Abstract
Objectives
Pulse pressure, a marker of arterial stiffness, and body composition are both risk factors for cardiovascular disease. Little is known about whether changes in body composition may be linked to future pulse pressure. We sought to determine whether change in amount of abdominal and thigh fat over 5 years predicted pulse pressure at 10 years.
Methods
Visceral fat as well as abdominal and thigh subcutaneous fat areas were measured by computed tomography at baseline and 5 years later in 284 Japanese Americans (mean age 49.3 years; 50.4% men) without hypertension, heart disease, and glucose-lowering medication use at baseline. Pulse pressure at 10 years was calculated as the difference between systolic blood pressure (BP) and diastolic BP measured with a mercury sphygmomanometer. The association between change in fat at 5 years and arterial pulse pressure at 10 years, adjusted for baseline pulse pressure, was examined using linear regression analysis.
Main results
Change in abdominal visceral fat area at 5 years was positively associated with 10-year pulse pressure independent of sex, 5-year change in body mass index (BMI), and baseline age, BMI, pulse pressure, abdominal visceral fat, smoking status, alcohol consumption, physical activity, HOMA-IR, and fasting plasma glucose. There were no significant associations between baseline amounts or change in abdominal or thigh subcutaneous fat areas and future pulse pressure.
Conclusions
The accumulation of abdominal visceral fat over time independently predicted future pulse pressure in Japanese Americans.
Keywords: arterial pulse pressure, intra-abdominal fat, body composition, prospective studies, epidemiology, vascular stiffness
Introduction
Arterial pulse pressure, defined as the difference between systolic blood pressure (BP) and diastolic BP, increases steeply after age 50 years due to decreased elasticity of large arteries [1]. Increased pulse pressure damages the elastic components of the vascular wall, thereby increasing the risk of atherosclerosis [2]. Increased pulse pressure is also associated with increased stress on the left ventricle, which can result in ventricular wall hypertrophy and failure [3]. Research has shown that increased pulse pressure is an independent prognostic marker of cardiovascular events not only in the elderly population, but also in younger normotensive subjects and in subjects with relatively low cardiovascular risk [4–7]. In the NHANES 1 study, an increase in pulse pressure of approximately 10 mmHg was associated with a 26% increase in the risk of cardiovascular death in those 25–45 years old, and about 10% in those 46–77 years old [8].
Obesity, generally defined by an overall excess of body fat, is associated with adverse cardiovascular health outcomes. Research has shown that individual variation in regional body fat distribution is an important factor explaining the metabolic heterogeneity of obesity and its related cardiovascular risk [9, 10]. In particular, visceral adiposity is a key driver of the cardiometabolic risk associated with obesity; in contrast, some research suggests that lower extremity fat depots may protect against cardiovascular disease [9, 11, 12]. A possible mechanism to explain the relationship between obesity and cardiovascular disease is remodeling of the vasculature, particularly arterial stiffness [13]. It has been reported that individuals with obesity have greater arterial stiffness [14–17], but this is not a consistent finding [18–20]. Several cross-sectional studies have demonstrated that abdominal adiposity is more strongly related to arterial stiffness than general obesity [16, 21, 22]. In addition, peripheral fat has been found to be inversely associated with arterial stiffness, as opposed to central fat [23–25]. However, little is known about the long-term effects of general and regional adiposity on pulse pressure. One study has shown that changes in body mass index (BMI) and waist-to-hip ratio over 24 years were not associated with changes in pulse pressure in women [26].
To our knowledge, there have been no reports on the association of change in the amount of fat in specific body locations with future pulse pressure. Therefore, we examined the relationship between change in computed tomography (CT) measured abdominal and thigh fat areas from baseline to 5 years and pulse pressure at 10 years.
Methods
Study subjects
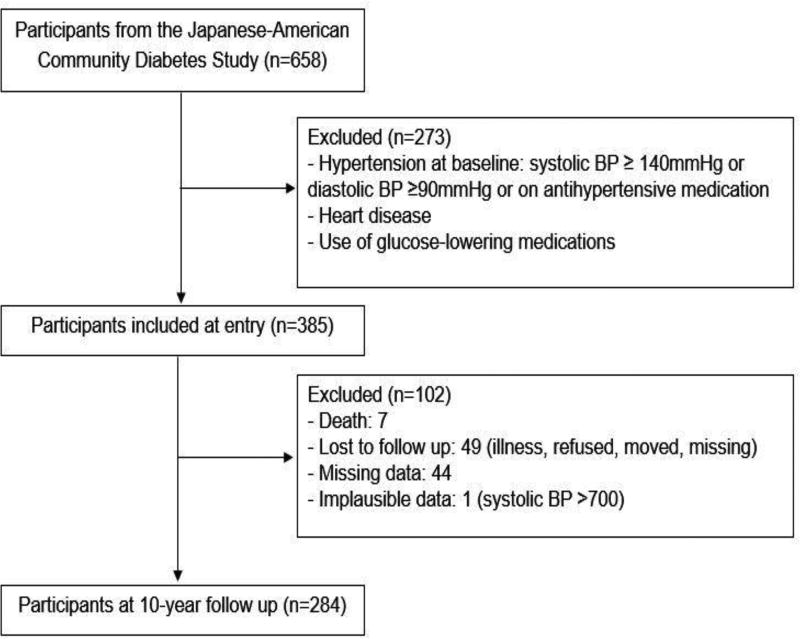
The study population consisted of Japanese American men and women enrolled in the Japanese American Community Diabetes Study (JACDS), a cohort of second- (Nisei) and third-generation (Sansei) Japanese Americans of 100% Japanese ancestry [27]. The hypothesis underlying the JACDS was that an interaction between ethnicity and environmental factors; that is, a Western lifestyle, caused pathophysiological changes that resulted in adiposity-related cardio-metabolic diseases. Participants were chosen from volunteers through community-wide recruitment and were representative of Japanese American residents of King County, Washington, in age distribution, residential distribution, and parental immigration pattern [27]. Participants were examined at 5 to 6 years and 10–11 years after a baseline evaluation. For the current analysis, subjects were removed from the original cohort of 658 subjects if at baseline they had a diagnosis of hypertension, defined by systolic blood pressure (BP) ≥140 mmHg, diastolic BP ≥90 mmHg, or the use of antihypertensive medications; had a diagnosis of heart disease; or were taking an oral glucose-lowering medication or insulin. Of the remaining 385 subjects, 102 were excluded for reasons show in Figure 1, leaving 284 for this analysis. The study received approval from the University of Washington Human Subjects Division and all subjects provided written informed consent.
Figure 1.
Flow diagram showing how subjects were selected for analysis
Clinical and laboratory examination
All evaluations were done at the General Clinical Research Center, University of Washington. A complete physical examination was performed at baseline. After a rest period of 30 minutes, BP was measured with a mercury sphygmomanometer in the recumbent position to the nearest 2 mmHg and reported as an average recording from the second and third of three consecutive measurements, Personal medical history and lifestyle factors were assessed using a standardized questionnaire. Smoking was classified into two groups (current smoker, past smoker/never smoked). Moderate alcohol intake was defined as consuming more than 6 g of ethanol per day [28]. The Paffenbarger physical activity index questionnaire was used to estimate physical activity level (usual kilocalories spent weekly) [29], and regular physical activity was defined as more than moderate intensity physical activity.
Blood samples were obtained after an overnight fast of 10 hours. A 75-g oral glucose tolerance test was used to define diabetes mellitus using American Diabetes Association criteria [30]. Plasma glucose was measured by the hexokinase method using an autoanalyzer (Department of Laboratory Medicine, University of Washington, Seattle, Washington). Plasma insulin was measured by radioimmunoassay by the Diabetes Research Center, University of Washington. Insulin sensitivity was estimated using the homeostasis model assessment insulin resistance (HOMA-IR) index calculated as [fasting serum insulin (µIU/mL) × fasting serum glucose (mg/dl)]/405[31]. Lipids were measured according to modified procedures of the Lipid Research Clinics (Northwest Lipid Research Laboratory, University of Washington).
Single (1-cm) CT scan slices were obtained of the abdomen at the level of the umbilicus and of the thigh at a level halfway between the greater trochanter and the superior margin of the patella as detailed previously [32]. CT scans were analyzed using density contour software. Areas corresponding to a density of −250 to −50 Hounsfield units (HU) were classified as adipose tissue. Change in CT-measured fat area was calculated as the difference between the 5-year and baseline values. The intra-observer variability for multiple measurements by a single observer of a single CT scan ranged from 0.2 to 1.4%.
Statistical analysis
Continuous variables are expressed as means ± standard deviation (SD), and categorical variables are expressed as numbers and percentages. Continuous variable distributions were tested for normality by the Kolmogorov-Smirnov test. Assessment of differences between two groups for continuous data was done by the independent t test and when data were not normally distributed by the Mann-Whitney U test. Chi-square test was used for categorical data. Pearson’s correlation analysis was used to estimate correlations between pulse pressure at 10-year and age and hemodynamic, biochemical, and body composition measures. Age- and sex-adjusted associations were performed with partial correlations. Stepwise multiple linear regression analysis was used to determine independent associations between pulse pressure at 10-year in relation to change in specific CT-measured fat depots between baseline and 5 years. The presence of multi-collinearity in multivariable models was evaluated using the variance inflation factor, with a value >5 suggesting its presence. The data were analyzed using SPSS software version 22.0 (SPSS Inc., Chicago, IL, USA). A two-sided p-value <0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of the study subjects are shown in Table 1. Men weighed more and had significantly greater BMI, systolic and diastolic BP, fasting plasma glucose, lower heart rate and less favorable lipid profiles than women. Mean pulse pressure did not differ by sex.
Table 1.
Baseline characteristics of the participants
| Characteristics | Total (n = 284) | Men (n = 143) | Women (n = 141) | P value |
|---|---|---|---|---|
| Age (years) | 49.3 ± 11.5 | 49.0 ± 11.1 | 49.6 ± 11.9 | 0.640 |
| Weight (kg) | 62.4 ± 11.8 | 70.3 ± 9.3 | 54.5 ± 8.3 | <0.001 |
| BMI (kg/m2) | 23.8 ± 3.1 | 24.9 ± 2.8 | 22.6 ± 3.1 | <0.001 |
| Systolic BP (mmHg) | 120.7 ± 10.2 | 123.5 ± 9.4 | 117.9 ± 10.2 | <0.001 |
| Diastolic BP (mmHg) | 73.4 ± 7.6 | 76.0 ± 6.8 | 70.9 ± 7.5 | <0.001 |
| Pulse pressure (mmHg) | 47.3 ± 7.5 | 47.5 ± 7.8 | 47.0 ± 7.2 | 0.584 |
| Pulse rate (beats/min) | 65.6 ± 8.0 | 63.1 ± 6.7 | 68.1 ± 8.4 | <0.001 |
| Current smoking (%) | 42 (14.8) | 22 (15.4) | 20 (14.2) | 0.776 |
| Moderate alcohol consumption (%) | 61 (21.5) | 48 (33.6) | 13 (9.2) | <0.001 |
| Regular physical activity (%) | 70 (24.6) | 52 (36.4) | 18 (12.8) | <0.001 |
| Diabetes mellitus, n (%) | 29 (10.2) | 15 (10.5) | 14 (9.9) | 0.876 |
| Fasting plasma glucose (mmol/l) | 5.24 ± 1.11 | 5.50 ± 1.42 | 4.97 ± 0.57 | <0.001 |
| 2-hour plasma glucose (mmol/l) | 7.55 ± 2.91 | 7.68 ± 3.44 | 7.41 ± 2.26 | 0.657 |
| Fasting insulin (pmol/l) | 92.4 ± 45.0 | 88.4 ± 42.2 | 96.5 ± 47.4 | 0.119 |
| HOMA-IR | 3.08 ± 1.92 | 3.12 ± 2.10 | 3.04 ± 1.73 | 0.765 |
| Total cholesterol (mmol/l) | 5.78 ± 1.01 | 5.93 ± 1.02 | 5.62 ± 0.97 | 0.008 |
| LDL cholesterol (mmol/l) | 3.57 ± 0.89 | 3.82 ± 0.92 | 3.32 ± 0.77 | <0.001 |
| Triglycerides (mmol/l) | 1.47 ± 1.24 | 1.73 ± 1.48 | 1.20 ± 0.88 | <0.001 |
| HDL cholesterol (mmol/l) | 1.55 ± 0.44 | 1.35 ± 0.33 | 1.75 ± 0.44 | <0.001 |
| Abdominal visceral fat area (cm2) | 70.3 ± 43.5 | 83.0 ± 45.3 | 57.4 ± 37.6 | <0.001 |
| Abdominal subcutaneous fat area (cm2) | 152.5 ± 73.7 | 131.9 ± 58.2 | 173.3 ± 81.7 | <0.001 |
| Thigh subcutaneous fat area (cm2) | 66.1 ± 32.6 | 46.2 ± 18.4 | 86.5 ± 31.3 | <0.001 |
BMI, body mass index; BP, blood pressure, HOMA-IR, homeostasis model assessment-insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Data are presented as means ± standard deviation (SD) or %.
Table 2 shows the correlation between pulse pressure at 10 years and baseline and change variables of interest. Age showed the highest correlation with future pulse pressure. Baseline abdominal visceral and subcutaneous fat areas were positively associated with 10-year pulse pressure, while change in abdominal subcutaneous fat area was inversely associated with this outcome in unadjusted analysis. However, these associations were no longer statistically significant after age and sex adjustment. Change in abdominal visceral fat area showed a positive correlation with 10-year pulse pressure after age and sex adjustment (β=0.144, p=0.016). In addition, systolic BP, pulse pressure, fasting plasma glucose, HOMA-IR, and BMI at baseline were positively associated with 10-year pulse pressure in both unadjusted and age- and sex-adjusted analyses.
Table 2.
Correlations between pulse pressure at 10-year follow-up and age, hemodynamic, biochemical, and body composition measures
| Unadjusted | P | Age and sex adjusted |
P | |
|---|---|---|---|---|
| Baseline: | ||||
| Age (year) | 0.628 | <0.001 | ||
| Systolic BP | 0.383 | <0.001 | 0.298 | <0.001 |
| Diastolic BP | 0.097 | 0.104 | 0.059 | 0.321 |
| Pulse pressure | 0.421 | <0.001 | 0.330 | <0.001 |
| Pulse rate | 0.024 | 0.693 | 0.054 | 0.368 |
| Fasting plasma glucose | 0.325 | <0.001 | 0.165 | 0.006 |
| 2-hour plasma glucose | 0.304 | <0.001 | 0.108 | 0.071 |
| Fasting insulin | 0.058 | 0.328 | 0.111 | 0.062 |
| HOMA-IR* | 0.147 | 0.013 | 0.144 | 0.015 |
| Total cholesterol | 0.243 | <0.001 | −0.002 | 0.967 |
| LDL cholesterol | 0.259 | <0.001 | 0.070 | 0.245 |
| Triglycerides | 0.012 | 0.842 | −0.017 | 0.780 |
| HDL cholesterol | −0.018 | 0.759 | −0.037 | 0.541 |
| BMI | 0.129 | 0.030 | 0.139 | 0.019 |
| Abdominal visceral fat area | 0.330 | <0.001 | 0.092 | 0.121 |
| Abdominal subcutaneous fat area | 0.159 | 0.007 | 0.097 | 0.103 |
| Thigh subcutaneous fat area | −0.071 | 0.231 | 0.008 | 0.900 |
| Change from baseline to 5 years: | ||||
| Change in abdominal visceral fat area | 0.046 | 0.436 | 0.144 | 0.016 |
| Change in abdominal subcutaneous fat area | −0.164 | 0.006 | 0.019 | 0.756 |
| Change in thigh subcutaneous fat area | 0.116 | 0.051 | 0.111 | 0.065 |
Data are expressed as standardized β. BMI, body mass index; BP, blood pressure, HOMA-IR, homeostasis model assessment-insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Log-transformed values were used for statistical analysis.
We next examined multivariable models to determine whether baseline and 5-year change in specific adipose depots independently predicted 10-year pulse pressure. Besides baseline age and pulse pressure, 5 year change in abdominal visceral fat area was independently associated with the future pulse pressure, adjusted for baseline age, sex, BMI, pulse pressure, abdominal visceral and subcutaneous fat areas, and thigh subcutaneous fat area, and 5 year change in abdominal and thigh subcutaneous fat area (Table 3, model 1). This relationship remained significant when further adjusted for smoking status, alcohol consumption, physical activity, HOMA-IR, fasting plasma glucose, and change in BMI (Table 3, models 2 & 3). The change at 5 years of neither abdominal nor thigh subcutaneous fat area was significantly related to future pulse pressure, using the same covariates shown in models 1–3 of Table 3 (data not shown). Fasting plasma glucose was also associated positively with future pulse pressure. In addition, when we performed multivariable linear regression analysis of the prediction of change in pulse pressure over 10 years instead of pulse pressure at 10 years, the results were similar (Supplementary Table 1).
Table 3.
Multivariable linear regression analysis of the prediction of pulse pressure at 10-year follow-up
| Variables | β* | P value | |
|---|---|---|---|
| Model 1 | Change in abdominal visceral fat area | 0.131 | 0.003 |
| Age | 0.564 | <0.001 | |
| Pulse pressure | 0.276 | <0.001 | |
| Model 2 | Change in abdominal visceral fat area | 0.131 | <0.001 |
| Age | 0.564 | <0.001 | |
| Pulse pressure | 0.276 | <0.001 | |
| Model 3 | Change in abdominal visceral fat area | 0.130 | 0.003 |
| Age | 0.529 | <0.001 | |
| Pulse pressure | 0.269 | <0.001 | |
| Fasting plasma glucose | 0.113 | 0.014 |
Standardized β.
Model 1: adjusted for demographics, overall, and regional body composition (variables in the model - age, sex, BMI, pulse pressure at baseline, baseline and changes in abdominal visceral fat area, abdominal subcutaneous fat area, and thigh subcutaneous fat area)
Model 2: Adjusted for variables included in Model 1 plus lifestyle behaviors (additional variables in the model - smoking, alcohol consumption, and physical activity)
Model 3: Adjusted for variables included in Model 2 plus insulin sensitivity, glycemia, and change in overall adiposity (additional variables in the model - HOMA-IR, fasting plasma glucose, and change in BMI)
A subset analysis was performed by excluding 11 subjects who initiated use of antihypertensive or heart disease medications during follow-up that might have affected pulse pressure and repeating the analyses that generated models 1–3 from Table 3. The 5-year change in abdominal visceral fat area remained an independent predictor of 10-year pulse pressure in this subset analysis (Supplementary Table 2).
We additionally examined the association between change in BMI from baseline to 5 years and 10-year pulse pressure after adjustment for age, sex, baseline BMI, pulse pressure, smoking status, alcohol consumption, physical activity, and did not find this to be significantly associated with future pulse pressure (data not shown).
Discussion
These prospective data demonstrate that an increase of abdominal visceral fat over 5 years is independently associated with increased pulse pressure at 10 years in Japanese Americans. This association was not seen with abdominal or thigh subcutaneous fat. The association between abdominal visceral fat area change and pulse pressure at 10 years could not be explained by overall or regional adiposity measures, glycemia, insulin sensitivity, and demographic or lifestyle factors included in regression models as covariates. Furthermore, this association could not be explained by abdominal visceral fat area change simply arising as a manifestation of overall BMI change, since we found no significant association between 5 year BMI change and 10-year pulse pressure.
Research examining the importance of BP as a cardiovascular risk factor has largely focused on assessing the effects of systolic and diastolic BP. However, when taken individually, these measurements do not represent the pulsatile component of blood pressure recommended for evaluating cardiovascular disease risk [33]. Greater pulse pressure has been associated with a higher risk of cardiovascular events, such as myocardial infarction, heart failure, stroke, and cardiovascular death in several population-based cohort studies [4, 6, 7, 34–36].
In this Japanese American population, we did not find an association between unadjusted abdominal visceral fat change over 5 years and pulse pressure after 10 years, but a significant association emerged for abdominal visceral fat change after adjustment for age and other covariates, including CT measures of other fat depots as well as general obesity assessed by BMI. These findings are consistent with the concept that visceral adiposity has a more important role in the development of arterial stiffness than other fat depots or an overall adiposity measure such as BMI.
Recent cross-sectional studies have demonstrated that greater abdominal visceral adiposity is associated with greater arterial stiffness independent of BMI in postmenopausal women with type 2 diabetes [37]. Visceral fat volume was also shown to be an independent correlate of arterial stiffness in severely obese subjects when controlling for subcutaneous fat volume [38]. With regard to overall adiposity, a prospective study by Benetos et al. [39] did not find BMI to be a significant determinant of arterial stiffness progression over 6-year follow-up in normotensive subjects with a mean age similar to that of our study population. However, Orr et al. [40] reported that weight gain increased large artery stiffness in young, nonobese men, and furthermore, the increase in arterial stiffness was associated with the amount of abdominal visceral fat gained independent of the amount of total body fat gained.
Although previous studies have suggested that peripheral fat as opposed to central fat may be favorably associated with less arterial stiffness, we did not find a negative association between thigh subcutaneous fat and change in pulse pressure. The “protective” association arose from cross-sectional research [23–25], and this may explain the difference between these results and the results we present from our prospective analysis.
Age was the strongest predictor of pulse pressure in our population. This association can be explained by the known association between older age and arterial stiffening which results in higher pulse pressure, while in younger subjects the magnitude of pulse pressure is mainly related to greater stroke volume [2]. Over 10 years, higher pulse pressure more likely reflects increased arterial stiffness rather than alterations in stroke volume. Also, other research has demonstrated a strong correlation between pulse pressure and pulse wave velocity, which directly measures the arterial BP wave and is considered the most reliable method of assessing arterial stiffness [41].
Consistent with previous research, in our study fasting plasma glucose was positively and independently associated with the future pulse pressure. Cay et al. [42] showed that subjects with impaired fasting glucose had significantly greater aortic pulse pressure compared with those with normal fasting glucose. In addition, other work has demonstrated a positive association between fasting plasma glucose levels with arterial stiffness in non-diabetic subjects [43, 44].
There are several possible mechanisms whereby an increase in abdominal visceral fat results in increased pulse pressure. The insulin resistant state that commonly accompanies greater visceral adiposity may induce endothelial dysfunction, stimulate proliferation of smooth muscle cells, and cause an increase in collagen synthesis and protein cross-linking [45–47], thereby increasing arterial stiffness. In our study, however, change in abdominal visceral fat remained associated with future pulse pressure after adjusting for HOMA-IR and fasting plasma glucose, implying that the influence of visceral adiposity on pulse pressure may be independent of insulin resistance or hyperglycemia. Another postulated mechanism is hypersecretion of components of the renin-angiotensin system more pronounced in visceral than subcutaneous adipose tissue [48, 49]. In addition, elevated circulating free fatty acids and proinflammatory cytokines secreted from visceral fat may lead to vascular damage and arterial stiffening [50].
Our study has several potential limitations. First, because the subjects were Japanese Americans, these results may not be generalizable to other ethnic groups. Second, although pulse pressure was calculated from blood pressure measurements taken mainly by one blinded observer with a standard protocol, it is possible that measurement error exists. Blood pressure was measured in the recumbent position, not the currently advised sitting position. Third, although we adjusted for known covariates, the potential for confounding by unmeasured factors exists given the observational research design. Despite these limitations, to our knowledge this is the first prospective study demonstrating that change in body fat composition, determined by CT scan, predicts future pulse pressure. Specifically, increases in abdominal visceral fat are associated with higher pulse pressure, independent of other measured fat depots. None of these other fat depots was associated with future pulse pressure.
In conclusion, our current findings provide novel evidence in support of the hypothesis that accrual of visceral adipose tissue may contribute to cardiovascular disease through increased arterial stiffness.
Supplementary Material
Acknowledgments
Source of Funding: National Institutes of Health grants DK-031170 and HL-049293. This work was supported by facilities and services provided by the Diabetes Research Center (DK-017047), Clinical Nutrition Research Unit (DK-035816), and the General Clinical Research Center (RR-000037) at the University of Washington.
We are grateful to the King County Japanese-American community for support and cooperation. VA Puget Sound Health Care System provided support for Drs. Boyko and Kahn’s involvement in this research.
Footnotes
Conflict of Interest: The funding entities had no role in the conduct of this study or interpretation of its results and the authors declare no conflict of interest.
Author Contributions
S.J.H. and E.J.B. made substantial contributions to the conception and design of the study, drafted the article, and provided approval for the final version. W.Y.F, D.L.L., and S.E.K., revised the article critically for important intellectual content and provided approval for the final version. S.J.H. and E.J.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 2.Dart AM, Kingwell BA. Pulse pressure--a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 3.Winston GJ, Palmas W, Lima J, Polak JF, Bertoni AG, Burke G, et al. Pulse pressure and subclinical cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Hypertens. 2013;26:636–642. doi: 10.1093/ajh/hps092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Madhavan S, Alderman MH. Pulse pressure: a predictor of cardiovascular mortality among young normotensive subjects. Blood Press. 2000;9:260–266. doi: 10.1080/080370500448641. [DOI] [PubMed] [Google Scholar]
- 6.Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieere P, et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. doi: 10.1161/01.hyp.30.6.1410. [DOI] [PubMed] [Google Scholar]
- 7.Benetos A, Rudnichi A, Safar M, Guize L. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998;32:560–564. doi: 10.1161/01.hyp.32.3.560. [DOI] [PubMed] [Google Scholar]
- 8.Domanski M, Norman J, Wolz M, Mitchell G, Pfeffer M. Cardiovascular risk assessment using pulse pressure in the first national health and nutrition examination survey (NHANES I) Hypertension. 2001;38:793–797. doi: 10.1161/hy1001.092966. [DOI] [PubMed] [Google Scholar]
- 9.Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 10.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 11.Sakai Y, Ito H, Egami Y, Ohoto N, Hijii C, Yanagawa M, et al. Favourable association of leg fat with cardiovascular risk factors. J Intern Med. 2005;257:194–200. doi: 10.1111/j.1365-2796.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 12.Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 13.Safar ME, Czernichow S, Blacher J. Obesity, arterial stiffness, and cardiovascular risk. J Am Soc Nephrol. 2006;17:S109–S111. doi: 10.1681/ASN.2005121321. [DOI] [PubMed] [Google Scholar]
- 14.Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42:468–473. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- 15.van Popele NM, Westendorp IC, Bots ML, Reneman RS, Hoeks AP, Hofman A, et al. Variables of the insulin resistance syndrome are associated with reduced arterial distensibility in healthy non-diabetic middle-aged women. Diabetologia. 2000;43:665–672. doi: 10.1007/s001250051356. [DOI] [PubMed] [Google Scholar]
- 16.Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, Tanaka H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr Metab Cardiovasc Dis. 2015;25:495–502. doi: 10.1016/j.numecd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues SL, Baldo MP, Lani L, Nogueira L, Mill JG, Sa Cunha R. Body mass index is not independently associated with increased aortic stiffness in a Brazilian population. Am J Hypertens. 2012;25:1064–1069. doi: 10.1038/ajh.2012.91. [DOI] [PubMed] [Google Scholar]
- 19.Tarnoki AD, Tarnoki DL, Bogl LH, Medda E, Fagnani C, Nistico L, et al. Association of body mass index with arterial stiffness and blood pressure components: a twin study. Atherosclerosis. 2013;229:388–395. doi: 10.1016/j.atherosclerosis.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Czernichow S, Bertrais S, Oppert JM, Galan P, Blacher J, Ducimetiere P, et al. Body composition and fat repartition in relation to structure and function of large arteries in middle-aged adults (the SU.VI.MAX study) Int J Obes (Lond) 2005;29:826–832. doi: 10.1038/sj.ijo.0802986. [DOI] [PubMed] [Google Scholar]
- 21.Wohlfahrt P, Somers VK, Cifkova R, Filipovsky J, Seidlerova J, Krajcoviechova A, et al. Relationship between measures of central and general adiposity with aortic stiffness in the general population. Atherosclerosis. 2014;235:625–631. doi: 10.1016/j.atherosclerosis.2014.05.958. [DOI] [PubMed] [Google Scholar]
- 22.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, et al. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. doi: 10.1161/01.hyp.38.3.429. [DOI] [PubMed] [Google Scholar]
- 23.Snijder MB, Henry RM, Visser M, Dekker JM, Seidell JC, Ferreira I, et al. Regional body composition as a determinant of arterial stiffness in the elderly: The Hoorn Study. J Hypertens. 2004;22:2339–2347. doi: 10.1097/00004872-200412000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira I, Snijder MB, Twisk JW, van Mechelen W, Kemper HC, Seidell JC, et al. Central fat mass versus peripheral fat and lean mass: opposite (adverse versus favorable) associations with arterial stiffness? The Amsterdam Growth and Health Longitudinal Study. J Clin Endocrinol Metab. 2004;89:2632–2639. doi: 10.1210/jc.2003-031619. [DOI] [PubMed] [Google Scholar]
- 25.Fantin F, Rossi AP, Cazzadori M, Comellato G, Mazzali G, Gozzoli MP, et al. Central and peripheral fat and subclinical vascular damage in older women. Age Ageing. 2013;42:359–365. doi: 10.1093/ageing/aft005. [DOI] [PubMed] [Google Scholar]
- 26.Kristjansson K, Sigurdsson JA, Lissner L, Sundh V, Bengtsson C. Blood pressure and pulse pressure development in a population sample of women with special reference to basal body mass and distribution of body fat and their changes during 24 years. Int J Obes Relat Metab Disord. 2003;27:128–133. doi: 10.1038/sj.ijo.0802190. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto WY, Leonetti DL, Kinyoun JL, Shuman WP, Stolov WC, Wahl PW. Prevalence of complications among second-generation Japanese-American men with diabetes, impaired glucose tolerance, or normal glucose tolerance. Diabetes. 1987;36:730–739. doi: 10.2337/diab.36.6.730. [DOI] [PubMed] [Google Scholar]
- 28.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- 29.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–S10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Shuman WP, Morris LL, Leonetti DL, Wahl PW, Moceri VM, Moss AA, et al. Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol. 1986;21:483–487. doi: 10.1097/00004424-198606000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 34.Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, et al. Pulse pressure and cardiovascular disease-related mortality: follow-up study of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 2002;287:2677–2683. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 36.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka KI, Kanazawa I, Sugimoto T. Reduced muscle mass and accumulation of visceral fat are independently associated with increased arterial stiffness in postmenopausal women with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2016;122:141–147. doi: 10.1016/j.diabres.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Arner P, Backdahl J, Hemmingsson P, Stenvinkel P, Eriksson-Hogling D, Naslund E, et al. Regional variations in the relationship between arterial stiffness and adipocyte volume or number in obese subjects. Int J Obes (Lond) 2015;39:222–227. doi: 10.1038/ijo.2014.118. [DOI] [PubMed] [Google Scholar]
- 39.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002;105:1202–1207. doi: 10.1161/hc1002.105135. [DOI] [PubMed] [Google Scholar]
- 40.Orr JS, Gentile CL, Davy BM, Davy KP. Large artery stiffening with weight gain in humans: role of visceral fat accumulation. Hypertension. 2008;51:1519–1524. doi: 10.1161/HYPERTENSIONAHA.108.112946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim EJ, Park CG, Park JS, Suh SY, Choi CU, Kim JW, et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: invasive study. J Hum Hypertens. 2007;21:141–148. doi: 10.1038/sj.jhh.1002120. [DOI] [PubMed] [Google Scholar]
- 42.Cay S, Ozturk S, Funda Biyikoglu S, Atak R, Balbay Y, Aydogdu S. Association of aortic pressures with fasting plasma glucose in patients with and without impaired fasting glucose. Blood Press. 2008;17:164–169. doi: 10.1080/08037050802218417. [DOI] [PubMed] [Google Scholar]
- 43.Shin JY, Lee HR, Lee DC. Increased arterial stiffness in healthy subjects with high-normal glucose levels and in subjects with pre-diabetes. Cardiovasc Diabetol. 2011;10:30. doi: 10.1186/1475-2840-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kawamoto R, Tabara Y, Kusunoki T, Abe M, Kohara K, Miki T. A slightly high-normal glucose level is associated with increased arterial stiffness in Japanese community-dwelling persons with pre-diabetes. Vasc Med. 2013;18:251–256. doi: 10.1177/1358863X13503192. [DOI] [PubMed] [Google Scholar]
- 45.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–582. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 46.Avena R, Mitchell ME, Neville RF, Sidawy AN. The additive effects of glucose and insulin on the proliferation of infragenicular vascular smooth muscle cells. J Vasc Surg. 1998;28:1033–8. doi: 10.1016/s0741-5214(98)70029-1. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 47.Ruiz-Torres A, Melon J, Munoz FJ. Insulin stimulates collagen synthesis in vascular smooth muscle cells from elderly patients. Gerontology. 1998;44:144–148. doi: 10.1159/000021998. [DOI] [PubMed] [Google Scholar]
- 48.Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 2008;10:93–98. doi: 10.1007/s11906-008-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Harmelen V, Elizalde M, Ariapart P, Bergstedt-Lindqvist S, Reynisdottir S, Hoffstedt J, et al. The association of human adipose angiotensinogen gene expression with abdominal fat distribution in obesity. Int J Obes Relat Metab Disord. 2000;24:673–678. doi: 10.1038/sj.ijo.0801217. [DOI] [PubMed] [Google Scholar]
- 50.Behn A, Ur E. The obesity epidemic and its cardiovascular consequences. Curr Opin Cardiol. 2006;21:353–360. doi: 10.1097/01.hco.0000231406.84554.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.