Abstract
Purpose and Method
This study examined functional connectivity of the default mode network (DMN) and examined brain–behavior relationships in a pilot cohort of children with chronic mild to moderate traumatic brain injury (TBI).
Results
Compared to uninjured peers, children with TBI demonstrated less anti-correlated functional connectivity between DMN and right Brodmann Area 40 (BA 40). In children with TBI, more anomalous less anti-correlated) connectivity between DMN and right BA 40 was linked to poorer performance on response inhibition tasks.
Conclusion
Collectively, these preliminary findings suggest that functional connectivity between DMN and BA 40 may relate to longterm functional outcomes in chronic pediatric TBI.
Keywords: Functional neuroimaging, mild traumatic brain injury, moderate traumatic brain injury, neural networks, response inhibition
Introduction
While many children who sustain severe traumatic brain injuries (TBI) experience long-lasting and pronounced deficits over in both cognitive and motor domains,1 children who sustain less severe TBIs often experience more subtle deficits, some of which appear to resolve with time.2 In particular, after mild to moderate TBI, motor deficits, which exist acutely, tend to resolve over time. However, the cognitive consequences of mild and moderate pediatric TBI may persist over a prolonged timeline, potentially affecting academic performance.3,4
With regard to motor sequelae, researchers observed that children with moderate TBI had acute deficits in fine and gross motor skills, but those same children performed within normal limits one year post-injury.4 In mild pediatric TBI, postural instability has been noted one month after injury,5 deficits in visual-motor response time and dynamic balance have been observed up to 12 weeks post-injury, 6,7 and dynamic balance issues and tremors have been reported for up to six months post-injury.8 Additionally, in a cohort of children with mild to moderate TBI, even subtle motor deficits that were present two months post-injury had largely resolved by one year post-injury.9
Conversely, with regard to cognitive function, researchers report longer-lasting deficits after mild–moderate TBI. Children with moderate TBI have been found to have working memory deficits at least one year post-injury,10 and children with mild–moderate TBI have been found to have theory of mind impairments one–five years post-injury.11 Attention can also be affected; in one study, at one year post-injury, parents reported new moderate to severe attention difficulties for 5% of children with mild TBI and 15% of children with moderate TBI.12 Children with TBI also have been observed to have lasting difficulties with response inhibition and inhibitory control.13–15 As these difficulties often negatively impact academic performance, the process of returning to school post-injury is a focus of pediatric TBI literature (see review:16).
Given the functional impairments resulting from milder forms of TBI, identifying the brain basis of performance deficits is useful for prognosis, management, and treatment of TBI, as it can guide therapeutic interventions and be used to evaluate change over time or as a result of treatment. Identifying the brain basis can be accomplished, in part, via functional neuroimaging. While in milder forms of TBI, traditional clinical neuroimaging tools (e.g., CT or conventional MRI) may fail to detect neural abnormalities, functional MRI (fMRI), which assesses changes in neural activity across time, is more sensitive to functional changes in brain activity due to TBI.17 Resting state fMRI (rs-fMRI) is completed, while a person is at rest and allows evaluation of functional connectivity of brain regions and networks.
With regard to functional connectivity, in the adult TBI rs-fMRI literature, much focus is allocated to the default mode network (DMN). The DMN, or task-negative network, is a well-established network of interacting brain regions that are most active when participants are not engaged in attention-demanding tasks.18 As a task-negative network, the DMN is optimally anti-correlated with task-positive regions of the brain.19 Thus, the DMN has been an important focus in TBI literature because it is a reliable and robust network, and DMN inhibition is integral to cognitive processes which are often disrupted after TBI.20
One approach in TBI research is to test for disruptions within the DMN. Deficits in functional connectivity within the DMN are linked to functional outcomes across the spectrum of TBI severity. In symptomatic adults two months post-mTBI, decreased posterior DMN connectivity was associated with cognitive dysfunction, whereas increased anterior DMN connectivity was associated with fewer psychological symptoms of anxiety and depression.21 In adults in chronic stages of mixed severity TBI (on average two years post-injury), hyper-connectivity within the DMN has been associated with deficits in sustained attention.22 Collectively, these studies suggested that altered connectivity within the DMN can be either compensatory or debilitating.
Researchers have also examined potential disruptions in functional connectivity between the DMN and other regions of the brain after TBI. Acute and sub-acute (i.e., four months post-injury) increases in functional connectivity between the DMN and frontal regions have been observed in adults with TBI.23,24 Similar to the consequences associated with changes within the DMN, increased connectivity between the DMN and other brain regions relates to behavioral outcomes. For example, in a population of adults one month post-mild TBI, increased connectivity between the DMN and a task positive network—comprised of bilateral dorsolateral prefrontal cortices and posterior parietal cortices and engaged during attention demanding tasks—was associated with memory deficits.25
Although the DMN is known to be well established in children,26 little literature exists specific to functional connectivity of the DMN in children with TBI. In previous work, whole-brain connectivity of the DMN was examined in children with mild to moderate TBI in the sub-acute (i.e., two months post-injury) stage of TBI recovery.27 In comparison with controls, children with TBI demonstrated a significant increase in connectivity between the DMN and the right dorsal premotor cortex. This anomalous connectivity was associated with motor performance; children in the subacute phase of TBI who had increased (more anomalous) connectivity between the DMN and premotor regions demonstrated poorer conscious and subconscious motor control.27 However, the persistence of this altered connectivity and long-term functional implications following pediatric TBI remains unknown.
Current objectives
The goal of this study was to examine whole-brain functional connectivity of the DMN and related brain–behavior associations in a pilot cohort of children in the chronic phase (one year) of TBI. In order to avoid potential circular analyses in evaluation of brain–behavior relationships 28,29 and to increase reproducibility of preliminary findings, we first used a voxel-based approach for identifying regions of between-groups differences in connectivity and then performed brain–behavior analyses using a region of interest (ROI) defined by Brodmann’s Areas (BA). We hypothesized that children with TBI would demonstrate significant differences from uninjured children in the whole-brain functional connectivity of the DMN. We secondarily hypothesized that children with TBI would show brain–behavior associations between functional connectivity and performance on tasks relevant to the localization of anomalous connectivity.
Method
Seventeen children aged 10–17 years at the time of mild–moderate TBI were recruited from outpatient pediatric brain injury rehabilitation clinics and enrolled in a longitudinal study of inhibitory control and processing speed after TBI. Participants were evaluated two months and twelve months post-injury. Participants were included if they sustained a distinct event of trauma resulting in mild complicated to moderate TBI characterized by at least one of the following: loss of consciousness lasting more than 15 minutes, post-traumatic amnesia (PTA) lasting at least one hour, or the presence of injury-related intracranial findings on clinical imaging. Exclusion criteria included overt motor impairments that limited participants’ ability to complete the assessment battery or, if at two months post-injury, PTA had not resolved. Glasgow Coma Scale (GCS) scores from the day of injury were infrequently available; therefore, severity of TBI was defined using the American Congress of Rehabilitation Medicine (ACRM) criteria based on duration of PTA (<24 hours = mild, 24 hours – 7 days = moderate, >7 days = severe) and the presence of injury-related intracranial findings on clinical computed tomography (CT) findings (no findings = mild TBI, findings = moderate or severe TBI) unless a GCS score was available and indicated a more severe injury.30 In determining severity, there is a bias toward greater severity; in other words, if one category indicates moderate TBI, but another category indicates severe TBI, the injury is classified as severe. The Johns Hopkins Medicine IRB approved this study, written informed consent was obtained from a parent or legal guardian, and assent was acquired from child participants.
Participants
Eleven of the seventeen total participants with TBI from the longitudinal study are included in the current analyses. Three other participants from the longitudinal study did not complete neuroimaging at either visit; two had dental hardware and one refused. Three participants who had neuroimaging data from two months post-injury were not included in the current data set: One participant did not return for any 12-month testing, and two participants did not have usable imaging data (one due to operator error and one due to participant motion). In the included 11 participants, both neuroimaging and behavioral data were collected at approximately 12 months post-injury (Mean (M) = 381 days; Range = 364 – 403 days). Of these 11 children, one child had a pre-morbid diagnosis of ADHD and a prior concussion, another child had pre-injury “executive functioning (EF) deficits” without a diagnosis of ADHD, and one child had sustained two prior concussions.
Comparison neuroimaging data from a single time point of testing were obtained for a control group comprised of 11 age-, sex-, and socioeconomic status (SES)—matched typically developing, uninjured children. SES status was determined for both groups using the Hollingshead Four-Factor Index of Social Status.31 Neurotypical controls were recruited with community advertisements (e.g., flyers, on-hold messages) and by word-of mouth, and none of these children participated as controls in prior evaluation of the imaging data from the TBI cohort two months post-injury.27 Exclusion criteria for controls included history of TBI, behavioral, or psychiatric concerns. One child had previously received support services for reading and math without need for ongoing support. No behavioral data were acquired from the control participants.
Procedure
Neuroimaging data were acquired (see image acquisition paragraph) from control participants during a single visit. Neuroimaging and behavioral data (see behavioral measures paragraphs) were acquired from TBI participants during a single visit approximately 12 months post-injury.
Image acquisition
Neuroimaging was completed on a 3T Philips scanner. T1, T2, and FLAIR images were used for clinical interpretation by a pediatric neuroradiologist. MPRAGE, a high resolution anatomical scan (8-channel head coil, TR = 7.99 ms, TE = 3.76 ms, Flip angle = 8°), was obtained for image co-registration, segmentation, and normalization processing. The duration of the resting state fMRI scan was 6 minutes 30 seconds (D-SENSE EPI, 8-channel head coil, TR = 2500 ms, TE = 30 ms, TI = 843.25 ms, Flip angle = 70°); participants were instructed to fixate on a centrally located fixation cross during scan acquisition.
Resting state fMRI processing and DMN connectivity maps
Data processing
Statistical Parametric Mapping (SPM12b) was used to complete standard image data preparation, processing, and analysis. Pre-processing of functional images, including reorientation, slice time correction, motion correction, co-registration, segmentation, and normalization, was performed using SPM12b and Matlab scripts. Using the aCompCor method,32 nuisance variables were estimated from the white matter and ventricles. Nuisance variables along with linearly detrended versions of the six motion parameters and their first derivatives estimated through backward differences were subsequently regressed from each voxel. Functional images were spatially smoothed using a 6-mm FWHM filter and then temporally filtered (bandpass 0.01–0.1 Hz). Image quality and alignment were confirmed; as reported above, one participant was excluded for excessive motion (> 3 mm of head displacement between frames); no participants were otherwise excluded for motion or other artifacts.
DMN connectivity maps
Intrinsic connectivity was evaluated for the DMN by examining whole-brain connectivity developed from the mean time course for each of the three network seeds; these seed maps were then averaged to develop a DMN map for each subject. About 6-mm radius 3D seeds were centered at locations identified in previous rs-fMRI studies.33,34 The three DMN seeds included the medial prefrontal cortex (MPFC, Talairach coordinates: −1, 47, −4), posterior cingulate cortex (PCC: −5, −49, 40), and the lateral parietal cortex (LP: −45, −67, 36). Seed coordinates were converted from Talairach to MNI space using the Lancaster transformation.35
Resting state fMRI connectivity analyses
Voxel based analyses
SPM12 second-level analyses were used to examine between-group differences in DMN connectivity maps. Each subject’s DMN map was entered into 2-sample t-tests to evaluate for differences between the TBI and control groups in DMN to whole brain connectivity. The voxel-level threshold was established at p < 0.01, and family-wise error (FWE) correction was used for multiple comparisons at a cluster-level threshold of p < 0.05 in accordance with random-field theory.36 Connectivity values for activated voxels were extracted using the MarsBar toolbox in SPM12.
Region of interest-based analyses
To avoid circular analyses in brain–behavior analyses,28,29 region of interests (ROI) were also examined in each group. The MNI coordinates of the peak activated voxel where cluster-level between-group differences were observed were entered into the WFU Pickatlas toolbox. 37,38 WFU Pickatlas was used to determine the region of origin of the peak activated voxel using the Brodmann’s Area (BA) atlas and to save out the BA Region of Interest (ROI) mask. This ROI mask includes both grey and white matter tissues; as a control experiment, we also created a mask that only included grey matter tissue. No discernible differences were observed in connectivity values using either mask, so we elected to use the standard grey and white matter ROI mask from WFU Pickatlas. The MarsBar toolbox was then used to convert this ROI mask to NifTI format and extract raw connectivity values from each voxel within the ROI; these raw connectivity values were averaged across the ROI to establish the connectivity between the DMN and ROI for each subject.
Behavioral measures
The longitudinal study from which these data were acquired was designed to examine inhibitory control and processing speed after pediatric TBI. Among the behavioral measures included in the larger study, the ones below were selected for use in the current brain–behavior analyses based on localization of between-group imaging findings. Data were available for all 11 participants with TBI.
Simple Go/No-Go
This task measures simple cognitive inhibitory control.39 Participants are instructed to make a button press response when a target is present and inhibit that response when a no-go-target item is present. This task has minimal cognitive demand as “go” targets are green and “no-go” targets are red. Commission rate, measuring the frequency of inhibition failures, was used as the primary outcome measure. Reaction time was used a secondary measure of motor speed.
Physical and Neurological Examination of Subtle Signs
The Physical and Neurological Examination of Subtle Signs (PANESS40) measures subtle signs of motor impairment during balance, gait, and timed basic motor functions. Poorer performance is represented by higher values. The PANESS has been shown to have good retest reliability.41 PANESS Total Overflow is a measure of subconscious motor disinhibition during balance, gait, and timed repetitive movements and was the primary outcome measure. Total Standard Deviation from Average (SFA) is an indicator of how a participant’s motor speed compares to age-based performance norms and was used as a secondary outcome measure.
Delis–Kaplan Executive Function System trail making test (D-KEFS; Delis, D.C., Kaplan, E., & Kramer, J.H. 2001)
In the Number/Letter Switching Condition of the DKEFS Trail Making task, participants are instructed to draw a line in sequential order, switching between numbers and letters. This is used to assess simple cognitive inhibitory control. Time to complete is recorded, and age-normed scaled scores were used in analysis.
Conflicting and contralateral motor tasks
These tasks assess conscious integrated cognitive and motor inhibition.42 In the Conflicting Motor Task, participants make a fist when the examiner lifts an index finger or lift their index finger when the examiner makes a fist. In the Contralateral Motor Task, an examiner taps one of the participants’ hands, and participants, whose eyes are closed, must lift the contralateral hand. Correct trials are counted, and the highest (i.e., best) possible score on both tasks is 50. Total scores on both tasks were included and analyzed separately.
Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000)
The BRIEF is a parent report measure of children and adolescents’ real world executive control, including inhibition. The BRIEF has two subscores: 1) Behavioral Regulation, which has three subscales: inhibit, shift, and emotional control and 2) Metacognition Index—initiate, working memory, plan/organize, organization of materials, and monitor. These two subscores comprise the Global Executive Composite (GEC) score. Higher GEC scores represent more difficulty with real world executive control, and the age-normed T-score was used in analysis.
Statistical analyses
SPSS was used for all analyses. P values <0.05 were considered significant; values between 0.05 and 0.10 were labeled as trend findings. Hypothesis 1: Between-group comparisons of ROI connectivity values were completed using independent sample Mann–Whitney U tests. Hypothesis 2: First, age of participants was examined to determine if it contributed to ROI connectivity or behavioral findings. Based on age–behavior findings presented below, partial correlations controlling for age were used to test for relationships between DMN and ROI connectivity values and behavioral performance in the TBI cohort. Due to the number of behavioral measures used in the study, Bonferroni corrections for multiple comparisons were used in examining brain–behavior relationships. Post hoc analyses: Within the TBI cohort, independent sample Mann–Whitney U tests were used to evaluate if severity was influencing connectivity values or behavioral performance. Additionally, all aforementioned analyses were run with and without the children from each group with pre-injury diagnoses/concerns and/or prior injuries, and no differences in findings were identified; therefore, data from all participants are included in results.
Results
Participant characteristics
The TBI group included 11 participants (five females), 10 right-handed and 1 left-handed, whose mean age at testing was 16.0 years; range was 12.6–18.7 years. Six sustained mild and five sustained moderate TBI. Of the children with moderate TBI, two children had abnormal findings on initial computed tomography (CT) scans (one with punctate hemorrhage and one with hemorrhagic contusion), two had PTA between 24 hours and 7 days post-injury, and one had a GCS score of 12; see Table 1 for detail. Additionally, two children with normal CT scans had findings on magnetic resonance imaging (MRI) which was acquired at the first study visit (both with single punctate abnormalities which were felt to be nonspecific and not definitively related to trauma). There were no significant differences in connectivity values or behavioral performance between the participants with mild versus moderate TBI (all p values > 0.177).
Table 1.
TBI group characteristics.
| TBI Group (N = 11) | |||||
|---|---|---|---|---|---|
| Age* | Sex | Premorbid conditions | Cause of TBI | Severity of TBI | Severity determined by: |
| 12.6 | F | None | Fall - bicycle | Mild | PTA < 24 hours |
| 12.8 | M | 2 Prior Concussions | Football | Mild | PTA < 24 hours |
| 13.4 | F | None | Struck by car | Moderate | GCS score of 12 |
| 14.3 | M | None | Basketball | Mild | PTA < 24 hours |
| 15.9 | F | None | Cheerleading | Mild | PTA < 24 hours |
| 16.8 | M | EF Deficits | Motor vehicle accident | Moderate | PTA b/w 24 hours - 7 days |
| 17.4 | M | None | Football | Moderate | PTA b/w 24 hours - 7 days |
| 17.8 | F | None | Struck by car | Moderate | Initial CT Findings |
| 18.0 | M | None | Rugby | Mild | PTA < 24 hours |
| 18.3 | M | None | Motor vehicle accident | Moderate | Initial CT Findings |
| 18.7 | F | ADHD Diagnosis | Fall horse | Mild | PTA < 24 hours |
Note.
At testing, in years.
The control group included 11 right-handed participants, six of whom were female. The mean age of the control group was 16.3 years; range was 12.7–18.9 years. No significant differences existed between the control group and TBI participants in age, sex, or SES. Findings were consistent when children with or without pre-injury diagnoses/concerns were included; therefore, data from all participants are included in following results.
Between-group differences in functional connectivity
In whole-brain contrasts, significant between-group difference in connectivity with the DMN was identified in one cluster (kE = 646) with the peak activated voxel (MNI coordinates 42, −42, 38) residing in right Brodmann Area 40 (BA 40), p < 0.001; see Figure 1. Post hoc evaluation revealed that, in the TBI group, the DMN was positively connected with this region (M = 0.067, SE = 0.037), whereas the control group showed negative connectivity between the DMN and this cluster (M = −0.177. SE = 0.038); see Figure 1.
Figure 1. Between-group differences in DMN and voxels of right BA 40.
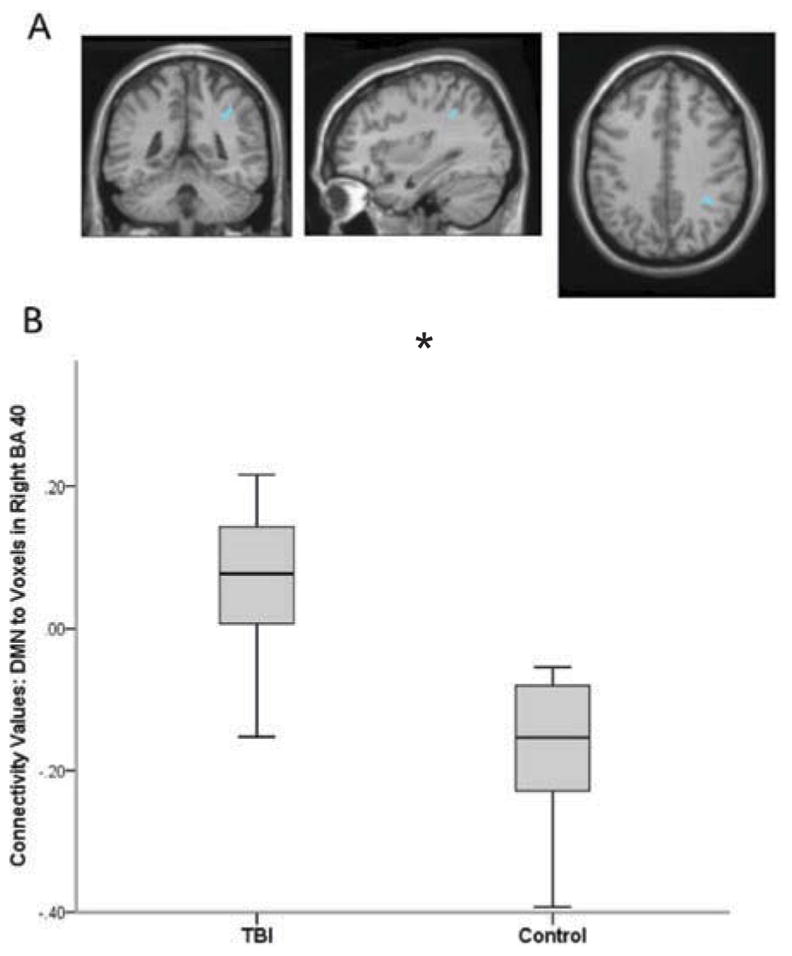
The TBI group had significantly greater connectivity between the DMN and voxels residing in Right BA 40 compared to uninjured controls. Figure 1a displays the anatomical location of the cluster with significant between-group difference in connectivity with the DMN. Figure 1b illustrates group differences in extracted connectivity values of DMN and BA 40 voxels. Error bars represent standard deviation.
Between-group comparison of extracted connectivity values between the DMN and ROI, right BA 40, revealed a trend toward a difference in connectivity, p = 0.056. Again, post hoc evaluation demonstrated that the TBI cohort had less negative connectivity (M = −0.055, SE = 0.020) than controls (M = −0.112, SE = 0.023) at the ROI level. With regard to age, there was a statistical trend in correlation with DMN-ROI connectivity values in the TBI cohort only, where age of participants was inversely related to ROI connectivity values; older children had more negative connectivity than younger children (p =0.086). This latter finding supports previous findings that anti-correlation between the DMN and BA 40 increases with development.33
Age–behavior relationships in the TBI group
Age of participants was significantly correlated with performance on some of the behavioral measures. There was a significant inverse relationship between age and Commission rate of the Simple Go/No-Go task, r = −0.783, p = 0.004, and a statistical trend between age and Reaction Time, r = −0.540, p = 0.086; older children had fewer commission errors and faster reaction time. A significant inverse relationship between age and PANESS Total Overflow was also observed, r = −0.603, p = 0.038; older children demonstrated less overflow than younger children. No relationship was observed between age and Timed SFA of the PANESS, p = 0.688. A significant positive relationship was observed between age and performance on the Conflicting Motor task, r = 0.793, p = 0.004, where older children had better performance than younger children. No significant relationship was observed between age and performance on the Contralateral Motor Task, p = 0.106. There were no significant relationships between age and performance on the D-KEFS Trail Making Task, Number/Letter Switching Condition, p = 0.293, nor with age and BRIEF GEC scores, p = 0.806, which is expected as these scores already account for age.
Brain–behavior relationships in TBI group
Partial correlations, controlling for age, revealed that the extracted connectivity values between DMN and BA 40 ROI were not significantly correlated with Commission Rate or Reaction Time on the Simple Go/No-Go task, Total Overflow or Total SFA on the PANESS, or D-KEFS Number/Letter Switching. There was a significant relationship between connectivity and BRIEF GEC, where more anomalous connectivity was associated with higher (poorer) scores (r = 0.671, p = 0.034), but this observation did not survive Bonferroni correction; see Table 2.
Table 2.
Correlations between DMN-BA 40 connectivity and behavioral tasks.
| Simple Go/No-Go | PANESS | D-KEFS | Motor tasks | BRIEF | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||
| Commissions rate | Reaction time | Total overflow | Total sfa | Num./let. switch | Conflicting | Contralateral | GEC | |
| uncorrected p | 0.300 | 0.627 | 0.582 | 0.616 | 0.967 | 0.005 | 0.003 | 0.034 |
| corrected p | 0.942 | 1.000 | 0.999 | 1.000 | 1.000 | 0.039 | 0.024 | 0.242 |
Note. These are partial correlation values after controlling for age.
Significant values (p < 0.05) in bold
Significant relationships were observed between the DMN and BA 40 ROI connectivity and performance on the Conflicting (r = −0.801, uncorrected p =0.005, corrected p = 0.039) and Contralateral (r = −0.823, uncorrected p = 0.003, corrected p = 0.024) Motor Tasks. Overall, more positive (more anomalous) connectivity was significantly associated with lower (worse) scores on these measures of inhibitory motor control; see Figure 2.
Figure 2. Relationship between DMN and BA 40 connectivity values and motor control.
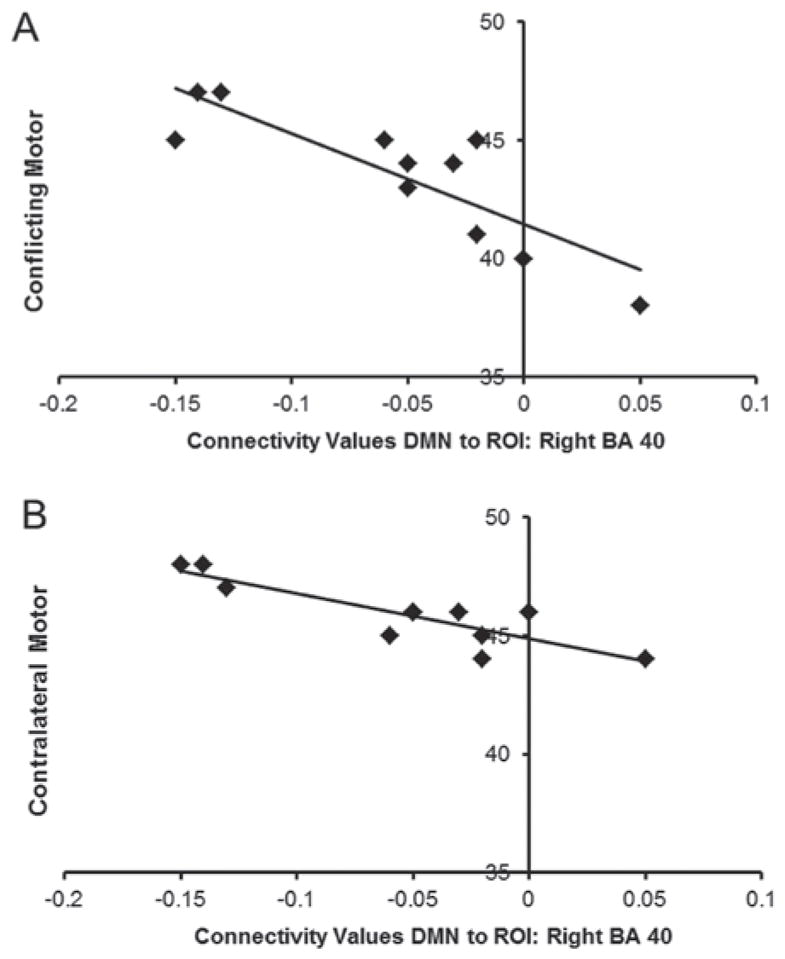
In the TBI group, more positive (more abnormal) connectivity of DMN and BA 40 was associated poorer inhibitory control as measured by the Conflicting (Figure 2a) and Contralateral (Figure 2b) motor tasks.
Discussion
We examined whole-brain functional connectivity of the DMN in the chronic phase (one year) of mild to moderate pediatric TBI. In comparison with healthy controls, children with TBI demonstrated increased connectivity between the DMN and voxels residing in Right BA 40. This anomalous connectivity pattern persisted when we examined DMN connectivity with an ROI of Right BA 40, although group differences were just above the margin of significance. To enhance reproducibility of these preliminary findings, we elected to examine brain–behavior relationships at the ROI level, as the ROI is easier to target and examine than specific activated voxels, which may change between cohorts in future studies. The localization of between-group differences to this higher level somatosensory association differs from the findings observed at two months post-injury, where between-group differences were identified in primary sensorimotor cortices.27 While to date, most longitudinal studies examining an overlapping cohort of individuals with TBI have followed individuals only through the subacute stages of recovery (i.e., up to three months post-injury), in a recent study, adults with mild TBI were followed for six months post-injury; similar to our findings, changing connectivity patterns were observed over time following injury, lending further support to evolution of functional connectivity during TBI recovery.43
Right BA 40 appears to be selectively engaged during tasks that require integrated information from various modalities. Researchers have observed Right BA 40 activation during complex grasping and concluded that Right BA 40 was likely responsible for delivering integrated feedback of proprioceptive and tactile information to the primary motor cortex.44 Right BA 40 is also engaged during tactile object recognition, again serving to integrate sensory and motor information.45 Furthermore, BA 40 activation has been directly linked to response inhibition,46,47 with its contribution to inhibitory behavior likely through the integration of sensory, cognitive, and motor information.
The conceptualization of BA 40 as important for integration of sensory, cognitive, and motor information is consistent with our brain–behavior findings. Specifically, we observed that functional connectivity between the DMN and BA40 was not related to measures of motor speed or subconscious motor control—tasks which do not rely upon integration of sensorimotor information. Additionally, there was no relationship between functional connectivity and simple cognitive inhibitory control. We did, however, observe a robust inverse relationship between DMN and Right BA 40 connectivity and tasks of inhibitory control that required conscious, complex, and integrated of cognitive and motor abilities (performance on Conflicting and Contralateral Motor Tasks). Children with TBI who had more typical connectivity patterns (anti-correlation of the DMN and BA 40) demonstrated better performance than children with more anomalous patterns of functional connectivity. As a task positive region, BA 40 is likely optimally anti-correlated with the DMN, and the brain–behavior findings may reflect disruption of optimal function of BA 40 due to interference from the DMN. These findings are consistent with adult TBI literature where less anti-correlation between the DMN and task positive regions is linked to poorer behavioral performance.25
A similar relationship was observed between functional connectivity and real-world executive control, as measured by the BRIEF parent report. Children with more atypical connectivity had greater reported difficulty with real-word executive function. This may represent that BA 40 plays a role of integrating multiple sensory inputs from the everyday environment to contribute to optimal real-world behavior. This brain–behavior relationship, however, did not survive corrections for multiple comparisons. This is not surprising because the BRIEF covers many domains and reflects real-world function; therefore, it is logical that the relationship between functional connectivity and performance is not as strong here as it was for an isolated task.
Collectively, these brain–behavior findings suggest that functional connectivity between DMN and BA 40 may have relevance for long-term functional outcomes in mild to moderate pediatric TBI. It is important to note that in this cohort of children, behavioral performance and ratings were good overall without marked deficits. This is consistent with the mild–moderate TBI literature where more readily measurable difficulties are seen early in recovery, but then, later in recovery, shift to more subtle, sometimes subjective, higher-level concerns.4–9
It is also important to highlight that these findings were observed one year post-injury. At this time point, we did not observe a relationship between functional connectivity and basic motor skills, but we did observe a robust relationship between functional connectivity and inhibitory control that relies upon integrated cognitive and motor skills. Our findings may represent chronic connectivity changes that mirror the adult TBI literature where hyper-connectivity within the DMN has been associated with cognitive and behavioral manifestations (e.g., sustained attention deficits) in chronic TBI.22 More broadly, it is possible that the anomalous functional connectivity observed here contributes to the neural basis of response inhibition deficits that are observed longitudinally across the pediatric TBI severity spectrum (for review: 15).
Together with previously published findings,27 our results suggest that rs-fMRI may be a meaningful tool with clinical implications for understanding the neural basis of function after milder forms of TBI. It is likely that the subtle differences in performance detected following TBI result from altered brain physiology, and, from a clinical perspective, these findings lend support to the children and families who report long-lasting changes in everyday performance and behavior, even when performance falls within the range expected for age. Furthermore, future studies like this that integrate neuroimaging and behavioral techniques could generate findings that have important clinical implications. Future findings in pediatric TBI could serve to inform interventions and help identify metrics that can be used to monitor change over the course of recovery or in response to treatment.
The primary limitation of this study is the small number of children in both the TBI and control cohorts. The participants within the TBI group had been followed longitudinally, and, consequently, we experienced participant attrition. However, this longitudinal approach was advantageous as it enabled us to observe that the functional connectivity of DMN one year post-injury was distinct from what was reported earlier in recovery.27 We were also limited by not having comparison behavioral data for the control group in this pilot study, though longitudinal imaging and behavioral assessment of controls are recommended for future studies. Finally, we note that some of the youth with TBI were athletes, and we did not control for sports participation or the possibility of subconcussive injuries. Recent studies suggest that subconcussive hits may relate to within-DMN connectivity, and so it is possible that this may have contributed to our findings.48,49
Overall, we were able to observe group differences in functional connectivity one year post-mild to moderate TBI and relate those functional connectivity differences to functional outcomes. These findings highlight the importance of larger scale studies to continue to examine how functional connectivity patterns relate to functional outcomes and identify characteristics that predict functional outcome after pediatric TBI, as early identification allows for earlier and more focused intervention efforts.
Conclusion
In summary, these results suggest that alterations exist in whole-brain connectivity of the DMN one year after mild–moderate TBI and these alterations correlate with performance on higher-level tasks requiring cognitive-motor integration. This lends support to the presence of subtle neural changes underlying the lasting cognitive and behavioral changes which are reported in some children with milder TBI. Additional work is needed to further understand how functional connectivity changes after milder TBI and how these changes affect long-term function.
Acknowledgments
Funding
This research was supported by the National Institutes of Health (J.S. & S.R., 5T32HD007414), (S.M., R01MH078160 & R01MH085328), (S.S., K23HD06161) and National Institute of Health National Center for Research Resources Clinical and Translational Science Awards Program (S.S., UL1TR001079-04).
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Massagli TL, Jaffe KM, Fay GC, Polissar NL, Liao S, Rivara JB. Neurobehavioral sequelae of severe pediatric traumatic brain injury: a cohort study. Archives of Physical Medicine and Rehabilitation. 1996;77(3):223–231. doi: 10.1016/s0003-9993(96)90102-1. [DOI] [PubMed] [Google Scholar]
- 2.Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI) Journal of Pediatric Psychology. 2008;33(7):707–718. doi: 10.1093/jpepsy/jsn006. [DOI] [PubMed] [Google Scholar]
- 3.Anderson V, Catroppa C. Recovery of executive skills following paediatric traumatic brain injury (TBI): a 2 year follow-up. Brain Injury. 2005;19(6):459–470. doi: 10.1080/02699050400004823. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe KM, Polissar NL, Fay GC, Liao S. Recovery trends over three years following pediatric traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 1995;76(1):17–26. doi: 10.1016/s0003-9993(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 5.Rhine T, Quatman-Yates C, Clark RA. A longitudinal examination of postural impairments in children with mild traumatic brain injury: implications for acute testing. Journal of Head Trauma Rehabilitation. 2017;32:E18–E23. doi: 10.1097/HTR.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon I, Swaine B, Friedman D, Forget R. Visuomotor response time in children with a mild traumatic brain injury. Journal of Head Trauma Rehabilitation. 2004;19(5):391–404. doi: 10.1097/00001199-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon I, Swaine B, Friedman D, Forget R. Children show decreased dynamic balance after mild traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2004;85(3):444–452. doi: 10.1016/j.apmr.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Dahl E, Emanuelson I. Motor proficiency in children with mild traumatic brain injury compared with a control group. Journal of Rehabilitation Medicine. 2013;45(8):729–733. doi: 10.2340/16501977-1188. [DOI] [PubMed] [Google Scholar]
- 9.Stephens J, Salorio C, Denckla M, Mostofsky S, Suskauer S. Subtle motor findings during recovery from pediatric traumatic brain injury: a preliminary report. Journal of Motor Behavior. 2017;49:1–7. doi: 10.1080/00222895.2016.1204267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roncadin C, Guger S, Archibald J, Barnes M, Dennis M. Working memory after mild, moderate, or severe childhood closed head injury. Developmental Neuropsychology. 2004;25(1–2):21–36. doi: 10.1080/87565641.2004.9651920. [DOI] [PubMed] [Google Scholar]
- 11.Robinson KE, Fountain-Zaragoza S, Dennis M, Taylor HG, Bigler ED, Rubin K, et al. Executive functions and theory of mind as predictors of social adjustment in childhood traumatic brain injury. Journal of Neurotrauma. 2014;31(22):1835–1842. doi: 10.1089/neu.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catroppa C, Anderson V. A prospective study of the recovery of attention from acute to 2 years following pediatric traumatic brain injury. Journal of the International Neuropsychological Society. 2005;11(1):84–98. doi: 10.1017/S1355617705050101. [DOI] [PubMed] [Google Scholar]
- 13.Leblanc N, Chen S, Swank PR, Ewing-Cobbs L, Barnes M, Dennis M, et al. Response inhibition after traumatic brain injury (TBI) in children: impairment and recovery. Developmental Neuropsychology. 2005;28(3):829–848. doi: 10.1207/s15326942dn2803_5. [DOI] [PubMed] [Google Scholar]
- 14.Ornstein TJ, Max JE, Schachar R, Dennis M, Barnes M, Ewing-Cobbs L, et al. Response inhibition in children with and without ADHD after traumatic brain injury. Journal of Neuropsychol. 2013;7(1):1–11. doi: 10.1111/j.1748-6653.2012.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinopoli KJ, Dennis M. Inhibitory control after traumatic brain injury in children. International Journal of Developmental Neuroscience. 2012;30(3):207–215. doi: 10.1016/j.ijdevneu.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semrud-Clikeman M. Pediatric traumatic brain injury: rehabilitation and transition to home and school. Applied Neuropsychology. 2010;17(2):116–122. doi: 10.1080/09084281003708985. [DOI] [PubMed] [Google Scholar]
- 17.Shin SS, Bales JW, Edward Dixon C, Hwang M. Structural imaging of mild traumatic brain injury may not be enough: overview of functional and metabolic imaging of mild traumatic brain injury. Brain Imaging Behaviour. 2017;11(2):591–610. doi: 10.1007/s11682-017-9684-0. [DOI] [PubMed] [Google Scholar]
- 18.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 20.Hayes JP, Bigler ED, Verfaellie M. Traumatic brain injury as a disorder of brain connectivity. Journal of the International Neuropsychological Society. 2016;22(2):120–137. doi: 10.1017/S1355617715000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, et al. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265(3):882–892. doi: 10.1148/radiol.12120748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. Journal of Neuroscience. 2011;31(38):13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iraji A, Benson RR, Welch RD, O’Neil BJ, Woodard JL, Ayaz SI, et al. Resting state functional connectivity in mild traumatic brain injury at the acute stage: independent component and seed-based analyses. Journal of Neurotrauma. 2015;32(14):1031–1045. doi: 10.1089/neu.2014.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Human Brain Mapping. 2011;32(11):1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sours C, Zhuo JC, Janowich J, Aarabi B, Shanmuganathan K, Gullapalli RP. Default mode network interference in mild traumatic brain injury: a pilot resting state study. Brain Research. 2013;1537:201–215. doi: 10.1016/j.brainres.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risen SR, Barber AD, Mostofsky SH, Suskauer SJ. Altered functional connectivity in children with mild to moderate TBI relates to motor control. Journal of Pediatric Rehabilitation Medicine. 2015;8(4):309–319. doi: 10.3233/PRM-150349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriegeskorte N, Lindquist MA, Nichols TE, Poldrack RA, Vul E. Everything you never wanted to know about circular analysis, but were afraid to ask. Journal of Cerebral Blood Flow & Metabolism. 2010;30(9):1551–1557. doi: 10.1038/jcbfm.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindquist MA, Mejia A. Zen and the art of multiple comparisons. Psychosomatic Medicine. 2015;77(2):114–125. doi: 10.1097/PSY.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ACRM. Definition of mild traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1993;8(3):86–87. [Google Scholar]
- 31.Hollingshead AA. Four-factor index of social status. 1975. [Google Scholar]
- 32.Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage. 2014;96:22–35. doi: 10.1016/j.neuroimage.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51(1):156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, et al. Bias between MNI and talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28(11):1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiebel SJ, Poline JB, Friston KJ, Holmes AP, Worsley KJ. Robust smoothness estimation in statistical parametric maps using standardized residuals from the general linear model. Neuroimage. 1999;10(6):756–766. doi: 10.1006/nimg.1999.0508. [DOI] [PubMed] [Google Scholar]
- 37.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 38.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 39.Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, et al. fMRI evidence that the neural basis of response inhibition is task-dependent. Brain Research, Cognitive Brain Research. 2003;17(2):419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 40.Denckla MB. Revised neurological examination for subtle signs (1985) Psychopharmacology Bulletin. 1985;21(4):773–800. [PubMed] [Google Scholar]
- 41.Vitiello B, Ricciuti AJ, Stoff DM, Behar D, Denckla MB. Reliability of subtle (soft) neurological signs in children. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28(5):749–753. doi: 10.1097/00004583-198909000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Shue KL, Douglas VI. Attention deficit hyperactivity disorder and the frontal lobe syndrome. Brain and Cognition. 1992;20(1):104–124. doi: 10.1016/0278-2626(92)90064-s. [DOI] [PubMed] [Google Scholar]
- 43.Bharath RD, Munivenkatappa A, Gohel S, Panda R, Saini J, Rajeswaran J, et al. Recovery of resting brain connectivity ensuing mild traumatic brain injury. Frontiers in Human Neuroscience. 2015;9:513. doi: 10.3389/fnhum.2015.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milner TE, Franklin DW, Imamizu H, Kawato M. Central control of grasp: manipulation of objects with complex and simple dynamics. Neuroimage. 2007;36(2):388–395. doi: 10.1016/j.neuroimage.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 45.Reed CL, Shoham S, Halgren E. Neural substrates of tactile object recognition: an fMRI study. Human Brain Mapping. 2004;21(4):236–246. doi: 10.1002/hbm.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barber AD, Caffo BS, Pekar JJ, Mostofsky SH. Effects of working memory demand on neural mechanisms of motor response selection and control. Journal of Cognitive Neuroscience. 2013;25(8):1235–1248. doi: 10.1162/jocn_a_00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbas K, Shenk TE, Poole VN, Breedlove EL, Leverenz LJ, Nauman EA, et al. Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting-state functional magnetic resonance imaging study. Brain Connectivity. 2015;5(2):91–101. doi: 10.1089/brain.2014.0279. [DOI] [PubMed] [Google Scholar]
- 49.Johnson B, Neuberger T, Gay M, Hallett M, Slobounov S. Effects of subconcussive head trauma on the default mode network of the brain. Journal of Neurotrauma. 2014;31(23):1907–1913. doi: 10.1089/neu.2014.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]


