Abstract
Background and Purpose
Capsaicin‐mediated modulation of taste nerve responses is thought to be produced indirectly by the actions of neuropeptides, for example, CGRP and substance P (SP), on taste cells implying they play a role in taste sensitivity. During the processing of gustatory information in taste buds, CGRP shapes peripheral taste signals via serotonergic signalling. The underlying assumption has been that SP exerts its effects on taste transmitter secretion in taste buds of mice.
Experimental Approach
To test this assumption, we investigated the net effect of SP on taste‐evoked ATP secretion from mouse taste buds, using functional calcium imaging with CHO cells expressing high‐affinity transmitter receptors as cellular biosensors.
Key Results
Our results showed that SP elicited PLC activation‐dependent intracellular Ca2+ transients in taste cells via neurokinin 1 receptors, most likely on glutamate–aspartate transporter‐expressing Type I cells. Furthermore, SP caused Type I cells to secrete GABA.
Conclusion and Implications
Combined with the recent findings that GABA depresses taste‐evoked ATP secretion, the current results indicate that SP elicited secretion of GABA, which provided negative feedback onto Type II (receptor) cells to reduce taste‐evoked ATP secretion. These findings are consistent with a role for SP as an inhibitory transmitter that shapes the peripheral taste signals, via GABAergic signalling, during the processing of gustatory information in taste buds. Notably, the results suggest that SP is intimately associated with GABA in mammalian taste signal processing and demonstrate an unanticipated route for sensory information flow within the taste bud.
Abbreviations
- GLAST
glutamate–aspartate transporter
- IP3
inositol 1,4,5‐trisphosphate
- NK1
neurokinin 1 receptor
- TRPV1
transient receptor potential vaniloid 1
Introduction
Taste signal transmission consists of cell–cell circuits via transmitters secreted by separate morphotypes of taste cells; these transmitters include 5‐HT (Kaya et al., 2004; Huang et al., 2005; Jaber et al., 2014), ATP (Finger et al., 2005; Huang et al., 2007; 2009; Murata et al., 2010), noradrenaline (NA) (Dvoryanchikov et al., 2007; Huang et al., 2008a) and GABA (Cao et al., 2009; Dvoryanchikov et al., 2011; Huang et al., 2012). Responding directly to sour (acid) taste stimuli (Huang et al., 2006, 2008b; Chang et al., 2010) and high concentrations of KCl (Huang et al., 2007; Oka et al., 2013), Type III (presynaptic) cells release 5‐HT, NA and GABA (Huang et al., 2007; 2008b; 2011a). Upon sweet or bitter taste stimulation, Type II (receptor) cells secrete ATP via membrane channels (Huang et al., 2007; Taruno et al., 2013). ATP acts on two targets within taste buds: (i) gustatory afferent fibres that propagate taste signals to the brain (Finger et al., 2005; Huang et al., 2011b; Vandenbeuch et al., 2015); and (ii) Type III (presynaptic) cells, which release 5‐HT and NA when stimulated (Huang et al., 2008b; 2009).
Early electrophysiological data revealed that capsaicin, an agonist of transient receptor potential vanilloid 1 (TRPV1) channels, modulates taste nerve responses to other taste compounds (Wang et al., 1995; Osada et al., 1997; Roper, 2014). This modulation was interpreted as being produced indirectly, via stimulation of capsaicin‐sensitive nerve terminals in the lingual epithelium that release stored neuropeptides [e.g. CGRP and substance P(SP)] onto taste cells by an axon reflex (Holzer, 1988; Maggi and Meli, 1988; Wang et al., 1995; Simon et al., 2003; Sato et al., 2012). Indeed, immunohistochemistry revealed that neuropeptides and TRPV1 coexist in the sensory neurons innervating the circumvallate papillae (Ishida et al., 2002). Despite these findings, our understanding of peptidergic modulation is limited.
SP, an 11 amino acid peptide member of the tachykinin family (Karagiannides et al., 2008), transmits nociceptive sensations. SP is produced in chemosensory neurons that contribute to chemesthesis, the general chemical sensitivity of mucus membranes in oronasal cavities perceived as pungency, irritation or heat (Green, 2012; Roper, 2014). In addition to evoking nociceptive responses to chemical irritants as part of a common chemical sense, the extensive innervation of those areas where taste buds are expected to be present may contribute to specific taste‐related chemical sensitivities (Nagy et al., 1982; Yamasaki et al., 1984; Montavon et al., 1996; Ishida et al., 2002; Sato et al., 2012). Several studies of polymodal nociceptors in taste buds, using electrophysiology (Simons et al., 2003; Talavera et al., 2005), Ca2+ imaging (Grant, 2012; Huang and Wu, 2015) and psychophysical testing (Simons et al., 2002; Kapaun and Dando, 2016), support the notion that oral nociceptive inputs on gustatory transmission may underlie the ability to modulate the perceived intensity of some taste qualities.
Here, we used Ca2+ imaging with cellular biosensors to test whether SP acts on taste cells to alter taste bud output, that is, ATP secretion. Our findings show that SP can shape the peripheral taste signals before their transmission across gustatory fibres.
Methods
Ethical approval
All procedures conducted followed the guidelines of the National Institute of Health (NIH Office of Animal Care and Use) and were approved by the Southern Illinois University Animal Care and Use Committee (Animal Welfare Assurance Number, A‐3078‐01). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Experimental animals
We used adult male C57BL/6J (https://www.jax.org/strain/000664) (n = 51) and GAD67‐GFP (https://www.jax.org/strain/006334) mice (n = 5) obtained from Jackson Laboratory. Animals (6–8 weeks old; average weight 23–26 g) were fed ad libitum and housed in a temperature‐controlled room (22 ± 1°C) under artificial illumination (lights on from 05:00 to 17:00 h) and 55% relative humidity. Mice were killed by exposure to CO2 followed by cervical dislocation. This procedure minimizes distress (NIH guideline, https://oacu.oir.nih.gov/sites/default/files/uploads/arac‐guidelines/rodent_euthanasia_adult.pdf). Tongues were then removed for further dissection.
Isolated taste buds and/or taste cells
Dispersed taste buds and taste cells were isolated as described previously (Huang and Wu, 2015; 2016). Briefly, we injected an enzyme cocktail containing 1 mg·mL−1 collagenase A (Roche, Indianapolis, IN, USA), 2.5 mg·mL−1 dispase II (Roche), 2 mg·mL−1 elastase (Worthington, Lakewood, NJ, USA) and 1 mg·mL−1 trypsin inhibitor (Sigma, St. Louis, MO, USA) beneath the epithelium surrounding circumvallate papillae and removed the lingual epithelium. Isolated taste buds were collected in glass micropipettes and transferred to a recording chamber (Warner Instrument, Hamden, CT, USA) with a glass coverslip base. To isolate single taste cells, individual taste buds were triturated in the recording chamber using a glass micropipette.
Ca2+ imaging
Taste cells located in the shallow recording chamber were loaded with 5 μM Fura 2‐AM (Invitrogen, Life Technologies, USA) following their isolation. During the experiments, taste buds and taste cells were continuously perfused with Tyrode's buffer, composed of the following (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, 10 Na‐pyruvate, 5 NaHCO3, pH 7.2, 310–320 Osm. For Ca2+‐free Tyrode's solution, MgCl2 was substituted for CaCl2. The conventional Ca2+ imaging recording was carried out using Indec Workbench v.6 software (INDEC Biosystem, Mountain View, CA, USA). Fura 2‐loaded cells were excited at 340 and 380 nm, and emission images were collected at ≥510 nm. The ratio of F340/F380 was converted to approximate [Ca2+]i as described by Grynkiewicz et al. (1985). The fluorescence ratios of free and Ca2+‐bound Fura 2 at 340 nM and the fluorescence ratios of free and Ca2+‐bound Fura 2 at 380 nM were determined using a Fura 2 Calcium Imaging Calibration Kit (Invitrogen, Life Technologies) (e.g. Huang et al., 2011a). The average baseline (resting) Ca2+ of taste cells in these experiments was 160 ± 42 nM (n = 39 cells), which corresponds well with values reported previously (Huang et al., 2011a; Huang and Wu, 2015; 2016). Our criteria for accepting Ca2+ responses for analysis are described fully in the previous publications (Huang et al., 2012; Huang and Wu, 2015). In brief, responses were quantified as peak minus baseline [Ca2+] (i.e. ∆[Ca2+]). We accepted Ca2+ responses only if they could be elicited repetitively in the same cell by the same stimulus, and evoked responses were at least 2× baseline [Ca2+] fluctuation.
Isolated taste buds and taste cells were stimulated by bath‐perfusion of ATP, KCl (50 mM, substituted equimolar for NaCl), taste mix and SP (up to 100 nM). Stimuli were bath‐applied for 30 s followed by a return to the buffer perfusion for at least 3–5 min between trials. This perfusion paradigm allows the stimuli to mix thoroughly within the recording chamber and to reach a final concentration in the bath followed by a complete washout. Moreover, this procedure produced reliable and consistent stimulus‐evoked responses from isolated taste cells, as described in Huang et al. (2009, 2011a) and Huang and Wu (2015, 2016). All experiments were conducted at room temperature.
Stimuli and solutions
All stimuli and pharmacological agents were made up in Tyrode's buffer. Isolated taste buds and taste cells were stimulated by bath‐perfusion of KCl (50 mM, substituted equimolar for NaCl), taste mix (10 μM cycloheximide, 1 mM sucralose, 0.1 mM SC45647, 1 mM denatonium) and SP (10 nM). Stimuli were bath‐applied for 30 s followed by a return to buffer perfusion for at least 3–5 min between trials. This perfusion paradigm allows the stimuli to mix thoroughly within the recording chamber followed by a sufficient washout. Moreover, this procedure produced reliable and consistent stimulus‐evoked responses from isolated taste cells.
Biosensor cells
GABA biosensors consisted of CHO cells stably expressing heteromeric GABAB receptors (GABAB1 and GABAB2 receptors) and the G‐protein α subunit, Gαqo5 (Huang et al., 2011a). We also used CHO cells expressing P2X2/P2X3 receptors (hereafter called ATP biosensors) (Huang et al., 2007; Huang 2011b). An aliquot of 5 μM Fura 2‐loaded biosensor cells (GABA or ATP) was transferred to the recording chamber containing isolated taste buds. Immediately after GABA or ATP biosensors had settled to the bottom of the chamber, they were probed with a single application of GABA (100 nM) or ATP (0.3 to 1 μM). Highly sensitive biosensors were drawn onto a fire‐polished glass micropipette to test transmitter release from taste buds (Figure 5A).
Figure 5.
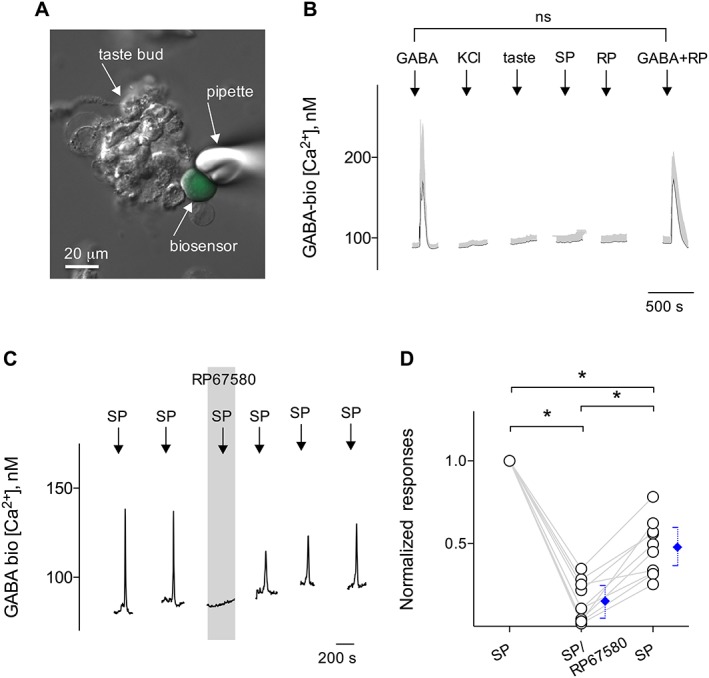
SP elicits GABA secretion from taste buds. CHO/GABAB cells (hereafter called GABA biosensors) were positioned against isolated taste buds to measure SP‐elicited transmitter release. (A) Micrograph of a biosensor cell abutted against an isolated taste bud in a living preparation. A Nomarski optics image and a fluorescence microscopy image were merged. (B) Sequential Ca2+ mobilization in CHO/GABAB cells was elicited by repeated applications of 100 nM GABA (↓, GABA) but not by depolarization with 50 mM KCl (↓, KCl), taste mix (↓, taste), SP (up to 100 nM) (↓, SP) or 100 nM RP67580 (↓, RP). Trace shows mean ± SEM (grey areas) of Ca2+ responses from GABA biosensor cells. Importantly, GABA biosensor cells (13 cells) retain robust Ca2+ responses to GABA in the presence of RP67580 (↓, GABA + RP). ns, not significant. (C) Traces showed robust responses from the biosensor cell positioned against a taste bud stimulated with 10 nM SP. RP67580 (0.1 μM) (present throughout the shaded area) reversibly reduced the biosensor responses evoked by stimulating taste buds repeatedly with SP. These results are consistent with SP evoked GABA release from taste buds. (D) Summary of SP‐elicited GABA secretion before, during and after the incubation of RP67580, plotted as in (C). Points represent normalized peak biosensor cell responses. Blue symbols show mean ± 95% confidence interval. *P < 0.05; significantly different as indicated; repeated measures ANOVA; n = 10 cells recorded in 10 experiments from nine mice.
Controls to establish the sensitivity and selectivity of GABA (Huang et al., 2011a) and ATP biosensors (Huang et al., 2007) were as described previously. Biosensor cells alone did not show the Ca2+ mobilization in response to bath‐applied KCl (50 mM) or taste stimuli used in the study as described previously (Huang et al., 2007; 2011a). Lastly, we verified that the Ca2+ responses of GABA biosensors were not affected by the pharmacological agents used in this study, including SP and RP67580, a neurokinin 1 (NK1) receptor antagonist, apart from CGP55845, a GABAB receptor antagonist, which was used to corroborate responses in biosensor cells generated by GABA receptors. Importantly, we verified that GABA biosensor cells retained full receptor agonist sensitivity (and thus Ca2+ responses) in the presence of RP67580 used in this study (see below Figure 5B).
Double immunostaining for NK1 receptors, glutamate–aspartate transporter (GLAST) and GABA
Isolated taste buds were fixed in 4% paraformaldehyde in PBS for 10–20 min at 4°C and immunostained following the procedures as described in Huang and Wu (2015, 2016). GLAST is exclusively expressed in Type I cells in vallate taste buds (Lawton et al., 2000; Vandenbeuch et al., 2013; Yoshida et al., 2015). To localize two different antigens, NK1 receptor/GLAST and/or GABA /GLAST, in isolated taste buds, a mixture of two primary antibodies generated in two different species against different antigens was used. We used rabbit anti‐NK1 (1:200; catalogue #ATR‐001; Alomone Labs, Jerusalem, Israel) for NK1 receptors (Muñoz et al., 2011), guinea pig anti‐GLAST (1:200; catalogue #AB‐2571717; Frontier Institute, Hokkaido, Japan) for Type I cells (Yoshida et al., 2015) and rabbit anti‐GABA (1:1500; catalogue #A2050; Sigma) for taste cell transmitter (Dvoryanchikov et al., 2011; Huang et al., 2011a). The immunoreactivity of NK1 receptors and GABA was determined by incubation with biotin–conjugated donkey anti‐rabbit IgG (1:200; catalogue #711–065‐152; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) followed by a tyramide reaction using a tyramide signal amplification (TSA) kit with Alexa Fluor 568 (catalogue #T‐20934; Molecular Probes, Life technologies, Eugene, OR, USA). GLAST primary antibody binding was revealed using a donkey anti‐guinea pig secondary antibody conjugated with Alexa Fluor 488 (1:200; catalogue #706–545‐148; Jackson ImmunoResearch Laboratories Inc.).
Briefly, fixed taste buds were washed with PBS and were blocked with 10% normal donkey serum for 1 h at room temperature. Primary antibodies were applied to taste buds for overnight at room temperature. Thereafter, for tyramide‐based detection, after incubation in the primary antiserum, the taste buds were washed three times for 10 min each in PBS followed by incubation in a biotin‐conjugated donkey anti‐rabbit secondary antibody (1:200) for 1 h at room temperature. The solutions from a Vectastain ABC kit (Vector Labs) were applied to the sections for 1 h at room temperature. After washes in PBS, a tyramide reaction using a TSA kit with Alexa Fluor 568 (Molecular Probes, Life technologies) was performed for 15 min. Double‐immunostaining was performed after the tyramide reaction. Donkey anti‐guinea pig secondary antibody (1:200) was applied to the taste buds for 0.5 h at room temperature. Taste buds were washed in PBS and coverslipped with ProLong Diamond Antifade Fluoromount (catalogue #P36961; Invitrogen, Life technologies). Negative controls were processed with the omission of primary antibodies. Alternatively, synthesizing NK1 receptor control antigen (0.1 μg·mg−1; Alomone Labs) and GLAST antigen proteins (1 μg·mL−1; Frontier Institute) were used in a blocking assay. In these control studies, neither NK1 receptor, GLAST nor GABA immunoreactivity was present. Images were obtained with an Olympus Optical laser‐scanning confocal microscope using Fluoview software.
Alternatively, taste buds, isolated from circumvallate papillae of GAD67‐GFP mice, were fixed for 10 min in 4% paraformaldehyde in 0.01 M PBS (pH 7.2–7.4) and immunostained following the procedures described in Dvoryanchikov et al. (2011) and Huang et al. (2011a). Taste buds were then rinsed three times in PBS and incubated with 10% normal goat serum for 1 h at room temperature. We used rabbit anti‐GABA (1:1500; catalogue #A2050; Sigma) for 60–90 min at room temperature. Immunoreactivity of GABA was determined by incubation with Alexa Fluor 568‐conjugated goat anti‐rabbit IgG (1:200; catalogue #A‐11011; Molecular Probes, Life Technologies) and then washed again three times in PBS. Negative controls were processed in parallel in every experiment with primary antibody omitted. No nonspecific fluorescence was detected. Images were obtained with an Olympus Optical laser‐scanning confocal microscope using Fluoview software.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). The experimental procedures or treatment and data analyses were carried out with randomization and blinding. In these studies, we performed a minimum of five independent experiments, where individual data points were based on at least technical duplicates each. Following acquisition, Ca2+ imaging recordings were viewed and analysed using Indec Workbench v.6 software (INDEC Biosystem). Converting ΔCa2+ to approximate [Ca2+]i was routinely calculated as described by Grynkiewicz et al. (1985). For statistical analyses, we used non‐parametric methods, including normalization of data, to reduce variability of baseline between independent experiments. The one‐way ANOVA and non‐parametric analyses were followed by Tukey's post hoc tests when F reached significance and Student's paired t‐tests were applied to determine whether changes in responses following a given treatment were significant. Data presented in graphs show means ±95% confidence interval. Significance is set at P < 0.05. GraphPad Prism (version 6.0, GraphPad Software Inc., San Diego, CA, USA) was used to perform the statistical analysis of in vitro experiments and to generate figures.
Materials
Drugs
All stimuli and pharmacological agents were made in Tyrode's buffer, and bath‐applied. SP, RP67580 and CGP55845 were obtained from Tocris (Park Ellisville, MO, USA). ATP, thapsigargin, U73122, bicuculline and GABA were purchased from Sigma.
Chemicals
The taste mix consisted of two bitter compounds, cycloheximide (10 μM) and denatonium (1 mM), as well as two sweet compounds, sucralose (1 mM) and SC45647 (0.1 mM). All compounds were purchased from Sigma.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d,e).
Results
SP mobilizes intracellular Ca2+ responses in taste cells
We tested whether SP excites taste bud cells to mobilize intracellular Ca2+ transients and which type(s) of taste cells are targets for the stimulation. Isolated taste cells were loaded with Fura 2, a Ca2+‐sensitive dye, and tested with bath‐applied SP. Stimuli, such as ATP, taste mix, KCl and SP, were applied sequentially to the bath medium in each experiment. Bath‐applying the lower concentration of SP, for example, 10 nM induced robust Ca2+ responses in a subset of taste cells (Figure 1A). Approximately 19% of isolated live taste cells (95 of 525) exhibited Ca2+ responses when stimulated with 10 nM SP. Taste cells were identified as Type II (receptor) or Type III (presynaptic) cells based on their responses to taste mix or KCl depolarization respectively (Figures 1B, C) (DeFazio et al., 2006; Huang et al., 2007; Vandenbeuch et al., 2010; Huang and Wu, 2015). We reasoned, however, that if the Type I taste cells were responsible for SP excitation, one might not necessarily observe significant taste‐ and KCl‐evoked Ca2+ oscillations. Unexpectedly, two independent subpopulations, 9 and 4% of SP‐responsive taste cells, also showed Ca2+ transients in response to taste mix (9 of 95) and 50 mM KCl (4 of 95) respectively. Nevertheless, our results suggest that SP acts on taste bud cells via the SP receptor, that is, the NK1 receptor, most likely on Type I cells, but also possibly on other types of taste cells, including Type II and Type III cells. Using the double‐immunostaining technique that evaluates Ca2+ imaging data, we refined these findings to examine whether Type I cells express NK1 receptors (as indeed the case, shown below). Figure 1D depicts the summary of these series of experiments and shows the relative proportions of taste cells that respond to SP.
Figure 1.
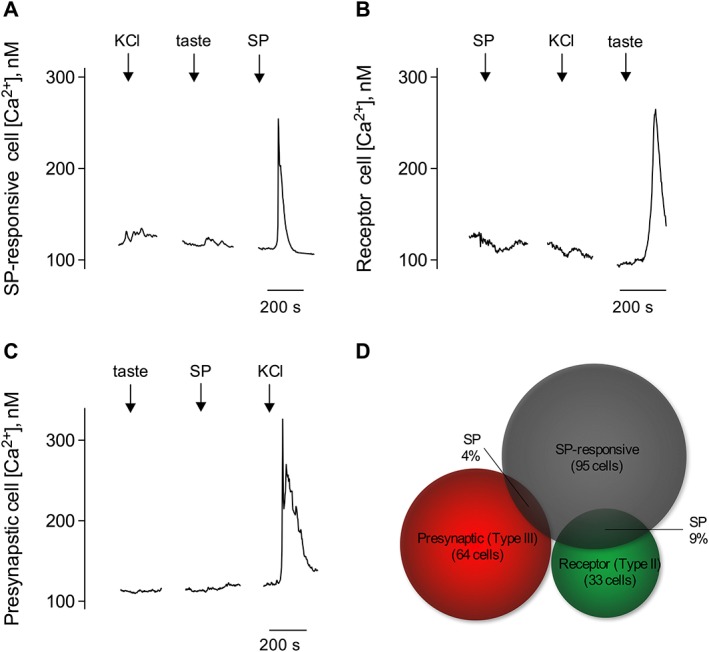
SP elicits intracellular Ca2+ transients in taste cells. Taste cells isolated from vallate taste buds were loaded with Fura 2, and their responses to stimuli were recorded by Ca2+ imaging. Sequentially, stimuli, such as taste mix (↓, taste), 50 mM KCl (↓, KCl) and 10 nM SP (↓, SP), were applied to the bath medium in each experiment. (A) Traces show Ca2+ recordings from an SP‐responsive taste cell, which did not respond to either taste mix or KCl depolarization. Immunofluorescence confocal microscopy revealed that SP acts on Type I cells via NK1 receptors (also named SP receptors) (see Figure 4A). (B) Individual receptor (Type II) cells showing robust taste‐evoked responses did not respond to SP. KCl depolarization did not elicit Ca2+ transients in receptor (Type II) cells. (C) Isolated presynaptic (Type III) cell showed KCl depolarization‐elicited Ca2+ responses but absence of intracellular transients in response to SP. (D) Venn diagrams representing the correlation between SP‐responsive taste cells (95 cells counted from five mice). Specifically, Type I cells and relative proportions of presynaptic (Type III) (4 of 64 cells) and receptor (Type II) (9 of 33 cells) taste cells that responded to SP.
SP causes Ca2+ transients through release from internal stores
RP67580, an NK1 receptor antagonist, significantly and reversibly reduced Ca2+ responses elicited by SP (10 nM), corroborating that the Ca2+ signals were caused by activation of NK1 receptors, also known SP receptors (Figures 2A, B). Because the intracellular Ca2+ transients in taste cells initiate from two different sources, influx from the extracellular Ca2+ and/or release from internal Ca2+ stores, we conducted a series of experiments to examine the source of Ca2+ responses during the stimulation. First, we stimulated taste cells with SP in the absence of extracellular Ca2+. Taste cells showed robust Ca2+ transients in response to SP when Ca2+ in the medium was replaced with Mg2+ (0 Ca), suggesting that SP‐elicited Ca2+ responses were caused by the release from internal Ca2+ stores (Figures 2C, D). The endoplasmic reticulum is a major intracellular organelle involved in the internal Ca2+ storage. Thus, we explored whether thapsigargin, a sarco/endoplasmic reticulum Ca2+‐ATPase (SERCA) inhibitor which depletes intracellular Ca2+ stores, abolished Ca2+ transients in the SP stimulated taste cells. As we expected, thapsigargin (1 μM, 5–10 min) significantly and irreversibly reduced SP‐elicited Ca2+ responses (Figures 3A, B). The PLC signalling cascade plays a crucial role in releasing the intracellular calcium from internal stores into the cytosol of taste cells (Huang et al., 2005; 2011b; Simon et al., 2006). We tested the effects of U73122, a broad‐spectrum PLC blocker, on SP‐elicited Ca2+ responses. Ca2+ imaging revealed that pretreating individual taste cells with U73122 (10 μM) significantly and irreversibly reduced SP‐elicited Ca2+ responses (Figures 3C, D). These results unambiguously indicated that the SP‐elicited Ca2+ transients were involved in the PLC‐mediated cascade. This clearly differs from the frank Ca2+ influx stimulated by KCl depolarization described in previous studies (Huang et al., 2007; 2009; 2012; Vandenbeuch et al., 2010; Huang and Wu, 2015). Collectively, these findings indicated that SP activates NK1 receptors and subsequently elicits PLC/IP3‐mediated Ca2+ release from intracellular stores.
Figure 2.
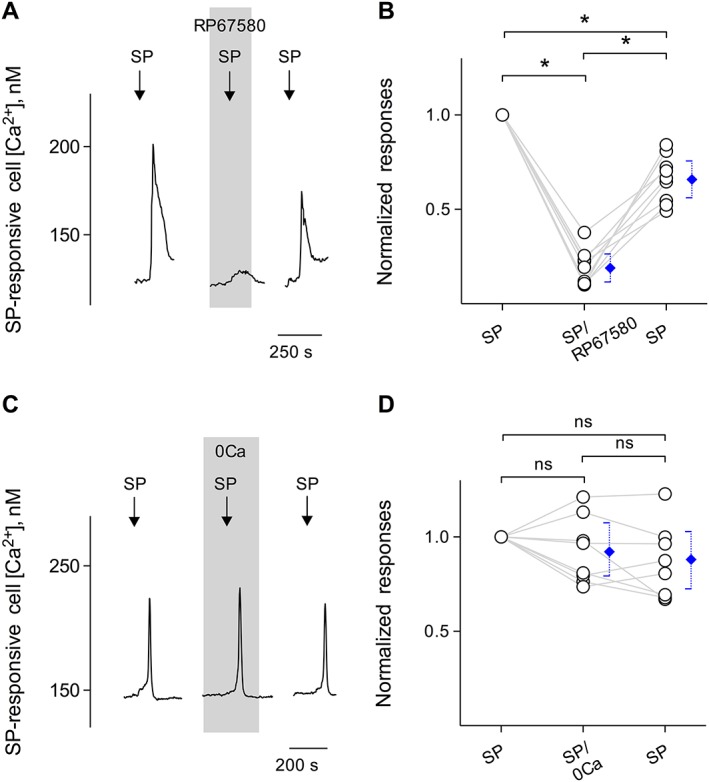
SP stimulates its receptors on Type I cells and induces Ca2+ release from intracellular stores. (A) Bath‐application of 10 nM SP elicits Ca2+ mobilization in Fura 2‐loaded taste cells (↓, SP). RP67580 (0.1 μM), an NK1 receptor antagonist (present throughout the shaded area), reversibly inhibited SP‐induced Ca2+ responses. (B) Summary of SP‐elicited Ca2+ responses before, during and after the presence of RP67580, plotted as in (A). Points represent normalized peak taste cell responses. Blue symbols show mean ± 95% confidence interval (95% CI). *P < 0.05; significantly different as indicated; repeated measures ANOVA; n= 9 cells from five mice. (C) SP‐elicited Ca2+ mobilization in an isolated taste cell was not affected when Ca2+ was eliminated from the bathing solution (0Ca, present throughout the shaded area). These findings indicate that SP‐elicited responses in taste cells were generated by the release of the intracellular Ca2+, consistent with the excitation of GPCRs. (D) Summary of experiments testing sources of SP‐elicited Ca2+ mobilization, plotted as in (C). Points indicate normalized peak taste cell responses. Blue symbols show mean ± 95% CI. ns, not significant; repeated measures ANOVA; n = 8 cells from five mice.
Figure 3.
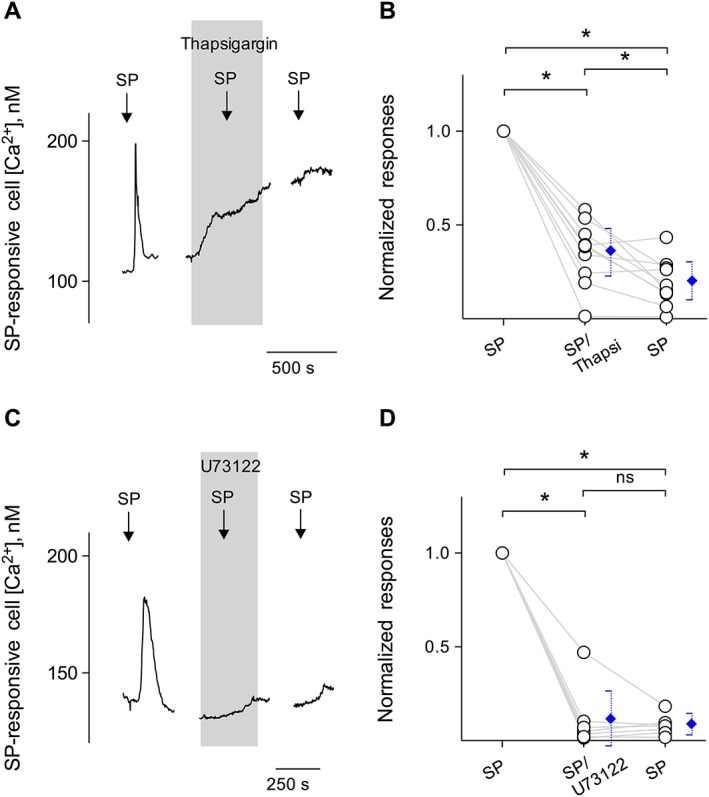
Intracellular Ca2+ release in Type I cells is via a PLC‐mediated pathway. (A) Bath‐application of 10 nM SP elicits Ca2+ mobilization in the taste cell (↓, SP). Treating Type I cells with thapsigargin (1 μM), a SERCA inhibitor (present throughout the shaded area), irreversibly reduced Ca2+ responses evoked by SP, consistent with Ca2+ store release mechanisms for this stimulus. (B) Summary of SP‐elicited Ca2+ responses before, during and after the incubation of thapsigargin, plotted as in (A). Points represent normalized peak taste cell responses. Blue symbols show mean ± 95% confidence interval (95% CI). *P < 0.05; significantly different as indicated; repeated measures ANOVA; n = 8 cells from five mice. (C) SP (10 nM)‐elicited Ca2+ mobilization in an isolated Type I cell was blocked when U73122 (10 μM) (present throughout the shaded area) was present in the bathing solution. Consistent with the blockage of the PLC‐mediated cascade, these findings indicate that SP‐elicited responses in Type I cells were due to Ca2+ release from intracellular stores. (D) Summary of SP‐elicited Ca2+ responses before, during and after the incubation of U73122, plotted as in (C). Points represent normalized peak taste cell responses. Blue symbols show mean ± 95% CI. *P < 0.05; significantly different as indicated; repeated measures ANOVA; n = 7 cells from five mice.
Type I cells express NK1 receptors
We used immunofluorescent confocal microscopy to investigate the expression of NK1 receptors on taste bud cells. Several laboratories have shown previously that GLAST is expressed only in Type I taste cells and thus a marker for Type I cells (Lawton et al., 2000; Vandenbeuch et al., 2013; Yoshida et al., 2015). To verify the expression of NK1 receptors on Type I taste cells, we double‐immunostained isolated and fixed vallate taste buds with antibodies directed against NK1 receptors and GLAST. As shown in Figure 4A, NK1‐immunoreactive taste cells were also immunostained with GLAST, indicating that these are Type I taste cells express NK1 receptors. Some NK1‐immunoreactive taste cells lack GLAST immunoreactivity, suggesting that other types of taste cells may express NK1 receptors as well. In this study, no specific immunoreactivity in taste cells was observed in these controls with the omission of primary antibodies. Nevertheless, confirmation that Type I cells prominently express NK1 receptors is in good correspondence with the finding of Type I cells that showed Ca2+ transients in response to SP in the Ca2+ imaging studies. Next, we refined these findings to examine whether SP elicits transmitter secretion from mouse vallate taste buds.
Figure 4.
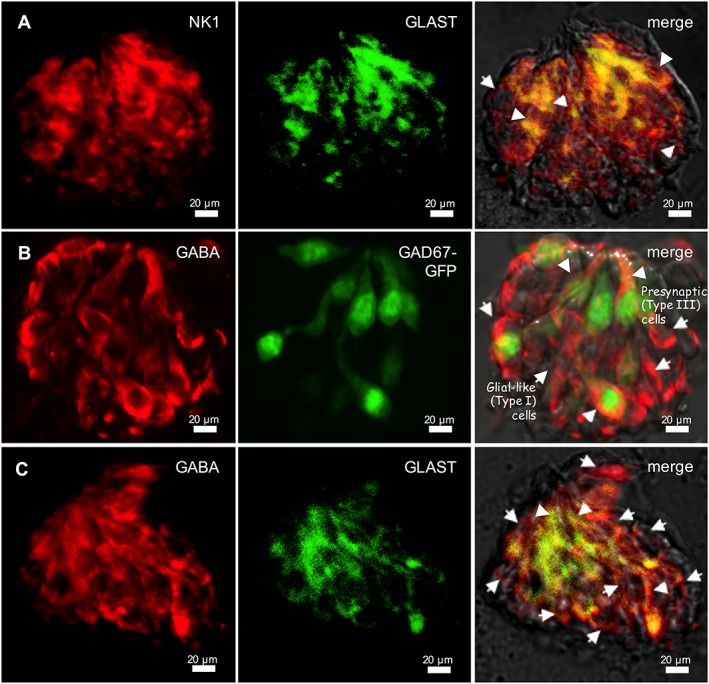
Type I taste cells express NK1 receptors and synthesize GABA. Nomarski optics images showing the view of individually isolated taste buds were merged with immunofluorescent confocal micrographs for these micrographs. (A) Double‐immunostaining of NK1 receptors (NK1; red) and GLAST, a marker of Type I cells (green), was performed on isolated and fixed taste buds. Several taste cells exhibit double labelling, suggesting that Type I cells express NK1 receptors (arrowheads). In addition to those obtaining double immunoreactivities, NK1‐immunoreactive taste cells without GLAST immunoreactivity were also revealed (arrow). The optical thicknesses (z‐stack) of confocal images are 12 μm. Bar = 20 μm. (B) GABA immunostaining of an isolated and fixed taste bud from a GAD67‐GFP mouse. Many GABA immunoreactive taste cells express GFP (arrowheads), but some do not. This latter category may be Type I cells (arrows), which express GAD65, a GABA synthetic enzyme (Dvoryanchikov et al., 2011). Bar = 20 μm. (C) Double‐immunostaining of GABA (red) and GLAST (green) on isolated and fixed taste buds. GABA immunoreactivity is in cells that express GLAST (arrowheads). In addition to those obtaining double immunoreactivities, Presynaptic (Type III) cells, GABA‐immunoreactive taste cells without GLAST immunoreactivity, were also observed (arrows). The optical thicknesses (z‐stack) of confocal images are 15 μm. Bar = 20 μm.
Type I cells synthesize GABA
In GAD67‐GFP mice, GFP is detected in about 75% of Type III cells of taste buds (Tomchik et al., 2007; Dvoryanchikov et al., 2011). GABA‐immunoreactive taste cells were either GAD65 expressing (i.e. Type I) or GAD67 expressing (i.e. Type III) (Dvoryanchikov et al., 2011; Huang et al., 2011a). In the present study, we observed GABA immunofluorescence in isolated taste buds in which Type III cells were marked with GFP and determined that some GABA immunoreactive cells lacked GFP, indicating these cells were most likely Type I cells (Figure 4B). To test the underlying assumption, we immunostained isolated and fixed vallate taste buds with antibodies directed against GABA and GLAST. As shown in Figure 4C, GABA‐immunoreactive taste cells were also immunostained with GLAST, indicating that these are Type I taste cells synthesizing GABA.
SP triggers GABA release from taste buds
We investigated whether transmitter release from SP‐stimulated taste buds is possible. Using Ca2+ imaging with GABA biosensor cells (Figure 5A), we tested whether SP evokes taste buds to release GABA. Parenthetically, in the absence of taste buds, GABA biosensor cells do not respond either to depolarization with bath‐applied KCl (50 mM; substituted for NaCl) or to bath‐applied SP (up to 100 nM) except GABA (100 nM). GABA biosensor cells maintained their responses to bath‐applied GABA when Ca2+ in the medium was replaced with Mg2+. This is consistent with the coupling of GABAB receptors and the G‐protein α subunit, Gαqo5, to intracellular Ca2+ release mechanisms (Huang et al., 2011a). GABA biosensor cells retained full receptor agonist sensitivity in the presence of RP67580, an NK1 receptor antagonist (Figure 5B).
Subsequently, we isolated taste buds from vallate papillae of the mouse tongue. GABA cells could be observed by immunostaining taste buds isolated for recording, verifying that their GABA content was maintained throughout the isolation procedure (see Figure 4B, C). As shown in Figure 5C, we attempted to detect GABA secretion upon SP stimulation each time after the release site was located (i.e. GABA biosensors abutted against taste buds showed Ca2+ transients while being stimulated). Figure 5C also showed that 10 nM SP was sufficient to trigger GABA secretion from taste buds. To verify that SP activated its cognate receptors on taste cells and subsequently elicited transmitter release, and thus GABA, we applied the selective NK1 receptor antagonist, RP67580 (100 nM) in the bath solution, and retested with SP. RP67580 significantly and reversibly blocked SP‐elicited biosensor responses (Figures 5C, D), indicating that the activation of NK1 receptors elicited GABA secretion.
In separate experiments, biosensor responses to SP were reversibly blocked by 10 μM CGP55845, a GABAB receptor antagonist (Figures 6A, B). Because NK1 receptors are targets of SP and because CGP55845 specifically blocks Ca2+ responses in GABA biosensors, as described in Huang et al. (2011a), these observations corroborated that the biosensor cell was detecting SP‐evoked GABA secretion from taste buds. To date, we have obtained successful biosensor recordings from 20 of 99 taste buds when testing SP stimulation. The success rate of measuring GABA release in these experiments was 20%, in good correspondence with the value described above that a limited number of individual Type I cells responded to SP. Lastly, we tested whether RP67580 reduced the GABA secretion from Type III cells, that is, KCl‐elicited GABA secretion. We detected no reduction of biosensor responses in the presence of RP67580 in the bath medium (Figures 6C, D). Collectively, these observations were fully consistent with the conclusion that SP triggered the secretion of GABA via the NK1 receptor, most likely on Type I cells, but also possibly on Type III cells.
Figure 6.
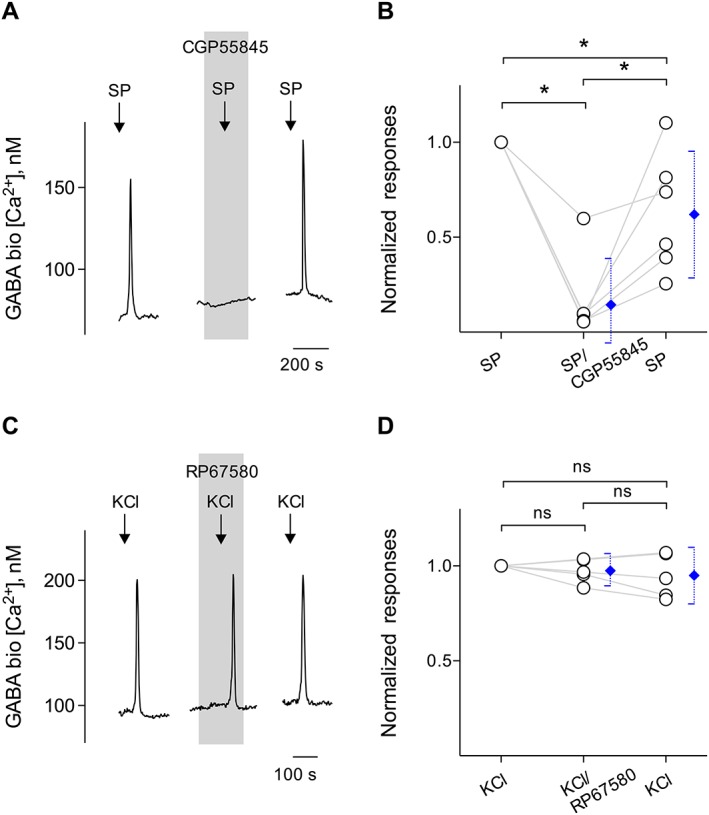
Stimulating taste buds with SP evokes GABA release. (A) Traces show robust responses from a biosensor positioned against a taste bud when the taste bud was stimulated with 10 nM SP (↓, SP). CGP55845 (10 μM), a GABAB receptor antagonist (present throughout the shaded area), reversibly abolished SP‐evoked biosensor responses. Withdrawing the biosensor from the taste bud eliminated all responses to SP (data not shown). (B) Summary of SP‐elicited GABA secretion before, during and after the incubation of CGP55845, plotted as in (C). Points represent normalized peak biosensor cell responses. Blue symbols show mean ± 95% confidence interval (95% CI). *P < 0.05; significantly different as indicated; repeated measures ANOVA; n = 6 cells recorded in six experiments from six mice. (C) In separate experiments, traces represent robust responses from the biosensor cell positioned against a taste bud when the taste bud was depolarized by 50 mM KCl (↓, KCl), that is, GABA secretion from presynaptic (Type III) cells. RP67580 in the bath medium (present throughout the shaded area) did not affect Ca2+ mobilization in the biosensor in response to KCl, consistent with the finding that RP67580 only blocked SP‐elicited GABA secretion from Type I cells. (D) Summary of KCl‐elicited GABA secretion before, during and after the incubation of RP67580, plotted as in (C). Points represent normalized peak biosensor cell responses. Blue symbols show mean ± 95% CI. ns, not significant; repeated measures ANOVA.; n = 5 cells recorded in five experiments from five mice.
SP‐induced GABA secretion inhibits ATP signalling in taste buds
Finally, we examined the role of SP during taste stimulation in whole taste buds where cell‐to‐cell communication remains intact. We hypothesize that GABA evoked by SP mediates inhibitory interactions within the taste bud during gustatory stimulation. Previous studies showed that taste stimulation elicits ATP release from intact taste buds isolated from lingual epithelium (Huang et al., 2007; 2011b; Murata et al., 2010; Dvoryanchikov et al., 2011; Huang and Wu, 2015; 2016). ATP is believed to be an important excitatory transmitter between taste buds and gustatory sensory afferent fibres (Finger et al., 2005; Huang et al., 2011b; Vandenbeuch et al., 2015). Additionally, GABA released from Type III cells provides the negative paracrine feedback onto Type II cells by activating GABAA as well as GABAB receptors and reducing taste‐evoked ATP secretion in taste buds (Dvoryanchikov et al., 2011; Huang et al., 2011a). We therefore measured taste‐evoked ATP secretion from intact taste buds using ATP biosensors (Huang et al., 2007) and tested whether SP‐induced GABAergic transmission alters taste‐evoked ATP secretion, as follows:
If so, this would provide proof that SP decreases gustatory responses in taste buds. Suramin, a broad‐spectrum P2 receptor antagonist, was used to verify the responses in ATP biosensor cells that were generated by activation of the P2X receptors expressed on biosensors (Huang et al., 2007; Huang and Wu, 2015). The success rate of measuring taste‐evoked ATP secretion in these experiments was 12% (18 out of 147), corresponding with rates found in previous studies (Huang et al., 2007; Huang and Wu, 2015). Figure 7 showed that 10 nM SP itself did not evoke ATP secretion from taste buds. Sweet/bitter‐evoked ATP secretion was significantly reduced when SP (10 nM) was added to the sweet or bitter taste stimuli (arrows, sweet+SP or bitter+SP in Figure 7A, C, respectively), consistent with SP‐evoked GABAergic inhibition. Repeating sweet/bitter plus SP stimulation in the presence of CGP55845 and bicuculline restored taste‐evoked ATP secretion. This is consistent with SP‐evoked GABA secretion contributing to depressed ATP output during taste stimulation. Our findings suggest that GABA indirectly mediated the inhibitory actions of SP on taste‐evoked ATP secretion.
Figure 7.
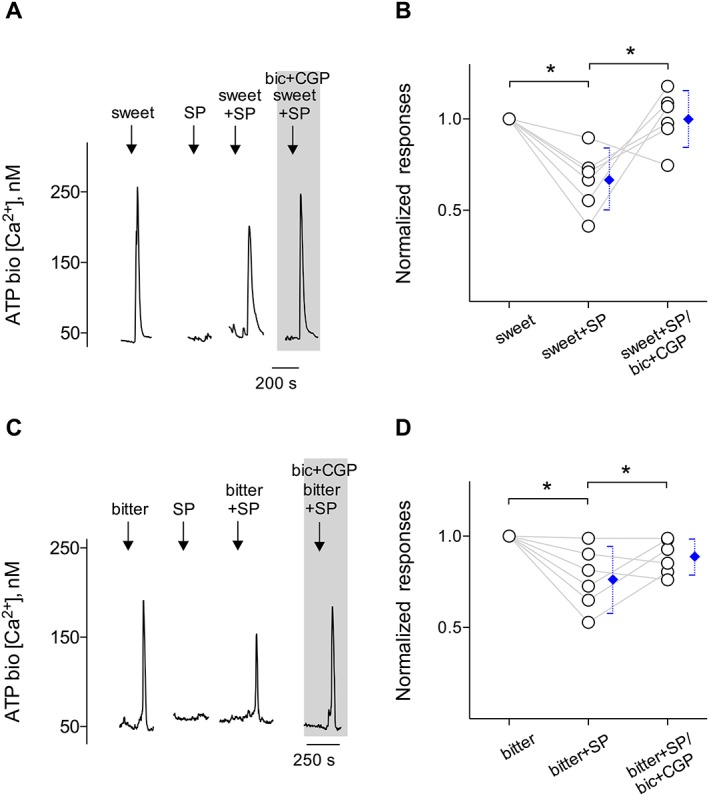
SP inhibits taste‐evoked ATP secretion from taste buds. CHO/ATPP2X2/P2X3 cells (hereafter called ATP biosensors) were used to monitor taste‐evoked ATP secretion from isolated taste buds. The extracellular GABA inhibits taste‐evoked ATP secretion from receptor (Type II) cells (Dvoryanchikov et al., 2011). We tested whether SP‐elicited GABA secretion from Type I cells depresses taste‐evoked ATP secretion. Whole taste buds were isolated from mouse vallate papillae to retain cell‐to‐cell communication between receptor (Type II) and Type I cells. Traces show robust responses from the biosensor positioned against an isolated taste bud when the taste bud was stimulated by sweet stimuli [A (↓, sweet)] or bitter stimuli [C (↓, bitter)], indicating ATP secretion from the taste bud. SP (10 nM) (↓, SP) itself did not evoke ATP secretion from taste buds. Taste‐evoked ATP secretion was moderately reduced by adding 10 nM SP with taste stimuli [A (↓, sweet+SP); C (↓, bitter+SP)]. Restoration of ATP secretion was observed by adding 10 μM bicuculline and 10 μM CGP55845, GABAA and GABAB receptor antagonists (‘bic + CGP’, present throughout the shaded area), to the bath. As expected, blocking GABA receptors restored SP‐inhibited ATP secretion, suggesting that SP plays as an inhibitory transmitter via the GABAergic signalling in taste buds. (B, D) Summary of SP inhibited sweet‐ or bitter‐evoked ATP secretion before and during the presence of ‘bic + CGP’ in the bath medium, plotted as in (A) and (C) respectively. Points represent normalized peak taste cell responses. Blue symbols show mean ± 95% confidence interval. *P < 0.05; significantly different as indicated; repeated measures ANOVA. (B) n = 6 cells recorded in six experiments from six mice; (D) n = 6 cells recorded in six experiments from six mice.
Discussion and conclusions
Our results document that (i) SP leads to Ca2+ release from internal stores via the activation of the PLC signalling cascade; (ii) a prominent action of SP is to evoke GABA release from Type I cells by acting on NK1 receptors expressed on these cells; and (iii) the net effect of SP stimulation alters taste‐evoked ATP secretion from taste buds.
Possible sources of SP
Previous studies indicated that lingual epithelia are rich in SP (Nagy et al., 1982; Nishimoto et al., 1982; Yamasaki et al., 1984; Astbäck et al., 1997; Ishida et al., 2002) as well as CGRP (Astbäck et al., 1997; Ishida et al., 2002; Huang and Wu, 2015) containing nerve fibres, especially in those areas where taste buds can be expected. The most likely source of peptidergic fibres in taste buds is as polymodal nociceptors, many of which express both SP and CGRP (Ishida et al., 2002). These sensory fibres mostly express TRPV1 receptors for chemesthetic activators such as capsaicin and a variety of other strong oral irritants (Osada et al., 1997; Ishida et al., 2002; Roper, 2014). Much evidence is now available that sensory neurons, which are excited by the environmental stimuli, can release stored transmitters via their terminals in the lingual epithelium (Maggi and Meli, 1988; Wang et al., 1995; Ishida et al., 2002; Simon et al., 2003; Sato et al., 2012). We anticipated that peptides may be released from peripheral axon terminals onto taste cells (Nagai et al., 1996; Finger and Simon, 2002; Simon et al., 2003; Sato et al., 2012). Their actions on the modulation of taste transmission have been studied recently. Specifically, our previous study showed that the action of CGRP on taste bud cells implies an inhibitory role in taste sensitivity (Huang and Wu, 2015). In the present study, our current findings indicated that SP modulates the signal output from taste buds.
SP‐evoked Ca2+ responses are associated with the PLC signalling cascade
Tissue‐specific signalling events are associated with the specific intracellular coupling and receptor phenotypes for the SP receptors, because SP operates via NK1 receptors (Douglas and Leeman, 2011; Diandong et al., 2014). Activating NK1 receptors triggers Gαs‐mediated activation of AC, with a subsequent increase in cAMP (Diandong et al., 2014). Alternatively, NK1 receptors can couple to Gαq‐mediated PLC, an enzyme responsible for the IP3‐induced Ca2+ release from intracellular stores as a second messenger (Miyano et al., 2010; Douglas and Leeman, 2011). Nevertheless, our Ca2+ imaging and immunostaining data revealed that Type I cells in vallate taste papillae express functional NK1 receptors. Our results are consistent with those of the previous work, which stated that the predominant secondary messenger system in Type I cells is represented by PLC (Grant, 2012). Type II and/or Type III cells may also express NK1 receptors because some taste cells showing NK1 immunoreactivity do not express GLAST. It remains to be seen whether providing this staining pattern by immunostaining the corresponding cell marker makes sense of that overlapping with NK1 receptors.
Previous studies have shown that there are two classes of taste bud cells directly involved in gustatory signalling. Type II cells detect and transduce sweet, bitter and umami compounds, and Type III cells respond directly to sour taste stimuli (Tomchik et al., 2007; Huang et al., 2008b). Although Type II and/or Type II cells only rarely respond to SP, the possibility that SP has a role in taste modalities cannot be ruled out. Intriguingly, Grant (2012) reported that activation of NK1 receptors could have an additive effect on umami responses in Type II cells, suggesting a possible mechanism by which SP‐mediated enhancement of taste may occur. Our data indicated that SP acts specifically on Type I cells, not globally on the taste bud. It is likely that peptidergic efferent input acts directly and prominently on paracrine transmission within taste buds, which has the potential to shape the final signals that taste buds transmit to the brain (see below).
SP shapes the signal output that taste buds transmit to the brain
Previous studies, using single cell RT‐PCR and immunofluorescence confocal microscopy, showed the distinct expression patterns of GABA synthetic enzymes in mouse vallate taste buds. For example, Type III cells express GAD67 and Type I cells express GAD65 (DeFazio et al., 2006; Starostik et al., 2010; Dvoryanchikov et al., 2011). Sour taste stimulation and KCl depolarization cause Type III taste bud cells to secrete GABA (Huang et al., 2011a). Recent studies reported that Type I cells may transduce the salt (Na+) taste (Vandenbeuch et al., 2008; Roper, 2015). We have no information on whether Type I cells contribute to the GABAergic transmission in taste buds during salt stimulation, but the unique feature of SP is that it appears to primarily affect Type I cells and to elicit the secretion of a signalling molecule, namely, GABA. Parenthetically, Vandenbeuch et al. (2008) reported that Type I cells lack voltage‐gated Ca2+ currents. In contrast to the requirement for Ca2+ influx for GABA secretion in Type III cells (Huang et al., 2011a), GABA secretion from Type I cells may be associated with the intracellular distribution of the Ca2+ responses to SP, which blocks Ca2+‐activated K+ channels leading to membrane depolarization, as is the case in airway smooth muscles (Deshpande et al., 2010). The effective mechanism for such secretion remains to be determined.
ATP is now widely acknowledged as the main gustatory transmitter within taste buds, acting on P2X receptors on afferent nerve fibre terminals (Bo et al., 1999; Finger et al., 2005; Huang et al., 2011b; Vandenbeuch et al., 2015). The combined actions of membrane depolarization, mediated by TRPM5, concurrent with an increased intracellular Ca2+ release from stores, mediated by a PLCß2‐IP3‐signalling cascade, are required to trigger ATP release from Type II cells (Pérez et al., 2002; Huang et al., 2005; Huang and Roper, 2010; Kinnamon, 2011). Type I cells themselves synthesize GABA, and thus, one might speculate that GABA released from Type I cells might also exert paracrine inhibitory feedback within taste buds. GABAergic inhibition in taste buds has been documented showing that GABA stabilizes or even hyperpolarizes taste bud cells (Cao et al., 2009) and reduces taste‐evoked ATP secretion from Type II cells by acting on GABAA and GABAB receptors expressed by these cells (Dvoryanchikov et al., 2011). We found that SP reduced sweet and/or bitter stimuli‐evoked ATP secretion from whole taste buds, which maintain intact cell‐to‐cell communication. Our data indicated SP‐elicited GABA secretion, which inhibits taste‐evoked ATP secretion from Type II cells. The significance of such inhibition in the intact system supports the notion that chemesthesis‐induced GABAergic inhibition is used in shaping responses to sweet and/or bitter qualities per se.
In summary, our results are consistent with a role for SP as an inhibitory transmitter that shapes taste signals via GABAergic signalling during the processing of gustatory information in taste buds. Collectively, combined with recent findings that CGRP depresses taste signals via serotonergic signalling (Huang and Wu, 2015), an interpretation of the peptidergic actions within taste buds is that it appears to be an important chemesthetic regulation of gustatory signal transmission, as theoretically it may relate to the processing of the gustatory information. Figure 8 summarizes this postulated scenario in the schematic taste bud.
Figure 8.
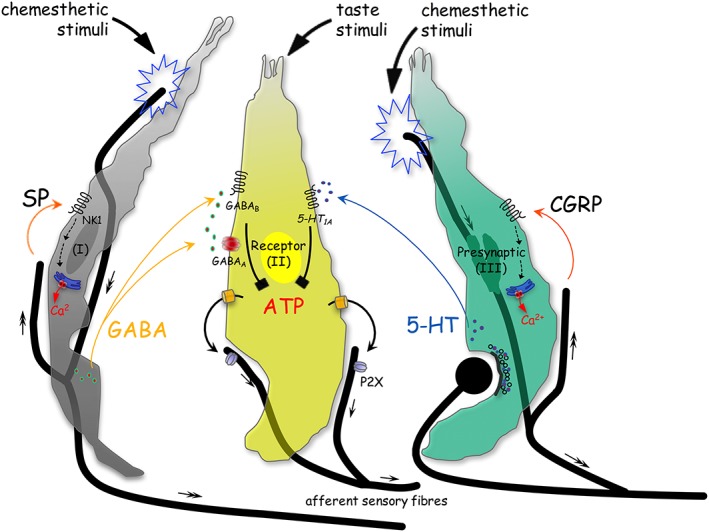
Schematic drawing shows the postulated scenario of SP, a putative efferent transmitter, in taste buds. Three distinct types of taste cells are shown, Type I (I), Type II (receptor) (II) and Type III (presynaptic) (III) cells. During sweet or bitter taste stimulation, ATP activates gustatory afferent fibres that propagate taste signals (small arrows) centrally (Finger et al., 2005; Huang et al., 2007; Jaber et al., 2014; Vandenbeuch et al., 2015). Chemesthetic stimuli activate sensory afferent fibres that propagate signals centrally (double‐headed arrows) and release SP and/or CGRP (orange curved arrows). The activation of the CGRP receptors triggers presynaptic (Type III) cells to elevate intracellular Ca2+ transients and to secrete 5‐HT, which inhibits ATP release from receptor (Type II) cells via serotonergic signalling pathways (black symbol) (Huang and Wu, 2015). Moreover, in the present study, the activation of NK1 receptors (NK1) triggers Type I cells to elevate intracellular Ca2+ transients and to secrete GABA, which reduces taste‐evoked ATP secretion through GABAergic signalling pathways. Collectively, combined with recent findings that CGRP suppresses taste signal transmission, an interpretation of the peptidergic actions within taste buds is that it appears to be an important chemesthetic regulation of gustatory signal transmission, as theoretically it may relate to the processing of the gustatory information.
Author contributions
A.Y.H. and S.Y.W. designed research; A.Y.H. and S.Y.W. performed research; A.Y.H. and S.Y.W. analysed data; A.Y.H. wrote the paper. All authors read and approved the final paper.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by SIUSOM research seed grant and Fellows Grant Award Program (FGAP) of American Association of Anatomists (AAA‐3419) to A.Y.H.
Huang, A. Y. , and Wu, S. Y. (2018) Substance P as a putative efferent transmitter mediates GABAergic inhibition in mouse taste buds. British Journal of Pharmacology, 175: 1039–1053. doi: 10.1111/bph.14142.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017b). The concise guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174: S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The concise guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017d). The concise guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017e). The concise guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astbäck J, Arvidson K, Johansson O (1997). An immunohistochemical screening of neurochemical markers in fungiform papillae and taste buds of the anterior rat tongue. Arch Oral Biol 42: 137–147. [DOI] [PubMed] [Google Scholar]
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G (1999). Localization of ATP‐gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport 10: 1107–1111. [DOI] [PubMed] [Google Scholar]
- Cao Y, Zhao FL, Kolli T, Hivley R, Herness S (2009). GABA expression in the mammalian taste bud functions as a route of inhibitory cell‐to‐cell communication. Proc Natl Acad Sci U S A 106: 4006–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang RB, Waters H, Liman ER (2010). A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci U S A 107: 22320–22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD et al (2006). Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 26: 3971–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS et al (2010). Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diandong H, Kefeng S, Weixin F, Moran W, Jiahui W, Zaifu L (2014). The role of Gαs in activation of NK92‐MI cells by neuropeptide substance P. Neuropeptides 48: 1–5. [DOI] [PubMed] [Google Scholar]
- Douglas SD, Leeman SE (2011). Neurokinin‐1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci 1217: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoryanchikov G, Tomchik SM, Chaudhari N (2007). Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol 505: 302–313. [DOI] [PubMed] [Google Scholar]
- Dvoryanchikov G, Huang YA, Barro‐Soria R, Chaudhari N, Roper SD (2011). GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci 31: 5782–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger TE, Simon SA (2002). The cell biology of lingual epithelia In: Finger TE, Silver WL, Restrepo D. (eds). The Neurobiology of Taste and Smell. Wiley‐Liss: New York, pp. 287–314. [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L et al (2005). ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310: 1495–1499. [DOI] [PubMed] [Google Scholar]
- Green BG (2012). Chemesthesis and the chemical senses as components of a “chemofensor complex”. Chem Senses 37: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J (2012). Tachykinins stimulate a subset of mouse taste cells. PLoS One 7: e31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450. [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P (1988). Local effector functions of capsaicin‐sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene‐related peptide and other neuropeptides. Neuroscience 24: 739–768. [DOI] [PubMed] [Google Scholar]
- Huang AY, Wu SY (2015). Calcitonin gene‐related peptide reduces taste‐evoked ATP secretion from mouse taste buds. J Neurosci 35: 12714–12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AY, Wu SY (2016). The effect of imiquimod on taste bud calcium transients and transmitter secretion. Br J Pharmacol 173: 3121–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE et al (2005). Mouse taste buds use serotonin as a neurotransmitter. J Neurosci 25: 843–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D et al (2006). The cells and logic for mammalian sour taste detection. Nature 442: 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2007). The role of pannexin 1 hemichannels in ATP release and cell‐cell communication in mouse taste buds. Proc Natl Acad Sci U S A 104: 6436–6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Roper SD (2008a). Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci 28: 13088–13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD (2008b). Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol 586: 2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Dando R, Roper SD (2009). Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci 29: 13909–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Roper SD (2010). Intracellular Ca2+ and TRPM5‐mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol 588: 2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Pereira E, Roper SD (2011a). Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS One 6: e25471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G et al (2011b). Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci 31: 13654–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Grant J, Roper S (2012). Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds. PLoS One 7: e30662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Ugawa S, Ueda T, Murakami S, Shimada S (2002). Vanilloid receptor subtype‐1 (VR1) is specifically localized to taste papillae. Brain Res Mol Brain Res 107: 17–22. [DOI] [PubMed] [Google Scholar]
- Jaber L, Zhao FL, Kolli T, Herness S (2014). A physiologic role for serotonergic transmission in adult rat taste buds. PLoS One 9: e112152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapaun CL, Dando R (2016). Deconvoluting physical and chemical heat: temperature and spiciness influence flavor differently. Physiol Behav 170: 54–61. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, Torres D, Tseng YH, Bowe C, Carvalho E, Espinoza D et al (2008). Substance P as a novel anti‐obesity target. Gastroenterology 134: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S (2004). A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol 286: R649–R658. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnamon SC (2011). Taste receptor signaling – from tongues to lungs. Acta Physiol 204: 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton DM, Furness DN, Lindemann B, Hackney CM (2000). Localization of the glutamate‐aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci 1122: 3163–3171. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A (1988). The sensory‐efferent function of capsaicin‐sensitive sensory neurons. Gen Pharmacol 19: 1–43. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyano K, Morioka N, Sugimoto T, Shiraishi S, Uezono Y, Nakata Y (2010). Activation of the neurokinin‐1 receptor in rat spinal astrocytes induces Ca2+ release from IP3‐sensitive Ca2+ stores and extracellular Ca2+ influx through TRPC3. Neurochem Int 57: 923–934. [DOI] [PubMed] [Google Scholar]
- Montavon P, Hellekant G, Farbman A (1996). Immunohistochemical, electrophysiological, and electron microscopical study of rat fungiform taste buds after regeneration of chorda tympani through the non‐gustatory lingual nerve. J Comp Neurol 367: 491–502. [DOI] [PubMed] [Google Scholar]
- Muñoz M, Bernabeu‐Wittel J, Coveñas R (2011). NK‐1 as a melanoma target. Expert Opin Ther Targets 15: 889–897. [DOI] [PubMed] [Google Scholar]
- Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF et al (2010). Action potential‐enhanced ATP release from taste cells through hemichannels. J Neurophysiol 104: 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Kim DJ, Delay RJ, Roper SD (1996). Neuromodulation of transduction and signal processing in the end organs of taste. Chem Senses 21: 353–365. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Goedert M, Hunt SP, Bond A (1982). The nature of the substance P‐containing nerve fibers in taste papillae of the rat tongue. Neuroscience 7: 3137–3151. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Akai M, Inagaki S, Shiosaka S, Shimizu Y, Yamamoto K et al (1982). On the distribution and origins of substance P in the papillae of the rat tongue: an experimental and immunohistochemical study. J Comp Neurol 207: 85–92. [DOI] [PubMed] [Google Scholar]
- Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS (2013). High salt recruits aversive taste pathways. Nature 494: 472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada K, Komai M, Bryant BP, Suzuki H, Goto A, Tsunoda K et al (1997). Capsaicin modifies responses of rat chorda tympani nerve fibers to NaCl. Chem Senses 22: 249–255. [DOI] [PubMed] [Google Scholar]
- Roper SD (2014). TRPs in taste and chemesthesis. Handb Exp Pharmacol 223: 827–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD (2015). The taste of table salt. Pflugers Arch 467: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H et al (2002). A transient receptor potential channel expressed in taste receptor cells. Nature Neurosci 5: 1169–1176. [DOI] [PubMed] [Google Scholar]
- Sato T, Nishishita K, Okada Y, Toda K (2012). Efferent fibers innervate gustatory and mechanosensitive afferent fibers in frog fungiform papillae. Chem Senses 37: 315–324. [DOI] [PubMed] [Google Scholar]
- Simon SA, de Araujo IE, Gutierrez R, Nicolelis MA (2006). The neural mechanisms of gustation: a distributed processing code. Nat Rev Neurosci 7: 890–901. [DOI] [PubMed] [Google Scholar]
- Simons CT, O'Mahony M, Carstens E (2002). Taste suppression following lingual capsaicin pretreatment in humans. Chem Senses 27: 353–365. [DOI] [PubMed] [Google Scholar]
- Simons CT, Boucher Y, Carstens E (2003). Suppression of central taste transmission by oral capsaicin. J Neurosci 23: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, Liu L, Erickson RP (2003). Neuropeptides modulate rat chorda tympani responses. Am J Physiol Regul Integr Comp Physiol 284: R1494–R1505. [DOI] [PubMed] [Google Scholar]
- Starostik MR, Rebello MR, Cotter KA, Kulik A, Medler KF (2010). Expression of GABAergic receptors in mouse taste receptor cells. PLoS ONE 5: e13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T, Droogmans G, Shigemura N, Ninomiya Y et al (2005). Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025. [DOI] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A et al (2013). CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495: 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD (2007). Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27: 10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC (2008). Amiloride‐sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Tizzano M, Anderson CB, Stone LM, Goldberg D, Kinnamon SC (2010). Evidence for a role of glutamate as an efferent transmitter in taste buds. BMC Neurosci 11: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE et al (2013). Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci U S A 110: 14789–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE et al (2015). Postsynaptic P2X3‐containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol 593: 1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Erickson RP, Simon SA (1995). Modulation of rat chorda tympani nerve activity by lingual nerve stimulation. J Neurophysiol 73: 1468–1483. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Kubota Y, Takagi H, Tohyama M (1984). Immunoelectron‐microscopic study on the fine structure of substance‐P‐containing fibers in the taste buds of the rat. J Comp Neurol 227: 380–392. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Noguchi K, Shigemura N, Jyotaki M, Takahashi I, Margolskee RF et al (2015). Leptin suppresses mouse taste cell responses to sweet compounds. Diabetes 64: 3751–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]


