lam TT, Lifton RP. Angiotensin II signaling via protein kinase C phosphorylates Kelch-like 3, preventing WNK4 degradation. Proceedings of the National Academy of Sciences. 2014;111(43):15556–15561. doi: 10.1073/pnas.1418342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AHJ, Fenton RA, Zietse R, Hoorn EJ. K+ -induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+ -Cl− cotransporter. American Journal of Physiology - Renal Physiology. 2013;305(8):F1177–F1188. doi: 10.1152/ajprenal.00201.2013. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83(5):811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 40.Take C, Ikeda K, Kurasawa T, Kurokawa K. Increased Chloride Reabsorption as an Inherited Renal Tubular Defect in Familial Type II Pseudohypoaldosteronism. N Engl J Med. 1991;324(7):472–476. doi: 10.1056/NEJM199102143240707. [DOI] [PubMed] [Google Scholar]
- 41.Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4. Proceedings of the National Academy of Sciences. 2013;110(19):7838–7843. doi: 10.1073/pnas.1304592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nature Genetics. 1996;12(1):24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 43.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol. 1999;277(5 Pt 2):F805–F812. doi: 10.1152/ajprenal.1999.277.5.F805. [DOI] [PubMed] [Google Scholar]
- 44.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol. 1994;104(4):693–710. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Hebert SC, Giebisch G. Renal K+ channels: structure and function. Ann Rev Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.