Abstract
The role of the p38 signaling pathway in the innate and adaptive immune responses has been well documented, especially in inflammatory cytokine production by dendritic cells (DCs). However, whether the p38 signaling pathway affects the important antigen (Ag) presentation function of DCs remains largely unknown. In this study, we reported that the deletion of p38α resulted in an impaired cross-presentation ability of CD8+ conventional DCs (cDCs) and a reduction in the direct presentation ability of CD8− cDCs ex vivo. Further study revealed that p38α had a crucial role in Ag processing by CD8+ cDCs but did not affect the Ag uptake or co-stimulation of T cells. Moreover, p38α deficiency led to reduced cross-priming of T cells in vivo. The production of the IL-12p40 and IL-12p70 cytokines by p38α-deficient cDCs was also significantly reduced. Our study identified a new role for p38α in modulating the important antigen cross-presentation function of DCs.
Keywords: antigen presentation, cross-presentation, dendritic cells, p38α
Introduction
Dendritic cells (DCs) are the most efficient antigen (Ag)-presenting cells and link innate and adaptive immunity. DCs have an important role in immune responses to infections and maintain immune tolerance to self tissues. Although all DCs have the ability to present Ags to naive T cells, they differ in their surface markers, localization and cytokine production.1 At steady state, DCs in the mouse lymphoid organs can be categorized as plasmacytoid DCs (pDCs) and conventional DCs (cDCs, CD8+ cDCs and CD8– cDCs).2 pDCs can produce a large amount of type-I interferon (IFN) upon activation but have a weak Ag presenting ability, whereas3 the cDCs are very efficient in Ag presentation and express high levels of the MHC-I and MHC- II molecules.4
DCs uptake Ag through endocytosis or phagocytosis.5 The Ags are degraded into peptides that form peptide/MHC complexes, which are expressed on the DC surface for presentation to T cells.6 Classically, endogenous Ag peptides are bound to MHC-I molecules for presentation to CD8+ T cells, whereas exogenous Ags are presented to CD4+ T cells via the MHC-II presentation pathway.7 These two Ag presentation pathways are known as direct presentation. Moreover, there is an interesting link between the two pathways, which is referred to as Ag cross-presentation, whereby exogenous Ags are presented by MHC-I molecules to CD8+ T cells to induce cytotoxic T lymphocyte (CTL) responses.8 Signals in addition to stimulation by the peptide-MHC-I complex are required for T-cell activation and proliferation, including co-stimulation, adhesion and cytokines.9 Although Ag presentation is a common function of DCs, different DC subsets use specialized Ag presentation pathways. Both CD8+ and CD8− cDCs capture exogenous Ags efficiently and present them through the MHC-II pathway, but only the CD8+ cDCs can cross-present extracellular Ag on MHC-I molecules.10 Moreover, the cross-presentation ability of different DC subsets can be regulated by certain cytokines involved in DC development and maturation,11 and immature CD8+ cDCs have a limited cross-presentation ability compared with mature CD8+ cDCs.12, 13 Although Ag cross-presentation has been described for many years, the molecules and signaling pathways that regulate this unique function of DCs are not understood completely.
Many studies have revealed that the p38 signaling pathway in DCs has essential roles in inducing innate and adaptive immune responses.14 A number of studies proposed a strong link between p38 signaling and inflammation.15 In particular, the p38 inhibitor almost completely abrogated the production of IL-6, IL-12p40, IL-12p70 and TNF-α from LPS-induced monocyte-derived DCs (MoDCs).16 The p38 pathway is required for the maturation of MoDCs because drug-induced inhibition of this pathway prevents LPS- and TNF-α-induced DC maturation.17 Moreover, TLR-induced activation of p38 might also participate in the regulation of DC Ag presentation.18 However, because targeted inactivation of the mouse p38α gene resulted in embryonic lethality,19 most studies are based on the use of pharmacological inhibitors and only limited data are available from these studies. Although p38α has been reported to program DCs to drive Th17 cell differentiation20 and regulate T-regulatory-cell functions,21, 22 the roles of p38α in DC Ag cross-presentation and T-cell activation have not been established.
In this study, we investigated the effect of genetic deletion of p38α on DC functions using DC-specific p38α-deficient mice. We found that the deletion of p38α resulted in an impaired cross-presentation ability of CD8+ cDCs and a reduction in the direct presentation ability of CD8− cDCs ex vivo. Moreover, p38α deficiency reduced cross-priming of T cells in vivo. Further analysis demonstrated that p38α deficiency impaired the Ag degradation process and antigen-peptide/MHC-I complex formation but did not affect Ag uptake or the expression of co-stimulatory molecules. The production of IL-12p40 and IL-12p70 by p38α-deficient cDCs was also significantly reduced. Overall, our study demonstrated for the first time an important role for p38α in Ag presentation by DCs at steady state.
Materials and methods
Mice
C57BL/6J mice were housed in a specific pathogen-free facility at Tsinghua University, Beijing, China. The p38αfl/fl mice were generated as previously described23 and backcrossed to the C57BL/6J background for at least eight generations. Floxed p38α mice were bred with CD11c-Cre (Jackson stock 008068, B6.Cg-Tg (Itgax-cre) 1-1Reiz/J) and Vav-cre (Jackson stock 008610, B6.Cg-Tg (Vav1-cre) A2kio/J) mice to generate DC-specific p38α-deficient (p38αΔDC) and hematopoietic cell-specific p38α-deficient mice (p38αΔHPC), respectively. The Cre-negative littermates were used as the controls. The OT-I transgenic mice (Jackson stock 003831, C57BL/6-Tg (TcraTcrb) 1100Mjb/J) and OT-II transgenic mice24 were maintained.
Isolation of splenic DCs
To isolate DCs from the spleen, the tissue was minced with scissors and digested with 0.1 mg/mL DNase I (Roche Molecular Biochemicals, Indianapolis, IN, USA) and 1 mg/ml collagenase III (Worthington Biochemical, Lakewood, NJ, USA) at 37 °C for 25 min. Then, light density cells were isolated in 1.077 g/cm3 Nycodenz (Axis-Shield, Scotland, UK) medium by centrifugation for 10 min at 1700g. The light density splenocytes were incubated with mAbs against CD3, CD90, TER119, Ly6G and CD19, followed by removal of non-DC cells using anti-immunoglobulin (Ig)-coated magnetic beads (Bangs Laboratories, Fishers, IN, USA).25 The enriched cells were stained with fluorescence-conjugated antibodies against DC-specific markers and sorted by flow cytometry.
Generation of BM-cultured DCs
To test the production of cytokines by DCs, BM cells were cultured in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin and streptomycin in the presence of recombinant murine Flt3L (200 ng/ml) for 7–8 d to generate DCs. To generate the DCs used in the T-cell proliferation assay, the BM cells were cultured with Flt3L for 7–8 d, with additional GM-CSF (2 ng/ml) added to the cultures on day 6. All cytokines were purchased from PeproTech (Rocky Hill, NJ, USA).
T-cell proliferation assay
The OT-I CD8+ and OT-II CD4+ T cells were isolated from the spleens of OT-I or OT-II transgenic mice through the depletion of red blood cells and immune-magnetic bead negative selection (for CD8+ T-cell isolation, the mAb cocktail contained mAbs targeting CD11b, F4/80, B220, CD19, TER119, Ly6G, MHC-II and CD4; for CD4+ T-cell isolation, the mAb cocktail contained mAbs targeting CD11b, F4/80, B220, CD19, TER119, Ly6G, MHC-II and CD8). The isolated T cells were labeled using the CFSE cell proliferation kit (Invitrogen, Carlsbad, CA, USA).
Splenic CD8+ cDCs and CD8− cDCs were sorted and incubated (1 × 105 cells/ml) with the ovalbumin (OVA) protein (100 μg/ml, Sigma, St Louis, MO, USA) or OVA peptide (OVA257–264, 1 ng/ml or OVA323–339, 1 μg/ml, Sigma) at 37 °C. After 2 h, the cells were washed twice. Different numbers of DCs were plated in 96-well round-bottom plates. CFSE-labeled OT-I and OT-II T cells (1 × 105 cells/well) were incubated with CD8+ cDCs and CD8− cDCs, respectively, in RPMI 1640 complete medium supplemented with 20 ng/ml GM-CSF. T-cell proliferation was analyzed by flow cytometry after 60–90 h.
Antigen presentation assay
DCs were isolated from the spleen and incubated with the OVA protein (100 μg/ml) for 2 h. Then, the free OVA protein was washed away and the cells were cultured for another 20 h. The DCs were stained using a biotin-conjugated anti-SIINFEKL/H-2Kb (eBioscience, San diego, CA, USA, eBio25-D1.16) antibody, followed by streptavidin-BV421 (eBioscience).
Antigen uptake assay
Splenic DCs were incubated with AF488-labeled OVA protein (5 μg/ml, Invitrogen) at 37 °C for 30 min. Uptake of the labeled OVA protein was measured by flow cytometry. DCs incubated on ice with the AF488-labeled OVA protein were used as the negative controls.
Antigen degradation assay
Latex beads (Polysciences, Warrington, PA, USA) were activated with 8% glutaraldehyde (Sigma) at room temperature for 4–6 h, coupled with the OVA protein (0.5 mg/ml) at 4 °C overnight, and blocked with glycine (0.5 M) at room temperature for 30 min.
DCs (0.5–1 × 106) were pulsed with the OVA-coated latex beads (cells:beads=1:3) in a 37 °C water bath for 30 min; then, phagocytosis was stopped with the addition of cold PBS. Non-internalized beads were removed by an FBS flotation gradient as follows. The cell suspension (1 ml) was layered over serum (2 ml) and centrifuged for 5 min at 150g. Then, the supernatant containing free beads was discarded. DCs were placed in RPMI 1640 complete medium supplemented with 20 ng/ml GM-CSF for the indicated times at 37 °C. Then, the cells were lysed with 200 μl of lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% NP40, and protease inhibitor cocktail, pH 7.4). The recovered latex beads were stained with a FITC-conjugated anti-OVA antibody (Rockland, Gilbertsille, PA, USA) at 4 °C for 30 min and finally analyzed by flow cytometry.
T-cell cross-priming assay in vivo
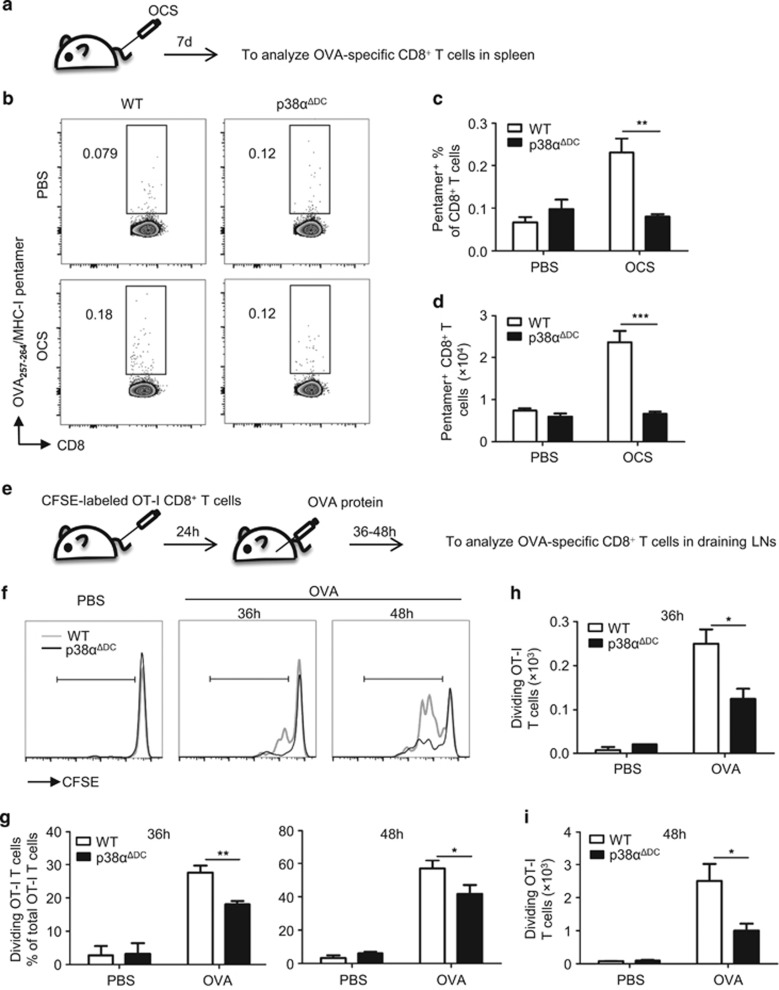
To prepare cell-associated Ags, red blood cells were removed from splenocytes. The splenocytes were irradiated with a 30 Gy X-ray and coated with the OVA protein (1 mg/ml) at 37 °C for 45 min; then, free Ag was washed away. The OVA-coated splenocytes (1 × 107 cells/mouse) were injected into the mice intravenously (i.v.). After 7 days, the mice were analyzed for the proliferation of OVA-specific CD8+ T cells in the spleen. To detect OVA-specific CD8+ T cells, the splenocytes were stained with the APC-labeled H-2Kb/SIINFEKL pentamer (Proimmune, Oxford, UK) for 30 min, followed by the anti-CD3 and anti-CD8 antibodies. The flow cytometry analysis was performed on the LSRII or LSRFortessa.
To examine the activation of T cells by soluble Ag in vivo, splenic CD8+ T cells were sorted from OT-I mice, stained with CFSE (Invitrogen), and then transferred (i.v. 7 × 105 cells/mouse) to the recipients. After 24 h, the recipients were injected with the OVA protein (hypodermic injection 100 μg/mouse) near both sides of the inguinal lymph nodes (50 μg/side). After 36–48 h, the mice were killed to analyze the proliferation of OVA-specific CD8+ T cells (gated on CFSE+ CD3+ CD8+ TCR Vα2+) in the inguinal lymph nodes.
Cell sorting by flow cytometry
Cells isolated from the BM or spleen were incubated with rat immunoglobulin (Jackson Laboratories, West Grove, PA, USA) for 10 min and then stained with antibodies at 4 °C for 30 min. The following mAbs were used for cell staining and sorting: PE-Cy7-conjugated CD11c (N418), PE-conjugated Siglec-H (eBio440c), CD3e (eBio500A2), CD40 (1C10), FITC-conjugated CD86 (GL-1), APC-conjugated CD172α (P84), CD80 (16-10A1), eFluor 450-conjugated CD8α (53-6.7), CD24 (M1/69), CD4 (GK1.5), MHC-II (M5/114.15.2), BV 605-conjugated CD11b (M1/70) and AF 700-conjugated CD8 (53-6.7). All of the antibodies were purchased from eBioscience, BD Biosciences (Palo Alto, CA, USA) or BioLegend (San Diego, CA, USA). Dead cells were discriminated in all experiments using 7-AAD (eBioscience) staining. The analysis was performed on the LSRII and LSRFortessa flow cytometers. Cell sorting was performed on the FACSAria II or FACSAria III instrument (BD). The purity of the sorted populations was routinely greater than 95%. The data analysis was performed on the single live cell gate using the FlowJo software (TreeStar, Ashland, OR, USA).
Cytokine production assays
BM-derived DCs (1 × 105 cells/well) or splenic DCs (5 × 104 cells/well) were seeded into 96-well plates and stimulated with TLR agonists, including 50 nM Pam2CSK4 (InvivoGen, San Diego, CA, USA, tlrl-pm2s-1), 50 nM Pam3CSK4 (InvivoGen, tlrl-pms), 100 μg/ml Poly (I:C) (InvivoGen, tlrl-pic), 100 ng/ml LPS (Sigma), 1 μg/ml R848 (InvivoGen, tlrl-r848), 10 nM ODN 1668 (AdipoGen, Liestal, Switzerland, IAX-200-001) and 1 μM ODN 2216 (AdipoGen, IAX-200-005). The medium used was RPMI 1640 supplemented with 10% FBS, 1% P/S (penicillin and streptomycin) and GM-CSF (20 ng/ml). Supernatants were collected after 24–36 h, and IL-12p40 and IL-12p70 were measured by ELISA. Recombinant mouse IL-12p70 (eBioscience, 14-8121) was used as the standard protein. Antibodies against mouse IL-12/IL-23p40 (eBioscience, 14-7125-85) and IL-12p70 (BioLegend, 511802) were used as the capture antibodies. Biotinylated monoclonal antibodies against the mouse IL12/IL23p40 (eBioscience, 13-7123) antibody were used in combination with streptavidin-HRP (Amersham, Little Chalfont, UK, RPN4401) for the ELISA analysis.
Real-time PCR for gene expression
The total RNA extraction and reverse transcription procedures were performed as previously described.26 Real-time PCR was performed with SYBR Green I on the 7900 real-time PCR detection system (Applied Biosystems, Grand Island, NY, USA) using the following conditions: denaturation, 95 °C, 15 s; annealing, 55 °C, 20 s; and extension, 72 °C, 25 s. The primers used for the mouse Nox2 gene were 5′-TGTGGTTGGGGCTGAATGTC-3′ and 5′-CTGAGAAAGGAGAGCAGATTTCG -3′. The primers used for the mouse TAP1 gene were 5′-GGACTTGCCTTGTTCCGAGAG-3′ and 5′-GCTGCCACATAACTGATAGCGA-3′. The primers used for the mouse GAPDH gene were 5′-TGTGTCCGTCGTGGATCTGA-3′ and 5′-CCTGCTTCACCACCTTCTTGA-3′.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5. The data were presented as the mean±s.e.m. Statistical significance was determined by the one-tailed unpaired Student’s t-test. Differences were considered statistically significant when P<0.05.
Results
p38α deficiency impaired the antigen cross-presentation ability of CD8+ cDCs
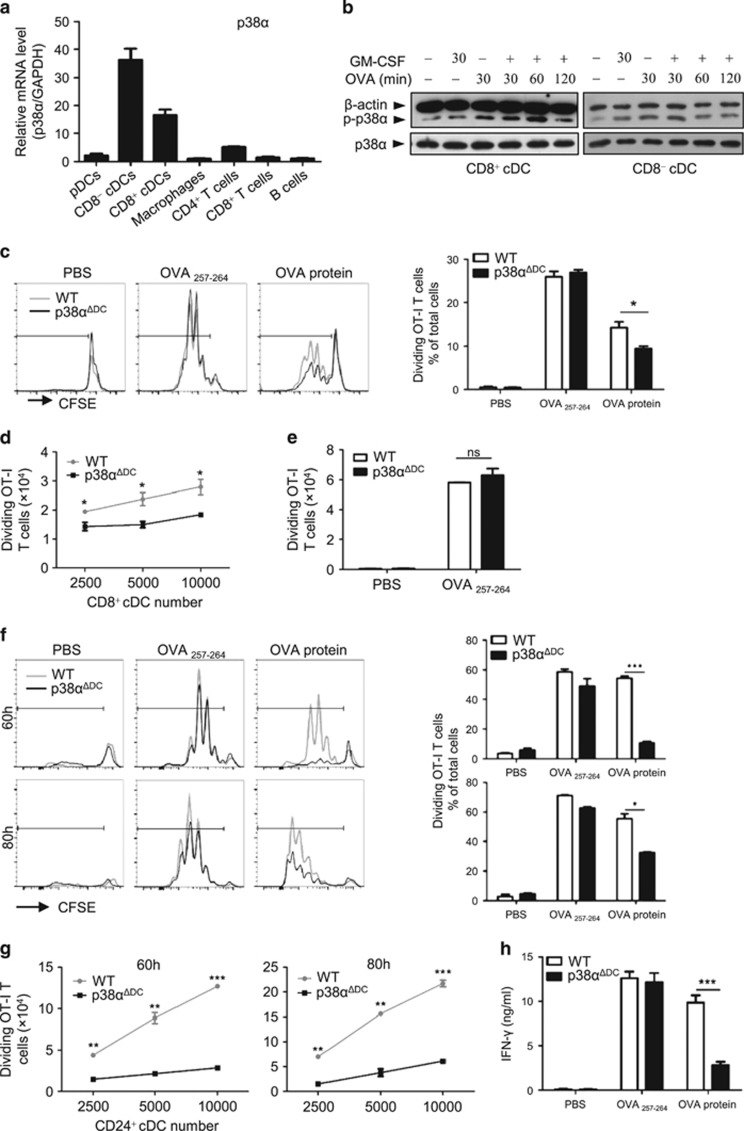
To investigate the role of p38α in immune cells, we first tested p38α expression in different immune cell types. We observed that p38α was highly expressed in splenic CD8+ cDCs and CD8− cDCs but weakly expressed in pDCs, macrophages, T cells (CD4+ and CD8+) and B cells (Figure 1a), which suggested that p38α might be important for cDC functions. Because the most important function of cDCs is Ag presentation, we examined whether the expression and phosphorylation of p38α were affected after cDCs encountered Ag. Splenic CD8+ and CD8− cDCs were cultured with the OVA protein or GM-CSF alone or in combination for various times; then, p38α phosphorylation (the activated form of p38α) was measured. Because DCs are short-lived cells and can rapidly lose their antigen presentation ability, GM-CSF was added to the culture medium to sustain DC viability and functionality. Both the CD8+ and CD8− cDCs showed significantly elevated p38α activation levels after the OVA and GM-CSF treatments, especially at 60 min for the CD8+ cDCs and 30 min for the CD8− cDCs (Figure 1b). Although p38α could be activated by either OVA or GM-CSF alone, the phosphorylated level of p38α in the OVA-treated (100 μg/ml) cDCs was much stronger than the level in the GM-CSF-treated (20 ng/ml) cDCs (Figure 1b). These data suggested that activation of p38 signaling might be important for antigen presentation by cDCs.
Figure 1.
The cross-presentation of soluble Ag by CD8+ cDCs from WT or p38αΔDC mice. (a) p38α was abundantly expressed in cDCs. The p38α mRNA levels were measured by real-time PCR in different immune cells. (b) p38α was activated in splenic CD8+ and CD8− cDCs after OVA treatment. DCs were sorted by flow cytometry and then stimulated with GM-CSF (20 ng/ml) and the OVA protein (100 μg/ml) as indicated. (c–e) Splenic CD8+ cDCs from WT or p38αΔDC mice were coated with OVA257–264 (1 ng/ml) or the OVA protein (100 μg/ml) for 2 h at 37 °C; then, the CD8+ cDCs (0.25–1 × 104/well) were incubated with CFSE-labeled OT-I CD8+ T cells (1 × 105/well) in a 96-well plate for 60 h. T-cell proliferation was measured by flow cytometry analysis. The medium used was RPMI 1640 supplemented with 10% FBS, 1% P/S (penicillin and streptomycin) and GM-CSF (20 ng/ml). The percentage (c) and the number (d) of dividing T cells were reduced during co-culture with OVA-pulsed CD8+ DCs from p38αΔDC mice. (e) The presentation abilities of OVA257–264 by CD8+ cDCs from WT and p38αΔDC mice were comparable. (f, g) Antigen presentation by Flt3L-supplemented BM cultured CD24+ cDCs from WT or p38αΔDC mice were measured as described in c–e. (g) The DC dose response of the cross-presentation of the OVA protein by CD24+ cDCs. (h) The IFNγ concentration in the supernatant after 24 h of co-culture of CD24+ cDCs and OT-I T cells (1 × 105/well). (a) The immune cells were isolated from 10 mice. The data were representative of two independent experiments, each including triplicate samples. (b) DCs were sorted from 15 pairs of mice. The data were representative of two independent experiments. (c–e) The CD8+ cDCs were sorted form 5 pairs of littermate mice. The results were representative of three independent experiments, including duplicate samples. (f–h) The CD24+ cDCs were derived from BM cells of three pairs of littermate mice. The results were representative of three independent experiments with duplicates. All of the results were presented as the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001.
To examine the involvement of p38α in DC functions, we investigated whether deletion of p38α in cDCs affected their Ag presentation ability. For this purpose, mice with a conditional p38α deletion in DCs (p38αΔDC mice) were generated by crossing CD11c-cre mice to p38αfl/fl mice. p38α expression was almost completely abrogated in the splenic pDCs, CD8+ cDCs and CD8− cDCs (Supplementary Figure S1a). Because splenic CD8+ cDCs are specialized for antigen cross-presentation, first we tested the effect of p38α deficiency on the cross-presentation ability of CD8+ cDCs. Splenic CD8+ cDCs were pulsed with the OVA protein or peptide and then incubated with carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled OT-I CD8+ T cells. The number of divided T cells (recognized by reduced CFSE fluorescence) after 60 h was used as a measure of the Ag cross-presentation ability of the CD8+ cDCs. The results showed that the percentage of dividing T cells was reduced during co-culture with OVA-pulsed CD8+ cDCs from the p38αΔDC mice compared with the WT mice (Figure 1c). Further analysis showed that the number of dividing OT-I CD8+ T cells was also decreased in the cultures with different numbers of p38α-deficient DCs (Figure 1d), indicating that the CD8+ cDCs from the p38αΔDC mice had a lower cross-presentation capacity than the CD8+ cDCs from the WT mice. However, the presentation of the OVA peptide by the CD8+ cDCs (which would not require Ag uptake and processing) from the p38αΔDC mice was comparable to the presentation of the CD8+ cDCs from the WT mice (Figures 1c and e), indicating that the antigen presentation ability per se was not affected. We also examined the effects of p38α deficiency on the ability of the CD8+ cDCs to directly present the OVA protein to the OT-II CD4+ T cells. No significant differences were observed between CD8+ cDCs from the p38αΔDC and WT mice in terms of the percentages (Supplementary Figure S1b) and numbers (Supplementary Figure S1c and d) of dividing T cells, suggesting that p38α had little effect on the direct presentation ability of the CD8+ cDCs.
To confirm the function of p38α in the regulation of the cDC Ag presentation ability, bone marrow (BM) cell-derived cDCs from the WT and p38αΔDC mice were utilized in the T-cell proliferation assay. The Flt3L-supplemented BM cultures generated three DC subsets, including pDCs, CD24+ cDCs (equivalent to splenic CD8+ cDCs) and CD24− cDCs (equivalent to splenic CD8− cDCs). Consistent with the observations in splenic cDCs, the cross-presentation ability of the CD24+ cDCs derived from p38αΔDC mouse BM cells was markedly reduced compared with the WT mouse cells (Figure 1f). Even with increasing numbers of OVA-pulsed CD24+ cDCs in culture, only low numbers of dividing T cells could be detected after 60 or 80 h (Figure 1g). Moreover, IFN-γ production was significantly reduced in the supernatant of the p38α-deficient CD24+ cDC and OT-I CD8+ T-cell co-culture (Figure 1h), which was another indication of impaired T-cell activation. We also performed a T-cell proliferation assay using splenic and BM-derived cDCs from hematopoietic cell-specific p38α-deficient (Vav-cre+/-p38αfl/fl, p38αΔHPC) mice and observed similar results (Supplementary Figures S1e and f). Overall, these data indicated an important role for p38α in Ag cross-presentation by CD8+ cDCs.
p38α deletion reduced the direct antigen presentation capacity of CD8− cDCs
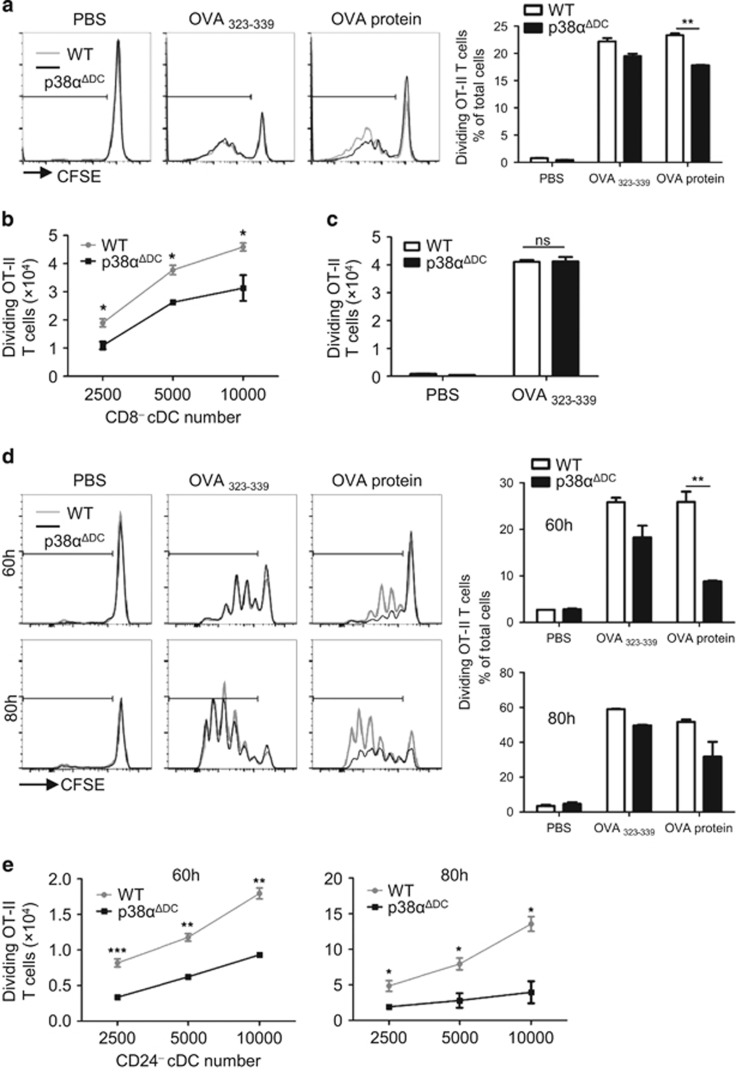
In addition to CD8+ cDCs, the mouse spleen contains CD8− cDCs, which have been shown to be efficient antigen presenting cells that can induce CD4+ T-cell activation. We investigated whether p38α deficiency affected the direct presentation ability of CD8− cDCs. Splenic CD8− cDCs were pulsed with the OVA protein or OVA323–339 and then incubated with OT-II CD4+ T cells for 85 h. The splenic CD8−cDCs from p38αΔDC mice showed a decreased Ag direct presentation capacity for the OVA protein (Figures 2a and b), whereas the presentation of the OVA peptide was not affected (Figures 2a and c). The unchanged ability to present a peptide antigen by the p38α-deficient CD8− cDCs suggested that the defective direct presentation capacity was not the result of the defective presentation of antigen peptides. Similarly, deletion of p38α reduced the direct presentation ability of CD24− cDCs derived from BM, which were equivalent to splenic CD8− cDCs (Figures 2d and e). In addition, we used CD8− cDCs from the spleen (Supplementary Figure S2a) and CD24− cDCs (Supplementary Figure S2b) derived from BM cells from the p38ɑΔHPC mice and found that their OVA protein presentation abilities were also decreased. These results indicated that p38α was an important regulator of Ag direct presentation by CD8− cDCs.
Figure 2.
The comparison of Ag direct presentation by CD8− cDCs from WT or p38αΔDC mice. (a–c) Splenic CD8− cDCs from the WT or p38αΔDC mice were pulsed with OVA323-339 (1 μg/ml) or OVA protein (100 μg/ml) for 2 h at 37 °C; then, the CD8− cDCs (0.25–1 × 104/well) and CFSE-labeled OT-II CD4+ T cells (1 × 105/well) were incubated together for 85 h. OT-II T-cell proliferation was measured by flow cytometry. The medium used was RPMI 1640 supplemented with 10% FBS, 1% P/S (penicillin and streptomycin) and GM-CSF (20 ng/ml). (a, b) The OT-II T-cell proliferation activated by OVA-pulsed p38α-deficient CD8− cDCs was reduced compared with the control cells. (c) Similar levels of OVA323–339 presentation by CD8− cDCs from WT and p38αΔDC mice. (d, e) BM-derived CD24− cDCs from WT or p38αΔDC mice were pulsed with OVA323-339 (1 μg/ml) or OVA protein (100 μg/ml) for 2 h; then, CD24− cDCs (0.25–1 × 104/well) and CFSE-labeled OT-II CD4+ T cells (1 × 105/well) were incubated together for 60 and 80 h. (a–c) The CD8− cDCs were sorted from five pairs of littermate mice. (d, e) The CD24− cDCs were derived from BM cells of three pairs of littermate mice. All of the in vitro experiments were repeated three times, and the results shown were representative. The results were presented as the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001.
p38α was not required for the upregulation of co-stimulatory molecules on cDCs
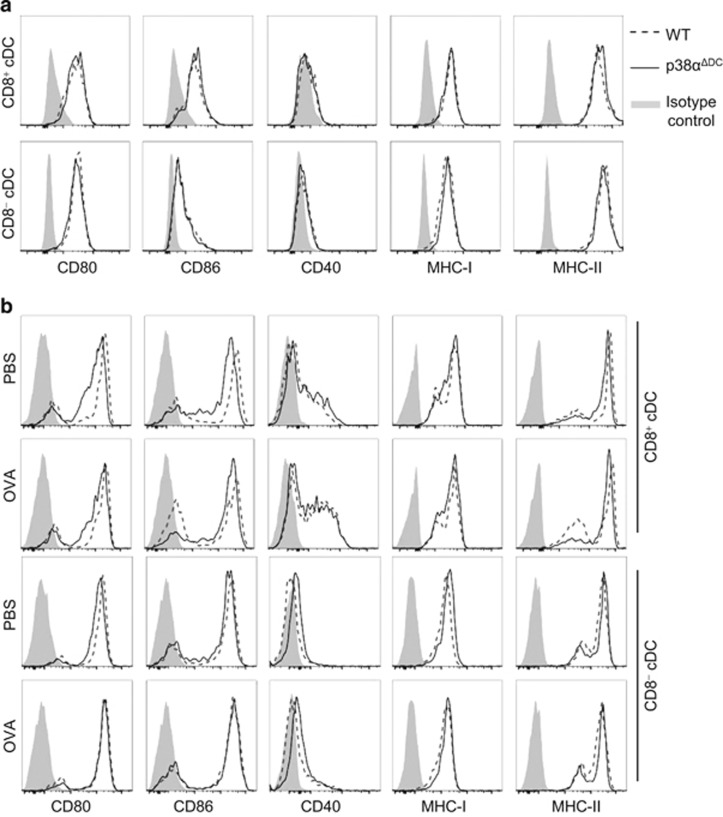
Antigen-specific T-cell activation by DCs requires the expression of several co-stimulatory molecules by DCs, such as CD80, CD86 and CD40. To investigate whether the reduction of T-cell proliferation in the co-cultures with Ag-pulsed splenic DCs from p38αΔDC mice was due to a defect in the expression of co-stimulatory and MHC molecules, we determined the CD80, CD86, CD40, MHC-I and MHC-II expression levels on splenic DCs from the p38αΔDC and WT mice. Comparable levels of these molecules were expressed by WT and p38αΔDC cDCs without any stimulation (Figure 3a). Moreover, when sorted splenic cDC subsets from the WT and p38αΔDC mice were pulsed with the OVA protein for 20 h (Figure 3b) or stimulated with a TLR agonist (Pam2 or CpG) for 16 h (Supplementary Figure S3a), the expression levels of the co-stimulatory and MHC molecules were also comparable between the p38α-deficient and WT cDCs after stimulation. This result suggested that the upregulation of these molecules by DCs did not require p38α.
Figure 3.
Co-stimulatory molecule expression by p38α-deficient DCs. (a) The expression of co-stimulatory and MHC molecules on splenic CD8+ cDCs and CD8− cDCs in WT and p38αΔDC mice without stimulation. The results were representative of three pairs of individuals. (b) The co-stimulatory molecule expression on splenic CD8+ cDCs and CD8− cDCs from WT or p38αΔDC mice after incubation with OVA for 20 h. The gray shadows were isotype controls. The medium used was RPMI 1640 medium supplemented with 10% FBS, 1% P/S (penicillin and streptomycin) and GM-CSF (20 ng/ml). The DCs were sorted from 5 pairs of littermate mice. The data were representative of two independent experiments, each including duplicated samples.
In addition to the expression of co-stimulatory molecules, the survival status of DCs could also affect the efficiency of DC antigen presentation. Therefore, we examined the survival status of splenic CD8+ and CD8− cDCs from the p38αΔDC and WT mice after stimulation with TLR agonists or a pulse with the OVA protein for various times. No significant differences in cell survival were observed between the cDCs from the p38αΔDC and WT mice (Supplementary Figures S3b and c), confirming that the differences in T-cell proliferation induced by the p38αΔDC cDCs were not due to increased cell death of the p38αΔDC DCs. Consistent with the OVA peptide presentation by DCs, these data suggested that p38α did not influence the DC Ag presentation ability at the co-stimulation level.
p38α deletion led to decreased Nox2 expression and excessive antigen degradation in the CD8+ cDCs
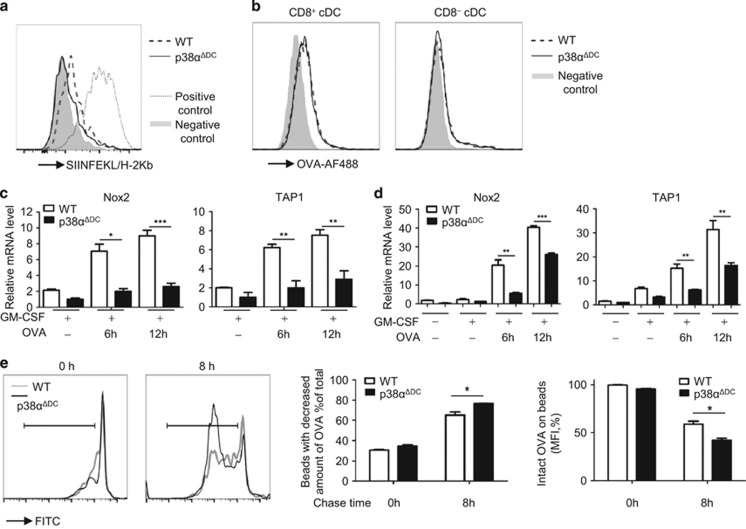
As shown above, p38α deficiency did not significantly affect the co-stimulation of T cells by DCs. Therefore, we speculated that p38α might regulate the cross-presentation of OVA by CD8+ cDCs. First, we compared the Ag peptide and MHC-I complex levels on the surface of the CD8+ cDCs from the p38αΔDC and WT mice using an antibody that bound to the OVA257–264/H-2Kb complex, which was the same OVA peptide/MHC-I complex recognized by the OT-I CD8+ T cells. The CD8+ cDCs were incubated with the OVA protein for 2 h, and free protein was washed away; then, the DCs were stained for the OVA257–264/H-2Kb complex on the surface 20 h later. The WT control CD8+ cDCs showed a moderate level of staining over the background (DCs without OVA protein incubation), whereas the p38α-deficient CD8+ cDCs showed very little positive staining above the background (Figure 4a). These results suggested that the decreased OT-I CD8+ T-cell proliferation induced by the p38α-deficient CD8+ cDCs was the result of decreased formation of the OVA257–264/H-2Kb complex on the cell surface, resulting in decreased cross-presentation of the OVA antigen by the p38α-deficient CD8+ cDCs.
Figure 4.
p38α had an important role in Ag processing. (a) Direct staining for the OVA peptide and MHC-I complex on the cell surface. The splenic CD8+ cDCs were incubated for 2 h with or without the OVA protein, washed, cultured for an additional 20 h, and then stained with SIINFEKL-H2Kb-biotin and streptavidin-PE. The dashed (WT) and solid (p38αΔDC) lines indicate staining with Ag pre-incubation; the gray shadow indicates background staining without Ag present during the pre-incubation (negative control) and the dotted line indicates staining with OVA257–264 pre-incubation (positive control). (b) Ag uptake into the cells. The splenic CD8+ or CD8− cDCs were incubated for 30 min at 37 °C with AF488-labeled OVA protein (5 μg/ml). The gray shadow indicates the negative control. The DCs were kept on ice for 10 min and then incubated with, OVA-AF488 on ice for 30 min. (c, d) Real time-PCR analysis of the of Nox2 and TAP1 mRNA levels in splenic CD8+ cDCs (c) and BM-derived CD24+ cDCs (d) from p38αΔDC mice and WT mice treated with GM-CSF (20 ng/ml) and OVA (100 μg/ml) as indicated. (e) Ag degradation was increased in the p38α-deficient CD24+ cDCs. The BM-derived CD24+ cDCs (1 × 106) were cultured with OVA-coated latex beads (3 × 106) for 30 min to allow phagocytosis, followed by removal of the free beads as described in the Methods. Then, the CD24+ cDCs were disrupted by lysis buffer immediately (0 h) or after another 8 h of culture (8 h). The recovered beads from cell lysis were stained with a FITC-conjugated anti-OVA antibody and the remaining OVA protein on beads was analyzed by flow cytometry. (a, b) The cDCs were sorted from five pairs of littermate mice. One of the two experiments with similar results is presented. The medium used was RPMI 1640 medium supplemented with 10% FBS, 1% P/S (penicillin and streptomycin) and GM-CSF (20 ng/ml). (c) The CD24+ cDCs were generated from BM cells from three pairs of littermate mice. (d) The CD8+ cDCs were sorted form 15 pairs of mice. (c, d) The data were representative of two independent experiments, each including triplicate samples. (e) The CD24+ cDCs were generated by BM cells from three pairs of littermate mice. The data were representative of two independent experiments, each including two repeats. The results were presented as the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001.
The reduced formation of the OVA peptide/MHC-I complex on the surface and the decreased Ag presentation by the p38α-deficient cDCs could be the result of decreased Ag uptake or deficient Ag processing by these cDCs. To test whether the Ag uptake was also affected by the p38α deficiency, splenic cDCs were isolated and incubated with the AF488-conjugated OVA protein at 37 °C for 30 min, and the uptake of the labeled OVA protein was measured by flow cytometry. Both the CD8+ and CD8− cDCs from the p38αΔDC mice showed similar uptake of the OVA protein into cells compared to that from WT mice (Figure 4b). These results indicated that the defect in Ag presentation by the p38α-deficient DCs was not at the Ag uptake stage.
To determine whether the defect in Ag cross-presentation by the p38α-deficient CD8+ cDCs was due to impaired Ag processing, we tested the effects of p38α on the expression of a few molecules important for Ag processing and presentation. We focused on two important molecules known to be involved in antigen processing during cross-presentation: NADPH oxidase 2 (Nox2), which is an essential regulator of phagosomal protease activity that limits Ag degradation, and transporter associated with Ag processing 1 (TAP1), which is an important transporter for Ag peptides into the endoplasmic reticulum (ER) or phagosome for subsequent loading onto MHC-I molecules. CD8+ and CD24+ cDCs were incubated with the OVA protein, followed by real-time PCR analysis to determine the Nox2 and TAP1 mRNA levels. As shown in Figures 4c and d, Nox2 and TAP1 expressions were significantly reduced in the CD8+ and CD24+ cDCs from the p38αΔDCmice compared with the cells from the WT mice after 6 or 12 h of incubation with OVA. Similar results were found in the CD24+ cDCs derived from BM cells from p38ɑΔHPC mice (Supplementary Figure S4a). After phagocytosis of the Ags, DCs execute an exquisitely controlled Ag degradation process to generate a sufficient quantity of potential peptides for T-cell recognition. Peptides with a suitable size for insertion into the MHC-I peptide binding groove are required for efficient Ag cross-presentation; these peptides are typically approximately eight to nine amino acids in length.7 Excessive Ag degradation would reduce the number of suitable peptides and diminish the potential epitopes for T-cell recognition. Nox2 was shown to regulate the phagosome pH in DCs to induce alkalization of the phagosomes (pH=7–7.5) via control of ROS (reactive oxygen species) production, which protected the Ags from excessive degradation by proteases (most of which have optimal activity at pH 5.5–6.5). Nox2-defective DCs showed enhanced phagosomal acidification, which led to elevated proteolytic activity of the proteases, immoderate antigen degradation, and defective cross-presentation. Next, we evaluated the functional consequences of decreased Nox2 expression on Ag degradation. We performed an Ag degradation assay using latex beads covalently coupled with the OVA protein, which could be selectively degraded in phagosomes. BM-derived CD24+ cDCs were pulsed with the beads for 30 min (pulse time). Then, the beads were washed away. The cells were incubated for another 8 h (chase time) and disrupted in lysis buffer for the isolation of the endocytosed beads. The amount of OVA protein remaining on the beads was measured with an OVA-specific antibody by flow cytometry. We observed that the degradation of the OVA protein was clearly increased in the p38α-deficient CD24+ cDCs, which exhibited a marked decrease in Nox2 expression over time compared with the wild-type DCs after a 30- min pulse and 8 h chase (Supplementary Figures S4b and e). Consequently, the excessive degradation of Ag might lead to inefficient antigen cross-presentation by p38α-deficient CD24+ cDCs. Taken together, our data suggested that the reduction of p38α-dependent expression of Nox2 at least partially accounted for the defective Ag cross-presentation ability of the p38α-deficient CD24+ cDCs.
p38α was required for cross-priming of antigen-specific CD8+ T lymphocytes in vivo
To confirm that the decreased Ag cross-presentation by p38α-deficient CD8+ cDCs in vitro also affected the activation of CD8+ T cells in vivo, we tested the activation of OVA-specific CD8+ T cells in vivo by injecting WT and p38αΔDC mice with OVA-coated X-ray-irradiated splenocytes and analyzed the proliferation of OVA-specific CD8+ T cells in the spleen after 7 days (Figure 5a). The results showed that the percentages (Figures 5b and c) and numbers (Figure 5d) of these OVA-specific T cells (gated on the CD8+ OVA257–264/H-2Kb pentamer+) were substantially reduced in the p38αΔDC mice compared with the WT mice, demonstrating that p38α was crucial for the cross-presentation of cell-associated antigens and the activation of cytotoxic T cells by CD8+ cDCs.
Figure 5.
p38α deficiency impaired cross-priming of T cells in vivo. (a) Splenocytes were irradiated by a 30 Gy X-ray, coated with OVA (1 mg/ml) at 37 °C for 45 min, and then free Ag was washed off. Then, the OVA-coated splenocytes (1 × 107 cells/mouse) were injected into the mice intravenously (i.v.). The proliferation of OVA-specific OT-I CD8+ T cells in the spleens of the injected mice was measured 7 days later using the APC-labeled MHC-I/SIINFEKL pentamer. The percentages (b, c) and numbers (d) of OVA-specific CD8+ T cells in the WT (n=5) and p38αΔDC mice (n=4) are presented. (e) CFSE-labeled OT-I CD8+ T cells (7 × 105cells/mouse) were transferred to the WT and p38αΔDC mice (i.v.). After 24 h, OVA protein (100 μg/mouse) was subcutaneously injected in the recipients at sites near the inguinal lymph nodes (OVA protein, 50 μg/site). A PBS injection was used as a negative control. After 36–48 h, two inguinal lymph nodes were collected from the recipients and analyzed for OVA-specific T-cell proliferation. The percentages (f, g) and numbers (h, i) of OVA-specific CD8+ T cells in the WT and p38αΔDC mice are presented (WT n=5 and p38αΔDC n=5 for the OVA protein-injected group; WT n=2 and p38αΔDC n=2 for the PBS-injected group). The results are presented as the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001.
To confirm the role of p38α in the cross-priming of T cells, we adoptively transferred CFSE-labeled OT-I CD8+ T cells to WT and p38αΔDC mice, which excluded potential defects of the CD8+ T cells in the p38αΔDC mice. After 24 h, the recipients were injected subcutaneously with the OVA protein. Within 36–48 h after OVA administration, the proliferation of OVA-specific OT-I T cells was determined in the draining lymph nodes of the recipients (Figure 5e). Rapid and robust proliferation of OVA-specific T cells was observed in the WT mice. In contrast, impaired proliferation of OVA-specific T cells (gated on CFSE+ CD3+ CD8+ TCR Vα2+) was observed in the p38-deficient mice (Figures 5f–i). These data confirmed that p38α was required for the efficient activation of T cells by both cell-associated and soluble Ags.
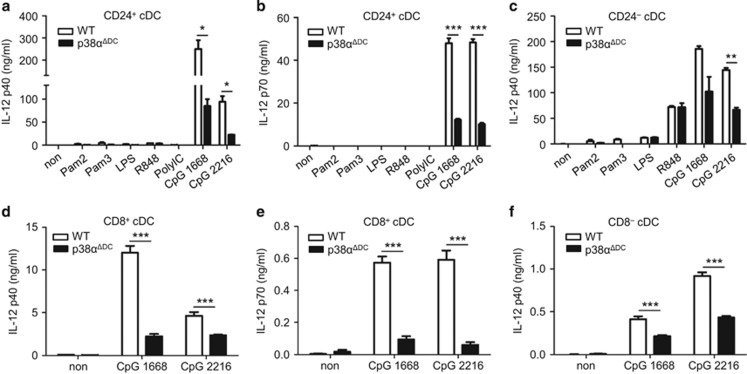
p38α was required for the normal production of IL-12 by cDCs
The cytokines produced by cDCs during antigen presentation also contribute to the activation of antigen-specific T cells. Therefore, we tested whether p38α also affected cytokine production by cDCs.
Among the mouse splenic cDCs, CD8+ cDCs are the major producers of IL-12p70, whereas CD8− cDCs produce a large amount of IL-12p40. IL-12p70 is composed of the IL-12p40 and IL-12p35 subunits.27 IL-12 can enhance CD8+ T-cell proliferation in antiviral CTL responses28 and can augment CTL activity through the increase in the perforin levels.29 To test whether p38α also regulated the production of IL-12, we analyzed TLR-triggered production of IL-12p40 and IL-12p70 by p38α-deficient cDCs. The CD24+ cDCs from the Flt3L-supplemented BM cultures were stimulated with a panel of TLR ligands in vitro, including Pam2 (TLR2/6), Pam3 (TLR2/1), polyIC (TLR3), LPS (TLR4), R848 (TLR7), CpG1668 and CpG2216 (TLR9). The CD24+ cDCs from the p38αΔDC mice produced considerably lower amounts of IL-12p40 and IL-12p70 (Figures 6a and b) in response to CpGs compared with the cells from the WT mice. Similarly, IL-12p40 production by the p38α-deficient CD24− cDCs was significantly reduced after stimulation by TLR9 agonists (Figure 6c). To confirm that similar results could be obtained with ex vivo DC subsets, we sorted splenic CD8+ and CD8− cDCs and stimulated them separately with CpGs. Similar results were observed with defective production of IL-12p40 and IL-12p70 by the p38α-deficient splenic CD8+ cDCs (Figures 6d and e) and impaired production of IL-12p40 by the p38α-deficient splenic CD8− cDCs (Figure 6f). Our results were in line with a previous report that p38 inhibitors reduced CD40 or LPS-induced production of IL-12 by MoDCs.30, 31 Therefore, we provided specific evidence for a role of p38α as an essential regulator of IL-12 production by cDCs.
Figure 6.
p38α deletion affected cytokine production by DCs in response to TLR stimulation. (a–c) Different subsets of BM-derived DCs from the WT and p38αΔDC mice were used to measure cytokine productions in response to the indicated TLR ligand stimulation. The production of IL-12p40 or IL-12p70 by CD24+ cDCs (a, b) and IL-12p40 by CD24− cDCs (c) after stimulation by TLR agonists for 36 h was measured by ELISA. Non-stimulated DCs were used as the negative controls. (d–f) Production of IL-12p40 /p70 by CpG-triggered splenic CD8+ cDCs and IL-12p40 by CD8− cDCs was examined by ELISA at 36 h. The medium used was RPMI 1640 supplemented with 10% FBS, 1% P/S (penicillin and streptomycin) and GM-CSF (20 ng/ml). (a–f) The results were representative of three independent experiments with at least two repeats. The data are presented as the mean±s.e.m. *P<0.05; **P<0.01; ***P<0.001.
Discussion
The p38 signaling pathway has been established as an important mediator of intracellular signaling during the innate and adaptive immune responses. Although the role of p38 signaling in DCs has been investigated in detail, most studies focused on its regulatory function in cytokine production. For instance, p38 inhibitors suppressed the production of IL-12p40, IL-12p70, IL-6 and TNF-a by MoDCs upon stimulation by the CD40 ligand16 or LPS,30 and the high-mobility group box protein 1 induced IL-6 secretion by MoDCs via a p38 MAPK-dependent pathway.32 These cytokines secreted by DCs are important for the differentiation and expansion of T cells.33, 34 Recent studies reported that p38α programmed DCs to promote Th17 differentiation during autoimmune neuroinflammation.20, 35 Deletion of p38α led to decreased IL-6 production but increased IL-27 expression by DCs, which impaired IL-17 and IL-23R expression in T cells.20, 35 Moreover, the DC-intrinsic p38α function was important for the differentiation of induced regulatory T cells (iTregs) and Th1 cells. p38α deficiency in mesenteric lymph node CD103+ DCs substantially diminished TGF-β2 expression, which impaired the generation of iTregs but promoted Th1 differentiation during mucosal immune tolerance.22 However, the role of p38 signaling in the most characteristic function of DCs (Ag presentation) has not been thoroughly investigated. Some studies reported that TLR signaling enhanced the DC Ag capture ability, which could be blocked by the p38 inhibitor SB203580.36 However, whether p38 MAPK also directly participated in Ag presentation of DCs at steady state without TLR activation was unknown. Therefore, in this study, we investigated the role of p38α in Ag presentation by DCs and reported for the first time that p38α had an important role in the cross-presentation of CD8+ cDCs and had a moderate effect on the direct presentation ability of CD8− cDCs. Conversely, the direct presentation ability of CD8+ cDCs was not significantly affected by p38α deficiency. This varying influence on the direct presentation ability of two cDC subsets might be due to the characteristics of the cells themselves because CD8− cDCs were reported to be more efficient than CD8+ cDCs at direct presentation of the soluble OVA protein via the MHC class II pathway.37, 38 Moreover, we found that p38α deficiency led to excessive Ag degradation and a subsequent reduction in Ag cross-presentation by CD8+ cDCs without affecting Ag uptake or co-stimulation. The dispensable role of p38α in Ag uptake was also demonstrated in other reports; for instance, the phagocytosis of citrobacter rodentium by DCs was not regulated by p38α.39 Furthermore, we demonstrated that p38α was important for the activation of OVA-specific CD8+ T cells in vivo. This finding was supported by other studies that demonstrated that the DC intrinsic function of p38α was crucial for hapten-specific CD8+ T-cell priming and skin inflammatory responses. This effect accounted for a much lower level of TLR2-triggered CCL7 by p38α-deficient DCs, which led to a reduced interaction between DC and T cells and resulted in failure of T-cell priming.40
The antigen cross-presentation is involved in many immune responses, including responses to viral infections, tumors and transplants.41, 42 Upon uptake, antigen proteins are partially degraded in phagosomes. Then, the antigens access the cytosol, where they are degraded into peptides by the proteasome. The peptides are transported by TAP1 into the ER lumen43 or phagosomes44, 45, 46 for loading onto MHC-I molecules.47 The function of TAP1 in antigen presentation has been extensively studied (for example, TAP1−/− DCs exhibited defective peptide translocation45 and OVA cross-presentation ability48 and TAP deficiency led to defective stable assembly and intracellular transport of MHC-I molecules, resulting in a failure to present cytosolic antigens to CD8+ cytotoxic T cells).49 Although p38α-deficient CD8+ cDCs had a defect in the upregulation of the TAP1 mRNA level during Ag presentation, this defect did not lead to defective MHC-I expression as observed in TAP1-deficient mice,49 suggesting that the reduced TAP1 mRNA level might not be the dominant factor leading to the defective cross-presentation by p38α-deficient CD8+ cDCs. For efficient antigen cross-presentation, peptides of suitable size with proper immunogenicity and a sufficient quantity must be generated under a precisely controlled process. The antigenic peptide for cross-presentation is approximately eight to nine amino acids in length, which is suitable for insertion into the MHC-I peptide binding groove.7 Exacerbated Ag degradation would damage potential T-cell epitopes and decrease the amount of antigenic peptides, which is detrimental for Ag cross-presentation. Recent studies demonstrated a pivotal role for Nox2 in the regulation of antigen degradation and the cross-presentation ability of DCs.50, 51 Nox2 generates superoxides in the lumens of phagosomes, which react with H+ to make hydrogen peroxide and ROS.52 Consequently, the DC phagosome is sustained in an alkalescent environment, leading to a low degree of proteolytic activity that helps to avoid excessive Ag degradation and to guarantee a sufficient array of peptides for presentation.53 Nox2−/− DCs of both mouse48 and human54 origin presented enhanced phagosomal acidification and excessive antigen degradation, resulting in markedly decreased antigen cross-presentation. However, the mechanism by which the expression or functions of Nox2 are regulated during Ag processing and presentation is not completely understood. Here we showed that deletion of p38α resulted in a reduction in the expression of Nox2 in CD8+ cDCs, which might lead to enhanced Ag degradation and inefficient antigen cross-presentation. In line with our results, a number of studies revealed that p38α had a role in the expression and activation of Nox2. In the heart and aorta, a p38 inhibitor significantly suppressed both Nox2 mRNA expression and ROS generation, which helped to protect the rat against the hypertension and organ damage induced by Angiotensin II.55 Moreover, p38 was required for activation of the NADPH oxidase (composed of Nox2, p67 phox, p22 phox and p47 phox) and the production of ROS induced by hyperoxia in human lung endothelial cells;56 p38 was also shown to regulate NADPH oxidase activity and O2− generation in bovine polymorphonuclear leukocytes.57 Activation of p38 mediated S100A8/A9 translocation, leading to Nox2 activation in neutrophils.58 In addition, studies of the hepatitis C virus nonstructural protein 3-induced oxidative burst demonstrated the involvement of p38 signaling in NADPH oxidase activation and the generation of ROS in human monocytes.59 All of these studies suggested an important role for p38-mediated Nox2 activation in various physiological processes. Our study provided new evidence for the importance of p38α-dependent Nox2 in DC functions, which at least partially accounted for the impaired Ag cross-presentation by DCs and the subsequent T-cell activation. Further studies are required to investigate how p38α exerts its effects on the expression and function of Nox2.
GM-CSF-induced MoDCs, which are equivalent to DCs under inflammatory conditions, were used in most previous studies.60 Our study examined the role of p38α in the functions of DCs in the mouse spleen or derived from Flt3L-BM cultures, which presented the majority of DCs in lymphoid tissues under a steady state.11, 61 Similar defects in Ag processing and presentation were observed in p38α-deficient DCs from both sources, confirming an important role for p38α in regulating the functions of DCs in lymphoid tissues.
In summary, our study provided evidence that p38α in DCs is a critical regulator of Ag cross-presentation and IL-12 production. The novel information obtained in our study should facilitate our understanding of the key molecular mechanisms that regulate the Ag presentation process and help with the development of novel therapeutic strategies for the treatment of infectious diseases and tumors.
Acknowledgments
This work was supported by grants from the Chinese Ministry of Science and Technology National Major Scientific Research Program (2015CB943200), the Key Project Grant from the National Natural Science Foundation of China (31330027), the National Basic Research Program of China (2015CB553800) and the Tsinghua Science Foundation (20111080963).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity 2007; 26: 741–750. [DOI] [PubMed] [Google Scholar]
- Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev 2010; 234: 45–54. [DOI] [PubMed] [Google Scholar]
- Hochrein H, O'Keeffe M, Wagner H. Human and mouse plasmacytoid dendritic cells. Hum Immunol 2002; 63: 1103–1110. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 2002; 17: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Martinez E, Planes R, Anselmi G, Reynolds M, Menezes S, Adiko AC et al. Cross-Presentation of cell-associated antigens by MHC class I in dendritic cell subsets. Front Immunol 2015; 6: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol 2012; 12: 557–569. [DOI] [PubMed] [Google Scholar]
- Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol 2011; 11: 823–836. [DOI] [PubMed] [Google Scholar]
- Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol 2008; 8: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med 2004; 199: 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol 2001; 166: 5327–5330. [DOI] [PubMed] [Google Scholar]
- Sathe P, Pooley J, Vremec D, Mintern J, Jin JO, Wu L et al. The acquisition of antigen cross-presentation function by newly formed dendritic cells. J Immunol 2011; 186: 5184–5192. [DOI] [PubMed] [Google Scholar]
- Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev 2010; 234: 18–31. [DOI] [PubMed] [Google Scholar]
- Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat Rev Immunol 2010; 10: 403–414. [DOI] [PubMed] [Google Scholar]
- Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev 2009; 228: 212–224. [DOI] [PubMed] [Google Scholar]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res 2005; 15: 11–18. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Uchi H, Urabe K, Chen Q, Furue M, Moroi Y. Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int Immunol 2004; 16: 1701–1709. [DOI] [PubMed] [Google Scholar]
- Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol 2001; 166: 3837–3845. [DOI] [PubMed] [Google Scholar]
- Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr Opin Immunol 2010; 22: 124–130. [DOI] [PubMed] [Google Scholar]
- Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S et al. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell 2000; 6: 109–116. [PubMed] [Google Scholar]
- Huang G, Wang Y, Vogel P, Kanneganti TD, Otsu K, Chi H. Signaling via the kinase p38alpha programs dendritic cells to drive TH17 differentiation and autoimmune inflammation. Nat Immunol 2012; 13: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang M, Wang S, Hong B, Wang Z, Li H et al. p38 MAPK-inhibited dendritic cells induce superior antitumour immune responses and overcome regulatory T-cell-mediated immunosuppression. Nat Commun 2014; 5: 4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Wang Y, Chi H. Control of T cell fates and immune tolerance by p38alpha signaling in mucosal CD103+ dendritic cells. J Immunol 2013; 191: 650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y et al. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol 2008; 180: 5075–5082. [DOI] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol 1998; 76: 34–40. [DOI] [PubMed] [Google Scholar]
- Xiao J, Zhou H, Wu N, Wu L. The non-canonical Wnt pathway negatively regulates dendritic cell differentiation by inhibiting the expansion of Flt3+ lymphocyte-primed multipotent precursors. e-pub ahead of print 8 June 2015 doi:10.1038/cmi.2015.39. [DOI] [PMC free article] [PubMed]
- Liu C, Zhang Y, Li J, Wang Y, Ren F, Zhou Y et al. p15RS/RPRD1A (p15INK4b-related sequence/regulation of nuclear pre-mRNA domain-containing protein 1A) interacts with HDAC2 in inhibition of the Wnt/beta-catenin signaling pathway. J Biol Chem 2015; 290: 9701–9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 2004; 202: 96–105. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Seder RA, Reddy A, Feldman MV. IL-12 in conjunction with dendritic cells enhances antiviral CD8+ CTL responses in vitro. J Clin Invest 1996; 98: 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom ET, Horvath JA. Cellular and molecular mechanisms of the IL-12-induced increase in allospecific murine cytolytic T cell activity. Implications for the age-related decline in CTL. J Immunol 1994; 152: 4242–4254. [PubMed] [Google Scholar]
- Yu Q, Kovacs C, Yue FY, Ostrowski MA. The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J Immunol 2004; 172: 6047–6056. [DOI] [PubMed] [Google Scholar]
- Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol 2003; 171: 4984–4989. [DOI] [PubMed] [Google Scholar]
- Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol 2004; 173: 307–313. [DOI] [PubMed] [Google Scholar]
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol 1995; 154: 5071–5079. [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med 2005; 202: 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Wang Y, Chi H. Regulation of TH17 cell differentiation by innate immune signals. Cell Mol Immunol 2012; 9: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Wallin RP, Matthews SP, Svensson HG, Zaru R, Ljunggren HG et al. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science 2004; 305: 1153–1157. [DOI] [PubMed] [Google Scholar]
- Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA 2006; 103: 10729–10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud MH, Proietto AI, Gartlan KH, Kitsoulis S, Curtis J, Wettenhall J et al. Signal regulatory protein molecules are differentially expressed by CD8− dendritic cells. J Immunol 2006; 177: 372–382. [DOI] [PubMed] [Google Scholar]
- Shim EJ, Bang BR, Kang SG, Ma J, Otsuka M, Kang J et al. Activation of p38alpha in T cells regulates the intestinal host defense against attaching and effacing bacterial infections. J Immunol 2013; 191: 2764–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritprajak P, Hayakawa M, Sano Y, Otsu K, Park JM. Cell type-specific targeting dissociates the therapeutic from the adverse effects of protein kinase inhibition in allergic skin disease. Proc Natl Acad Sci USA 2012; 109: 9089–9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 1994; 264: 961–965. [DOI] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol 2001; 19: 47–64. [DOI] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 1995; 267: 243–246. [DOI] [PubMed] [Google Scholar]
- Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci USA 2003; 100: 12889–12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 2003; 425: 397–402. [DOI] [PubMed] [Google Scholar]
- Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 2003; 425: 402–406. [DOI] [PubMed] [Google Scholar]
- Shepherd JC, Schumacher TN, Ashton-Rickardt PG, Imaeda S, Ploegh HL, Janeway CA Jr et al. TAP1-dependent peptide translocation in vitro is ATP dependent and peptide selective. Cell 1993; 74: 577–584. [DOI] [PubMed] [Google Scholar]
- Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol 1997; 27: 280–288. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell 1992; 71: 1205–1214. [DOI] [PubMed] [Google Scholar]
- Rybicka JM, Balce DR, Chaudhuri S, Allan ER, Yates RM. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J 2012; 31: 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 2006; 126: 205–218. [DOI] [PubMed] [Google Scholar]
- Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity 2009; 30: 544–555. [DOI] [PubMed] [Google Scholar]
- Lam GY, Huang J, Brumell JH. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol 2010; 32: 415–430. [DOI] [PubMed] [Google Scholar]
- Mantegazza AR, Savina A, Vermeulen M, Perez L, Geffner J, Hermine O et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 2008; 112: 4712–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC et al. Effects of p38 MAPK inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol 2007; 49: 362–368. [DOI] [PubMed] [Google Scholar]
- Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ et al. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 2003; 284: L26–L38. [DOI] [PubMed] [Google Scholar]
- Yamamori T, Inanami O, Nagahata H, Cui Y, Kuwabara M. Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes. FEBS Lett 2000; 467: 253–258. [DOI] [PubMed] [Google Scholar]
- Schenten V, Melchior C, Steinckwich N, Tschirhart EJ, Brechard S. Sphingosine kinases regulate NOX2 activity via p38 MAPK-dependent translocation of S100A8/A9. J Leukoc Biol 2011; 89: 587–596. [DOI] [PubMed] [Google Scholar]
- Bureau C, Bernad J, Chaouche N, Orfila C, Beraud M, Gonindard C et al. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J Biol Chem 2001; 276: 23077–23083. [DOI] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 2007; 7: 19–30. [DOI] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M et al. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol 2005; 174: 6592–6597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.