Abstract
The last decade has seen major advances in neuroscience tools allowing us to selectively modulate cellular pathways in freely moving animals. Chemogenetic approaches such as designer receptors exclusively activated by designer drugs (DREADDs) permit the remote control of neuronal function by systemic drug administration. These approaches have dramatically advanced our understanding of the neural control of behaviour. Here, we review the different techniques and genetic approaches available for the restriction of chemogenetic receptors to defined neuronal populations. We highlight the use of a dual virus approach to target specific circuitries and the effectiveness of different routes of administration of designer drugs. Finally, we discuss the potential caveats associated with DREADDs including off‐target effects of designer drugs, the effects of chronic chemogenetic receptor activation and the issue of collateral projections associated with DREADD activation and inhibition.
Abbreviations
- AAVs
adeno‐associated viral vectors
- BBB
blood–brain barrier
- CAMKII
calmodulin‐dependent protein kinase II
- CAVs
canine adenoviruses
- CNO
clozapine N‐oxide
- DREADDs
designer receptors exclusively activated by designer drugs
- hM3D
human M3 muscarinic DREADD
- hM4D
human M4 muscarinic DREADD
- HSV
herpes simplex viral
- hSyn
human synapsin
- KORD
κ‐opioid‐based receptor designer receptor exclusively activated by designer drugs
- LGICs
ligand‐gated ion channels
- NAc
nucleus accumbens
- RASSL
receptor activated solely by a synthetic ligand
- SalB
salvinorin B
- VTA
ventral tegmental area
Introduction
During the last decade, there has been a revolution in neuroscience techniques that have resulted in increasingly precise methods to manipulate neural systems in awake, behaving animals. Understanding the relationship between brain function and behaviour is critical for the advancement of both neuroscience research and targeted medication development. Chemogenetics refers to the technique that allows for the reversible remote control of cell populations and neural circuitry via systemic injection or microinfusion of an activating ligand (Alexander et al., 2009; Armbruster et al., 2007). The chemogenetic technique uses engineered receptors and biologically inert ligands to achieve this aim. Unlike optogenetics, which has the ability to control cells and neural circuitry with light, the use of designer drugs makes chemogenetics simple to use, removing the need for optical fibre probes and tethers. While the temporal resolution of chemogenetics is lower than optogenetics, this relatively non‐invasive technique is still effective for functional mapping, cell‐type‐specific manipulations and multiplexed control of neurons.
In this review, we will evaluate the different strategies that have been used to restrict chemogenetic receptors to defined neuronal populations. We will also highlight the use of a dual virus approach at targeting projection neurons and the effectiveness of different routes of administration of designer drugs. Finally, we discuss the potential caveats associated with chemogenetics including off‐target effects of designer drugs as well as the issue of collateral projections associated with chemogenetic activation and inhibition.
Development of chemogenetic receptors
Necessary conditions for chemogenetic interrogation of brain function
Chemogenetic receptors are used to selectively modulate the activity of defined neuronal populations, primarily through a systemic drug injection. To be an effective behavioural neuroscience tool, a chemogenetic receptor must meet the following conditions: (i) the modified receptor must not be receptive to any endogenous ligand; (ii) the modified receptor needs to have minimal or no endogenous activity in the absence of ligand binding; and (iii) the modified receptor must have a high affinity for the ligand that has no pharmacological activity at other endogenous receptors (Urban and Roth, 2015).
Early chemogenetic receptors
GPCRs are at the forefront of current chemogenetic practice. The earliest evidence for the use of specifically engineered GPCRs was by Strader et al. (1991) who substituted a single amino acid residue to mutate the β‐adrenoceptor to become activated by catechol‐containing esters and ketones, compounds that do not activate endogenous β‐adrenoceptors. This paper introduced the idea of modifying endogenous receptors, altering their specificity and binding properties, so that they can be activated at a time point chosen by the experimenter. Coward et al. (1998) subsequently developed GPCRs that responded solely to synthetic ligands and termed these receptors RASSLs (receptors activated solely by a synthetic ligand). One limitation of RASSLs was that the synthetic ligands often had high affinity at the native receptor (Coward et al., 1998). In addition, when RASSL expression was very high, they were found to have endogenous activity in the absence of ligand binding (Sweger et al., 2007), limiting their applicability in vivo.
Directed molecular evolution
Approaches to protein engineering are critical for designer receptor development, involving gene shuffling, random mutagenesis and analysis of structure and sequence (see Steiner and Schwab, 2012). RASSLs were generated using a rational design approach whereby receptors were generated with deliberate mutations at key residues important for native ligand binding (Strader et al., 1991; Coward et al., 1998). Dong et al. (2010) used a novel approach to receptor engineering, termed directed molecular evolution, to generate the next generation of chemogenetic receptors. The directed evolution of GPCRs that are exclusively activated by certain ligands involved the generation of a random mutagenesis library of amino acid substitutions in the DNA for muscarinic receptors, through error‐prone PCR (Dong et al., 2010). This was followed by screening the resultant population of mutant receptors for the ability to be activated by a designer ligand. This selection step was done by hijacking the natural signalling pathway of endogenous GPCRs in yeast to make GPCR activity critical for survival of the yeast host cell. The biologically inert ligand clozapine N‐oxide (CNO) was selected as the designer ligand to activate the modified GPCR, and thus, the yeasts were screened for growth in medium with CNO. Multiple rounds of mutagenesis and selection were employed until receptors were identified that exhibited high affinity for clozapine and CNO but not ACh (Armbruster et al., 2007). This novel approach to receptor engineering resulted in a new class of RASSLs termed DREADDs (designer receptors exclusively activated by designer drugs) (Armbruster et al., 2007).
Types of chemogenetic receptors
Human muscarinic DREADDs and κ‐opioid receptor DREADD
The muscarinic‐based DREADDs meet the necessary criteria to be effective tools for behavioural neuroscience research. They are insensitive to the endogenous ligand (ACh), they have low constitutive activity, and they have orders of magnitude greater sensitivity to the ligand CNO compared with the endogenous ligand (Armbruster et al., 2007). Different muscarinic‐based DREADDs have been developed that can either increase neuronal activity (Alexander et al., 2009) or decrease neuronal activity (Armbruster et al., 2007), and the mechanism of action for both requires the action of associated G proteins. The three main types of signalling pathways for muscarinic‐based DREADDS are Gq, Gi and Gs. The Gq DREADD increases neuronal firing by stimulating phospholipase C, releasing intracellular calcium stores (Conklin et al., 2008). Gs DREADDs are less commonly used, stimulating cAMP production (Conklin et al., 2008). Gi DREADDs inhibit cAMP production (Urban and Roth, 2015). Electrophysiological in vivo recordings of DREADD‐expressing neurons show an initial effect of systemic CNO administration on neuronal activity after 5–10 min, with peak activity demonstrated after 45–50 min, and effects can last up to 9 h (Alexander et al., 2009; Guettier et al., 2009). The solubility of CNO varies depending on concentration and source. For example, CNO obtained from the National Institutes of Health (NIH) appears to be less soluble, requiring DMSO concentrations of up to 15% in a 10 mg·mL−1 solution (Raper et al., 2017). However, 2 mg·mL−1 CNO obtained from NIH has been dissolved in sterile PBS (Carvalho Poyraz et al., 2016). CNO from Biomol International has been shown to dissolve in 0.9% saline at concentrations of up to 10 mg·mL−1 (Guettier et al., 2009).
One limitation from an experimental design perspective is that both excitatory and inhibitory receptors are activated by the same ligand, and therefore, selective manipulation of neurons within the same animal is not possible. Recently, Vardy et al. (2015) addressed this limitation by developing an inhibitory κ‐opioid‐based receptor DREADD (KORD) activated by the ligand salvinorin B (SalB), a metabolite of the κ‐opioid receptor agonist salvinorin A. SalB has limited solubility, dissolving in 100% DMSO, but is faster acting than CNO, affecting neuronal activity in vivo within a few minutes after systemic administration and lasting approximately 1 h (Vardy et al., 2015). KORD permits multiplexed control of diverse neuronal populations within the same animal, expression of both the human M3 DREADD (hM3D) and KORD in the same population of neurons is possible, and behaviour can be bidirectionally controlled by systemic application of each DREADD ligand (Vardy et al., 2015). Marchant et al. (2016b) showed that systemic injection of SalB in rats with ventral tegmental area expression of KORD reduces locomotor behaviour, demonstrating its efficacy in vivo in rats.
Ligand‐gated G proteins: allatostatin neuropeptide receptor
Other methods for reversible inhibition of neural activity involve the use of the insect allatostatin neuropeptide receptor. The G‐protein coupled Drosophila allatostatin receptor is activated by the peptide ligand allatostatin and has been used to silence neuronal activity (Lechner et al., 2002). Allatostatin is soluble in saline and water and is fast acting (Haettig et al., 2013). Electrophysiological recordings of allatostatin receptor‐transduced neurons show neuronal silencing within minutes of bath application of allatostatin (Tan et al., 2006), which is recovered within minutes following washout (Menuet et al., 2017; Tan et al., 2006). Haettig et al. (2013) used this technique to inactivate CA1 hippocampal interneurons and showed impairments in long‐term memory for object location. Additionally, Menuet et al. (2017) used the allatostatin receptor to show a functional link between respiratory modulation of BP and hypertension via the inhibition of rostral ventrolateral medulla adrenergic (C1) neurons. Importantly though, allatostatin does not cross the blood–brain barrier (BBB), limiting its clinical potential.
Ligand‐gated ion channels
Another method for controlling neuronal activity in vivo is with the use of ligand‐gated ion channels (LGICs) (Magnus et al., 2011). LGICs permit the control over ion conductance, allowing for the activation or inhibition of neurons (Magnus et al., 2011). This strategy developed chimeric LGICs with unique conductance properties originating from combinations of ligand binding domains and ion pore domains (Magnus et al., 2011). It requires intracranial injection of a virus encoding a pharmacologically selective actuator molecule (PSAM) element. An i.p. injection of the inert pharmacologically selective effector molecule binds to the LGIC causing activation or inhibition of PSAM‐expressing neurons (Simonds et al., 2014). Simonds et al. (2014) used this technique to demonstrate the involvement of leptin receptor‐expressing neurons in the dorsomedial hypothalamus in increasing BP and heart rate in mice.
Modified receptors that are activated by ivermectin may be especially well suited for future clinical trials compared with muscarinic receptor‐based DREADDs, because ivermectin is currently a FDA approved anti‐parasitic drug. Lerchner et al. (2007) developed a glutamate and ivermectin‐gated chloride channel that could be activated by systemic ivermectin administration in vivo. However, this channel had low expression levels. Lynagh et al. (2010) improved upon this design by identifying the A288G mutation of the human α1 glycine receptor, which had increased expression and ivermectin sensitivity. Islam et al. (2017) have also developed glycine receptor chloride channels, members of the LGIC family. Ivermectin has a t 1/2 of approximately 24 h in humans (Edwards et al., 1988). However, neuronal silencing and reversal following ivermectin administration in animals is relatively slow with onset occurring within hours and lasting up to several days (Lerchner et al., 2007). Additionally, ivermectin is insoluble in water but can be dissolved in methanol, high concentrations of ethanol, propylene glycol and DMSO (Lerchner et al., 2007).
Viral methods for chemogenetic receptor expression
Types of viral vectors and promoters
The type of viral vector and promoter used may affect the neuronal transduction and expression of DREADDs. Typically, DREADD expression in behavioural neuroscience experiments is mediated by viral vector‐induced neuronal transfection. The type of viral vector used depends on the experimental question. One of the most common methods is to use intracranial injections of recombinant adeno‐associated viral vectors (AAVs)‐encoding DREADDs for neuronal transfection. AAVs were developed to improve transduction capability and tropism by using capsid genes from other AAV serotypes (Gao et al., 2002). AAVs are relatively non‐toxic and achieve long‐term (months to year) expression (Morsy et al., 1998). There are several different serotypes of AAV, and each serotype has different transduction and retrograde transport efficiencies depending on the infected brain region (Aschauer et al., 2013; Nair et al., 2015). Lentiviral and herpes simplex viral (HSV) vectors are also used to transduce DREADD expression in vivo (Ferguson et al., 2011; Mahler et al., 2014). Lentiviral vectors appear to have greater transduction properties compared with AAV; however, they have poor retrograde transport capabilities (Blomer et al., 1997). HSV vectors provide specific neuronal transduction along with highly efficient retrograde transport. However, transduction using HSV vectors is typically lower than AAV or lentiviruses (Palella et al., 1989; Soudais et al., 2001).
The type of promoter chosen depends on the type of cell the experimenter is trying to target. In regard to cell‐type‐specific promoters, the size of the genetic material required to target these cells is important. This is also dependent on the type of viral vector chosen, with HSV vectors having the greatest capacity for multiple gene cassettes (Nair et al., 2015). Several commercially available promoters are commonly used to examine the behavioural response to DREADD manipulations. For example, the human synapsin (hSyn) promoter is pan‐neuronal, whereas calmodulin‐dependent protein kinase II (CAMKII) predominately targets excitatory neurons, although not always (Jennings et al., 2013; Yizhar et al., 2011; Yau and Mcnally, 2015). There are also some specific promoters such as human glial fibrillary acidic protein, which is expressed in astrocytes (Yizhar et al., 2011). However, it is common to find that the in vitro specificity of expression based on gene promoters do not always translate faithfully to the in vivo models.
Strategies to restrict chemogenetic receptor expression to neuronal subtypes
A key advantage of using viral vectors to mediate DREADD expression occurs when it is combined with Cre systems to restrict DREADD expression in genetically defined neuronal populations. Cre‐dependent viral vectors permit restriction of DREADDs in neurons defined by the expression of specific genetic markers. Atasoy et al. (2008) designed an AAV system that uses a FLip and EXcise approach (Schnutgen et al., 2003), similar to double‐floxed inverted open reading frame, to restrict expression of viral‐transduced DNA in Cre‐expressing neurons. This technique has been widely adopted because of its efficiency in restricting expression of DREADD receptors to cells that express Cre. However, an important caveat with Cre systems that should be taken into account is the risk of ‘tumour‐causing’ off‐target effects in vivo (Janbandhu et al., 2014). Another method for expressing DREADDs in specific neuronal subtypes is with the use of transgenic mice expressing DREADD receptors. Farrell et al. (2013) developed this model expressing the Gs DREADD specifically in striatopallidal neurons.
Circuit‐specific uses of chemogenetics
Like optogenetics, chemogenetics can be used for selective interrogation of neuronal circuitry and manipulation of behavioural output. Two different strategies have been developed that allow experimenters to achieve this. One way is through local intracranial administration of the activating ligand (Figure 1). Typically, DREADD expression is induced through non‐selective expression of DREADDs in the projection region, using AAV with promoters such as hSyn or CAMKII. In addition, intracranial cannulas are implanted above the projection target region. To date, several studies have used local infusions of CNO into the projection target region to cause selective manipulation of only the DREADD‐expressing terminals in the target region (Lichtenberg et al., 2017; Mahler et al., 2014; McGlinchey and Aston‐Jones, 2017; Stachniak et al., 2014; Venniro et al., 2017). For example, Ge et al. (2017) used this approach to show that inactivation of entorhinal cortex terminals in the dorsal dentate gyrus significantly decreased context‐induced reinstatement of heroin seeking. While this technique has significant advantages to examine the neural circuitry of complex behaviours, the invasive methodology limits its clinical applicability. The use of high (1 mM) concentrations of CNO for microinfusions may lead to off‐target effects (Gomez et al., 2017). However, there have been no reports of general locomotor deficits with this intracranial dose of CNO (Ge et al., 2017; Mahler et al., 2014; McGlinchey and Aston‐Jones, 2017; Venniro et al., 2017).
Figure 1.
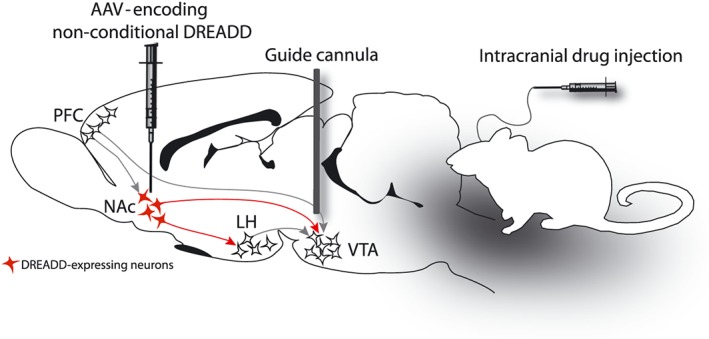
Interrogation of neuronal circuitry using chemogenetics: local intracranial administration of the activating ligand. Intracranial injection of DREADD ligands can be used to selectively manipulate neuronal circuits and behavioural output. In this sagittal rat brain schematic, an AAV‐encoding non‐conditional DREADD is injected into the NAcs, and intracranial guide cannulas are implanted above the VTA. Intracranial infusion of the DREADD ligand into VTA will change the activity of DREADD‐expressing terminals in VTA.
Another approach for circuit‐specific modulation of neuronal activity with chemogenetics uses a dual viral–vector approach (Figure 2). In these experiments, the Cre vector is a retrograde transport type and is injected into a brain region that has anatomical connectivity with the brain region that receives the Cre‐dependent vector. One advantage of this approach is that selective manipulation of neurons defined by their anatomical projection is possible through a systemic drug injection. The extent of retrograde labelling varies across brain regions depending on the serotype that is used (Aschauer et al., 2013). Canine adenoviruses (CAVs) were pioneered by Kremer et al. (2000) as an alternative to human adenoviruses, because they display effective retrograde transport properties (Soudais et al., 2001). Boender et al. (2014) used CAV to show that this approach is feasible for DREADD control over nucleus accumbens (NAc) projecting VTA neurons. More recently, Foldi et al. (2017) also used the CAV approach in VTA → NAc projection neurons and showed that excitation of this projection increased food intake, ameliorating activity‐based anorexia‐induced weight loss. This CAV–DREADD technique has also been used in other neural pathways. For example, Burgos‐Robles et al. (2017) inhibited the projection from the basolateral amygdala to the prelimbic cortex using a combination of CAV and the human M4 (hM4Di) DREADD to demonstrate a functional role for this pathway in fear‐associated memories. The AAV serotype 2/5 has also been shown to have retrograde tropism (Aschauer et al., 2013). Marchant et al. (2016a,b) used a similar approach to restrict KORD expression in ventral subiculum neurons that project to the NAc shell. They found that KORD‐mediated inhibition of these projection neurons decreased context‐induced relapse to alcohol seeking after punishment‐imposed abstinence (Marchant et al., 2016a).
Figure 2.
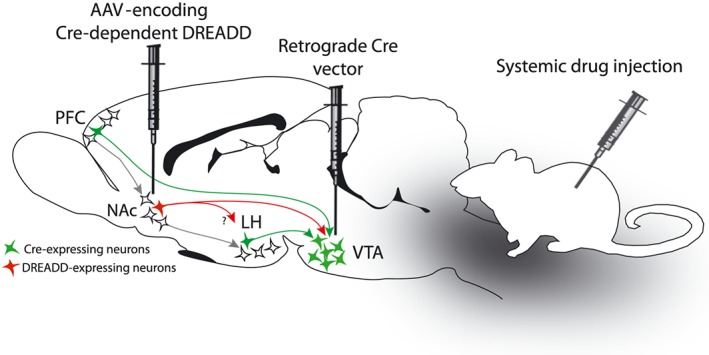
Interrogation of neuronal circuitry using chemogenetics: dual viral vector approach. A dual‐virus approach may be used to manipulate neurons defined by their anatomical projections. In this sagittal rat brain schematic, an AAV‐encoding Cre‐dependent DREADD is injected into the NAcs, and a retrograde vector‐encoding Cre is injected into the VTA. With this arrangement, Cre expression can be expected in the inputs to VTA, such as NAc, prefrontal cortex (PFC) and lateral hypothalamus (LH). However, chemogenetic receptor expression will be confined to NAc → VTA neurons. Systemic injection of the DREADD ligand will affect the activity of NAc → VTA neurons. However, this manipulation will also affect collateral projections of NAc → VTA neurons, such as to LH, if they exist.
Recently, Tervo et al. (2016) used a directed evolution approach to develop an AAV serotype specifically selected for retrograde transport. The serotype they developed (rAAV2‐retro) was shown to label projection neurons at least as effectively as the traditional retrograde tracer FluoroGold. The application of this retrograde AAV serotype is likely to increase the effectiveness of circuit‐specific manipulations using chemogenetics. The efficiency of DREADD expression within a specific pathway is likely to determine the magnitude of the behavioural effect that can be observed by chemogenetic manipulations. It is interesting to note that the dual‐virus approach has also been tested in non‐human primates. Oguchi et al. (2015) used Macaca fuscata monkeys and showed that Cre‐dependent expression of a reporter protein (mCherry) is found in prefrontal cortex neurons that project to the caudate nucleus in monkeys with a highly efficient retrograde gene transfer (HiRet) lentivirus injected into the caudate nucleus.
Chemogenetic control in the spinal cord and periphery
Chemogenetics may also be used to manipulate cells (neurons and other cells) outside the brain. Karadimas et al. (2016) demonstrated that chemogenetic (hM3Dq) activation of lumbar glutamatergic cells in mice resulted in greater locomotor ability following cervical spinal cord injury compared with controls. Additionally, Miller et al. (2017) showed that chemogenetic (hM4Di) inhibition of sensory cells expressing Nav1.8 channels, in the dorsal root ganglion, reduced early stage osteoarthritis‐associated pain in mice. DREADDs have also been used in pancreatic cells, chemogenetic (hM3Dq/Ds) stimulation of pancreatic beta‐cell signalling has been shown to increase insulin release (Guettier et al., 2009; Nakajima and Wess, 2012). Finally, Jaiswal and English (2017) showed successful chemogenetic transduction of motoneurons in the lateral gastrocnemius muscle following an i.m. injection of hM3Dq DREADD. These studies together highlight the potential for the use of DREADDs in many types of cells, beyond the CNS.
Effective designer drug administration
Systemic CNO administration
One of the key advantages of chemogenetics is that remote control of defined neuronal populations is possible with a systemic drug injection. However, there is substantial variability in the literature in terms of the effective dose of CNO used to achieve a behavioural effect. The type of DREADD used (Gq, Gi or Gs), as well as the size of the target brain region, can influence the required ligand dose. For example, hM4D is often found to be less effective at inhibiting neuronal activity compared with the ability of hM3Dq to activate neurons. As a consequence, experiments with hM4D often require greater doses of CNO to induce behavioural effects (Farrell and Roth, 2013; Yau and Mcnally, 2015; Mahler et al., 2014). One possible reason for this is that it may be easier to cause a behavioural effect than to interfere with its expression.
Intracranial and intracerebroventricular CNO administration
Circuit‐specific manipulation is also possible via intracranial injections of CNO. Chemogenetic receptors are expressed throughout the neuronal cell body, axons and terminals, and local application of CNO in the terminal projection regions enables researchers to isolate chemogenetic manipulation to pathways. Mahler et al. (2014) used this approach to selectively modulate the activity of ventral pallidum output pathways in animal models of relapse to cocaine‐seeking. They infused a synapsin‐driven lentivirus‐encoding non‐conditional hM4Di DREADD into different ventral pallidum subregions (rostral or caudal), and cannulas were implanted above the VTA. On test for reinstatement of extinguished cocaine seeking, CNO was infused into the VTA (1 mM), selectively inhibiting these pathways. They found that cue‐induced reinstatement, but not cocaine‐priming‐induced reinstatement, was decreased by inhibition of the rostral ventral pallidum → VTA pathway but not the caudal ventral pallidum → VTA pathway. This result further exemplifies the specificity of intracranial injection of CNO (see Non‐specific effects of the ligands section). Mahler et al. (2014) show that the actions of CNO are specific to DREADD‐expressing neurons with intra‐VTA injections of CNO in the caudal ventral pallidum of rats having no effect on reinstatement.
Finally, i.c.v. injections of CNO may have more applicability for experiments examining the effect of chronic chemogenetic activation of neuronal populations. Nakajima et al. (2016) have performed i.c.v. injections of CNO (1 μg) into mice with hM3Ds expression in agouti‐related peptide neurons and examined food intake. They found that i.c.v. CNO caused a long‐lasting effect on food intake and have argued that i.c.v. administration of CNO yielded more consistent results compared with systemic CNO administration.
Oral administration of CNO
CNO can also be administered p.o., via food or water, for studies examining chronic activation of DREADD‐expressing neuronal populations. Using this method of voluntary CNO administration, saccharin or other sweeteners are often added to the CNO water to make it more palatable, introducing potential confounding factors in some studies. In a study examining the role of the NAc core in binge alcohol drinking in mice, Cassataro et al. (2014) used a dose of 0.1 mg·mL−1 CNO in tap water. They found that the mice consumed approximately 3 mL·day−1, resulting in a dose approximating 10 mg·kg−1 over 24 h. This dose was found to be sufficient to decrease ethanol consumption in mice expressing hM4Di in the NAc and to increase ethanol consumption in mice expressing hM3Dq in the NAc. This method is particularly attractive because of the non‐invasive test method, which reduces stress particularly in cases of chronic or repeated testing with CNO (Jain et al., 2013). Furthermore, CNO has been reported to retain effectiveness 5–10 h after a systemic injection (Alexander et al., 2009). Thus, the time course of administration in drinking water might be considered comparable with systemic injections.
Use of chemogenetics in non‐human primates
The translational nature of DREADDS and its therapeutic potential in humans is highlighted by several non‐human primate studies. Importantly, these studies have shown repeatable changes in reward‐related behaviours following repeated DREADD‐induced inactivation of several brain regions including the orbitofrontal cortex and rostromedial caudate (Eldridge et al., 2016; Nagai et al., 2016). These primate studies have also demonstrated the importance of anatomical connectivity and functional interactions using the hM4Di DREADD in combination with MRI scans (Grayson et al., 2016). A recent study by Raper et al. (2017) examined the pharmacokinetics of s.c. CNO administration in rhesus monkeys. They found that CNO readily metabolizes to clozapine in monkeys, which may interfere with the behavioural interpretation of DREADD‐based experiments in both humans and non‐human primates. In both humans and rodents, CNO is also metabolized to clozapine (Chang et al., 1998; Gomez et al., 2017). It may be that effective DREADD manipulation in non‐human primates and rodents will require low doses of clozapine. Given that muscarinic‐based DREADDs have 100‐fold greater sensitivity to clozapine than CNO (Armbruster et al., 2007), it remains possible that there is a therapeutic window for selective manipulation of DREADDs with a dose of clozapine that has minimal off‐target effects on the many receptors that clozapine acts on. Finally, it is important to note that other DREADD ligands exist. For example, Chen et al. (2015) suggest the use of perlapine, a hypnotic agent, or compound 21, which both have greater selectivity for hM3Dq over the native hM3 receptor. However, in vivo testing for off‐target effects of perlapine is yet to be conducted.
Potential caveats associated with chemogenetics
Collateral projections in circuit‐specific DREADD experiments
The dual‐virus approach has the advantage that manipulation of neurons defined by their anatomical projections is possible with a systemic drug injection. However, one limitation of this approach is that collateral projections are also included in the manipulation. For example, using the dual‐virus approach, while the DREADD‐expressing neurons do by definition project to the target region where the retrograde Cre virus was injected, any collateral projections would also contain DREADDs (Figure 2). Thus, systemic drug injection of the chemogenetic ligand has the potential to alter the activity of more than one projection. This factor will vary depending on the circuit that is being interrogated and whether the transduced neurons have extensive collateral projections. For example, in the case of ventral subiculum projections to the NAc shell, Marchant et al. (2016a) used immunolabelling for the hemagglutinin tag for KORD to show that terminal expression was highest in the NAc shell, the site of retrograde Cre injection, with minimal observation of terminals in other known outputs of the ventral subiculum. Studies in other pathways, such as output pathways of the basolateral amygdala (Beyeler et al., 2016), have been similarly analysed and extensive collateralization has been found in some pathways (e.g. basolateral amygdala → ventral hippocampus), but not others (e.g. basolateral amygdala → central amygdala).
The extent to which this is a limiting factor for this approach remains to be shown. It raises interesting questions about whether information routing in the brain does occur within single circuits or whether activation of the collateral output targets are also necessary to mediate function. In vivo, neurons that have collateral projections do not discriminate between these output projections. Therefore, collateral projections are a critical part of normal brain function, where output projections exert their function through modulation of activity in all downstream nuclei, rather than in just one output target. Nevertheless, the use of intracranial ligand injections to selectively isolate circuit projections addresses the limitation of collateral projections.
Non‐specific effects of the ligands
Chemogenetic receptors are sensitive to otherwise pharmacologically inert ligands. Recent debate has surrounded the potential for non‐DREADD mediated effects of CNO or other ligands. In the example of human muscarinic DREADDs, clozapine is a major metabolite of CNO, a prototype atypical antipsychotic drug frequently used for the treatment of schizophrenia and other psychotic‐related disorders (Geddes et al., 2000). A recent study by MacLaren et al. (2016) showed that small doses of CNO (1 mg·kg−1) altered the startle response to a loud acoustic stimulus, and larger doses (5 mg·kg−1) reduced amphetamine‐induced hyperlocomotion in rats that do not express any DREADD receptors. However, it should be noted that while CNO altered these two behaviours in the absence of DREADD receptors, several other behaviours, including spontaneous locomotion and prepulse inhibition, were not affected. These data show that CNO is not entirely pharmacologically inert and that an effective dose of CNO must be established where off‐target effects are minimalized. MacLaren et al. (2016) suggest that the inclusion of behavioural control groups, specifically a CNO‐treated group without DREADD virus, will go a long way to address this limitation.
Until recently, the precise in vivo action of CNO had not been fully investigated. Gomez et al. (2017) recently reported no evidence that CNO crosses the BBB, in contrast to the findings of Ji et al. (2016). These data suggest that activation of DREADDs in vivo is likely to be mediated by metabolism of CNO to clozapine, which readily crosses the BBB. Furthermore, Gomez et al. (2017) showed that clozapine has a much higher affinity for the hM4D than CNO, which was demonstrated initially by Armbruster et al. (2007). One interpretation of these data is that a major premise of the muscarinic‐based chemogenetic approach is compromised, because it is in fact clozapine that is causing activation of the muscarinic‐based DREADDs. Based on this, Gomez et al. (2017) suggest that subthreshold doses of clozapine may be suitable for in vivo DREADD experiments, rather than CNO itself. This may result in confounding behavioural effects given clozapine has affinity for several serotonergic and dopaminergic receptors (Meltzer, 1994). However, concerns regarding this caveat should be tempered because the affinity of clozapine for muscarinic‐based DREADDs is substantially higher than for native receptors, and an effective dose‐window is achievable because of this. However, the inclusion of a control group without DREADD expression is a critical for interpretation of chemogenetic experiments. One potentially relevant consequence of this finding is that intracranial injections (see Intracranial and intracerebroventricular CNO administration section) avoid this complication. Because CNO is injected directly into the brain, the actions on DREADDs are more likely to be mediated by CNO, rather than clozapine.
Effects of chronic drug administration and DREADD activation
The most common use of chemogenetics is for acute manipulation of neuronal function to identify a critical role of the neuronal population expressing chemogenetic receptors, in a specific behaviour. This approach has provided, and will continue to provide, important findings in terms of basic neuroscience. However, any potential clinical applications will rely on chronic or repeated drug administration and receptor activation. With this in mind, the effect of chronic activation of muscarinic‐based DREADDs, which exert their actions through G‐protein coupled signalling cascades, is a factor that can be given greater focus. While chronic (daily for 4 weeks) administration of CNO (i.p.; 1 mg·kg−1), stimulating beta cells expressing hM3Dq, has been used to examine high‐fat‐diet‐induced diabetes in mice (Jain et al., 2013), the off‐target behavioural effects of chronic CNO were not examined. Urban et al. (2016) sought to examine the long‐term effects of serotonergic neuron stimulation assessing the effect of both acute (one 2 mg·kg−1 i.p. injection) and chronic (5 mg·kg−1·day−1 for 3 weeks in drinking water) activation of these neurons in 5HT transporter‐Cre mice. Interestingly, they found that chronic administration effectively reduced anxiety‐like behaviours. These clinically relevant results highlight the different behavioural effects that follow acute or chronic stimulation of a population of neurons. Furthermore, they suggest that chronic administration of CNO can induce important neural adaptations that acute experiments are not able to detect. In summary, the effects of chronic DREADD ligand administration, as well as chronic receptor activation, are critical factors that need to be extensively studied before the translational potential of chemogenetics is realized.
Conclusion and future directions
The chemogenetic technique has allowed for significant progress in the basic neuroscience mechanisms of animal behaviour. Because this approach is relatively less invasive than optogenetics, it may be favoured for clinical application. The use of AAV in clinical studies has precedent, with studies involving transgene expression in patients with Parkinson's disease (Christine et al., 2009). Thus, at the very least, viral vector transfection is not a limiting factor for the translatability of this technique. Indeed, recent advances have been made, which enable efficient transduction and non‐invasive gene delivery throughout the central and peripheral nervous systems via i.v. AAV injection (Chan et al., 2017). However, important caveats regarding the specificity of existing DREADD ligands, as well as the lack of evidence that chronic DREADD activation will yield the same effects as acute activation, limit immediate translatability. Nevertheless, this technique has significant future potential for basic neuroscience discoveries into the neural control of animal behaviour.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank Daniel Scott and Andrew Lawrence for their comments on the draft of this manuscript. This work was supported by the National Health and Medical Research Council project grant 1105741 and the Victorian State Government Operational Infrastructure Scheme.
Campbell, E. J. , and Marchant, N. J. (2018) The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. British Journal of Pharmacology, 175: 994–1003. doi: 10.1111/bph.14146.
Contributor Information
Erin J Campbell, Email: erin.campbell@florey.edu.au.
Nathan J Marchant, Email: n.marchant@vumc.nl.
References
- Alexander GM, Rogan SC, Abbas AI, armbruster BN, Pei Y, Allen JA et al (2009). Remote control of neuronal activity in transgenic mice expressing evolved G protein‐coupled receptors. Neuron 63: 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to Pharmacology 2017/18: G protein‐coupled receptors. Brit J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017b). The Concise Guide to Pharmacology 2017/18: Ligand‐gated ion channels. Brit J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to Pharmacology 2017/18: Enzymes. Brit J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007). Evolving the lock to fit the key to create a family of G protein‐coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 104: 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S (2013). Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 8: e76310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM (2008). A FLEX switch targets channelrhodopsin‐2 to multiple cell types for imaging and long‐range circuit mapping. J Neurosci 28: 7025–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GG et al (2016). Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron 90: 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH (1997). Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol 71: 6641–6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boender AJ, De Jong JW, Boekhoudt L, Luijendijk MC, Van Der Plasse G et al (2014). Combined use of the canine adenovirus‐2 and DREADD‐technology to activate specific neural pathways in vivo . PLoS One 9: e95392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos‐Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos‐Guasp WA, Nieh EH et al (2017). Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci 20: 824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho Poyraz F, Holzner E, Bailey MR, Meszaros J, Kenney L, Kheirbek MA et al (2016). Decreasing striatopallidal pathway function enhances motivation by energizing the iniation of goal‐directed action. J Neurosci 36: 5988–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassataro D, Bergfeldt D, Malekian C, Van Snellenberg JX, Thanos PK, Fishell G et al (2014). Reverse pharmacogenetic modulation of the nucleus accumbens reduces ethanol consumption in a limited access paradigm. Neuropsychopharmacology 39: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL et al (2017). Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20: 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YW et al (1998). Reversible metabolism of clozapine and clozapine N‐oxide in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 22: 723–739. [DOI] [PubMed] [Google Scholar]
- Chen X, Choo H, Huang XP, Yang X, Stone O, Roth BL et al (2015). The first structure–activity relationship studies for designer receptors exclusively activated by designer drugs. ACS Chem Nerosci 6: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA et al (2009). Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 73: 1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin BR, Hsiao EC, Claeysen S, Dumuis A, Srinivasan S, Forsayeth JR et al (2008). Engineering GPCR signaling pathways with RASSLs. Nat Methods 5: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward P, Wada HG, Falk MS, Chan SD, Meng F, Akil H et al (1998). Controlling signaling with a specifically designed Gi‐coupled receptor. Proc Natl Acad Sci U S A 95: 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Rogan SC, Roth BL (2010). Directed molecular evolution of DREADDs: a generic approach to creating next‐generation RASSLs. Nat Protoc 5: 561–573. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dingsdale A, Helsby N, Orme ML, Breckenridge AM (1988). The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution. Eur J Clin Pharmacol 35: 681–684. [DOI] [PubMed] [Google Scholar]
- Eldridge MAG, Lerchner W, Saunders RC, Kaneko H, Krausz KW, Gonzalez FJ et al (2016). Disruption of relative reward value by reversible disconnection of orbitofrontal and rhinal cortex using DREADDs in rhesus monkeys. Nat Neurosci 19: 37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ et al (2013). A Gαs DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology 38: 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MS, Roth BL (2013). Pharmacosynthetics: reimagining the pharmacogenetic approach. Brain Res 1511: 6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PEM, Dong Y et al (2011). Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14: 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldi CJ, Milton LK, Oldfield BJ (2017). The role of mesolimbic reward neurocircuitry in prevention and rescue of the activity‐based anorexia (ABA) phenotype in rats. Neuropsychopharm 42: 2291–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM (2002). Novel adeno‐associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A 99: 11854–11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge F, Wang N, Cui C, Li Y, Liu Y, Ma Y et al (2017). Glutamatergic projections from the entorhinal cortex to dorsal dentate gyrus mediate context‐induced reinstatement of heroin seeking. Neuropsychopharmacology 42: 1860–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J, Freemantle N, Harrison P, Bebbington P (2000). Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta‐regression analysis. BMJ 321: 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa‐Shah P, Rodriguez LA et al (2017). Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DS, Bliss‐Moreau E, Machado CJ, Bennett J, Shen K, Grant KA et al (2016). The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron 91: 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettier JM, Gautam D, Scarselli M, Ruiz De Azua I, Li JH, Rosemond E et al (2009). A chemical–genetic approach to study G protein regulation of β cell function in vivo . Proc Natl Acad Sci U S A 10: 19197–19202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Sun Y, Wood MA, Xu X (2013). Cell‐type specific inactivation of hippocampal CA1 disrupts location‐dependent object recognition in the mouse. Learn Mem 20: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al 2018. The IUPHAR/BPS guide to pharmacology in 2018: updates and expansion to encompass the new guide to immunopharmacology. doi: https://doi.org/10.1093/nar/gkx1121. [DOI] [PMC free article] [PubMed]
- Islam R, Zhang Y, Xu L, Sah P, Lynch JW (2017). A chemogenetic receptor that enhances the magnitude and frequency of glycinergic inhibitory postsynaptic currents without inducing a tonic chloride flux. ACS Chem Nerosci 8: 460–467. [DOI] [PubMed] [Google Scholar]
- Jain S, Ruiz De Azua I, Lu H, White MF, Guettier JM, Wess J (2013). Chronic activation of a designer Gq‐coupled receptor improves beta cell function. J Clin Invest 123: 1750–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal PB, English AW (2017). Chemogenetic enhancement of functional recovery after sciatic nerve injury. Eur J Neurosci 45: 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janbandhu VC, Moik D, Fassler R (2014). Cre recombinase induces DNA damage and tetraploidy in the absence of LoxP sites. Cell Cycle 13: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL et al (2013). Distinct extended amygdala circuits for divergent motivational states. Nature 496: 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Kaneko H, Minamimoto T, Inoue H, Takeuchi H, Kumata K et al (2016). Multimodal imaging for DREADD‐expressing neurons in living brain and their application to implantation of iPSC‐derived neural progenitors. J Neurosci 36: 11544–11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadimas SK, Satkunendrarajah K, Fehlings MG (2016). 179 chemogenetic stimulation of the lumbar locomotor network enhances motor function following experimental cervical spinal cord injury: translational relevance for a novel therapeutic strategy. Neurosurgery 63 (Suppl 1): 171. [Google Scholar]
- Kremer EJ, Boutin S, Chillon M, Danos O (2000). Canine adenovirus vectors: An alternative for adenovirus‐mediated gene transfer. J Virol 74: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Lein ES, Callaway EM (2002). A genetic method for selective and quickly reversible silencing of mammalian neurons. J Neurosci 22: 5287–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchner W, Xiao C, Nashmi R, Slimko EM, Van Trigt L, Lester HA et al (2007). Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin‐gated Cl− channel. Neuron 54: 35–49. [DOI] [PubMed] [Google Scholar]
- Lichtenberg NT, Pennington ZT, Holley SM, Greenfield VY, Cepeda C, Levine MS et al (2017). Basolateral amygdala to orbitofrontal cortex projections enable cue‐triggered reward expectations. J Neurosci 37: 8374–8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynagh T, Lynch JW (2010). An improved ivermectin‐activated chloride channel receptor for inhibiting electrical activity in defined neuronal populations. J Biol Chem 285: 14890–14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, Espana RA et al (2016). Clozapine N‐oxide administration produces behavioral effects in Long–Evans rats: implications for designing DREADD experiments. eNeuro 3 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM (2011). Chemical and genetic engineering of selective ion channel–ligand interactions. Science 333: 1292–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J et al (2014). Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 17: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Whitaker LR, Harvey BK, Kaganovsky K, Adhikary S et al (2016a). Role of ventral subiculum in context‐induced relapse to alcohol seeking after punishment‐imposed abstinence. J Neurosci 36: 3281–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Whitaker LR, Bossert JM, Harvey BK, Hope BT, Kaganovsky K et al (2016b). Behavioral and physiological effects of a novel κ‐opioid receptor‐based DREADD in rats. Neuropsychopharmacology 41: 402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, Aston‐Jones G (2017). Dorsal hippocampus drives context‐induced cocaine seeking via inputs to lateral septum. Neuropsychopharm: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY (1994). An overview of the mechanism of action of clozapine. J Clin Psychiatry 55: 47–52. [PubMed] [Google Scholar]
- Menuet C, Le S, Dempsey B, Connelly AA, Kamar JL, Jancovski N et al (2017). Excessive respiratory modulation of blood pressure triggers hypertension. Cell Metab 25: 739–748. [DOI] [PubMed] [Google Scholar]
- Miller RE, Ishihara S, Bhattacharyya B, Delaney A, Menichella DM, Miller RJ et al (2017). Chemogenetic inhibition of pain neurons in a mouse model of osteoarthritis. Arthritis & Rheumatology 69: 1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy MA, Gu M, Motzel S, Zhao J, Lin J, Su Q et al (1998). An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci U S A 95: 7866–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Kikuchi E, Lerchner W, Inoue K, Eldridge MAG, Kaneko H et al (2016). PET imaging‐guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat Commun 6: 13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Smirnov D, Neumaier JF (2015). DREADD'ed addiction: using designer receptors to delineate neural circuits underlying drug‐seeking behaviors In: Thiel G. (ed). Designer Receptors Exclusively Activated by Designer Drugs. Humana Press: New York, pp. 129–146. [Google Scholar]
- Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O et al (2016). Gs‐coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun 8: 10268–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Wess J (2012). Design and functional characterization of a novel, arrestin‐biased designer G protein‐coupled receptor. Mol Pharmacol 82: 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguchi M, Okajima M, Tanaka S, Koizumi M, Kikusui T, Ichihara N et al (2015). Double virus vector infection to the prefrontal network of the macaque brain. PLoS One 10: e0132825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palella TD, Hidaka Y, Silverman LJ, Levine M, Glorioso J, Kelley WN (1989). Expression of human HPRT mRNA in brains of mice infected with a recombinant herpes simplex virus‐1 vector. Gene 80: 137–144. [DOI] [PubMed] [Google Scholar]
- Raper J, Morrison RD, Daniels JS, Howell L, Bachevalier J, Wichmann T et al (2017). Metabolism and distribution of clozapine‐N‐oxide: implications for nonhuman primate chemogenetics. ACS Chem Neurosci 8: 1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnutgen F, Doerflinger N, Calleja C, Wendling O, Chambon P, Ghyselinck NB (2003). A directional strategy for monitoring Cre‐mediated recombination at the cellular level in the mouse. Nat Biotechnol 21: 562–565. [DOI] [PubMed] [Google Scholar]
- Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM et al (2014). Leptin mediates the increase in blood pressure associated with obesity. Cell 159: 1404–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudais C, Laplace‐Builhe C, Kissa K, Kremer EJ (2001). Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo . FASEB J 15: 2283–2285. [DOI] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM (2014). Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus > midbrain pathway for feeding behavior. Neuron 82: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner K, Schwab H (2012). Recent advances in rational approaches for enzyme engineering. Comput Struct Biotechnol J 2: e201209010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader CD, Gaffney T, Sugg EE, Candelore MR, Keys R, Patchett AA et al (1991). Allele‐specific activation of genetically engineered receptors. J Biol Chem 266: 5–8. [PubMed] [Google Scholar]
- Sweger EJ, Casper KB, Scearce‐Levie K, Conklin BR, McCarthy KD (2007). Development of hydrocephalus in mice expressing the Gi‐coupled GPCR Ro1 RASSL receptor in astrocytes. J Neurosci 27: 2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M et al (2006). Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron 51: 157–170. [DOI] [PubMed] [Google Scholar]
- Tervo DG, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD et al (2016). A designer AAV variant permits efficient retrograde access to projection neurons. Neuron 92: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Roth BL (2015). DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Ann Rev Pharmacology and Toxicity 55: 399–417. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D et al. (2016). Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41: 1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy E, Robinson JE, Li C, Olsen RH, Diberto JF, Giguere PM et al (2015). A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86: 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL et al (2017). The anterior insular cortex → central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron 96: 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JO, Mcnally GP (2015). Pharmacogenetic excitation of dorsomedial prefrontal cortex restores fear prediction error. J Neurosci 35: 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011). Optogenetics in neural systems. Neuron 71: 9–34. [DOI] [PubMed] [Google Scholar]


