Abstract
Background and Purpose
The ω‐6 fatty acid‐derived lipid mediators such as prostanoids, thromboxane and leukotrienes have well‐established roles in regulating both inflammation and smooth muscle contractility. Resolvins are derived from ω‐3 fatty acids and have important roles in promoting the resolution of inflammation, but their activity on smooth muscle contractility is unknown. We investigated whether resolvin E1 (RvE1), resolvin D1 (RvD1) and resolvin D2 (RvD2) can modulate contractions of isolated segments of rat thoracic aorta (RTA) or human pulmonary artery (HPA) induced by the α1‐adrenoceptor agonist phenylephrine or the stable thromboxane A2 mimetic U46619.
Experimental Approach
Contractile responses in RTA and HPA were measured using wire myography. Receptor expression was investigated by immunohistochemistry.
Key Results
Constriction of RTA segments by U46619, but not by phenylephrine, was significantly inhibited by pretreatment for 1 or 24 h with 10–100 nM RvE1, RvD1 or RvD2. The inhibitory effect of RvE1 was partially blocked by a chemerin receptor antagonist (CCX832). RvE1 at only 1–10 nM also significantly inhibited U46619‐induced constriction of HPA segments, and the chemerin receptor, GPR32 and FPR2/ALX were identified in HPA smooth muscle.
Conclusion and Implications
These data suggest that resolvins or their mimetics may prove useful novel therapeutics in diseases such as pulmonary arterial hypertension, which are characterized by increased thromboxane contractile activity.
Abbreviations
- BLT1
LTB4 receptor
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FPR2/ALX
formyl peptide receptor 2/lipoxin A4 receptor
- HPA
human pulmonary artery
- KPSS
potassium physiological salt solution
- PE
phenylephrine
- PSS
physiological salt solution
- PUFA
polyunsaturated fatty acid
- RTA
rat thoracic aorta
- Rv
resolvin
- SPM
specialized pro‐resolving lipid mediator
Introduction
Inappropriate smooth muscle contraction is central to chronic vascular diseases such as pulmonary and systemic hypertension. Many lipid mediators derived from ω‐6 polyunsaturated fatty acids (PUFAs) are vasoactive; LTD4 and thromboxane A2 are both potent vasoconstrictors, whilst PGI2 (prostacyclin) is a vasodilator. Specialized pro‐resolving lipid mediators (SPM) including the resolvins are derived from the ω‐3 PUFAs eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) (Serhan et al., 2000; Serhan et al., 2002).
They have important roles in the resolution of inflammation, either via their own GPCRs or by modulating GPCRs for ω‐6 PUFA (Serhan et al., 2015). For example, resolvin E1 (RvE1) (5S,12R,18R–trihydroxy‐6Z,8E,10E,14Z,16E–EPA; Arita et al., 2005) enhances the phagocytosis of apoptotic neutrophils via its chemerin receptor (Ohira et al., 2010) and also inhibits the infiltration of neutrophils by antagonizing LTB4 at BLT1 receptors (Arita et al., 2007). Resolvin D1 (RvD1) (7S,8R,17S–trihydroxy‐4Z,9E,11E,13Z,15E,19Z–DHA; Sun et al., 2007) has been shown to bind to two GPCRs, namely, the orphan receptor, GPR32, and the lipoxin receptor, FPR2/ALX (Krishnamoorthy et al., 2010). Evidence that resolvin D2 (RvD2) (7S, 16R, 17S–trihydroxy‐4Z, 8E, 10Z, 12E, 14E, 19Z–DHA; Spite et al., 2009) binds to orphan receptor GPR18 expressed on human leukocytes was recently demonstrated, whilst GPR18‐knockout mice displayed reduced phagocytotic clearance of bacteria and a lack of resolution (Chiang et al., 2015).
RvE1, RvD1 and RvD2 have been shown to influence vascular smooth muscle cell phenotype, including chemotaxis, proliferation and migration (Ho et al., 2010; Miyahara et al., 2013). More recently, RvD1 loaded into biodegradable wraps was found to reduce neointimal hyperplasia, likely due in part to the reduced proliferation and migration of smooth muscle cells seen in vitro (Wu et al., 2017). Importantly, receptors for all three resolvins have been identified in smooth muscle (Ho et al., 2010; Miyahara et al., 2013; Watts et al., 2013; Hiram et al., 2015). However, little is known about whether resolvins can modulate the contractility of vascular smooth muscle. In this study, we investigated whether RvE1, RvD1 and RvD2 can directly modulate the contractility of intact segments of rat thoracic aorta (RTA) and human pulmonary artery (HPA) in vitro.
Methods
Animal tissue retrieval
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). All housing, care and procedures were carried out in accordance with institutional guidelines. Rats were chosen based on previously published work undertaking successful wire myography experiments with RTA. Rats were housed in standard housing conditions with 0–1 cage companions. Rats [total of 17 male Wistar rats (Charles River, UK or in‐house stock), weighing between 200 and 300 g, aged 6–12 months] were killed by an increased concentration of CO2 and subsequent cervical dislocation. The RTA was removed and cut into adjacent segments ready for experimentation.
Human tissue retrieval
HPA segments were obtained from samples donated by patients with informed consent who were undergoing thoracic surgery at Southampton General Hospital. Samples were obtained following review and approval by the institutional review committee (Ethical permission: Southampton & SW Hants LREC 08/H0502/32 or REC Reference Number 14/SC/0186). HPAs were dissected out and cut into adjacent segments ready for experimentation (Table 1).
Table 1.
Characteristics of patients from whom samples were obtained for wire myography
| Number of samples | Average age (years) | F/M | Average FEV1/FVC |
|---|---|---|---|
| 10 | 66.5 ± 0.84 | 5/5 | 0.64 ± 0.01 |
FEV1, forced expiratory volume in 1 s; FVC: forced vital capacity.
General wire myography procedures
Wire myography was carried out using multi wire myograph system 610 M from Danish Myo Technology. Segments were mounted on the wire myograph as described previously (Pike et al., 2014). Briefly, segments, which had been cleaned of surrounding tissue, were carefully slid onto pins on the myograph jaw and bathed in physiological salt solution (PSS). Paired adjacent segments from the same animal or human sample were used across the multiple chambers during a single experiment, eliminating the need for sample randomisation. Operator blinding was not carried out since a single individual undertook all experimental work and data are quantitative and not subjective. Based on both published data and preliminary studies in our laboratory, a baseline tension of 1.5 g was set for both RTA and HPA. Tension was permitted to plateau before confirming functional integrity by a contractile response to potassium PSS (KPSS). Concentration–response curves are displayed as a percentage of the KPSS response of that individual tissue segment, whilst reversal of preconstriction experiments are expressed as a percentage relaxation to account for small differences in segment size and therefore the amount of contractile smooth muscle present.
Resolvin pretreatment and constriction of arteries with U46619 or phenylephrine
Adjacent segments of freshly isolated RTA or HPA (2 mm length; 800 μm diameter) were incubated in culture plates in DMEM‐F12 (+10% newborn calf serum; + penicillin and streptomycin) with or without RvE1 (0.1–300 nM), RvD1 (1–100 nM) or RvD2 (1–100 nM) for 1 or 24 h at 37°C and 5% CO2. In some experiments, the chemerin receptor antagonist CCX832 (100 nM) (Chemocentryx) or vehicle was added 15 min before subsequent resolvin incubation. Segments were mounted on the wire myograph in PSS (described in detail above) and then constricted with cumulative concentrations of the stable thromboxane mimetic U46619 (RTA 1–1000 nM; HPA 0.1–1000 nM) or phenylephrine (PE) (10 nM to 30 μM).
Immunohistochemistry
Segments of HPA were fixed in 10% neutral buffered formalin for 24 h then processed and embedded in paraffin wax. Sections (4 μm) were immunostained with primary antibodies for chemerin receptor (ab150491, Abcam, UK), GPR32 (ab61429, Abcam, UK) or FRP2/ALX (ab101702, Abcam UK) and visualized with an AEC chromogen and Mayer's haematoxylin.
Reversal of artery preconstriction
Isolated RTA segments (2 mm length; 800 μm diameter) bathed in PSS were pre‐constricted with an 80% submaximal concentration (3 μM) of PE; once a stable contractile plateau had been established, the muscarinic antagonist ACh (10 μM) was used to confirm the ability of the constricted segments to relax. Segments were then washed with PSS and constricted with an 80% submaximal concentration of either PE (3 μM) or U46619 (100 nM), then RvD1, RvD2 or RvE1 (100 nM) was applied with changes in tension monitored for the following 10 min.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). All data were analysed using GraphPad Prism Version 6 (GraphPad Software Inc., La Jolla, CA, USA). The threshold for statistical significance was P < 0.05. Concentration–response curves are reported as mean ± SEM and were analysed using a two‐way repeated measures ANOVA with Sidak's multiple comparisons correction, with the exception of data shown in Figure 3A. These data were analysed using an ordinary two‐way ANOVA owing to some missing values at 0.1 and 0.3 nmol·L−1 U46619 since the cumulative response curve was extended part way through the study to account for the unexpected increased response to U46619 in HPA compared to RTA. Reversal experiments are reported as medians and, where appropriate, analysed using a Kruskal–Wallis test with Dunn's multiple comparisons correction. In some experiments (Figures 1E, 2A, B and 5A), limited animal availability and time constraints resulted in n < 5. No statistical analysis has been performed on these data sets. In Figure 5A, experimental loss on one occasion has resulted in unequal group sizes. No statistical analysis was performed on this data set.
Figure 1.
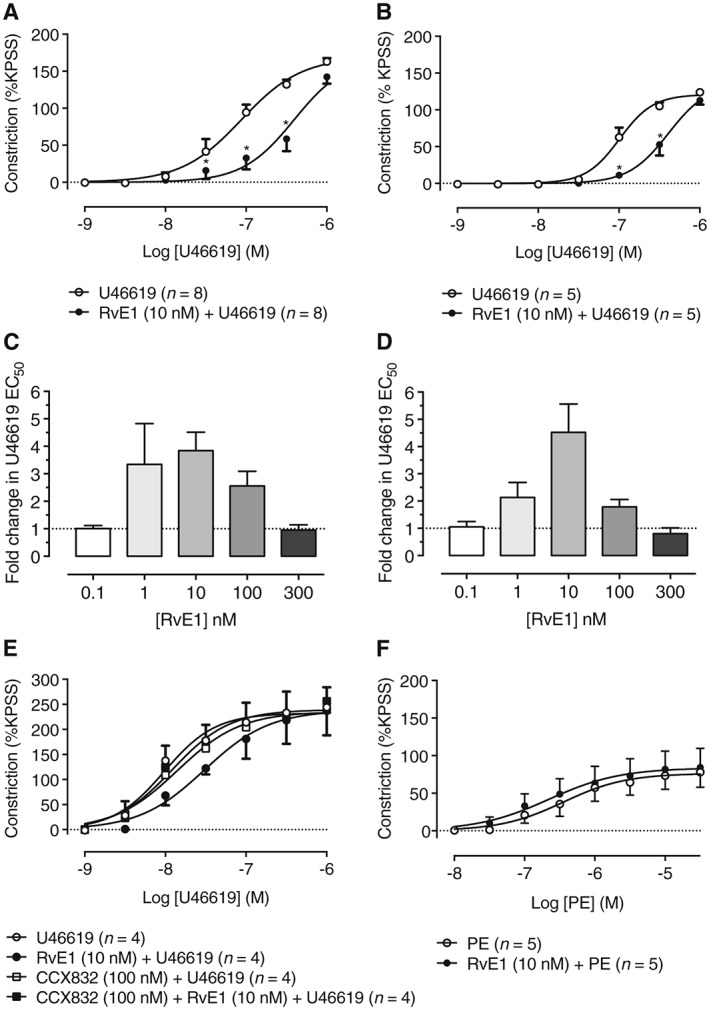
Pretreatment with RvE1 concentration‐dependently inhibits U46619‐induced constriction of RTA segments. (A) Pretreatment with RvE1 at a concentration of 10 nM for 1 h significantly inhibited constriction of RTA segments induced by cumulative concentrations of U46619 (n = 8). (B) U46619‐induced constriction of RTA segments was also inhibited by RvE1 (10 nM) pretreatment for 24 h (n = 5). (C) Inhibition of U46619‐induced constriction was dependent on the concentration of RvE1 (0.1–300 nM) used during pretreatment for 1 h or (D) 24 h. In both instances, the greatest shift in U46619 EC50 occurred at 10 nM RvE1. (E) The compound, CCX832, is a novel antagonist of the chemerin receptor, a receptor for RvE1. At 100 nM, CCX832 alone had no effect on U46619‐induced constriction of RTA segments. When added 15 min before a 1 h pretreatment with RvE1 (10 nM, n = 4), CCX832 reduced the inhibition by RvE1 of U46619‐induced constriction at both 10 and 30 nM of U46619, suggesting that the inhibitory action of RvE1 is mediated by chemerin receptors. (F) RvE1 (10 nM) pretreatment for 1 h did not affect constriction of RTA segments induced by the α1‐adrenoceptor agonist PE, indicating a selective inhibitory activity of RvE1 against the thromboxane mimetic U46619.
Figure 2.
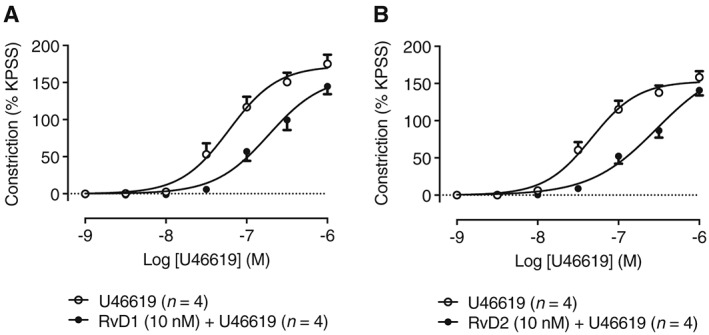
Effect of D‐series resolvins on U46619‐induced constriction of RTA segments. Pretreatment for 1 h with 10 nM concentrations of (A) RvD1 or (B) RvD2 reduced constriction of RTA segments induced by cumulative concentrations of U46619 (n = 4).
Figure 5.
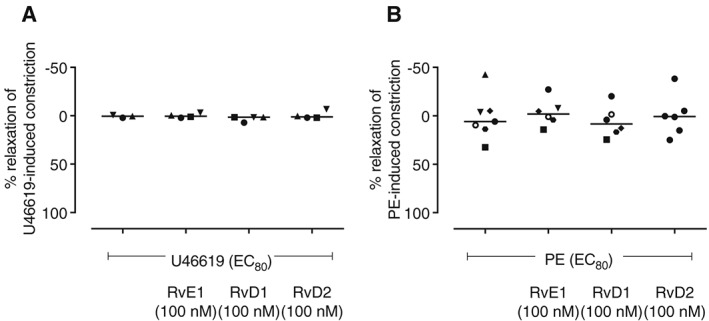
Resolvins E1, D1 and D2 do not reverse constriction of RTA. RTA segments were preconstricted with a submaximal (EC80) concentration of agonist and then treated with RvD1, RvD2 or RvE1 (100 nM). The resolvins had no vasodilator effect on RTA preconstricted with either (A) U46619 or (B) PE.
Materials
Resolvins E1, D1 and D2 were purchased from Cambridge Bioscience (Cambridge, UK). U46619 was purchased from Tocris Bioscience (Abingdon, UK). ACh and PE were purchased from Sigma‐Aldrich (Dorset, UK). Antibodies against the chemerin, GPR32 and FPR2/ALX receptors were purchased from Abcam (Cambridge, UK).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Results
Pretreatment with RvE1 inhibits U46619‐induced constriction of RTA
The stable thromboxane mimetic U46619 (1–1000 nM) constricted RTA in a concentration‐dependent manner (Figure 1). Pretreatment for 1 h with RvE1 (10 nM) significantly inhibited U46619‐induced constriction of RTA segments, increasing the U46619 EC50 by 3.8‐fold compared to control (Figure 1A). Similar inhibition of contractility was seen after pretreatment with RvE1 (10 nM) for 24 h, with the U46619 EC50 being increased 4.5‐fold (Figure 1B). To determine the maximal inhibitory concentration of RvE1, RTA segments were pretreated with RvE1 concentrations from 0.1 to 300 nM for 1 or 24 h then constricted with U46619 (1–1000 nM). In each case, the inhibitory effect of RvE1 was concentration‐dependent, forming bell‐shaped response curves with maximal inhibition occurring at 10 nM (Figure 1C, D).
Effect of the chemerin receptor antagonist CCX832 on inhibition of U46619‐induced contractility of RTA and the effect of RvE1 on RTA constriction induced by phenylephrine (PE)
RvE1 does not compete with U46619 for the thromboxane TP receptor (Dona et al., 2008), but it is an agonist for chemerin receptors (Ohira et al., 2010). We therefore explored whether the chemerin receptor antagonist CCX832 (Watts et al., 2013) can block the inhibitory effect of RvE1 on U46619‐induced constriction. RTA segments were pretreated with RvE1 (10 nM) in the presence or absence of CCX832 (100 nM) before constriction with U46619 (1–1000 nM). CCX832 reduced the inhibitory effect of RvE1 on U46619‐induced constriction (Figure 1E), indicating its dependence on chemerin receptors. To further explore the inhibitory effect of RvE1 on contractility, RTA segments were pretreated with RvE1 (10 nM, 1 h) before constriction with cumulative concentrations of the α1‐adrenoreceptor agonist PE (0.01–30 μM). RvE1 pretreatment had no effect on PE‐induced constriction (Figure 1F).
D‐series resolvins also inhibit U46619‐induced constriction of RTA segments
Experiments were also performed to determine whether the D‐series resolvins RvD1 (10 nM) or RvD2 (10 nM) can modulate contractility of RTA segments to U46619 (1–1000 nM). U46619‐induced RTA constriction was reduced by 1 h of pretreatment with either RvD1 or RvD2 (Figure 2A, B respectively).
RvE1 also inhibits U46619‐induced constriction of human pulmonary artery (HPA)
In experiments analogous to those in RTA segments, the ability of RvE1 to modulate vascular contractility was investigated in HPA segments (Figure 3). Pretreatment of HPA with RvE1 (10 nM) for 1 h significantly impaired HPA constrictions induced by U46619 (0.1–1000 nM) (Figure 3A). The RvE1 inhibitory activity of RvE1 followed a bell‐shaped curve with maximal inhibition being an eightfold increase in U46619 EC50 seen at a concentration of 1 nM RvE1 (Figure 3B).
Figure 3.
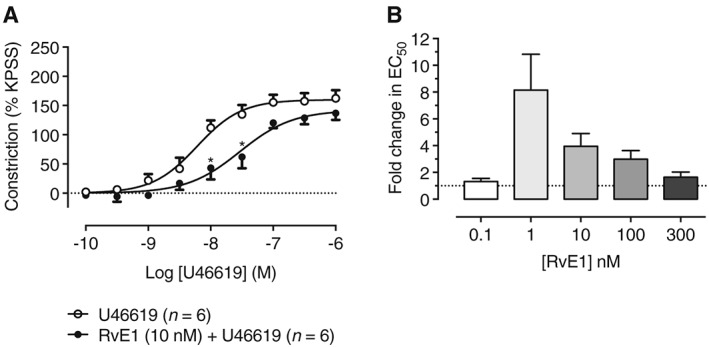
Pretreatment with RvE1 concentration‐dependently inhibits U46619‐induced constriction of HPA. (A) Pretreatment with RvE1 at a concentration of 10 nM for 1 h significantly inhibited constriction of HPA segments induced by cumulative concentrations of U46619 (n = 6). (B) Pretreatment of HPA segments with various concentrations of RvE1 (0.1–300 nM) for 1 h significantly inhibited constriction induced by cumulative concentrations of U46619 (1–1000 nM), with the greatest shift in U46619 EC50 occurring at 1 nM RvE1, suggesting greater sensitivity of HPA compared with RTA segments.
Expression of resolvin receptors in human pulmonary artery
Isolated HPA immunostained with antibodies against the chemerin receptor, GPR32 and FPR2/ALX demonstrated expression of these receptors in both the vascular endothelium and smooth muscle (Figure 4).
Figure 4.
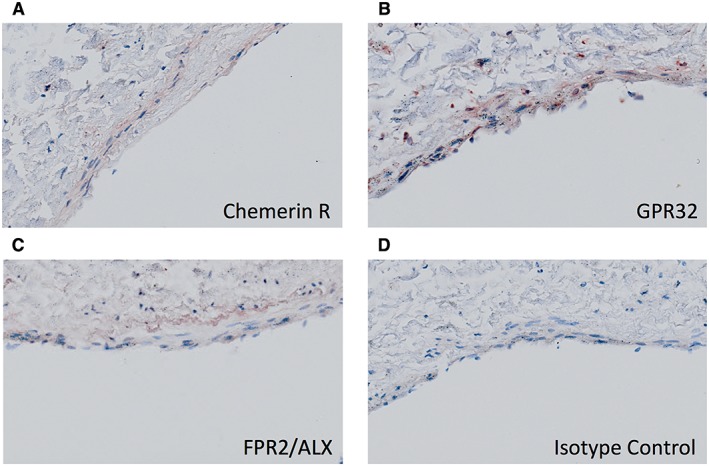
Expression of resolvin receptors in HPA. Immunohistochemistry of formalin‐fixed paraffin sections showed expression of (A) the chemerin receptor (RvE1 receptor) (B) RvD1 receptor GPR32 and (C) RvD1 receptor FPR/ALX in the vascular endothelium and smooth muscle of HPA segments. (D) HPA isotype control.
Resolvins D1, D2 and E1 do not relax pre‐constricted RTA segments
Having established the inhibitory effect of pretreatment for 1 or 24 h on RTA and HPA contractility, we next explored whether the addition of resolvins can reverse an 80% submaximal pre‐constriction of RTA segments induced by U44619 (100 nM) or PE (3 μM). RvE1, RvD1 and RvD2 (100 nM) each had no effect on RTA segments pre‐contracted with either PE (Figure 5A) or U46619 (Figure 5B). In contrast, constriction of RTA segments induced by PE were completely reversed within 10 min of the addition of ACh (10 μM) (Supporting Information Figure S1), probably acting via muscarinic receptors.
Discussion and conclusions
Lipid mediators derived from ω‐6 fatty acids include highly potent pro‐inflammatory and vasoactive mediators such as LTD4, thromboxane A2 and prostacyclin. SPMs such as the E‐series and D‐series Rv derived from ω‐3 fatty acids are important mediators in the resolution of inflammation (Serhan et al., 2015), but their ability to modulate contraction of vascular smooth muscle is unknown. In the present study, we investigated the ability of RvE1, RvD1 and RvD2 to prevent or reverse contractions of RTA and HPA segments induced in vitro by the stable thromboxane mimetic U46619 and the α1‐adrenoceptor agonist PE.
Using wire myography of intact arterial segments, our study shows for the first time that pretreatment of either RTA or HPA segments for only 1 h with nM concentrations of RvE1 significantly inhibited constrictions induced by U46619. The effect of RvE1 was concentration‐dependent in each tissue with bell‐shaped inhibition curves showing maximal inhibition at 10 nM in RTA (Figure 1) and 1 nM in HPA (Figure 3), diminishing gradually to zero inhibition at 300 nM. A published study may have failed to detect a direct inhibitory effect of RvE1 on HPA contractility due to their use of a concentration (300 nM) shown to be inactive in our study, although this concentration was reported to inhibit hyperresponsiveness of HPA induced by inflammatory mediators (Hiram et al., 2015). Notably, the authors found RvE1 capable of inhibiting the inflammatory mediator‐induced increase in phosphorylation of contractile proteins such as CPI‐17 (C‐kinase potentiated protein phosphatase‐1 inhibitor Mr = 17 kDa I‐17). It is possible that the results seen in our study are the result of a decrease in the sensitivity of the smooth muscle contractile proteins. Together, these studies suggest that resolvins can directly prevent smooth muscle contraction at low concentrations and prevent the induction of chronic hyperresponsiveness at higher concentrations. Incidentally, bell‐shaped concentration–response curves with resolvins have been shown a number of times previously (Spite et al., 2009; Oh et al., 2011; Clària et al., 2012).
The findings that the inhibitory effect of RvE1 in RTA and HPA segments is apparent after only 1 h of pretreatment and that it is not enhanced in RTA by longer pretreatment (24 h) suggest that the effect is not dependent on protein synthesis but is rather a direct action either on the thromboxane (TP) receptor activated by U46619 or on the thromboxane signalling pathways leading to contraction. The former is unlikely as RvE1 can inhibit U46619‐induced platelet aggregation but does not displace U46619 from TP receptors, as determined by radioligand binding experiments (Dona et al., 2008). The finding that RvE1 did not inhibit RTA constriction induced by PE (Figure 1F) indicates that it is selective for TP receptor signalling; this may have important implications in the regulation of vascular contractility by thromboxane in cardiovascular disease, including pulmonary hypertension. Further experiments with other lipid and non‐lipid contractile agonists will better define the selectivity of resolvin actions on vascular contractility.
The chemerin receptor antagonist CCX832 reduced the ability of RvE1 to inhibit RTA constriction induced by U46619 (Figure 1E), indicating that chemerin receptors are required and sufficient for the action of RvE1 in this tissue. As well as the E‐series resolvins, we further showed that the ability of RvE1 to suppress U46619‐induced vascular contractility is shared by the D‐series resolvins RvD1 and RvD2 and that the D‐series resolvins were similarly active in the low nM range (Figure 2A, B). D‐series resolvins do not act on the chemerin receptor, suggesting that U46619‐induced contractility is susceptible to inhibition by multiple resolvin receptor‐dependent pathways. RvD1 is an agonist at two GPCRs, the FPR2/ALX receptor and GPR32, and RvD2 may act at the orphan receptor GPR18 (Chiang et al., 2015). Given the ability of all three resolvins to inhibit U46619‐induced constriction, it is likely that their corresponding GPCRs have signalling pathways that converge on TP receptor signalling to produce physiological antagonism of vascular contractility. Immunohistochemical experiments confirmed the expression of the chemerin receptor, GPR32 and FPR2/ALX in the vascular endothelium and smooth muscle of HPA sections (Figure 4A–C), and others have shown that the chemerin receptor is also expressed in RTA tissue (Watts et al., 2013). Interestingly, this latter study also demonstrated chemerin receptor‐dependent contraction of RTA by chemerin‐9, a nonapeptide derived from chemerin (Wittamer et al., 2004). More recently, the same group demonstrated the Gαi dependence of this contraction, with downstream activation of both src and ρ kinase (Ferland et al., 2017). Whilst this may seem contradictory to the findings in this study, the activation of the same GPCR to generate opposing effects is demonstrated with the activation of FPR2 by both serum amyloid A and annexin A1 to give pro‐inflammatory and anti‐inflammatory actions. It is possible that chemerin and RvE1 are interacting with the chemerin receptor in distinct ways to trigger separate downstream signalling pathways. This concept is explored in the review by Cash et al. (2014). Together, these studies may reflect a direct effect of resolvins acting at their respective GPCRs on vascular smooth muscle or perhaps an indirect action mediated by inhibition of the release of thromboxane or modulation of other vasoactive mediators via resolvin GPCRs on endothelial cells. Assays of eicosanoid and other mediator release from endothelium‐intact and denuded vessels should be performed to explore these possibilities. The expression of GPR18 was not investigated in this study, and to our knowledge, it is yet to be investigated in vascular tissues.
Finally, experiments using RTA segments pre‐contracted with U46619 or PE showed that RvE1, RvD1 and RvD2 were unable to reverse contractions to these agonists (Figure 5), although contractions were readily reversible with ACh (Supporting Information Figure S1). This may suggest a mechanism similar to that reported for the ability of RvD1 to prevent, but not reverse, histamine‐induced mucin secretion by conjunctival goblet cells, in which GPR32 activation by the resolvin led to inactivation of H1 histamine receptors due to phosphorylation by intracellular kinases (Li et al., 2013). Intriguingly, previous research has demonstrated the ability of RvE1 to attenuate the phosphorylation of the platelet‐derived growth factor receptor PDGFRβ under both basal and stimulated conditions, providing further evidence of GPCR crosstalk (Ho et al., 2010).
In summary, this study is the first to show that low nM concentrations of RvE1, RvD1 and RvD2 can prevent constriction in rat and human arteries induced by a thromboxane mimetic. Resolvins and stable mimetics of these specialized proresolving mediators may have dual therapeutic activities both to resolve inflammation and to prevent inappropriate vascular contractility in cardiovascular disease.
Author contributions
M.J. collected the data, prepared the figures and wrote the main manuscript text. A.P.S., C.T. and J.A.W. supervised the work and reviewed the manuscript. All authors contributed to experimental design and data interpretation.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 This supporting figure is supplied to illustrate the ability of constricted RTA segments to relax in response to the known vasodilator, ACh. Individual segments were preconstricted with a submaximal concentration of PE, before being treated with ACh.
Acknowledgements
The chemerin receptor antagonist CCX832 was kindly supplied by Chemocentryx. We thank the Histochemical Research Unit (Faculty of Medicine, University of Southampton) for technical assistance. We are extremely grateful to all the patients who donated samples, as well as the nurses, surgeons, pathologists and research colleagues who made tissue retrieval possible. This work was supported by a PhD studentship jointly funded by the Gerald Kerkut Charitable Trust and The University of Southampton Faculty of Medicine.
Jannaway, M. , Torrens, C. , Warner, J. A. , and Sampson, A. P. (2018) Resolvin E1, resolvin D1 and resolvin D2 inhibit constriction of rat thoracic aorta and human pulmonary artery induced by the thromboxane mimetic U46619. British Journal of Pharmacology, 175: 1100–1108. doi: 10.1111/bph.14151.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S et al (2005). Stereochemical assignment, antiinflammatory properties, and receptor for the omega‐3 lipid mediator resolvin E1. J Exp Med 201: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun Y‐P, Elangovan S, Chiang N, Serhan CN (2007). Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 178: 3912–3917. [DOI] [PubMed] [Google Scholar]
- Cash JL, Norling LV, Perretti M (2014). Resolution of inflammation: targeting GPCRs that interact with lipids and peptides. Drug Discov Today 19: 1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Dalli J, Colas RA, Serhan CN (2015). Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 212: 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clària J, Dalli J, Yacoubian S, Gao F, Serhan CN (2012). Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J Immunol 189: 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A et al (2008). Resolvin E1, an EPA‐derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 112: 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland DJ, Darios ES, Neubig RR, Sjögren B, Truong N, Torres R et al (2017). Chemerin‐induced arterial contraction is Gi‐ and calcium‐dependent. Vascul Pharmacol 88: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiram R, Rizcallah E, Marouan S, Sirois C, Sirois M, Morin C et al (2015). Resolvin E1 normalizes contractility, Ca2+ sensitivity and smooth muscle cell migration rate in TNF‐α‐ and IL‐6‐pretreated human pulmonary arteries. Am J Physiol ‐ Lung Cell Mol Physiol 309: L776–L788. [DOI] [PubMed] [Google Scholar]
- Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AHK, Pande R et al (2010). Aspirin‐triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol 177: 2116–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee C‐H, Yang R et al (2010). Resolvin D1 binds human phagocytes with evidence for proresolving receptors. PNAS 107: 1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N et al (2013). Resolvin D1 and aspirin‐triggered resolvin D1 regulate histamine‐stimulated conjunctival goblet cell secretion. Mucosal Immunol 6: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Runge S, Chatterjee A, Chen M, Mottola G, Fitzgerald JM et al (2013). D‐series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J 27: 2220–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SF, Pillai PS, Recchiuti A, Yang R, Serhan CN (2011). Pro‐resolving actions and stereoselective biosynthesis of 18S E‐series resolvins in human leukocytes and murine inflammation. J Clin Invest 121: 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T, Arita M, Omori K, Recchiuti A, Van Dykeand TE, Serhan CN (2010). Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem 285: 3451–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KC, Davis SA, Collins SA, Lucas JSA, Inskip HM, Wilson SJ et al (2014). Prenatal development is linked to bronchial reactivity: epidemiological and animal model evidence. Sci Rep 4: 4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K (2000). Novel functional sets of lipid‐derived mediators with antiinflammatory actions generated from omega‐3 fatty acids via cyclooxygenase 2–nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med 192: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G (2002). Resolvins: a family of bioactive products of omega‐3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196: 1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Dalli J (2015). The resolution code of acute inflammation: novel pro‐resolving lipid mediators in resolution. Semin Immunol 27: 200–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA et al (2009). Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461: 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y‐P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E et al (2007). Resolvin D1 and its aspirin‐triggered 17R epimer. Stereochemical assignments, anti‐inflammation properties, and enzymatic inactivation. J Biol Chem 282: 9323–9334. [DOI] [PubMed] [Google Scholar]
- Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B et al (2013). Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol 33: 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittamer V, Grégoire F, Robberecht P, Vassart G, Communi D, Parmentier M (2004). The C‐terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem 279: 9956–9962. [DOI] [PubMed] [Google Scholar]
- Wu B, Mottola G, Chatterjee A, Lance KD, Chen M, Siguenza IO et al (2017). Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. J Vasc Surg 65: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 This supporting figure is supplied to illustrate the ability of constricted RTA segments to relax in response to the known vasodilator, ACh. Individual segments were preconstricted with a submaximal concentration of PE, before being treated with ACh.


