Abstract
Interleukin-17 (IL-17), IL-21, IL-22 and IL-23 can be grouped as T helper 17 (Th17)-related cytokines because they are either produced by Th17/Th22 cells or involved in their development. Here, we review Th17-related cytokines/Th17-like cells, networks/signals and their roles in immune responses or immunity against Mycobacterium tuberculosis (Mtb) infection. Published studies suggest that Th17-related cytokine pathways may be manipulated by Mtb microorganisms for their survival benefits in primary tuberculosis (TB). In addition, there is evidence that immune responses of the signal transducer and activator of transcription 3 (STAT3) signal pathway and Th17-like T-cell subsets are dysregulated or destroyed in patients with TB. Furthermore, Mtb infection can impact upstream cytokines in the STAT3 pathway of Th17-like responses. Based on these findings, we discuss the need for future studies and the rationale for targeting Th17-related cytokines/signals as a potential adjunctive treatment.
Keywords: immunotherapy, miRNA, STAT, Th17-related cytokines
Introduction
Tuberculosis (TB) is now one of 10 most frequent causes of death and the top killer in infectious diseases due to the HIV/AIDS epidemics and the increased spread of multidrug-resistant TB (MDR-TB).1 Mycobacteria tuberculosis (Mtb), the causative agent of TB, is an intracellular microorganism that lives in macrophages and lung epithelial cells.2 Cell-mediated immunity has a crucial role in the control of Mtb infection and ultimately determines whether Mtb infection is cleared, latent or active with TB consequences. Approximately one-third of the world's population has been infected by Mtb, but only 5–10% of them will eventually become ill with TB.3 However, persons with compromised immune systems, such as those living with HIV, malnutrition or diabetes, have a much higher risk of developing TB.1
Although cellular immune responses can inhibit or limit bacterial growth, they can also damage host tissues. It is therefore critical to maintain the cellular immune response balance.4 To achieve this balance, the host uses some strategies, such as producing cytokines, to monitor and mediate effector cell function.5 Cytokines are important in cell signaling and can affect the behavior of adjacent cells. In Mtb infection, the complex interaction between the immune system and pathogen is closely related to the production of various levels of cytokines, which contribute to determining outcomes of the infection.6
T helper 17 (Th17)-related cytokines comprise interleukin-17A (IL-17A)/IL-17F, IL-21, IL-22 and IL-23, which are produced by Th17/Th22 cells or involved in their development. Th17 cells differentiate with the induction of IL-6, transforming growth factor-β (TGF-β) and IL-1β and are expanded by IL-23 via the STAT3 signaling pathway.7 Th17 cytokines can be produced by CD4+ T, CD8+ T, γδ T, natural killer T (NKT) and NK cells and can regulate effector functions of other immune cells after Mtb infection.8 Cellular signal pathways of Th17-related cytokines may be key modulators of adaptive immune responses.9 Th17-related cytokines can also trigger the production of anti-microbial peptides involved in the defense against bacterial pathogens.9
In this article, we review Th17-related cytokines, networks/signals and their roles in immune responses or immunity against Mtb infection. We also outline studies showing how Mtb microorganisms manipulate Th17-related cytokine pathways and upstream cytokines of the STAT3 signal in primary TB. Furthermore, we discuss the evidence that immune responses of the STAT3 signal pathway and Th17-like T-cell subsets are dysregulated or destroyed in patients with TB. Finally, we discuss future studies in TB research.
Th17-Related cytokines/Th17-like cells and their roles in Mtb infection
IL-17 and Th17 cells
IL-17 family cytokines contain six members, IL-17A–17F. Among them, IL-17A and IL-17F share a similar structure and have similar roles in the immune response against Mtb infection.10, 11 The roles of other members of the IL-17 family are currently not known in Mtb infection.
In patients with chronic TB, IL-17A production appears to be decreased. The production of IL-17A by peripheral blood mononuclear cells (PBMCs) isolated from chronic TB patients is significantly lower than PBMCs isolated from healthy control (HC) subjects. Under the ex vivo purified protein derivative stimulation, PBMCs from TB patients also secrete lower levels of IL-17A than those isolated from HC subjects. The decrease in IL-17A production, correlated with the exhaustion of T cells, may be due to the overexposure to Mtb antigens and hyperexpression of the exhausted marker programmed death-1 (PD-1).12, 13 Increased PD-1 expression appears to be relevant to the depressed production of IL-17A in TB because anti-PD-1 antibodies can enhance IL-17A production by Mtb-stimulated CD4+ T cells of TB patients.14 Anti-TB therapy can decrease PD-1 expression and increase IL-17A production by CD4+ T cells.15, 16
IL-17A is a protective cytokine against mycobacteria infection in the host; suppressing IL-17A production will increase TB susceptibility.17 In fact, there is a decreased risk for TB development related to the IL-17A–197A allele, AA genotype and A carrier (AG/AA).18 IL-17A is involved in the formation and stability of granulomas by increasing chemokine production, which helps recruit inflammatory cells migrating to the Mtb-infected sites.10, 19 The immune recall response of CD4+ T cells producing IL-17 occurs simultaneously with the expression of the chemokines of CXCL11, CXCL10 and CXCL9 and facilitates pulmonary recruitment of Th1 cells and anti-TB immunity.20 Virtually, IL-17RA, the A subset of the IL-17A receptor, can mediate the expression of CXCL-1 and CXCL-5, which are important for recruiting neutrophils moving to the lungs of Mtb-infected mice.21 Notably, when the IL-17A gene is knocked out in mice, granulomas in the mycobacteria-infected lung fail to mature, and the expression of adhesion molecules of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 decreases, which leads to an impaired protective response.19 Furthermore, the adoptive transfer of γδ T cells, which are the dominating IL-17A-producing cells in the lung granuloma, can rebuild granuloma in the IL-17A knockout (KO) mice.19
The immune protective role of IL-17F in Mtb infection is similar to IL-17A, which is 50% homologous to IL-17F in structure.17 The sequence variant of IL-17F is also correlated with susceptibility to TB.22 Interestingly, we have recently demonstrated that IL-17F and IL-17A can induce the recall response and effector function of TB phosphoantigen-specific Vγ2Vδ2 T cells after Bacillus Calmette–Guérin (BCG) immunization and Mtb infection in nonhuman primates,23 suggesting a role of IL-17 in adaptive γδ T-cell responses.
Interleukin-21
Th17/22-like γδ T cells, which express the transcription factor RORγt (retinoic acid receptor-related orphan receptor-γt) and IL-23R (IL-23 receptor), produce not only IL-17 and IL-22 but also IL-21 under the stimulation of IL-1β and IL-23; participation of the T-cell receptor is not necessary.7 After stimulation with Mtb antigens, NKT cells isolated from TB patients also produced IL-21 and other cytokines such as IL-17, interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and IL-2.24, 25 IL-21-expressing NKT cells showed an effector memory phenotype and expressed CXCR5.25 However, the main sources producing IL-21 are activated CD4+ T cells with the induction of Mtb-specific peptides.26 IL-21 signaling plays important roles in host resistance to Mtb infection.27 In TB patients, circulating levels of IL-21 are significantly diminished compared with latent tuberculosis infection (LTBI) or HC individuals.27, 28
The IL-21/ IL-21R signaling pathway has pleiotropic effects on immunity and has an important role in T-cell immune responses against Mtb infection because it contributes to augment CD8+ T-cell priming and improve T-cell accumulation in the lungs, enhancing the production of effector cytokines.27 IL-21 signaling may also inhibit exhaustion of T cells since more CD4+ and CD8+ T cells expressing T-cell immunoglobulin and mucin domain 3 (TIM-3) and PD-1 are observed in chronically infected IL-21R−/− mice.12 These IL-21R KO mice show an increased susceptibility to Mtb infection, characterized by earlier mortality and higher lung bacterial burden compared with wild-type (WT) mice.27
Circulating T follicular helper (Tfh) cells can also produce IL-21 and have important roles in immunity to infections.29 The frequencies of Tfh cell subsets induced by Mtb antigen are significantly lower in TB patients than those in LTBI subjects.30 Similarly, frequencies of antigen-induced IL-21-producing Tfh cells are also obviously lower in TB patients, with diminished circulating levels of IL-21.30 Although IL-21 is associated with the expansion of B cells and helps B cells secrete antibodies of immunoglobulin G (IgG) and IgA, it may also participate in local immune responses for fighting against Mtb infection.25
IL-22 and Th22 cells
T cells and NK cells are the major sources of IL-22. Accumulating data suggest that Th17 and Th22 are two distinct cell subsets in humans and nonhuman primates.31 In TB patients, the IL-22 concentration in the serum is lower than that in LTBI subjects, and anti-TB treatment could enhance IL-22 antigen-specific responses in active TB patients.32, 33, 34 Although frequencies of Th22 cells in the blood of TB patients are low, the IL-22 protein level in bronchoalveolar lavage (BAL) fluid is high.35
IL-22 induces the production of anti-bacterial peptides including β-defensins, lipocalin and regenerating islet-3-γ from lung epithelial cells and monocytes and macrophages to kill pathogens.36 In addition, macrophages express the IL-22 receptor within TB granulomas in the lungs, and IL-22 can activate macrophages to mediate mycobacterial control and induce TNF-α production (refs 31, 37, 38, data not shown).
Surprisingly, the productions of IL-22 and IFN-γ are reciprocally related. The expression of IL-22 is significantly increased in the shortage of IFN-γ likewise, the production of IFN-γ is enhanced when monoclonal anti-IL-22 antibodies inhibit IL-22 signaling.39 For example, Vγ2Vδ2 T cells activated by phosphoantigen HMBPP ((E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate) can increase IFN-γ production and downregulate a potential over-reactive IL-22 response in lymphocytes from BAL fluid, blood and lymph nodes.31 The production of endogenous IFN-γ by HMBPP-activated Vγ2Vδ2 T cells appears to be the underlying mechanism since adding anti-IFN-γ-neutralizing antibody can abrogate or reduce the capability of HMBPP-induced Vγ2Vδ2 T cells to decrease IL-22 production.40, 41 Similarly, anti-IL-22 monoclonal antibody treatment can increase the expression of IFN-γ.39, 42 IL-22-producing cells may regulate protection against Mtb infection. Primary Mtb infection in nonhuman primates induces significant increases in T cells, producing IL-22. Moreover, these IL-22-producing cells are more apparent in the lungs than in the lymphoid tissues and blood.31, 40, 43 With the help of confocal microscopy and immunohistochemistry, IL-22-producing T cells can be detected in situ in lung TB granulomas.44 Appreciable numbers of mature IL-22-producing T cells in lung TB granulomas suggest that they may have an important role in protective immune responses to Mtb infection, despite the fact that an over-reactive IL-22 or Th22 response may also contribute to TB pathology.31 The hypothesis of protective IL-22/Th22 responses in TB is also supported by the observation that Th22 cells carrying membrane-borne IL-22 and IL-22 released by NK cells from Mtb-infected individuals can inhibit the intracellular growth of Mtb bacilli.38, 40
Moreover, the rs2227473 single-nucleotide polymorphism (SNP) in IL-22 is related to the risk of pulmonary TB, and the G allele is the risk factor.45 This SNP may have a significant role in the protective immune process against TB by affecting the IL-22 expression of PBMCs.45
Despite a lack of conclusive in vivo evidence,46 the above findings suggest that Th22 or IL-22 may contribute to protection against Mtb infection.38, 40, 42 Currently, functions and dysfunctions of IL-22 and Th22 cells in human TB remain incompletely characterized. Further in-depth studies may help elucidate the roles of IL-22/Th22 in anti-TB immunity or TB pathology.
Interleukin-23
IL-23 is a heterodimeric cytokine comprised of an IL-12B (IL-12p40) subunit and the IL-23A (IL-23p19) subunit, and its functional receptor includes IL-12Rβ1 and IL-23R. IL-23 is mainly produced by antigen-presenting cells.20 In vitro, Mtb can induce monocyte-derived dendritic cells (DCs) and human alveolar macrophages to produce IL-23.47, 48, 49 In vivo, the expression of IL-23α is enhanced in PBMCs in nonhuman primates at the early stage of mycobacteria infection; later, its expression decreases to the normal level.50 Moreover, levels of IL-12p40, one subunit of IL-23, are higher in the serum of TB patients than in HCs and decrease after anti-TB treatments.48, 50
IL-23 could mediate its effects on both innate and adaptive arms of the immune system that express the IL-23R.48 Th17 cells are the representative T-cell subset that vigorously responds to IL-23.51 The IL-23/IL-17 axis appears to act as an important modulator of immune responses associated with all phases of Mtb infection, with protective roles reported in a mouse TB model.20
TCR γδ T cells can be one of the main sources of IL-17 responding to IL-23 stimulation.52 Our studies recently showed that IL-23 and other Th17-related cytokines can induce proliferation and expansion of Vγ2Vδ2 T cells in the presence of HMBPP.23 Mycobacteria vaccination/infection of macaques enhances the ability of IL-23 to expand HMBPP-activated Vγ2Vδ2 T cells, and those expanded cells have multieffector functions for producing cytokines of IL-17, IL-22, IL-2 and IFN-γ.23 Autocrine production of IFN-γ and IL-2 can, in turn, enhance IL-23/HMBPP-stimulated recall-like expansion of Vγ2Vδ2 T cells.23, 53 The STAT3-dependent signal pathway is involved in the IL-23 expansion of Vγ2Vδ2 T cells.48, 54, 55 Data from studies using PBMCs of TB patients show that the IL-23-IL-17 axis is likely dysregulated or damaged by overexposure to stimulation of Mtb antigens in chronic infection.56, 57 This will be discussed in detail below.
In summary, these findings demonstrate that Th17-related cytokines and Th17/Th22 cells may be devoted to immune responses to Mtb infection and may be involved in protective immunity against primary Mtb infection. There are some experimental data implicating dysregulated immune responses of selected Th17-related cytokines in patients with TB.
Mtb microorganisms may manipulate Th17-related cytokine signaling pathways for their surviving benefits in primary Tb
In the primary Mtb infection phase, STAT3 pathways are stimulated in phagocytes (monocytes/macrophages),58 but their protective effects are suppressed by the products of Mtb and/or by immunosuppressive cytokines from Mtb-stimulated host cells.59, 60, 61 The STAT3 pathway and Th17-related cytokines appear to be influenced by Mtb infection in the following complex aspects.
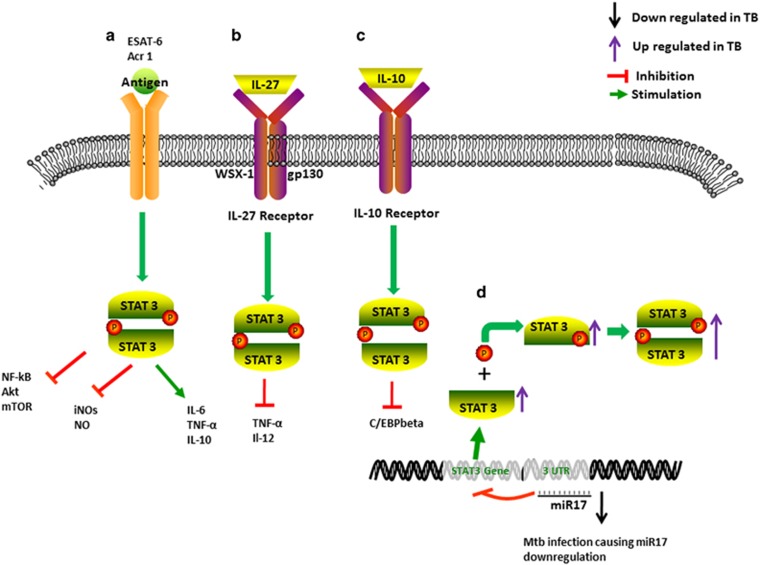
After infection, virulent Mtb organisms survive in phagocytes, and Mtb antigens directly simulate the STAT3 signal pathway to regulate host immunity. The early secreted antigenic target of 6 kDa (ESAT-6) is an essential virulence factor, stimulating macrophages to produce IL-6 via STAT3 activation (Figure 1).62 It induces phosphorylation and DNA binding of STAT3, which can be blocked by STAT3 inhibitors.62 The induced IL-6 is responsible for the suppression of Th1 responses and the suppression of Mtb-infected and Mtb-uninfected bystander macrophage responses to IFN-γ, which induces autophagy in Mtb-infected macrophages.63, 64 Mtb antigens expressed in latency, such as α-crystalline 1 (Acr1), can also interfere with the differentiation of DCs by targeting STAT3 pathways (Figure 1).65 Continuous activation of STAT3 would inhibit the translocation of nuclear factor-κB (NF-κB) in DCs treated by Acr1.65 Thus, Mtb in latently infected individuals could use these strategies to survive and antagonize the attempts of eradication by the anti-TB immune system.
Figure 1.
Primary Mtb infection exploits STAT3-dependent signals in phagocytes for bacterial survival benefits. (a) Mtb antigens ESAT-6 and α-crystalline 1 (Acr1) activate the STAT3 signal pathway to induce the production of IL-6 and IL-10 and inhibit the translocation of NF-κB, Akt and mTOR in phagocytes in primary Mtb infection. This also represses the expression/synthesis of iNOS and NO, favoring Mtb survival. (b) Mtb-induced IL-27 induces STAT3 phosphorylation and inhibits the production of TNF-α and IL-12 in activated macrophages. (c) IL-10 exerts its effects in a partly STAT3-dependent manner, inhibiting the production of C/EBP-beta, with potential enhancement of HIV-1 replication, as observed in THP-1 cells. (d) Mtb infection facilitates STAT3 expression and phosphorylation by decreasing the repression of miR-17. ESAT-6, early secreted antigenic target of 6 kDa; IL, interleukin; iNOS, inducible nitric oxide synthase; NO, nitric oxide; Mtb, Mycobacterium tuberculosis; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; STAT3, signal transducer and activator of transcription 3; TB, tuberculosis; TNF-α, tumor necrosis factor-α.
Mtb infection induces the production of immunosuppressive cytokines including IL-10 to affect STAT3 activation.66 After Mtb infection, IL-10 levels and STAT3 and pSTAT3 expression levels increase significantly in the first week. The production of IL-10 is strongly correlated with the expression of STAT3 and pSTAT3 proteins.67 In vitro, IL-10 can regulate the protective phenotype in Mtb-infected phagocytes including monocyte-derived macrophages, phorbol myristate acetate-treated THP-1 cells and human alveolar macrophages.68 IL-10 plays its immunosuppressive effects on this early response of Mtb-infected macrophages in a partly STAT3-dependent manner. The inhibitory activity of Mtb would be reversed when IL-10 is neutralized through the addition of soluble IL-10 receptor.66 The interaction of Mtb with differentiating monocytes rapidly activates the STAT pathway, which likely participates in IL-10 gene expression.69 STAT3 activation leads to the inhibition of cytokines IL-6, IFN-γ, TNF-α and MIP-1β (macrophage inflammatory protein-1β).70 In the primary infection phase, STAT3 also represses the expression/synthesis of inducible nitric oxide synthase and nitric oxide, which are important factors that kill intracellular Mtb.2, 71
Unlike virulent Mtb, the TB vaccine BCG can drive effective Th1-cell responses dependent on Th17-related cytokines to defeat IL-10 inhibitory effects induced by bacteria. Prostaglandin-E2 induced by BCG promotes IL-10 expression while simultaneously inducing IL-23 production and differentiation of Th17 cells. The ability of IL-17 to decrease IL-10 and increase IL-12 production admits the generation of protective Th1-cell responses and subsequent vaccine-induced protection against Mtb challenge. Thus, the IL-23/IL-17 pathway could overcome IL-10-mediated inhibition to drive Th1-cell responses.51
IL-27, another immunosuppressive cytokine, can also modulate the STAT3 pathway.72 In vitro, IL-27 induces STAT3 phosphorylation and inhibits activated macrophages to produce TNF-α and IL-12 and regulate the Th1 response during Mtb infection.72 Furthermore, IL-27 can modulate excessive inflammation via a feedback mechanism.72, 73 Whether this can be observed in vivo remains to be investigated in TB.
Mtb infection may manipulate or utilize the STAT3 pathway by inducing the differentiation of monocytes toward an immunosuppressive (M2-like) macrophage activation program.70 These M2-like macrophages are characterized by the phenotype of CD16+CD163+MerTK+pSTAT3+ and can function as an immunomodulator.67 This process relies on STAT3 activation and shows a detrimental role in TB.67 There is a significant connection between the progression of the disease and the copiousness of the CD16+CD163+MerTK+pSTAT3+ cells.67
Mtb infection could also regulate the STAT3 effect by mediating the expression of microRNAs (miRNAs) targeted on STAT3.55, 74 Mtb infection causes the downregulation of miR-17 and corresponding upregulation of its target STAT3 to suppress autophagy,74, 75 which has an important role in the bacterial burden control.76 Forced expression of miR-17 reduces the production of STAT3 and regulates autophagy.74
Thus, in vitro or animal studies demonstrate that during primary TB, Mtb microorganisms can manipulate the STAT3 pathway for their surviving benefits using the following strategies: producing Mtb products, inducing immunosuppressive cytokine/inflammatory macrophage induction and altering specific miRNAs targeting STAT3.
Immune responses of the STAT3 pathway and Th17-like cells are dysregulated or destroyed in Tb patients
In TB patients, the chronic Mtb infection appears to destroy the STAT3 signal pathway in T cells, leading to selective impairing of the signal effect of IL-23.53, 59 IL-23 is a positive regulator of the STAT3 signal pathway and can act on both the differentiation of CD4 Th0 to IL-23R-expressing Th17 cells and the maturation of γδ T cells, which constitutively express IL-23R and are important sources to produce IL-17.20
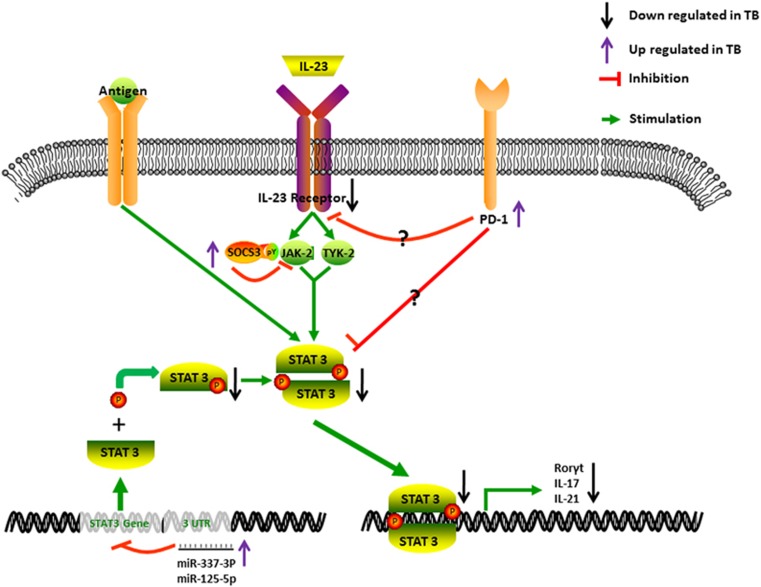
CD4 T cells of TB patients are demonstrated to secrete less IL-17 under stimulation of Mtb than do those of LTBI subjects,48 although Mtb-stimulated monocytes from TB patients express similar amounts of IL-23p19 mRNA and protein as those from LTBI subjects. Mtb-stimulated CD4+ T cells from TB patients express less IL-23R and pSTAT3 than those from LTBI subjects.16 This may be correlated with highly expressed PD-1 because a blockade by anti-PD-1 antibodies can enhance IL-23R and pSTAT3 expression and IL-17 production by Mtb-stimulated CD4+ T cells from TB patients.77 Anti-TB therapy can decrease PD-1 expression and increase the expression of IL-17, IFN-γ, pSTAT3 and IL-23R. Chronic TB may depress the STAT3 signal pathway, leading to a decrease in the expression of IL-23R and production of IL-17 by CD4+ T cells in TB patients (Figure 2).
Figure 2.
Responses of the STAT3 signal pathway and Th17-like T cells that are dysregulated or impaired in patients with TB. In TB patients, T-cell exhaustion coincides with a high expression of PD-1 and downregulation of the IL-23 receptor. Thus, stimulation with IL-23 and Mtb antigens cannot induce adequate expression of STAT3. The phosphorylation of STAT3 is also decreased. Mechanistically, two miRNAs targeting STAT3 are highly expressed, and they could inhibit the expression and phosphorylation of STAT3. Consequently, the production of IL-17 is reduced. IL, interleukin; miRNA, microRNA; Mtb, Mycobacterium tuberculosis; PD-1, programmed death-1; RORγt, retinoic acid receptor-related orphan receptor-γt; STAT3, signal transducer and activator of transcription 3; TB, tuberculosis; Th17, T helper 17.
Interestingly, our recent studies in humans have shown that TB patients exhibit selective impairing of the IL-23 signal effect on the TB phosphoantigen HMBPP-specific γδ T-cell subset, with a consequence of IL-23-targeted exhaustion of Vγ2Vδ2 T-cell responses.53, 78, 79 Such selective impairing of the IL-23 signaling effect can be linked to depressed expression and phosphorylation of STAT3 and the overexpression of antagonizing factor SOCS3.60, 61 The downregulation of the STAT3 signal pathway in Vγ2Vδ2 T cells correlates with remarkable increases in two miRNAs targeting STAT3 (Figure 2). hsa-miR-337-3p and hsa-miRNA-125b-5p are expressed much higher in Vγ2Vδ2 T cells from TB patients compared with those from HC subjects with a BCG vaccination history (Figure 2). Most strikingly, the downregulation of hsa-miR-337-3p and hsa-miRNA-125b-5p using an miRNA sponge allows for detectable recovery of IL-23-induced expansion of Vγ2Vδ2 T cells in TB patients and their effector functions for producing anti-TB cytokines of IFN-γ and IL-17A.53
By contrast, the IL-2 signaling pathway does not appear to be disrupted in TB because IL-2+HMBPP still can expand Vγ2Vδ2 T cells in peripheral blood from TB patients.53, 80 Although IL-2 synergizes or facilitates the IL-23-induced expansion of Vγ2Vδ2 T cells, the IL-2 blockade cannot completely abrogate the IL-23-induced expansion.53 This notion is also supported by the STAT3 blockade data demonstrating that STAT3 has an important role in the expansion of Vγ2Vδ2 T cells by IL-23, but not IL-2.80, 81, 82 It is interesting to demonstrate that TB can selectively impair IL-23 signaling but spare IL-2 effects on Vγ2Vδ2 T cells.53, 80 Such selective impairing of the IL-23 effect may occur as a result of persistent exposure of Vγ2Vδ2 T cells to phosphoantigen HMBPP or IL-23 during chronic TB infection.
Unlike CD4+ T cells, TB-driven impairing of the IL-23-induced expansion of HMBPP-specific Vγ2Vδ2 T cells cannot be explained by T-cell exhaustion linked to PD-1 signaling. Although Ab blocking of the PD-1 pathway can reverse the exhaustion of αβ T cells linked to PD-1 expression,83 this blockade cannot restore IL-23 induced expansion of Vγ2Vδ2 T cells despite our use of two different sources of PD-1 Abs capable of recovering from T-cell exhaustion.
Mtb infection regulates the expression of upstream cytokines in the STAT3 pathway, driving Th17-like responses
Cytokines of TGF-β, IL-6 and IL-1β are regulatory mediators upstream of the STAT3 pathway. These upstream cytokines have been shown to undergo significant changes in Mtb infection, although it is unknown how each contributes to dysregulation of Th17-like responses in TB. Below are the reported changes in these cytokines in Mtb infection, mostly in patients with TB.
Transforming growth factor-β
TGF-β belongs to the TGF superfamily produced by all white blood cell lineages.84 Costimulation of TGF-β with IL-6 is required for Th17 cell differentiation.85 The TGF-β1 content in the serum of TB patients is higher than that in HC controls, and the serum level of TGF-β1 is directly related to the bacterial load and radiological severity.86 Moreover, PBMCs from TB patients produce more TGF-β than those from LTBI under Mtb antigen stimulation. The overexpression of TGF-β in TB patients appears to be transient, and the values return to normal ranges at the end of 3 months of treatment in sequential studies. Although TGF-β can act as an anti-inflammatory cytokine, the immunological significance of changes in this cytokine of TB patients remains to be investigated.
Interleukin-6
IL-6 could initiate early proinflammatory responses, such as to induce the differentiation of T cells producing IL-17. Compared with HC subjects, TB patients have higher baseline levels of serum IL-6.87 Four months after anti-TB drug treatment, IL-6 levels rapidly decrease and stabilize in TB patients. These results indicate that IL-6 may participate in the regulation of immune responses to Mtb infection.87
Interleukin-1β
IL-1β is part of an 11-member IL-1 cytokine family and signals through IL-1R.88 IL-1β is mainly produced by monocytes and DCs and is vital to the Th17 response to Mtb.88 IL-1β functions as the innate Th17-polarizing cytokine and determines the outcome of the Th17 response to Mtb and its antigen fractions.89 The amounts of secreted IL-1β are significantly correlated with Th17 responses,90 and exogenous replenishment of IL-1β is sufficient to markedly increase the Th17 response by the Mtb cytoplasmic fraction.89 Moreover, the receptor of IL-1β and IL-18 receptor engagement induce an Mtb antigen-specific Th1/Th17 immune response.91, 92
Notably, although these upstream cytokines of the STAT3 pathway are highly expressed in TB patients, STAT3-driven immune responses of Th17 cells and dominant Ag-specific Vγ2Vδ2 T-cell subsets are dysregulated or impaired. This may be attributed to the dysfunction or destruction of the STAT3 pathway, which is characterized by decreases in expression and phosphorylation of STAT3 and marked increases of microRNAs that downregulate STAT3, leading to no or a reduced response to IL-23 or Th17-related cytokines.
Th17-related cytokines in Tb pathology
TB pathogenesis is critically related to the extent of inflammation.93 Therefore, Th17 responses in TB are presumed to participate in pathology according to their activities, such as neutrophil recruitment and promoting inflammatory responses in infection sites,93, 94 which will cause serious tissue damage in redundancy neutrophils and high degrees of inflammation.93 Recently, it was reported that there are more IL-17-producing T cells and IL-2- and IL-10-producing T cells in the lung granulomas of latent Mtb-infected macaques with a high risk of reactivation than those in low-risk animals identified by positron emission tomography CT.95 Therefore, high lung inflammation is associated with TB reactivation from LTBI.
Moreover, IL-22 is also suggested to participate in inducing TB pathology.96 IL-22 was readily identified in disease sites of pericardial and pleural effusions of human TB, and the levels of IL-22 are associated with the levels of matrix metalloproteinase-9, which degrades the extracellular matrix components, causing pathology.96 Because TB is a complex disease, cytokine-induced protective or pathological consequences are critically related to the balance of hemostasis. Based on the present data, the roles of Th17-related cytokines cannot be simply defined as ‘good’ or ‘bad’. Further detailed study is necessary to describe the features of the development of these cytokines in local disease sites and define their roles in TB pathology or protection.
Future studies and perspectives
It is important to note that most of the above observations describing dysregulated responses of Th17-related cytokines, STAT3 and Th17-like cells are made in the setting of primary Mtb infection or the utilization of blood samples from TB patients. This raises a critical question as to whether those findings can indeed closely represent immune responses or immunopathogenesis occurring in the pulmonary compartment or lung tissue of TB patients. This is not a trivial question because TB is a chronic, complex disease that is accompanied by pathological processes in the lung tissue. More investigations in the lung and pulmonary compartment of TB patients will display many aspects distinct from what is observed in the blood. In fact, there are compelling lines of evidence that Th17-related cytokines and Th17-like cells are remarkably different from those observed in the blood in TB.31, 97 Thus, in-depth studies using pulmonary compartment samples from TB patients are necessary. It would also be useful to conduct mechanistic studies of TB immunology/pathobiology in lungs using an improved nonhuman primate TB model mimicking chronic TB in humans.
Th17-related cytokines could contribute to immune protection against primary Mtb infection, and their expression levels are decreased in TB patients. Such decreases may be related to the exhaustion of T cells as a result of prolonged overexposure to the stimulation of Mtb antigens in chronic infection. The production of Th17-related cytokines is dependent on the STAT3 signal pathway, and this pathway is dysregulated or damaged in TB infection, with decreases in the expression and phosphorylation of STAT3 and marked increases in microRNAs targeting STAT3. Such dysregulated or deficient conditions may provide a rationale for adjunctive replacement treatment targeting Th17 responses. This notion is supported by recent reports demonstrating that treatments with IL-2 and others can result in beneficial results for attenuating chronic non-tuberculosis mycobacteria (NTM) pulmonary disease.98, 99, 100, 101
Conversely, the overproduction of Th17-related cytokines in TB patients may be detrimental and contribute to TB pathology. If advanced studies confirm that IL-22 or other related cytokines have an active role in TB pathology in the lungs, immune interventions controlling for the overproduction of IL-22 or others may be explored as a potential adjunctive host-directed therapy.13, 42, 91, 96
Acknowledgments
This work was supported by the following research grants: The National Key Research and Development Program of China (2016YFA0502204); the National Institutes of Health R01 grants (NIH R01 HL64560/OD015092/HL129887 to ZWC).
Footnotes
The authors declare no conflict of interest.
References
- Scott L, da Silva P, Boehme CC, Stevens W, Gilpin CM. Diagnosis of opportunistic infections: HIV co-infections—tuberculosis. Curr Opin HIV AIDS 2017; 12: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awuh JA, Flo TH. Molecular basis of mycobacterial survival in macrophages. Cell Mol Life Sci 2017; 74: 1625–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmer RM, Nahid P, Hopewell PC. Latent tuberculosis infection. N Engl J Med 2002; 347: 1860–1866. [DOI] [PubMed] [Google Scholar]
- Fan L, Shen H, Huang H, Yang R, Yao L. Impairment of Wnt/β-catenin signaling in blood cells of patients with severe cavitary pulmonary tuberculosis. PLoS One 2017; 12: e0172549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garib FY, Rizopulu AP. T regulatory cells as part of strategy of immune evasion by pathogens. Biochemistry (Moscow) 2015; 80: 957–971. [DOI] [PubMed] [Google Scholar]
- Stenger SNK, Modlin RL. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J Immunol 1998; 161: 3582–3588. [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Brereton CF, Sweeney CM, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009; 31: 331–341. [DOI] [PubMed] [Google Scholar]
- Song X, He X, Li X, Qian Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cell Mol Immunol 2016; 13: 418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papotto PH, Ribot JC, Silva-Santos B. IL-17+ γδ T cells as kick-starters of inflammation. Nat Immunol 2017; 18: 604–611. [DOI] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol 2007; 178: 3786–3796. [DOI] [PubMed] [Google Scholar]
- Tateosian NL, Pellegrini JM, Amiano NO, Rolandelli A, Casco N, Palmero DJ et al. IL17A augments autophagy in Mycobacterium tuberculosis-infected monocytes from patients with active tuberculosis in association with the severity of the disease. Autophagy 2017; 13: 1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Vidyarthi A, Amir M, Mushtaq K, Agrewala JN. T-cell exhaustion in tuberculosis: pitfalls and prospects. Crit Rev Microbiol 2017; 43: 133–141. [DOI] [PubMed] [Google Scholar]
- Rai PK, Chodisetti SB, Nadeem S, Maurya SK, Gowthaman U, Zeng W et al. A novel therapeutic strategy of lipidated promiscuous peptide against Mycobacterium tuberculosis by eliciting Th1 and Th17 immunity of host. Sci Rep 2016; 6: 23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy S, Dhiman R, Barnes PF, Paidipally P, Tvinnereim A, Bandaru A, VL et al. Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation with Mycobacterium tuberculosis. J Infect Dis 2011; 203: 1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Shi H, Gao Y, Liu Q, Liu Y, Wu J et al. The characteristic profiles of PD-1 and PD-L1 expressions and dynamic changes during treatment in active tuberculosis. Tuberculosis (Edinb) 2016; 101: 146–150. [DOI] [PubMed] [Google Scholar]
- Bandaru A, Devalraju KP, Paidipally P, Dhiman R, Venkatasubramanian S, Barnes PF et al. Phosphorylated STAT3 and PD-1 regulate IL-17 production and IL-23 receptor expression in Mycobacterium tuberculosis infection. Eur J Immunol 2014; 44: 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segueni N, Tritto E, Bourigault M-L, Rose S, Erard F, Bert ML et al. Controlled Mycobacterium tuberculosis infection in mice under treatment with anti-IL-17A or IL-17F antibodies, in contrast to TNFα neutralization. Sci Rep 2016; 6: 36923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano M, Moraes MO, Rodenbusch R, Carvalho CX, Delcroix M, Mousquer G et al. Single nucleotide polymorphisms in IL17A and IL6 are associated with decreased risk for pulmonary tuberculosis in Southern Brazilian Population. PLoS One 2016; 11: e0147814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida YO, Umemura M, Yahagi A, O’Brien RL, Ikuta K, Kishihara K et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol 2010; 184: 4414–4422. [DOI] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007; 8: 369–377. [DOI] [PubMed] [Google Scholar]
- Lombard R, Doz E, Carreras F, Epardaud M, Vern YL, Buzoni-Gatel D et al. IL-17RA in non-hematopoietic cells controls CXCL-1 and 5 critical to recruit neutrophils to the lung of mycobacteria-infected mice during the adaptive immune response. PLoS One 2016; 11: e0149455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danda D, Goel R, Danda S, Mohan H, Joseph G, Kabeerdoss J et al. Interleukin-17F and interleukin-6 gene polymorphisms in Asian Indian patients with Takayasu arteritis. Hum Immunol 2017; S0198-8859: 30070–30078. [DOI] [PubMed] [Google Scholar]
- Hongbo Shen YW, Chen CY, Frencher J, Huang D, Yang E, Ryan-Payseur B et al. Th17-related cytokines contribute to recall-like expansion/effector function of HMBPP-specific Vγ2Vδ2 T cells after M. tuberculosis infection or vaccination. Eur J Immunol 2015; 45: 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian S, Cheekatla S, Paidipally P, Tripathi D, Welch E, Tvinnereim AR et al. IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mycobacterium tuberculosis. Mucosal Immunol 2016; 10: 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Li Z, Fu X, Yu S, Lao S, Yang B. Antigen-specific human NKT cells from tuberculosis patients produce IL-21 to help B cells for the production of immunoglobulins. Oncotarget 2015; 6: 28633–28645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Jiang Y, Lao S, Yang B, Yu S, Zhang Y et al. Mycobacterium tuberculosis-specific IL-21+IFN-γ+CD4+ T cells are regulated by IL-12. PLoS One 2016; 11: e0147356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booty MG, Barreira-Silva P, Carpenter SM, Nunes-Alves C, Jacques MK, Stowell BL et al. IL-21 signaling is essential for optimal host resistance against Mycobacterium tuberculosis infection. Sci Rep 2016; 6: 36720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessis WJD, Kleynhans L, Plessis ND, Stanley K, Malherbe ST, Maasdorp E et al. The Functional response of B cells to antigenic stimulation: a preliminary report of latent tuberculosis. PLoS One 2016; 11: e0152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol 2007; 179: 8180–8190. [DOI] [PubMed] [Google Scholar]
- Kumar NP, Sridhar R, Hanna LE, Banurekha VV, Nutman TB, Babu S. Decreased frequencies of circulating CD4+ T follicular helper cells associated with diminished plasma IL-21 in active pulmonary tuberculosis. PLoS One 2014; 9: e111098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC et al. Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog 2010; 6: e1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zeng G, Yang Q, Zhang J, Zhu X, Chen Q et al. Anti-tuberculosis treatment enhances the production of IL-22 through reducing the frequencies of regulatory B cell. Tuberculosis (Edinb) 2014; 94: 238–244. [DOI] [PubMed] [Google Scholar]
- Lee M-R, Tsai C-J, Wang W-J, Chuang T-Y, Yang C-M, Chang L-Y et al. Plasma biomarkers can predict treatment response in tuberculosis patients, a prospective observational study. Medicine (Baltimore) 2015; 94: e1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan J, Pandey S, Filion LG, Angel JB, Kumar A, Cameron DW. Comparison of interferon-γ-, interleukin (IL)-17- and IL-22-expressing CD4 T cells, IL-22-expressing granulocytes and proinflammatory cytokines during latent and active tuberculosis infection. Clin Exp Immunol 2012; 167: 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol 2008; 180: 1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon A, Jouan Y, Brea D, Gueugnon F, Dalloneau E, Baranek T et al. Neutrophil proteases alter the interleukin-22-receptor-dependent lung antimicrobial defence. Eur Respir J 2015; 46: 771–782. [DOI] [PubMed] [Google Scholar]
- Yao S, Huang D, Chen CY, Halliday L, Wang RC, Chen ZW. CD4+ T cells are required to contain early extrathoracic TB dissemination and sustain multi-effector functions of CD8+ T and CD3− lymphocytes. J Immunol 2014; 192: 2120–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LVM et al. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 2009; 183: 6639–6645. [DOI] [PubMed] [Google Scholar]
- Søndergaard JN, Laursen JM, Rosholm LB, Brix S. Mycobacterium tuberculosis promotes Th17 expansion via regulation of human dendritic cells toward a high CD14 and low IL-12p70 phenotype that reprograms upon exogenous IFN-γ. Int Immunol 2014; 26: 705–716. [DOI] [PubMed] [Google Scholar]
- Zeng G, Chen CY, Huang D, Yao S, Wang RC, Chen ZW. Membrane-bound IL-22 after de novo production in tuberculosis and anti-Mycobacterium tuberculosis effector function of IL-22+ CD4+ T cells. J Immunol 2011; 187: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Huang Y, Qiao D, Zeng G, Cai J. Depletion of IL-22 during culture enhanced antigen-driven IFN-γ production by CD4+T cells from patients with active TB. Immunol Lett 2013; 150: 48–53. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A et al. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol 2010; 184: 4378–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyou Qiu DH, Chen CY, Wang R, Shen L, Shen Y, Hunt R et al. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1α, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-β, TIM1, and TLR2 but low antigen-specific cellular responses. J Infect Dis 2008; 198: 1514–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treerat P, Prince O, Cruz-Lagunas A, Muñoz-Torrico M, Salazar-Lezama MA, Selman M et al. Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol 2017; 10: 1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Chen X, Chan L, Zhang M, Zhu B, Wang L et al. An SNP selection strategy identified IL-22 associating with susceptibility to tuberculosis in Chinese. Sci Rep 2011; 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutay N, Håkansson G, Alaridah N, Hallgren O, Westergren-Thorsson G, Godaly G. Mycobacteria bypass mucosal NF-kB signalling to induce an epithelial anti-inflammatory IL-22 and IL-10 response. PLoS One 2014; 9: e86466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C-S, Song C-H, Lee J-S, Jung S-B, Oh J-H, Park J et al. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell Microbiol 2006; 8: 1158–1171. [DOI] [PubMed] [Google Scholar]
- Fazila N, Mat C, Zhang X, Guzzo C, Gee K. Interleukin-23-induced interleukin-23 receptor subunit expression is mediated by the Janus kinase/signal transducer and activation of transcription pathway in human CD4 T cells. J Interferon Cytokine Res 2011; 31: 363–371. [DOI] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med 2008; 205: 1447–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leepiyasakulchai C, Taher C, Chuquimia OD, Mazurek J, Söderberg-Naucler C, Fernández C et al. Infection rate and tissue localization of murine IL-12p40-producing monocyte-derived CD103(+) lung dendritic cells during pulmonary tuberculosis. PLoS One 2013; 8: e69287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M et al. Interleukin-23 dependent IL-17 drives Th1 responses following Mycobacterium bovis BCG vaccination. Eur J Immunol 2012; 42: 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Matsumura Y, Hatano S, Noguchi N, Murakami T, Iwakura Y et al. IL-21 inhibits IL-17A-producing cd T-cell response after infection with Bacillus Calmette-Gue'rin via induction of apoptosis. Innate Immunity 2016; 22: 588–597. [DOI] [PubMed] [Google Scholar]
- Shen H, Gu J, Xiao H, Liang S, Yang E, Yang R et al. Selective destruction of interleukin 23-induced expansion of a major antigen-specific γδ T-cell subset in patients with tuberculosis. J Infect Dis 2017; 215: 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Li YY, He W-F, Zhang Z-Z, Zhou Q, Liu X et al. Interplay between microRNAs and the STAT3 signaling pathway in human cancers. Physiol Genomics 2013; 45: 1206–1214. [DOI] [PubMed] [Google Scholar]
- Ambros V. microRNAs: tiny regulators with great potential. Cell 2001; 107: 823–826. [DOI] [PubMed] [Google Scholar]
- Brent S, McKenzie RAKADJC. Understanding the IL-23-IL-17 immune pathway. Trends Immunol 2006; 27: 17–23. [DOI] [PubMed] [Google Scholar]
- Shen L, Shen Y, Huang D, Qiu L, Sehgal P, Du GZ et al. Development of Vgamma2Vdelta2+ T cell responses during active mycobacterial coinfection of simian immunodeficiency virus-infected macaques requires control of viral infection and immune competence of CD4+ T cells. J Infect Dis 2004; 190: 1438–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong ZWZ, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science (New York, NY) 1994; 264: 95–98. [DOI] [PubMed] [Google Scholar]
- Huang G, Yan H, Ye S, Tong C, Ying Q-L. STAT3 phosphorylation at tyrosine 705 and serine 727 differentially regulates mouse ESC fates. Stem Cells 2014; 32: 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J, Suessmuth Y, Scott LM, Nahlik K, McMullin MF, Constantinescu SN et al. SOCS3 tyrosine phosphorylation as a potential bio-marker for myeloproliferative neoplasms associated with mutant JAK2 kinases. Haematologica 2009; 94: 576–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Gan L, Zhou Z, Jin W, Sun C. SOCS3 promotes inflammation and apoptosis via inhibiting JAK2/STAT3 signaling pathway in 3T3-L1 adipocyte. Immunobiology 2015; S0171-298500025-X. [DOI] [PubMed] [Google Scholar]
- Jung BG, Wang X, Yi N, Ma J, Turner J, Samten B. Early secreted antigenic target of 6-kDa of Mycobacterium tuberculosis stimulates IL-6 production by macrophages through activation of STAT3. Sci Rep 2017; 7: 40984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushanam V, Solache A, Ting L-M, Escaron CJ, Zhang JY, Ernst JD. Innate inhibition of adaptive immunity: Mycobacterium tuberculosis-induced IL-6 inhibits macrophage responses to IFN-gamma. J Immunol 2003; 171: 4750–4757. [DOI] [PubMed] [Google Scholar]
- Lienard J, Movert E, Valfridsson C, Sturegård E, Carlsson F. ESX-1 exploits type I IFN-signalling to promote a regulatory macrophage phenotype refractory to IFNγ-mediated autophagy and growth restriction of intracellular mycobacteria. Cell Microbiol 2016; 18: 1471–1485. [DOI] [PubMed] [Google Scholar]
- Siddiqui KF, Amir M, Gurram RK, Khan N, Arora A, Rajagopal K, Agrewala JN et al. Latency-associated protein Acr1 impairs dendritic cell maturation and functionality: a possible mechanism of immune evasion by Mycobacterium tuberculosis. J Infect Dis 2014; 209: 1436–1445. [DOI] [PubMed] [Google Scholar]
- Arcos J, Sasindran SJ, Moliva JI, Scordo JM, Sidiki S, Guo H et al. Mycobacterium tuberculosis cell wall released fragments by the action of the human lung mucosa modulate macrophages to control infection in an IL-10-dependent manner. Mucosal Immunol 2016; 10: 1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastrucci C, Bénard A, Balboa L, Pingris K, Souriant S, Poincloux R et al. Tuberculosis is associated with expansion of a motile, permissive and immunomodulatory CD16(+) monocyte population via the IL-10/STAT3 axis. Cell Res 2015; 25: 1333–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Neri PA, López-Rincón G, Mancilla-Jiménez R, Toro-Arreola SD, Muñoz-Valle JF, Fafutis-Morris M et al. Prolactin modulates cytokine production induced by culture filtrate proteins of M. bovis through different signaling mechanisms in THP1 cells. Cytokine 2015; 71: 1. [DOI] [PubMed] [Google Scholar]
- Labzin LI, Lauterbach MAR, Latz E. Interferons and inflammasomes: cooperation and counterregulation in disease. J Allergy Clin Immunol 2016; 38: 37–46. [DOI] [PubMed] [Google Scholar]
- Polgar NCV, Szabo M, Zambo V, Melegh BI, Sumegi K, Nagy G, Tulassay Z, Melegh B. Investigation of JAK2, STAT3 and CCR6 polymorphisms and their gene-gene interactions in inflammatory bowel disease. Int J Immunogenet 2012; 39: 247–252. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Lovewell RR, Olive AJ, Zhang G, Wang W, Eugenin E et al. Nitric oxide prevents a pathogen-permissive granulocytic inflammation during tuberculosis. Nat Microbiol 2017; 2: 17072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-J, Xu L-L, Zhou Q, Cui A, Wang X-J, Zhai K et al. Recruitment of IL-27-producing CD4(+) T cells and effect of IL-27 on pleural mesothelial cells in tuberculous pleurisy. Lung 2015; 193: 539–548. [DOI] [PubMed] [Google Scholar]
- Hölscher C, Hölscher A, Rückerl D, Yoshimoto T, Yoshida H, Mak T et al. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol 2005; 174: 3534–3544. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sahu SK, Kumar M, Jana K, Gupta P, Gupta UD et al. MicroRNA 17-5p regulates autophagy in Mycobacterium tuberculosis-infected macrophages by targeting Mcl-1 and STAT3. Cell Microbiol 2016; 18: 679–691. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Kondo Y, Kondo S. Roles of mTOR and STAT3 in autophagy induced by telomere 3' overhang-specific DNA oligonucleotides. Autophagy 2007; 3: 496–498. [DOI] [PubMed] [Google Scholar]
- Deretic V, Delgado M, Vergne I, Master S, Haro SD, Ponpuak M et al. Autophagy in immunity against Mycobacterium tuberculosis: a model system to dissect immunological roles of autophagy. Curr Top Microbiol Immunol 2009; 335: 169–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Zhong S, Ma Z, Kong H, Medvec A, Ahme R et al. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci USA 2013; 110: E2480–E2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L et al. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science (New York, NY) 2002; 295: 2255–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnevillea M, Chen ZW, Déchanet-Mervillec J, Eberld M, Fourniée JJ, Jamesonf JM et al. Chicago 2014—30years of γδ T cells. Cell Immunol 2015; 296: 3–9. [DOI] [PubMed] [Google Scholar]
- de Jong R, Janson AA, Faber WR, Naafs B, Ottenhoff TH. IL-2 and IL-12 act in synergy to overcome antigen-specific T cell unresponsiveness in mycobacterial disease. J Immunol 1997; 159: 786–793. [PubMed] [Google Scholar]
- Valle-Mendiola A, Weiss-Steider B, Rocha-Zavaleta L, Soto-Cruz I. IL-2 enhances cervical cancer cells proliferation and JAK3/STAT5 phosphorylation at low doses, while at high doses IL-2 has opposite effects. Cancer Invest 2014; 32: 115–125. [DOI] [PubMed] [Google Scholar]
- Chen CY, Huang D, Yao S, Halliday L, Zeng G, Wang RC et al. IL-2 simultaneously expands Foxp3+ T regulatory and T effector cells and confers resistance to severe tuberculosis (TB): implicative Treg-T effector cooperation in immunity to TB. J Immunol 2012; 199: 4278–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan L, Chen X, Liu A, Zhang Y, Guo X, Yan S et al. PD-1 blockade can restore functions of T-Cells in Epstein–Barr virus-positive diffuse large B-cell lymphoma in vitro. PLoS One 2015; 10: e0136476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile JI, Iatcovsky DK, Romero MM, Balboa L, Monteserin J, Ritacco V et al. Mycobacterium tuberculosis multi-drug-resistant strain M induces IL-17+ IFNγ− CD4+ T cell expansion through an IL-23 and TGF-β-dependent mechanism in patients with MDR-TB tuberculosis. Clin Exp Immunol 2017; 187: 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowska E, Roży A, Jaguś P, Wiatr E, Gawryluk D, Chorostowska-Wynimko J et al. Cryptogenic organizing pneumonia: IL-1β, IL-6, IL-8, and TGF- β1 serum concentrations and response to clarithromycin treatment. Adv Exp Med Biol 2016; 911: 77–85. [DOI] [PubMed] [Google Scholar]
- Chowdhury IH, Ahmed AM, Choudhuri S, Sen A, Hazra A, Pal NK et al. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Mol Immunol 2014; 62: 159–168. [DOI] [PubMed] [Google Scholar]
- Clifford V, Zufferey C, Street A, Denholm J, Tebruegge M, Curtis N. Cytokines for monitoring anti-tuberculous therapy: a systematic review. Tuberculosis 2015; 95: 217–218. [DOI] [PubMed] [Google Scholar]
- Stephen-Victor E, Sharma VK, Das M, Karnam A, Saha C, Lecerf M et al. IL-1β, but not programed death-1 and programed death ligand pathway, is critical for the human Th17 response to Mycobacterium tuberculosis. Front Immunol 2016; 7: 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the Thelper 17 response in humans. Immunol Rev 2008; 226: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathamuthu GR, Moideen K, Baskaran D, Banurekha VV, Nair D, Sekar G et al. Tuberculous lymphadenitis is associated with enhanced baseline and antigen-specific induction of type 1 and type 17 cytokines and reduced interleukin-1β (IL-1β) and IL-18 at the site of infection. Clin Vaccine Immunol 2017; 24: e00045–00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I et al. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol 2007; 179: 1178–1189. [DOI] [PubMed] [Google Scholar]
- Desel C, Werninghaus K, Ritter M, Jozefowski K, Wenzel J, Russkamp N et al. The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS One 2013; 8: e53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyadova IV, Panteleev AV. Th1 and Th17 cells in tuberculosis: protection, pathology, and biomarkers. Mediat Inflamm 2015; 2015: 854507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol 2007; 179: 7791–7799. [DOI] [PubMed] [Google Scholar]
- Lin PL, Maiello P, Gideon HP, Coleman MT, Cadena AM, Rodgers MA et al. PET CT identifies reactivation risk in cynomolgus macaques with latent M. tuberculosis. PLoS Pathog 2016; 12: e1005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Wilkinson KA, Kalsdorf B, Roberts T, Diacon A, Walzl G et al. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis (Edinb) 2011; 91: 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Huang D, Chen CY, Wang R, Shen L, Shen Y et al. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1alpha, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-beta, TIM1, and TLR2 but low antigen-specific cellular responses. J Infect Dis 2008; 198: 1514–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Huang D, Yao S, Halliday L, Zeng G, Wang RC et al. IL-2 simultaneously expands Foxp3+ T regulatory and T effector cells and confers resistance to severe tuberculosis (TB): implicative Treg-T effector cooperation in immunity to TB. J Immunol 2012; 188: 4278–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrom J, Taher TE, Muhyaddin MS, Clanchy FI, Mangat P, Jawad AS et al. Harnessing the therapeutic potential of Th17 cells. Mediators Inflamm 2015; 2015: 205156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Yao S, Huang D, Wei H, Sicard H, Zeng G et al. Phosphoantigen/IL2 expansion and differentiation of Vgamma2Vdelta2 T cells increase resistance to tuberculosis in nonhuman primates. PLoS Pathog 2013; 9: e1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez LE, Stevens P, Kolonoski P, Wu M, Young LS. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J Immunol 1989; 143: 2996–3000. [PubMed] [Google Scholar]