Abstract
In the United States (US), there are high levels of disengagement along the HIV care continuum. We sought to characterize the heterogeneity in research studies and interventions to improve care engagement among people living with diagnosed HIV infection. We performed a systematic literature search for interventions to improve HIV linkage to care, retention in care, reengagement in care and adherence to antiretroviral therapy (ART) in the US published from 2007-mid 2015. Study designs and outcomes were allowed to vary in included studies. We grouped interventions into categories, target populations, and whether results were significantly improved. We identified 152 studies, 7 (5%) linkage studies, 33 (22%) retention studies, 4 (3%) reengagement studies, and 117 (77%) adherence studies. ‘Linkage’ studies utilized 11 different outcome definitions, while ‘retention’ studies utilized 39, with very little consistency in effect measurements. The majority (59%) of studies reported significantly improved outcomes, but this proportion and corresponding effect sizes varied substantially across study categories. This review highlights a paucity of assessments of linkage and reengagement interventions; limited generalizability of results; and substantial heterogeneity in intervention types, outcome definitions, and effect measures. In order to make strides against the HIV epidemic in the US, care continuum research must be improved and benchmarked against an integrated, comprehensive framework.
Keywords: HIV care continuum, HIV linkage to care, HIV retention in care, HIV reengagement, HIV adherence
Introduction
In 2015, the White House released an updated comprehensive National HIV/AIDS Strategy (NHAS) [1], and outlined specific measures to assess progress along the HIV care continuum. It is increasingly recognized that effective approaches to ending HIV in the United States (US) will require comprehensive strengthening of multiple components of the HIV care continuum [2]. While recent models have suggested that improved retention of HIV-positive persons in care is critical to reducing transmission [3, 4], large numbers of people living with HIV (PLHIV) in the US remain unaware of their infection (~13% of all PLHIV), unlinked to care, disengaged from care (~61% of all PLHIV), incompletely adherent to antiretroviral therapy (ART), and virologically unsuppressed (~70% of all PLHIV) [5, 6]. While the NHAS characterizes federal and local implementation strategies to address these gaps, it does not specify particular programs to implement in given areas with particular populations. To date, the majority of scientific effort has been dedicated to improving each stage of the HIV care continuum independently—each of which has been reviewed in recent years, including by the Centers for Disease Control and Prevention’s Prevention Research Synthesis (PRS) team [7–13]. The PRS project provides a vital resource for the identification of evidence-based interventions to improve steps of the HIV care continuum. However, these evidence-based interventions have variability in their magnitude of effect, sustainability of effects, costs (or lack of assessment of costs) and heterogeneity in study design and outcomes assessments.
The next step in designing a comprehensive approach to HIV prevention and engagement is to build upon prior work [13] by synthesizing the literature on interventions to strengthen the HIV continuum of care, in order to describe the heterogeneity of studied interventions’ approaches, costs, study designs, and study outcomes. Characterizing these heterogeneities will lay the foundation for decision-makers to develop a common framework for assessing HIV care continuum interventions, while helping researchers to better understand the evidence gaps we most urgently need to fill. For example, the extent to which currently studied interventions could achieve the NHAS goals is unknown, and without a common framework it will remain a challenge to evaluate.
As such, we performed a broad-based systematic review of all published interventions designed to strengthen the HIV care continuum after HIV diagnosis, with the aim of collecting evidence for prioritization of interventions, direction of future research, and evaluation of interventions to improve health outcomes and prevent HIV across the continuum of care.
Methods
We conducted a systematic review of English language literature to comprehensively characterize interventions designed to improve HIV care engagement in the US after HIV diagnosis. We conducted separate searches for the following care continuum steps: initial linkage to care (for those newly diagnosed with HIV), care retention (for those currently in care), care reengagement (for those previously in care), and medication adherence while on ART (for those in care and prescribed ART) (see Fig. 1 for a schematic of the steps of the HIV care continuum addressed). Our primary objectives were to characterize and describe the spectrum of HIV care continuum interventions for different target populations, and to characterize the heterogeneity of outcomes measured among such studies. Our secondary objectives were to describe the efficacy and whether costs are reported for identified interventions.
Fig. 1.
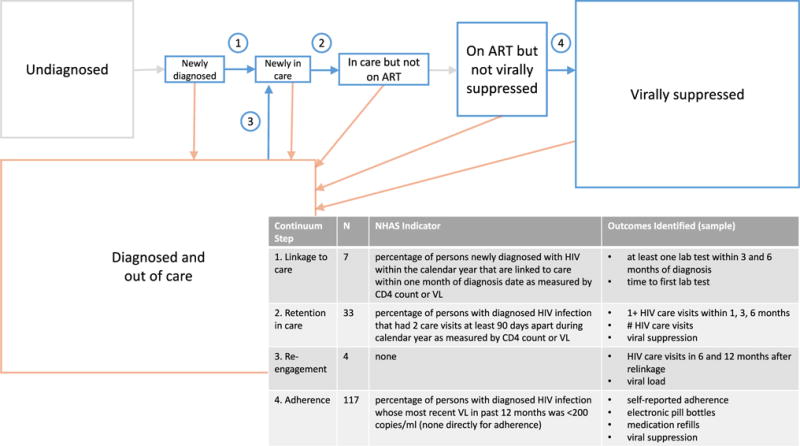
Schematic of HIV care continuum in the United States indicating steps of interest in review, and summary of outcomes in review. Each box’s area is proportionally sized to the United States population of people living with HIV in 2012 [5, 173]. Boxes representing very small proportions of the population (Newly Diagnosed and Newly in Care) are enlarged for purposes of display. Table displays the continuum steps of interest in review, number of studies included in review (N), NHAS indicator relevant to the continuum step, and a sampling of the outcomes identified in included studies in the review (for full list of outcomes identified, please see Supplemental Table)
Search Strategy and Study Selection
We systematically searched MEDLINE and EMBASE for citations published from 1 Jan 2007 to 17 June 2015. A list of keywords was created around the domains of interest (see Appendix 1 for complete search strategy). The reference lists of 64 reviews found in our search were evaluated to identify any manuscripts meeting inclusion criteria that were not found in our search strategy.
The overall target population for the review was people living with diagnosed HIV infection in the US. We included all study participants regardless of age, gender, and ethnicity. We included studies that had explicitly defined study populations exposed to an intervention, and a comparator population that did not receive an intervention. This review did not seek to evaluate any specific intervention, and interventions included were any biomedical, behavioral, health system, or policy strategy that sought to increase engagement in the four areas listed above. We excluded studies evaluating or comparing specific ART regimens or dosing frequencies, those without a comparator group, those without a defined intervention, and those without at least one quantifiable outcome (i.e., qualitative analyses). We included prospective and retrospective cohort studies, pre-post studies, and randomized clinical trials (RCTs); we excluded cross-sectional studies, mathematical modeling studies, and studies without empirically collected patient data. We included any study-defined outcomes and measures of intervention efficacy and quantified the variability in these outcome definitions.
Two reviewers independently evaluated titles, and then performed a review of abstracts to identify potentially relevant studies. We then conducted full-text review according to the established inclusion and exclusion criteria. Two reviewers abstracted the following data from all included studies: dates of study, location, intervention and comparator description, target population, eligibility criteria, study design, sample size, description of outcome measures, results, and any cost data. We utilized the Cochrane Collaboration’s tool to assess quality of included RCTs [14] and the Newcastle-Ottawa quality assessment scale for observational studies [15].
Data Analysis
Studies were classified based on the HIV care continuum step that the intervention sought to modify. We defined linkage studies as those targeting a population newly diagnosed with HIV and not yet in care, in which the outcome included time required to initiate care or percentage of the population establishing initial care within a defined period of time after diagnosis. Retention in care studies were those in which the target population was in care at the intervention initiation, with outcomes measuring any event that provided evidence of care engagement. By contrast, we defined reengagement studies to be those in which the target population was previously HIV-diagnosed (not newly diagnosed) but not currently engaged in HIV care. Adherence studies were defined to include populations currently in care and prescribed ART, with outcomes dependent on the degree of receipt and/or medication adherence to daily ART. If a publication included outcomes that fit our definitions for more than one care continuum step, it was included in multiple categories.
Anticipating significant heterogeneity of study outcomes within and across stages of the care continuum, we did not seek to perform meta-analyses. Rather, we approached summarization and characterization of data along the following domains. First, for each step in the care continuum we sought to qualitatively categorize interventions based on similarities of modality or approach utilized. We grouped education and behavioral/counseling interventions separately with systematic (non-individualized) interventions described as education, while behavioral/counseling interventions were those that appeared to be centered on a client-specific exchange. We additionally categorized the target populations included within intervention studies: general population, people who use drugs (PWUD) (any use), men who have sex with men (MSM), prison/jail, adolescents/youth, women, homeless population, other, and when possible racial/ethnic minorities. Next, we quantified the number of different study outcomes and effect measures reported across studies. Many studies reported multiple outcomes, and these were counted separately. Finally, we summarized intervention efficacy by characterizing the study outcomes as being significantly improved or not, as defined by each individual study (a study counted as having significant results if at least one outcome was statistically significantly improved in study defined statistical analysis). When possible we compiled effect sizes in tabular format and/or in descriptive analysis. For each step of the HIV care continuum, we additionally assessed whether study outcomes could be compared to metrics outlined in the updated NHAS progress indicators (i.e. % linking to care within one month, % retained in care among those diagnosed, % virally suppressed among those diagnosed). Finally, we also reported on whether costs and cost-effectiveness data were provided.
Results
Our search yielded 5786 articles, of which 152 were included in the final analysis (Fig. 1 and Supplemental Figure). Among included studies, 7 (5%) presented data on ‘linkage’, 33 (22%) on ‘retention‘, 4 (3%) on ‘reengagement’, and 117 (77%) on ‘adherence’, with some categorized to multiple care continuum steps (Supplemental Figure).
Linkage
Among 7 studies that targeted newly diagnosed HIV-positive persons (shown in Table 1), 4 (57%) were cohort studies while the remainder utilized a pre-post study design; there were no RCTs identified. None of the 7 ‘linkage’ studies assessed costs of their intervention. The target population in each of the studies (7/7, 100%) was the general population of PLHIV, without further targeting of risk groups.
Table 1.
Included linkage to care studies, systematic review of interventions to impact engagement along the HIV care continuum in the United States, 2007–2015
| Study | Intervention type |
Intervention description | Comparator | Target population |
Significant results? |
Outcomes | Intervention results |
Comparator results |
Cost data? |
|---|---|---|---|---|---|---|---|---|---|
| Cohort studies | |||||||||
| Bocour et al. 2013 [40] | CM | Field Services Unit provided brief case management-like services as part of partner services | SOC | G | Y |
1. Linkage to care within 3 months (CD4, VL as proxy for visit) 2. Established in care among those linked (2 lab tests >90 days apart) |
79% 87% |
66% 84% |
N |
| Willis et al. 2013 [41] | CM | HIV medical case management for linkage | SOC | G | N | 1. Linkage to care (based on CD4 and/or VL) at 3 months 2. Linkage to care (based on CD4 and/or VL) at 6 months |
1. PEMS data source–80%; Surveillance data source–72% 2. PEMS data source–83 %; Surveillance data source–80% |
1.PEMS–76%; Surveillance 80% 1.PEMS–81%; Surveillance 85% |
N |
| Keller et al. 2011 [42] | HT | Oral-based point-of-service testing | Conventional tests | G | N | Linkage/engagement in care within 6 months (2 CV after post-test counseling visit) | 52% | 42% | N |
| Craw et al. 2008 [43] | CL | HIV medical care co-located at facility that participants received the ARTAS-II intervention | Non-co-located | G | Y | AOR for HIV medical care in past 6 months | AOR = 3.0 (95% Cl: 1.9 to 4.9) | Ref | N |
| Pre-post studies | |||||||||
| Mugavero 2008 [44] | CM | Orientation visit within 5 days of initial visit to clinic | Pre-period | G | Y | % ‘No show’ at initial visit | 19% | 31% | N |
| Castel et al. 2014 [45] | P | City-wide initiative to implement routine opt-out testing and complete linkage with confirmatory positive test (2006–2009) | Pre-period (2005–2006) | G | Y | Linkage to care within 3 months of diagnosis (based on CD4 test or VL) | 76% | 51% | N |
| Onyeajam et al. 2013 P [46] | P | Routine, opt-out HIV testing (2008–2010) in clinical settings for all adults 13–64 years old (implemented in 2007 in South Carolina) | Pre-period (2004–2006) | G | Y |
1. AOR to take >12 months to be in care (Intervention vs. Comparator) 2. AOR viral suppression at 12 months (Intervention vs. Comparator) |
AOR = 0.42 (95% Cl 0.34–0.51) AOR = 8.4 (95% Cl 7.0–10.2) |
Ref Ref |
N |
Bold rows represent studies with statistically significant improvements in linkage to care as defined by the study authors. When authors report multiple outcomes, these are shown independently in different rows
AOR adjusted odds ratio, CI confidence interval, CV clinic visit, Ref Reference, VL viral load, SOC standard of care
Intervention types include: CM case management, HT HIV testing modality, P policy, CL co-located services
Target populations: G general
PEMS Program Evaluation and Monitoring System. Study assessed outcomes from two data sources
Overall, we found no two studies that measured identical linkage to care outcomes (Supplemental Table), and no studies (0/7, 0%) reported data that would allow direct comparison against the current NHAS progress indicator for linkage to care targets (i.e. percent linked to care within one month of diagnosis date—see Fig. 1 for relevant NHAS indicators), though one study did directly assess viral suppression (NHAS indicator 6). Most studies (5/7, 71%) identified the proportion of patients that ‘established’ care after new diagnosis—measures of establishing care included documentation of a clinic visit, laboratory test, or ART initiation within a set period of time (varied between 3 months and 1 year). Effect sizes of interventions were largely reported as an absolute comparison of proportions (5/7, 71%), or relative measures such as hazard ratios or odds ratios (3/7, 43%). Alternatively, one study (14%) reported outcomes as a continuous measure of time until first visit.
Interventions were broadly categorized as involving case management (3/7, 43%, e.g. providing a case manager until an individual established HIV care), policy changes (2/7, 29%, e.g. routine opt-out HIV testing), change in HIV testing modality (1/7, 14%, e.g. rapid vs conventional tests), and co-location of care (1/7, 14%, e.g. HIV medical care co-located with ARTAS II site). Efficacy of these differing strategies was mixed. Two (2/3, 66%) case management interventions showed statistically significant improvements in linkage to care, two (2/2, 100%) policy interventions and the one co-location of services study also showed improvement. However, the overall effect size of these interventions was modest and ranged from an incremental 3% to 24% linked compared to standard of care.
Retention
Among 33 total studies that targeted patients already established in care, 31 (94%) studies evaluated 35 separate interventions addressing retention in care, while the remaining two (6%) addressed only the costs of such interventions. Of the 31 intervention studies, 13 (42%) were RCTs, while 10 (32%) were cohort studies, and 8 (26%) were pre-post study designs. The target populations studied included PWUD (13% of studies), adolescents/youth (10%), young black and Latino MSM (6%), prison or jail populations (6%), and other target populations (23%); 42% lacked a pre-specified target risk group.
Overall, these 31 studies utilized 39 different measures to evaluate the impact of retention in care interventions, with each study measuring from 1–5 (median = 2) outcomes (Supplemental Table). These study outcomes could be broadly grouped into four categories: change in clinic visits within given period of time, change in the number of laboratory tests (such as CD4 count) within a given period of time, change in ART prescriptions or ART usage, and change in viral load/viral suppression. Follow-up times at which the outcome assessments were made varied across studies. Methodology for reporting effect sizes was heterogeneous. Some studies reported dichotomized data (23/31, 74%) based on the number or percentage of the study participants that met the study definitions for being retained in care over the study period, while others reported continuous data (13/31, 42%, e.g. mean number or proportion of clinic visits kept). Eleven (35%) reported on the downstream care continuum target of viral suppression. The current NHAS progress indicator related to retention in care (NHAS indicator 5) seeks to engage (cross-sectionally) 90% of all diagnosed persons in care. Among the 31 intervention studies, 19 (61%) provided results in a format (i.e. proportion in care among intervention group) that would allow some comparison to this NHAS progress indicator, while only 3 (10%) used the precise outcome of retention (two care visits, 90 days apart, within the calendar year) listed by the NHAS.
Interventions studied by investigators were diverse (Table 2). The majority of interventions focused on implementing novel technology (7/31, 23%, example: text message appointment reminders, interactive clinical decision-support with alerts for poor patient outcomes), case management or outreach (8/31, 26%, example: enhanced personal contact, medical case management), or counseling/behavior modification strategies (8/31, 26%, example: motivational interviewing, peer mentoring).
Table 2.
Included retention in care intervention studies, systematic review of interventions to impact engagement along the HIV care continuum in the United States, 2007–2015
| Study | Intervention type |
Intervention description | Comparator | Target population |
Significant results? |
Outcomes | Intervention results |
Comparator results |
Cost data?a |
|---|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | |||||||||
| Gardner et al. 2014 [16] | CMO | 1. Enhanced personal contact (EC) (12 month) | SOC | G | Y |
1. Visit constancy, % keeping 1 visit for 3 consecutive quarters 2. Visit Adherence, % kept appointments |
56% 73% |
46% 67% |
Y [47] |
| CMO, E | 2. EC plus basic HIV education (EC+ skills) | SOC | Y |
1. Visit constancy, % keeping 1 visit for 3 consecutive quarters 2. Visit Adherence, % kept appointments |
56% 71% |
46% 67% |
|||
| Gwadz et al. 2015 [48] | BC | Motivational interviewing, support partner, navigation, support groups | SOC | YBL-MSM | N | % attended at least one primary care visit in past 6 mo | 60% | 48% | N |
| Konkle-Parker et al. 2014 [17] | E, BC | One-on-one educational sessions, motivational interviewing, social motivation, and adherence enhancing devices (6 month) | SOC | G | N | % with a visit in each 4-month block over 12 months: year prior to, and year of study | 34%, 18% | 46%, 20% | N |
| Konkle-Parker et al. 2012 [18] | E, BC | One-on-one sessions and phone calls including: HIV education, peer video about adherence, motivational interviewing, feedback, communication training and adherence enhancing devices (3 visits) | SOC | G | N | 1. Mean number of missed medical visits 2. % Viral suppression |
0.79 75% |
1.11 78% |
N |
| Lucas et al. 2010 [20] | IS | Clinic-based buprenorphine/naloxone (12 months) | Referred out for opioid tx | PWUD | Y |
1. Number of visits 2. Months receiving ART |
3.5 11 months |
3.0 12 months |
N |
| MacGowan et al. 2015 [19] | E | Education and skills building (4 sessions pre-release [3 months]; 2 sessions post-release [3 months]) | SOC | PJ | N | % reporting healthcare at HIV clinic: baseline, 3-month follow-up | 63%, 84% | 44%, 63% | N |
| Naar-King et al. 2009 [49] | BC | Motivational interviewing by peer outreach workers (2 sessions) | Masters-level staff outreach | AY | N | Gap score (1 point per quarter with missed appointment) change pre to post intervention | −1.50 gaps | −1.06 gaps | N |
| Norton et al. 2014 [22] | T | Text message appointment reminders | SOC | G | N | Next appointment attendance | 72% | 81% | N |
| Proeschold-Bell et al. 2010 [23] | T, C | Health information exchange between ancillary care sites and medical care providers (2 years) | SOC | G | N | 1. % suppressed viral load at baseline, 12 and 24 months 2. % prescribed ART at baseline, 12 and 24 mo |
56%, 54%, 55% 72%, 79%, 81% |
42%, 49%, 54% 72%, 76%, 79% |
N |
| Purcell et al. 2007 [50] | BC | Intervention to develop participants as informal peer mentors (10 sessions) | Video discussion | PWUD | N | HIV care 2+ times in past 6 months: baseline, 6 & 12 mo | 71%, 71%, 69% | 69%, 72%, 64% | N |
| Robbins et al. 2012 [21] | T, C | Interactive clinical decision-support system that generates alerts to notify HIV outpatient providers of poor patient outcomes (1 year) | Static alerts | G | Y | Rate of 6-month suboptimal follow-up (events per 100 patient years) | 20.6/100 patient years | 30.1/100 patient years | N |
| Wohl et al. 2011 [51] | CMO | Bridging case management following release from prison (3 months prior and 6 months after release) | SOC | PJ | N | Linkage/Continuation of care (at least one medical appointment) by week 4, 12, 24 post release | 65%, 88%, 91% | 54%, 78%, 89% | N |
| Wolitski et al. 2010 [52] | H | Immediate HOPWA rental assistance | Customary housing services | O | N | % any medical care in the past 6 months at: baseline, 6, 12, and 18-month follow-up | 85%, 70%, 77%, 78% | 80%, 71%, 72%, 73% | N |
| Cohort studies | |||||||||
| Altice et al. 2011 [53] | IS | Integration of HIV care with BUP/NX treatment for 3–4 quarters (Evaluated over 12 months) | BUP/NX for <3 quarters | PWUD | Y* |
1. ART initiation among those not on ART at baseline 2. Viral suppression among those not on ART at baseline |
β = 1.34 (95% Cl 1.18, 1.53) β = 1.25 (95% Cl 1.10, 1.42) |
Ref Ref |
N |
| Bogart et al. 2012 [54] | BC, CMO | Treatment Advocacy Program including counseling and motivational interviewing, patient–provider relationship building, referrals to necessary services | SOC | G | N | Odds of attending at least one medical visit and not reporting any missed visits (3 months) | AOR = 2.64 (95% Cl 0.67, 10.36) | Ref | N |
| Cabral et al. 2007 [55] | CMO | Outreach, advocacy and some support services (9+ contacts within 3 months) | No intervention contacts in first 3 months of program | G | Y | % with gap in care (over 12 months) | 22% | 39% | N |
| Cunningham et al. 2011 [56] | IS | Integrated HIV and opioid addiction treatment with buprenorphine (6 months) | Non-integrated opioid tx | PWUD | Y |
1. Median clinic visits (6 mo) 2. Lab tests (≥ 2 CD4 counts) (6 months) 3. % VS (6 months) |
8 53% 47% |
2 8% 0% |
N |
| Davila et al. 2013 [57] | IS | 1. Centralized multidisciplinary youth clinic (C) | De-centralized | AY | Y |
1. Over 12 months: “Adequate” visit constancy (3 quarters with at least one visit) 2. No gap in care >180 days between visits |
57% 80% |
31% 83% |
N |
| IS, BC, E | 2. Centralized multidisciplinary youth clinic with youth-specific support groups and education activities (12 months) (C + ES) | Centralized | Y |
1. Over 12 months: “Adequate” visit constancy (3 quarters with at least one visit) 2. No gap in care >180 days between visits |
65% 96% |
57% 80% |
|||
| Hanna et al. 2013 [58] | P | 1. ADAP state characteristics—state funding provided to the annual ADAP budget | No state funding | G | Y | ART initiation within 6 months of ART eligibility | 58% | 39% | N |
| P | 2. ADAP state characteristics—no use of ADAP waiting lists in the state | Waiting lists | N | ART initiation within 6 months of ART eligibility | 55% | 73% | |||
| Himelhoch et al. 2009 [59] | IS | 6+ mental health visits | No mental health visits | O | Y | Discrete time survival analysis ART discontinuation (AOR comparing intervention to comparator) |
6–11 visits/yr AOR 0.78 (95% Cl 0.61–1.00); 12+ visits/year 0.60 (95% Cl 0.45–0.80) |
Ref | N |
| Schranz et al. 2015 [60] | C | Hospital-based HIV clinics | Community-based HIV clinics | G | N | 1. Retention in care (2+ outpatient visits separated by 90+ days in year) 2. Retained and on ART 3. Retained on ART and suppressed |
82% 72% 59% |
85% 74% 59% |
N |
| Terzian et al. 2015 [61] | H | NYC HOPWA provided rental assistance, housing placement assistance, and supportive permanent housing (receiving intervention during 2011) | No housing assistance | O |
Y N |
1. Retention in care (2 + lab tests >3 months apart over 12 months) 2. % viral suppression (at least once in 12 months) |
94% 78% |
82% 82% |
N |
| Willis et al. 2013 [62] | CMO | HIV medical case management (MCM) (fiscal year 2010) | Non-MCM funded facility | G | Y | Retention in care (2+ lab tests 3+ months apart) % (AOR) | 76% (AOR 4.13 [1.93–8.85]) | 60% (Ref) | N |
| Pre-post studies | |||||||||
| Andersen et al. 2007 [62] | Tr | 1. Transportation only [TO] (12 mo) | Pre-period | W | N | Mean number of medical visits for 6 months pre-(comparator), 6 months post-intervention, 12 months post-intervention | 1.5, 1.1 mean visits | 1.3 mean visits | N |
| Tr, BC | 2. Transportation Plus Personalized Counseling [TO Plus] (12 months of Transportation with initial 6 months of counseling) | Pre-period | Y | Mean number of medical visits for 6 months pre-(comparator), 6 months post-intervention, 12 months post-intervention | 1.6, 1.0 mean visits | 1.1 mean visits | |||
| Enriquez et al. 2008 [63] | C | Bilingual/bicultural team (12 months post) | 12 months pre-period | O | Y |
1. Mean clinic visits 2. % viral suppression (end of 12 months pre- and post-periods) |
5.30 visits 53% |
2.81 visits 21% |
N |
| Henry et al. 2012 [64] | T | Automated telephone reminders 2 weeks prior to regularly scheduled HIV clinic appointments (6 months) | 6 months pre-period | O | Y* | % missed appts overall (% missed among non-depressed) | 24% (18%) | 24% (23%) | N |
| Hightow-Weidman et al. 2011 [65] | T, CMO | Social marketing campaign, intensified outreach, tightly linked medical-social support network (3 years) | 3 year pre-period | YBL-MSM | Y | % of visits attended (OR) | 80% (OR 2.58[95% Cl 1.34–4.98]) | 67% (Ref) | N |
| Irvine et al. 2015 [66] | CMO | HIV Care Coordination Program included outreach, case management, multidisciplinary care team communication, patient navigation, adherence support and health promotion (1 year) | 12 months pre-period | G | Y |
1. % engaged in care (≥ 2 laboratory tests 90 days apart) 2. % viral suppression (on most recent test in second half of 12-month period) |
91% 51% |
74% 32% |
N |
| Saifu et al. 2012 [67] | T | Telemedicine clinic encounters for HIV (2009–2011) and HCV patients (2010–2011) at “spoke” sites with healthcare providers at “hub” sites | In-person clinic visits (pre-period starting 2008) | O | Y | Appointment completion | 76% | 61% | N |
| Shade et al. 2015 [68] | T, C | 6 Health Information Technology Interventions at 6 sites (6 months) | 6 months pre-period | G | Y* |
1. % engaged in care (≥ 1 visit or lab test over 6 months) 2. % ART prescription 3. % viral suppression across 6 intervention sites |
83–96% 75–92% 11–68% |
65–100% 73–89% 3–62% |
N |
| Gardner et al. 2012 [69] | C | Clinic-wide printed reminder materials and verbal messages used by clinic staff (12 months) | 12 months pre-period | G | Y |
1. % keeping next 2 visits 2. Mean % of visits kept |
53% 70% |
49% 68% |
Y [70] |
Bold rows represent study outcomes with statistically significant improvements in retention in care as defined by the study authors. When authors report multiple interventions and/or outcomes, these are shown in different rows. When possible, the timing of outcome measurement is indicated; if not listed, indicates unclear or unreported timing. In the results column, we present only selected representative analyses and results from each study and some outcomes and subgroup analyses are not shown
ADAP AIDS Drug Assistance Program, AOR adjusted odds ratio, appt appointment, BUP/NX buprenorphine/naloxone, CI confidence interval, OR odds ratio, Ref Reference, SOC standard of care, tx treatment
Intervention types include: BC behavioral/counseling, CMO case management/outreach, C clinic-wide (such as a clinic-wide social marketing campaign, integration of care across specialties, or shared medical records), E education, H housing assistance, IS integration of services, P policy, T technology-based, Tr transportation
Target populations include: AY adolescents/youth, G general, MSM men who have sex with men, O other, PJ prison/jail, PWUD people who use drugs
Y in significant results column indicates that any significant result was presented and does not imply that all analyses were significant. Y* indicates that results were significant in subgroup analyses only, or within only some sites
Two studies assessing the costs of parent efficacy studies were published separately
A low proportion of studies that evaluated behavior modification/counseling (1/7, 14%) found significant improvements in care retention. By contrast, integration of services (5/5, 100%), and to a lesser extent, case management (5/7, 71%), technology (5/7, 71%) and clinic-based interventions (4/6, 67%) all had higher proportions of potentially efficacious interventions according to the effect measures and outcomes chosen by the study investigators. The heterogeneity of these interventions, target groups, and effect measures precluded meta-analysis. Overall, the effect sizes of interventions with significant results (bold rows, Table 2) were modest, and few (3/31) assessed outcomes longitudinally over more than 1 year. Of the included studies and interventions that could be assessed against the NHAS progress indicator 5, only 4/19 (21%) reported achieving retention of 90% of study participants at 1 year (or the end of their study period).
Among the most rigorously designed studies (RCTs), only 3/13 (23%) reported significantly improved retention among the intervention groups. None (0/5, 0%) of the RCTs that centered on behavioral or counseling interventions demonstrated significant impact on care retention. The effect sizes and interventions among RCTs suggesting improvements in retention in care varied. Gardner et al. implemented an intervention for education and enhanced personal contact for a general HIV clinic population over 12 months and found that visit adherence (defined as proportion kept out of all scheduled primary care visits) increased from 67% to 73% [16]. By contrast, however, three other RCTs evaluating educationally focused interventions found no significant effect [17–19]. Lucas et al. found that clinic-based substance abuse treatment modestly improved the number of clinic visits over the twelve month study period from 3 to 3.5 when comparing control to intervention arms [20]. Robbins et al. studied a technology-based decision support intervention to alert providers to poor patient outcomes and found a 9.5 per 100 person year reduction in the rates of 6 month suboptimal follow-up [21]; two other technology-centered interventions, however, showed no significant change in retention in care [22, 23].
Reengagement
Few studies (4) assessed reengagement in care (see Table 3), and none of these studies addressed costs. One of these was a cohort study, while the other three employed a pre-post study design. All four studies addressed general populations of PLHIV who were out of care. Intervention types were variable, and included provider notifications, policy (routine opt-out HIV screening), case management, collaboration between clinics and health departments, and navigation-like interventions. Reengagement studies reported on 8 different outcomes; three of the four studies reported viral load or viral suppression as one of their outcomes. Three of these four studies found significant improvements in engagement in care following the intervention, though these significant impacts included a range of effect sizes (such as a 5% increase in re-linkage [24], 11.2% increase in viral suppression [25], and 3.9–5.4% reduction in no care in the past 6 months [26]).
Table 3.
Included reengagement studies, systematic review of interventions to impact engagement along the HIV care continuum in the United States, 2007–2015
| Studies | Intervention type | Intervention description | Comparator | Target population | Significant results? | Outcomes | Intervention results | Comparator results | Cost data? |
|---|---|---|---|---|---|---|---|---|---|
| Magnus et al. 2012 [71] | PN | Automatic notification to providers when out of care PLHIV registers at care facility, decision support to staff to engage patients in HIV care | Previously experienced delayed entry into care | G | N | 1. AOR VL > 10,000 copies/ml 2. AOR ART Prescription |
1.72 (95% CI 0.95–3.14) 0.86 (95% CI 0.38–1.93 |
Ref Ref |
N |
| Flash et al. 2015 [25] | P, CM | Routine opt-out HIV screening and nonmedical case management by service linkage workers—evaluated among those previously tested positive | Pre-period | G | Y |
1. Engaged in care (HIV primary care visit in 6 months) 2. Viral suppression |
59%, 34% | 41%, 23% | N |
| Bove et al. 2015 [24] | C | HIV clinic & local health department collaboration to identify disengaged patients and relink with a linkage specialist | Historical cohort | G | Y | Relinked in one year | 15% | 10% | N |
| Bradford et al. 2007 [26] | N | Navigation-like interventions | Pre-period | G | Y |
1. No care in the past 6 months for 6 months pre- (comparator), 6 months post-intervention, 12 months post-intervention 2. Viral Suppression at baseline (comparator), 6 months, 12 months |
5%, 8% 54%, 53% |
12% 35% |
N |
Bold rows represent studies with statistically significant improvements in reengagement in care as defined by the study authors. When authors report multiple interventions and/or outcomes, these are shown independently in different rows
AOR adjusted odds ratio. CI confidence interval. Ref reference
Intervention types include: C collaboration between clinics and health departments, CM case management, N navigation, P policy, PN provider notifications
Target populations include: G general
Adherence
Among 117 total studies evaluating interventions addressing medication adherence among patients in care, 111 adherence studies evaluated efficacy alone, 4 addressed costs alone, and 2 addressed both efficacy and costs. Of the 113 efficacy studies, 65 (57%) were RCTs, while 20 (18%) were cohort studies, and 28 (25%) were pre-post study designs. Nearly half of studies (53/113, 47%) targeted a general population of PLHIV taking ART, while 23 (20%) targeted PWUD, 9 (8%) targeted adolescents/youth and 8 (7%) targeted women. Only 2 (2%) studies exclusively recruited MSM, and an additional 2 (2%) exclusively recruited racial minorities. Interventions ranged from adjunctive treatment for drug use, to active reminder systems (using technology) to clinic-wide interventions that were not individually targeted (Table 4).
Table 4.
Included adherence studies by intervention type, systematic review of interventions to impact engagement along the HIV care continuum in the United States, 2007–2015
| Intervention type | Count | Intervention examples | Study design | Target populationab | % Reporting viral suppression | Significant results? (%) | % with cost data | Studies |
|---|---|---|---|---|---|---|---|---|
| Drug use treatment | 5 | Integrated BUP/NX tx for opioid dependence | RCT: 1 Cohort: 3 Pre-post: 1 |
PWUD-PJ: 2 PWUD-W: 1 Other: 1 PWUD: 1 |
60 | 60 | 0 | [53, 72–75] |
| Financial incentives | 6 | Contingency management | RCT: 4 Pre-post: 2 |
Other: 1 PWUD: 5 |
67 | 83 | 17 [76] | [76–81] |
| Structural | 5 | Ecosystem intervention, harm reduction housing program | RCT: 3 Cohort: 1 Pre-post: 1 |
General: 1 Other: 2 Prison/Jail: 1 PWUD: 1 |
20 | 80 | 0 | [82–86] |
| Pharmacy-based | 10 | HIV-specialized pharmacies, pharmacist-managed drug-optimization, pharmacist adherence counseling | Cohort: 6 Pre-post: 4 |
General: 9 AY: 1 |
50 | 70 | 0 | [31, 32, 87–94] |
| Active reminder devices | 7 | Phone adherence support reminders, SMS reminders, two-way SMS messaging, Wisepill device with immediate phone adherence counseling on missed dose | RCT: 5 Pre-post: 2 |
General: 3 Other: 1 AY: 2 MSM: 1 |
14 | 71 | 14 [95] | [96–102] |
| Passive reminder devices | 5 | Pillboxes | RCT: 3 Cohort: 1 Pre-post: 1 |
General: 3 PWUD: 1 Homeless: 1 |
80 | 60 | 0 | [17, 18, 103–105] |
| Treatment supporter | 7 | Visits from community health workers, visits from advanced practice nurse to community, adherence support partner, peer support | RCT: 7 | General: 5 Other: 1 MSM: 1 |
28 | 57 | 14 [106] | [102, 107–112] |
| DAART | 15 | Directly administered ART in health care vans, methadone clinics, during hospitalization | RCT: 8 Cohort: 1 Pre-post: 6 |
General: 3 PWUD: 8 Prison/Jail: 1 AY: 2 BPWUD: 1 |
93 | 60 | 0 | [28, 33, 34, 104, 113–123] |
| Clinic-based | 5 | Clinic-wide social marketing campaign, multi-disciplinary care team, shared medical record, adherence data shared with provider | RCT: 1 Cohort: 2 Pre-post: 2 |
General: 5 | 20 | 60 | 0 | [124–128] |
| Behavioral/counseling | 37 | Motivational interviewing, cognitive behavioral therapy, peer mentoring, group and individual counseling, delivered via phone and in person | RCT: 29 Cohort: 3 Pre-post: 5 |
General: 19 Other: 5 AY: 2 Women: 5 Black/Latino: 1 |
43 | 51 | 5 [27, 129] | [17, 18, 27, 30, 48, 50, 54, 56, 78, 101, 130-156] |
| Education | 13 | PDA video, multimedia education with quizzes, interactive computer-based tutorial, pill swallowing demonstration, audio music program, group and one-on-one educational sessions, printouts | RCT: 9 Pre-post: 4 |
General: 10 AY: 2 Women: 1 |
46 | 31 | 8 [157] | [17, 18, 29, 111, 158-166] |
| Depression treatment | 4 | SSRIs (including direct administration), collaborative care, therapy groups | RCT: 3 Cohort: 1 |
Other: 3 Homeless: 1 |
50 | 25 | 0 | [73, 167–169] |
| Case management | 2 | Transitional care coordination when leaving prison, medical case management | Cohort: 1 Pre-post: 1 |
General: 1 Prison/Jail: 1 |
50 | 0 | 0 | [41, 170] |
| Other | 2 | Unannounced pill counts, journal writing | RCT: 1 Cohort: 1 |
General: 1 Women: 1 |
0 | 0 | 0 | [171, 172] |
Viral suppression is as defined by the authors and thus varies between studies (cutoffs ranged from 20-400 copies/ml as the limit of detection). The “Significant results” column indicates studies that found any significant results in primary or secondary analyses. Studies were allowed to belong to multiple “Intervention types” if the implemented intervention contained more than one component
BUP/NX buprenorphine/naloxone, PDA personal digital assistant, RCT randomized clinical trial, SSRI selective serotonin reuptake inhibitor, tx treatment, AY = adolescents/youth, MSM = men who have sex with men, PWUD = people who use drugs, BPWUD Black people who use drugs, PWUD-PJ people who use drugs among prison/jail populations, PWUD-W women who use drugs. Other people with mental health diagnoses, veterans, those who abuse alcohol, and high-risk insurance pool members
Outcomes of interest ranged from adherence measures to biological outcomes, with 50% reporting on viral suppression as an outcome. Adherence was measured in numerous ways, including self-report [using several measures, including the visual analog scale (VAS) and the AIDS clinical trial group (ACTG) questionnaire], electronic pill bottles [such as Medication Event Monitoring System (MEMS) caps], pill counting, medication refills, and presence of antiretrovirals in specimens (such as hair or plasma). Some of these measures included a time component (proportion of doses taken on time), and measures used a variety of recall periods (3 days, 7 days, 3 weeks, etc.).
Efficacy of adherence interventions was extremely varied across and within intervention types (Table 4). While the greatest number of studies focused on counseling (37, 33%), half (51%) of these reported improved adherence and the remainder indicated no improvement or worsened adherence. NHAS progress indicator 6 calls for achieving 80% viral suppression among those diagnosed. In these cohorts of individuals that were diagnosed (and in care), 8 studies (15% of the 52 studies for which it was possible to assess) reported intervention viral suppression results that met this target. Assessing virologic outcomes was limited by methodologic issues—for example, cutoffs for viral suppression varied from <20 to <400 copies/ml (limiting comparability), some patients met criteria for viral suppression at some time points but not longitudinally, and some studies reported viral loads but did not formally assess viral suppression. Among the 8 studies that achieved at least 80% virologic suppression [27–34], the types of interventions varied [3 directly administered ART (DAART), 2 behavioral/counseling, 2 pharmacy-based and 1 education], as did the target population being addressed (6 general, 1 women, 1 PWUD), and the type of study design (4 RCTs, 1 cohort, 3 pre-post). Nonetheless, only four of these studies [27, 29, 32, 34] found a significant improvement in viral suppression in the intervention group versus the comparator group.
Study Quality
The quality of included studies varied substantially. Among the 74 included RCTs, most (63/74) reported random sequence generation though some (10/74) did not clearly describe their randomization method. Few studies clearly described concealment of treatment allocation (18/74). Very few RCTs (9/74) were completely blinded/-masked (largely due to the nature of interventions assessed), though few (21/74) used outcome assessors blinded/masked to treatment assignment either. A high proportion of RCTs (31/74) were deemed to be at high risk of bias due to attrition from the studies. Among 73 observational studies, most (64/73) were assessed to be somewhat or truly representative of the population of interest. Most (49/73) collected outcomes by linking to medical records, with just over a quarter using self-reported outcomes (20/73). Observational studies also had high levels of attrition, with only 32/73 having over 90% follow-up. Among 31 cohort studies, most (26/31) accounted for confounding in their analyses.
Discussion
Successful strengthening of the care continuum will require combination of, and prioritization between, different interventions—tasks that are difficult without common metrics to evaluate the effect of interventions on outcomes. Our review highlights the tremendous degree of heterogeneity across existing studies to improve the HIV continuum of care. We found a high degree of heterogeneity when defining outcomes of interest, as well as in measuring effect sizes of interventions. Moreover, only 3/38 linkage and retention studies offered care engagement results in a format that could be compared to external metrics of success set forth in the newest NHAS. These elements of study heterogeneity threaten our ability to effectively select the combinations of interventions likely to have the greatest effect. Additionally, models suggest that as little as 3.3% of HIV transmissions in the US occur as a result of individuals on ART but not virally suppressed (the population targeted by adherence interventions) whereas the population diagnosed but not retained in care contribute as much as 61.3% of infections [35]. We reveal a disconnect between the areas of the HIV continuum of care where greatest impact could be achieved (retention and reengagement) and the areas where intervention evidence is strongest (adherence, representing 77% of studies identified). We identify a paucity of evidence to guide interventions targeting persons at high risk (e.g., MSM, transgender individuals), a gap which has been identified previously [36, 37]. Further, we identify few studies that present cost data, despite the widespread understanding that funders and health departments need these data in an era of largely flat budgets. These findings suggest a need for a consensus process to develop more clear guidance to the HIV research community about which types of interventions are in greatest need of study, which populations should be targeted, and which outcomes should be measured in order to develop a more effective and coordinated HIV response in the US.
Overall, we found few consistent themes to suggest efficacy of specific interventions, or broader intervention types. Among studies of individuals in care, we found that a majority of studies that used integrated services (such as co-located substance abuse treatment with HIV treatment), case management, technology (such as provider alerts and automated messages to patients) and clinic-based interventions (such as clinic-wide messaging and bilingual care teams) had significantly improved results in retention. Financial incentives, active reminder devices, structural and pharmacy-based interventions had the highest proportion of significantly improved results among the included adherence studies. The CDC’s Prevention Research Synthesis compendium provides an excellent resource for policymakers looking to select an evidence-based intervention in their population of interest [13]. We note that given the heterogeneity in study populations and locally specific intervention details, that it is difficult to draw any conclusions about the generalizability of these intervention types across settings.
While the NHAS has outlined specific targets, which are laudable in outlining objective goals for providers, program managers, and researchers to assess progress along the HIV care continuum, we found that these metrics are mismatched to recent research efforts in important ways. For example, NHAS indicators for care retention and viral suppression (indicators 5 and 6) have denominators of all individuals who are diagnosed with HIV, while in general, retention and adherence studies have denominators of people who are in care. In order for researchers or program managers to easily compare their results of a retention or adherence intervention to these measures, they would have to extrapolate to the proportion diagnosed instead of directly comparing their data.
Beyond the challenges to comparing intervention effects with NHAS indicators, we found no consensus in how to define or measure any stage of engagement along the HIV care continuum. First, we found 11 different outcomes used in the literature to assess initial linkage to care, and 39 different outcomes among retention in care studies. This heterogeneity makes it very difficult to compare interventions or intervention types, and precludes our ability to draw any solid conclusions regarding intervention efficacy. While work has been done to consolidate definitions [38], this is not resulting in consolidation among researchers. Second, achieving 80% viral suppression among those diagnosed is part of the NHAS (indicator 6), but only 1/7 (14%) studies of linkage interventions, 11/31 (35%) studies of retention interventions, and only 56/113 (50%) studies of adherence interventions evaluated improvements in achieving the ultimate goal of viral suppression. By measuring ‘upstream’ effects, it is not possible to assess the net benefit of a care continuum intervention, which may be compromised by gaps in downstream care engagement (i.e., interventions showing incremental increases in initial linkage to care may have reduced net benefits due to poor downstream longitudinal retention in care). Third, compounding the challenge in evaluating the literature is the lack of standardization in how intervention efficacy is assessed. Beyond variability in defining outcomes of interest, researchers assessed intervention effects in a varied manner; as such, we were unable to provide pooled estimates of incremental benefit for any specific interventions. Finally, engagement in HIV care is dynamic over time and continuous retention and viral suppression is needed to reduce transmission and improve health [39]. As such, cross-sectional or short-term assessments of care engagement may misrepresent longitudinal efficacy. In our review, we found that very few studies assessed outcomes beyond a few months.
Throughout our analysis, we have highlighted the relative distribution of RCTs to observational studies, recognizing that observational study designs may have greater risk of some biases. However, it is important to note that causal inference methods have made tremendous strides to allow observational studies to better approximate randomized designs through novel analytic methods and designs which take advantage of “natural experiments.” Such novel methods of analysis or study designs would be a benefit to the care continuum research community, and appear to be under-utilized. For instance, propensity score matching, a method to make groups exposed and unexposed to an intervention as comparable as possible in observational studies, was used by only 4 cohort studies out of 31 included in our review. Other methods that take advantage of natural experiments or changes over time, such as instrumental variables, regression discontinuity, and interrupted time series could also help in providing valid causal inference regarding the effect of studied interventions. Given some of the resource and practical challenges of incorporating randomized trial designs into implementation research on care continuum interventions, investigators may wish to consider incorporation of alternative novel study designs and analytic methods in the future.
Given our findings, we recommend several steps be taken in order to improve the evidence base for strengthening HIV care engagement in the US. First, researchers, health departments and funders must come to consensus on definitions for linkage, retention and reengagement in care, and ART adherence for the purposes of scientific investigation. Second, we would propose that irrespective of the step in the care continuum being targeted, that a central outcome of interest (in addition to the proximal impact of an intervention) is the incremental number or percentage of individuals achieving viral suppression (over a defined period of time, such as a year). Since directly studying viral suppression may not be feasible in a study of ‘upstream’ interventions (such as linkage interventions), we propose that a tool be developed to aid researchers to translate upstream interventions to viral suppression outcomes. Such a tool or calculator would incorporate locally specific care continuum data to allow translation of incremental impact from upstream interventions into an incremental change in viral suppression; while such an approach would require several assumptions and have limitations, it would allow a common framework to measure care continuum interventions. By utilizing a consensus downstream effect measure, one begins to be able to compare absolute levels of benefit of interventions at varying stages in the care continuum. Third, studies of novel care continuum interventions need carefully constructed study designs. We found that over 1/3 of studies identified for full text review in this study (see Appendix for PRISMA diagram) were excluded because of a lack of a defined comparator group (i.e. one armed studies) or lack of quantifiable outcomes. Studies should clearly define the intervention, population under study, and distinguish the intervention group from a comparator group unexposed to the intervention, while clearly defining the comparator (for instance, “standard of care” needs to be explicitly defined). While qualitative information about interventions is vital to understanding aspects of acceptability, usability, and scalability, quantifiable outcomes and standard measures of effect size should be established. Fourth, the vast majority of interventions (95%) addressed only a single step of the care continuum, which fails to capture that care is lifelong and involves ongoing engagement and movement between continuum “steps.” Therefore, we recommend that where possible, integration of different steps of the care continuum be assessed. In conjunction with this suggestion, we acknowledge that within a given step of the care cascade, multiple intervention modalities could be implemented; thus far, a minority of studies implemented multi-modal interventions. It is unclear whether combination interventions will result in independent effects, and as such testing combination interventions should be prioritized. Moreover, while many of the absolute effect sizes for interventions were small, these effects could offer meaningful impact when scaled-up at a population level; future studies may consider incorporation of modeling approaches that allows one to extrapolate the potential impact of HIV care continuum strengthening at a population level. Fifth, we recommend that observational designs increasingly use appropriate causal inference methods and study designs where possible to allow greater confidence in the results of these studies. Finally, as the care continuum is dynamic over time, we propose that reporting of longitudinal measures of care continuum outcomes be prioritized. As an example, reporting the incremental change in the rate of loss from care per unit time (as a metric for evaluating interventions to improve retention in care) would allow a more comprehensive understanding of intervention efficacy; currently most reported outcomes in the studies identified in this review were limited by their cross-sectional nature and short time period for follow up.
Our review has several important limitations. First, in order to offer a comprehensive picture of all research studying interventions to strengthen the HIV continuum of care, we present studies of highly varying quality (for instance RCTs vs pre-post design, and with substantial variability within each of those design types) without an attempt to exclude low-quality studies. Other reviews (with different purposes) restrict themselves exclusively to interventions with a high-quality evidence base and the CDC Prevention Research Synthesis Team has compiled this data to promote best practices, despite limited data [7, 9, 13]. Second, since our purpose was to characterize the breadth of HIV care continuum interventions, we also were not able to evaluate any specific set of interventions (e.g. those related to adherence) in substantial depth. Other systematic reviews [7–10] have aimed to fill this gap. Third, due to the substantial variability in outcomes, study design, and target populations, we were unable to conduct a meta-analysis. The heterogeneity that we characterize, however, can help motivate HIV researchers to unify their methodology to enable future research to be more easily summarized. Additionally, we had no limit for the year of implementation of the study (only for year of publication) so some findings may be less relevant for the current care context.
In conclusion, this systematic review of over 150 interventions to strengthen the continuum of HIV care in the US highlights a small number of effective interventions but more importantly reveals tremendous heterogeneity in methodology and outcome assessment. Furthermore, there is a paucity of data on linkage and reengagement, less evidence on retention than adherence, and few studies targeting populations experiencing the highest incidence of HIV in the US (MSM, particularly black and Latino MSM). If we are to develop a coordinated strategy to achieve the ambitious NHAS targets, increased attention must be paid to filling the biggest gaps in the current continuum of care. Researchers must also report outcomes in a standardized fashion—with focus on the ultimate outcome of viral suppression—that will enable combination and prioritization. The current piecemeal approach to HIV continuum research must be improved; by looking at the HIV continuum of care from an integrated, top-down perspective, a more comprehensive strategy can be created to end the HIV epidemic in the US.
Supplementary Material
Acknowledgments
This work was supported by Cooperative Agreement Number 5U38PS004646 by the National Center for HIV, Viral Hepatitis, STD and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention, as part of the NCHHSTP Epidemiologic and Economic Modeling Cooperative Agreement. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. The authors would like to thank the Applied Public Health Advisory Group, a collaborative set of state and local public health professionals convened for this project, for their guidance and expertise. We would like to specifically thank Dr. Greg Felzein, Thomas Betrand, Nanette Benbow, Dr. Jane Kelly, Dr. David Holtgrave and Dr. Jim Curran for reviewing this paper and providing feedback.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10461-017-1687-8) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors declare no conflict of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.The Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. 2015 https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf. Accessed 1 Feb 2016.
- 2.Sidibé M, Zuniga JM. Leveraging HIV treatment to end AIDS, stop new HIV infections, and avoid the cost of inaction. Clin Infect Dis. 2014;59(Suppl 1):S3–6. doi: 10.1093/cid/ciu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah M, Perry A, Risher K, et al. Effect of the US National HIV/AIDS Strategy targets for improved HIV care engagement: a modelling study. Lancet HIV. 2016;3(3):e140–6. doi: 10.1016/S2352-3018(16)00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah M, Risher K, Berry SA, Dowdy DW. The epidemiologic and economic impact of improving HIV testing, linkage, and retention in care in the United States. Clin Infect Dis. 2016;62(2):220–9. doi: 10.1093/cid/civ801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frieden TR, Foti KE, Mermin J. Applying public health principles to the HIV epidemic—how are we doing? N Engl J Med. 2015;373(23):2281–7. doi: 10.1056/NEJMms1513641. [DOI] [PubMed] [Google Scholar]
- 6.Bradley H, Hall HI, Wolitski RJ, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV– United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Charania MR, Marshall KJ, Lyles CM, et al. Identification of evidence-based interventions for promoting HIV medication adherence: findings from a systematic review of U.S.-based studies, 1996–2011. AIDS Behav. 2014;18(4):646–60. doi: 10.1007/s10461-013-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crepaz N, Baack BN, Higa DH, Mullins MM. Effects of integrated interventions on transmission risk and care continuum outcomes in persons living with HIV: meta-analysis, 1996–2014. AIDS. 2015;29(18):2371–83. doi: 10.1097/QAD.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higa DH, Crepaz N, Mullins MM, Prevention Research Synthesis Project Identifying best practices for increasing linkage to, retention, and re-engagement in HIV medical care: findings from a systematic review, 1996-2014. AIDS Behav. 2016;20(5):951–66. doi: 10.1007/s10461-015-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higa DH, Marks G, Crepaz N, Liau A, Lyles CM. Interventions to improve retention in HIV primary care: a systematic review of U.S. studies. Curr HIV/AIDS Rep. 2012;9(4):313–25. doi: 10.1007/s11904-012-0136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liau A, Crepaz N, Lyles CM, et al. Interventions to promote linkage to and utilization of HIV medical care among HIV-diagnosed persons: a qualitative systematic review, 1996-2011. AIDS Behav. 2013;17(6):1941–62. doi: 10.1007/s10461-013-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathes T, Pieper D, Antoine SL, Eikermann M. Cost-effectiveness of adherence interventions for highly active antiretro-viral therapy: a systematic review. Int J Technol Assess Health Care. 2013;29(3):227–33. doi: 10.1017/S0266462313000317. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Prevention Resarch Synthesis. 2015 http://www.cdc.gov/hiv/dhap/prb/prs/. Accessed 20 May 2016.
- 14.Higgins JPT, Altman DG, Stern JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org/. Accessed 15 Aug 2015. [Google Scholar]
- 15.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute (OHRI); 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 15 Aug 2015. [Google Scholar]
- 16.Gardner LI, Giordano TP, Marks G, et al. Enhanced personal contact with HIV patients improves retention in primary care: a randomized trial in 6 US HIV clinics. Clin Infect Dis. 2014;59(5):725–34. doi: 10.1093/cid/ciu357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konkle-Parker DJ, Amico KR, McKinney VE. Effects of an intervention addressing information, motivation, and behavioral skills on HIV care adherence in a southern clinic cohort. AIDS Care. 2014;26(6):674–83. doi: 10.1080/09540121.2013.845283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konkle-Parker DJ, Erlen JA, Dubbert PM, May W. Pilot testing of an HIV medication adherence intervention in a public clinic in the Deep South. J Am Acad Nurse Pract. 2012;24(8):488–98. doi: 10.1111/j.1745-7599.2012.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacGowan RJ, Lifshay J, Mizuno Y, Johnson WD, McCormick L, Zack B. Positive Transitions (POST): evaluation of an HIV prevention intervention for HIV-positive persons releasing from correctional facilities. AIDS Behav. 2015;19(6):1061–9. doi: 10.1007/s10461-014-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas GM, Chaudhry A, Hsu J, et al. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: a randomized trial. Ann Intern Med. 2010;152(11):704–11. doi: 10.1059/0003-4819-152-11-201006010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins GK, Lester W, Johnson KL, et al. Efficacy of a clinical decision-support system in an HIV practice: a randomized trial. Ann Intern Med. 2012;157(11):757–66. doi: 10.7326/0003-4819-157-11-201212040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton BL, Person AK, Castillo C, Pastrana C, Subramanian M, Stout JE. Barriers to using text message appointment reminders in an HIV clinic. Telemed J E-Health. 2014;20(1):86–9. doi: 10.1089/tmj.2012.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proeschold-Bell RJ, Belden CM, Parnell H, Cohen S, Cromwell M, Lombard F. A randomized controlled trial of health information exchange between human immunodeficiency virus institutions. J Public Health Manag Pract. 2010;16(6):521–8. doi: 10.1097/PHH.0b013e3181df78b9. [DOI] [PubMed] [Google Scholar]
- 24.Bove J, Golden MR, Dhanireddy S, Harrington RD, Dombrowski JC. Outcomes of a clinic-based, surveillance-informed intervention to relink patients to HIV care. J Acquir Immune Defic Syndr. 2015;70(3):262–8. doi: 10.1097/QAI.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flash CA, Pasalar S, Hemmige V, et al. Benefits of a routine opt-out HIV testing and linkage to care program for previously diagnosed patients in publicly funded emergency departments in Houston, TX. J Acquir Immune Defic Syndr. 2015;69(Suppl 1):S8–15. doi: 10.1097/QAI.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford JB, Coleman S, Cunningham W. HIV System Navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(Suppl 1):S49–58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 27.Gross R, Bellamy SL, Chapman J, et al. Managed problem solving for antiretroviral therapy adherence: a randomized trial. JAMA Intern Med. 2013;173(4):300–6. doi: 10.1001/jamainternmed.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross R, Tierney C, Andrade A, et al. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009;169(13):1224–32. doi: 10.1001/archinternmed.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hersch RK, Cook RF, Billings DW, et al. Test of a web-based program to improve adherence to HIV medications. AIDS Behav. 2013;17(9):2963–76. doi: 10.1007/s10461-013-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holstad MM, DiIorio C, Kelley ME, Resnicow K, Sharma S. Group motivational interviewing to promote adherence to antiretroviral medications and risk reduction behaviors in HIV infected women. AIDS Behav. 2011;15(5):885–96. doi: 10.1007/s10461-010-9865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horberg MA, Hurley LB, Silverberg MJ, Kinsman CJ, Quesenberry CP. Effect of clinical pharmacists on utilization of and clinical response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(5):531–9. doi: 10.1097/QAI.0b013e318031d7cd. [DOI] [PubMed] [Google Scholar]
- 32.Ma A, Chen DM, Chau FM, Saberi P. Improving adherence and clinical outcomes through an HIV pharmacist’s interventions. AIDS Care. 2010;22(10):1189–94. doi: 10.1080/09540121003668102. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell CG, Freels S, Creticos CM, Oltean A, Douglas R. Preliminary findings of an intervention integrating modified directly observed therapy and risk reduction counseling. AIDS Care. 2007;19(4):561–4. doi: 10.1080/09540120601040813. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen JL, Haug NA, Larios S, et al. Directly administered antiretroviral therapy: pilot study of a structural intervention in methadone maintenance. J Subst Abuse Treat. 2012;43(4):418–23. doi: 10.1016/j.jsat.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–96. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 36.Magnus M, Jones K, Phillips G, 2nd, et al. Characteristics associated with retention among African American and Latino adolescent HIV-positive men: results from the outreach, care, and prevention to engage HIV-seropositive young MSM of color special project of national significance initiative. J Acquir Immune Defic Syndr. 2010;53(4):529–36. doi: 10.1097/QAI.0b013e3181b56404. [DOI] [PubMed] [Google Scholar]
- 37.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr. 2012;60(1):77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 38.Keller SC, Yehia BR, Eberhart MG, Brady KA. Accuracy of definitions for linkage to care in persons living with HIV. J Acquir Immune Defic Syndr. 2013;63(5):622–30. doi: 10.1097/QAI.0b013e3182968e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colasanti J, Kelly J, Pennisi E, et al. Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clin Infect Dis. 2016;62(5):648–54. doi: 10.1093/cid/civ941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bocour A, Renaud TC, Udeagu CC, Shepard CW. HIV partner services are associated with timely linkage to HIV medical care. AIDS. 2013;27(18):2961–3. doi: 10.1097/QAD.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 41.Willis S, Castel AD, Ahmed T, Olejemeh C, Frison L, Kharfen M. Linkage, engagement, and viral suppression rates among HIV-infected persons receiving care at medical case management programs in Washington, DC. J Acquir Immune Defic Syndr. 2013;64(Suppl 1):S33–41. doi: 10.1097/QAI.0b013e3182a99b67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keller S, Jones J, Erbelding E. Choice of rapid HIV testing and entrance into care in Baltimore City sexually transmitted infections clinics. AIDS Patient Care STDS. 2011;25(4):237–43. doi: 10.1089/apc.2010.0298. [DOI] [PubMed] [Google Scholar]
- 43.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 44.Mugavero MJ. Improving engagement in HIV care: what can we do? Top HIV Med. 2008;16(5):156–61. [PubMed] [Google Scholar]
- 45.Castel AD, Greenberg AE, Befus M, et al. Temporal association between expanded HIV testing and improvements in population-based HIV/AIDS clinical outcomes, District of Columbia. AIDS Care. 2014;26(6):785–9. doi: 10.1080/09540121.2013.855296. [DOI] [PubMed] [Google Scholar]
- 46.Onyeajam DJ, Eke R, Stephens TG, Duffus WA. Time to linkage to care and viro-immunologic parameters of individuals diagnosed before and after the 2006 HIV testing recommendations. South Med J. 2013;106(4):257–66. doi: 10.1097/SMJ.0b013e31828d967c. [DOI] [PubMed] [Google Scholar]
- 47.Shrestha RK, Gardner L, Marks G, et al. Estimating the cost of increasing retention in care for HIV-infected patients: results of the CDC/HRSA retention in care trial. J Acquir Immune Defic Syndr. 2015;68(3):345–50. doi: 10.1097/QAI.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gwadz M, Cleland CM, Applegate E, et al. Behavioral intervention improves treatment outcomes among HIV-infected individuals who have delayed, declined, or discontinued antiretroviral therapy: a randomized controlled trial of a novel intervention. AIDS Behav. 2015;19(10):1801–17. doi: 10.1007/s10461-015-1054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naar-King S, Outlaw A, Green-Jones M, Wright K, Parsons JT. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–73. doi: 10.1080/09540120802612824. [DOI] [PubMed] [Google Scholar]
- 50.Purcell DW, Latka MH, Metsch LR, et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46(Suppl 2):S35–47. doi: 10.1097/QAI.0b013e31815767c4. [DOI] [PubMed] [Google Scholar]
- 51.Wohl DA, Scheyett A, Golin CE, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15(2):356–64. doi: 10.1007/s10461-010-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolitski RJ, Kidder DP, Pals SL, et al. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493–503. doi: 10.1007/s10461-009-9643-x. [DOI] [PubMed] [Google Scholar]
- 53.Altice FL, Bruce RD, Lucas GM, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S22–32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bogart LM, Wagner GJ, Mutchler MG, et al. Community HIV treatment advocacy programs may support treatment adherence. AIDS Educ Prev. 2012;24(1):1–14. doi: 10.1521/aeap.2012.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cabral HJ, Tobias C, Rajabiun S, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care STDS. 2007;21(Suppl 1):S59–67. doi: 10.1089/apc.2007.9986. [DOI] [PubMed] [Google Scholar]
- 56.Cunningham CO, Sohler NL, Cooperman NA, Berg KM, Litwin AH, Arnsten JH. Strategies to improve access to and utilization of health care services and adherence to antiretroviral therapy among HIV-infected drug users. Subst Use Misuse. 2011;46(2–3):218–32. doi: 10.3109/10826084.2011.522840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davila JA, Miertschin N, Sansgiry S, Schwarzwald H, Henley C, Giordano TP. Centralization of HIV services in HIV-positive African-American and Hispanic youth improves retention in care. AIDS Care. 2013;25(2):202–6. doi: 10.1080/09540121.2012.689811. [DOI] [PubMed] [Google Scholar]
- 58.Hanna DB, Buchacz K, Gebo KA, et al. Association between U.S. state AIDS Drug Assistance Program (ADAP) features and HIV antiretroviral therapy initiation, 2001–2009. PLoS ONE. 2013;8(11):e78952. doi: 10.1371/journal.pone.0078952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Himelhoch S, Brown CH, Walkup J, et al. HIV patients with psychiatric disorders are less likely to discontinue HAART. AIDS. 2009;23(13):1735–42. doi: 10.1097/QAD.0b013e32832b428f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schranz AJ, Brady KA, Momplaisir F, Metlay JP, Stephens A, Yehia BR. Comparison of HIV outcomes for patients linked at hospital versus community-based clinics. AIDS Patient Care STDS. 2015;29(3):117–25. doi: 10.1089/apc.2014.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terzian AS, Irvine MK, Hollod LM, Lim S, Rojas J, Shepard CW. Effect of HIV housing services on engagement in care and treatment, New York City, 2011. AIDS Behav. 2015;19(11):2087–96. doi: 10.1007/s10461-015-1003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersen M, Hockman E, Smereck G, et al. Retaining women in HIV medical care. J Assoc Nurses AIDS Care. 2007;18(3):33–41. doi: 10.1016/j.jana.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 63.Enriquez M, Farnan R, Cheng AL, et al. Impact of a bilingual/bicultural care team on HIV-related health outcomes. J Assoc Nurses AIDS Care. 2008;19(4):295–301. doi: 10.1016/j.jana.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Henry SR, Goetz MB, Asch SM. The effect of automated telephone appointment reminders on HIV primary care no-shows by veterans. J Assoc Nurses AIDS Care. 2012;23(5):409–18. doi: 10.1016/j.jana.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping them in “STYLE”: finding, linking, and retaining young HIV-positive black and Latino men who have sex with men in care. AIDS Patient Care STDS. 2011;25(1):37–45. doi: 10.1089/apc.2010.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irvine MK, Chamberlin SA, Robbins RS, et al. Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clin Infect Dis. 2015;60(2):298–310. doi: 10.1093/cid/ciu783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saifu HN, Asch SM, Goetz MB, et al. Evaluation of human immunodeficiency virus and hepatitis C telemedicine clinics. Am J Manag Care. 2012;18(4):207–12. [PubMed] [Google Scholar]
- 68.Shade SB, Steward WT, Koester KA, Chakravarty D, Myers JJ. Health information technology interventions enhance care completion, engagement in HIV care and treatment, and viral suppression among HIV-infected patients in publicly funded settings. J Am Med Inf Assoc. 2015;22(e1):e104–11. doi: 10.1136/amiajnl-2013-002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gardner LI, Marks G, Craw JA, et al. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clin Infect Dis. 2012;55(8):1124–34. doi: 10.1093/cid/cis623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardner LI, Marks G, Wilson TE, et al. Clinic-wide intervention lowers financial risk and improves revenue to HIV clinics through fewer missed primary care visits. J Acquir Immune Defic Syndr. 2015;68(4):472–6. doi: 10.1097/QAI.0000000000000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magnus M, Herwehe J, Gruber D, et al. Improved HIV-related outcomes associated with implementation of a novel public health information exchange. Int J Med Inform. 2012;81(10):e30–8. doi: 10.1016/j.ijmedinf.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Kapadia F, Vlahov D, Wu Y, et al. Impact of drug abuse treatment modalities on adherence to ART/HAART among a cohort of HIV seropositive women. Am J Drug Alcohol Abuse. 2008;34(2):161–70. doi: 10.1080/00952990701877052. [DOI] [PubMed] [Google Scholar]
- 73.Sacks S, McKendrick K, Vazan P, Sacks JY, Cleland CM. Modified therapeutic community aftercare for clients triply diagnosed with HIV/AIDS and co-occurring mental and substance use disorders. AIDS Care. 2011;23(12):1676–86. doi: 10.1080/09540121.2011.582075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS ONE. 2012;7(5):e38335. doi: 10.1371/journal.pone.0038335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J Urban Health. 2010;87(4):592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnett PG, Sorensen JL, Wong W, Haug NA, Hall SM. Effect of incentives for medication adherence on health care use and costs in methadone patients with HIV. Drug Alcohol Depend. 2009;100(1–2):115–21. doi: 10.1016/j.drugalcdep.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farber S, Tate J, Frank C, et al. A study of financial incentives to reduce plasma HIV RNA among patients in care. AIDS Behav. 2013;17(7):2293–300. doi: 10.1007/s10461-013-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore BA, Rosen MI, Wang Y, et al. A remotely-delivered CBT and contingency management therapy for substance using people with HIV. AIDS Behav. 2015;19(Suppl 2):156–62. doi: 10.1007/s10461-014-0990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petry NM, Weinstock J, Alessi SM, Lewis MW, Dieckhaus K. Group-based randomized trial of contingencies for health and abstinence in HIV patients. J Consult Clin Psychol. 2010;78(1):89–97. doi: 10.1037/a0016778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosen MI, Dieckhaus K, McMahon TJ, et al. Improved adherence with contingency management. AIDS Patient Care STDS. 2007;21(1):30–40. doi: 10.1089/apc.2006.0028. [DOI] [PubMed] [Google Scholar]
- 81.Sorensen JL, Haug NA, Delucchi KL, et al. Voucher reinforcement improves medication adherence in HIV-positive methadone patients: a randomized trial. Drug Alcohol Depend. 2007;88(1):54–63. doi: 10.1016/j.drugalcdep.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feaster DJ, Brincks AM, Mitrani VB, Prado G, Schwartz SJ, Szapocznik J. The efficacy of Structural Ecosystems Therapy for HIV medication adherence with African American women. J Fam Psychol. 2010;24(1):51–9. doi: 10.1037/a0017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feaster DJ, Mitrani VB, Burns MJ, et al. A randomized controlled trial of Structural Ecosystems Therapy for HIV medication adherence and substance abuse relapse prevention. Drug Alcohol Depend. 2010;111(3):227–34. doi: 10.1016/j.drugalcdep.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hawk M, Davis D. The effects of a harm reduction housing program on the viral loads of homeless individuals living with HIV/AIDS. AIDS Care. 2012;24(5):577–82. doi: 10.1080/09540121.2011.630352. [DOI] [PubMed] [Google Scholar]
- 85.Johnston SS, Juday T, Seekins D, Espindle D, Chu BC. Association between prescription cost sharing and adherence to initial combination antiretroviral therapy in commercially insured antiretroviral-naive patients with HIV. J Manag Care Pharm. 2012;18(2):129–45. doi: 10.18553/jmcp.2012.18.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reznick OG, McCartney K, Gregorich SE, Zack B, Feaster DJ. An ecosystem-based intervention to reduce HIV transmission risk and increase medication adherence among inmates being released to the community. J Correct Health Care. 2013;19(3):178–93. doi: 10.1177/1078345813486442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DuChane J, Clark B, Hou J, Fitzner K, Pietrandoni G, Duncan I. Impact of HIV-specialized pharmacies on adherence to medications for comorbid conditions. J Am Pharm Assoc (2003) 2014;54(5):493–501. doi: 10.1331/JAPhA.2014.13165. [DOI] [PubMed] [Google Scholar]
- 88.Henderson KC, Hindman J, Johnson SC, Valuck RJ, Kiser JJ. Assessing the effectiveness of pharmacy-based adherence interventions on antiretroviral adherence in persons with HIV. AIDS Patient Care STDS. 2011;25(4):221–8. doi: 10.1089/apc.2010.0324. [DOI] [PubMed] [Google Scholar]
- 89.Hirsch JD, Rosenquist A, Best BM, Miller TA, Gilmer TP. Evaluation of the first year of a pilot program in community pharmacy: HIV/AIDS medication therapy management for Medi-Cal beneficiaries. J Manag Care Pharm. 2009;15(1):32–41. doi: 10.18553/jmcp.2009.15.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirsch JD, Gonzales M, Rosenquist A, Miller TA, Gilmer TP, Best BM. Antiretroviral therapy adherence, medication use, and health care costs during 3 years of a community pharmacy medication therapy management program for Medi-Cal beneficiaries with HIV/AIDS. J Manag Care Pharm. 2011;17(3):213–23. doi: 10.18553/jmcp.2011.17.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.March K, Mak M, Louie SG. Effects of pharmacists’ interventions on patient outcomes in an HIV primary care clinic. Am J Health Syst Pharm. 2007;64(24):2574–8. doi: 10.2146/ajhp070048. [DOI] [PubMed] [Google Scholar]
- 92.Murphy P, Cocohoba J, Tang A, Pietrandoni G, Hou J, Guglielmo BJ. Impact of HIV-specialized pharmacies on adherence and persistence with antiretroviral therapy. AIDS Patient Care STDS. 2012;26(9):526–31. doi: 10.1089/apc.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khan M, Song X, Williams K, Bright K, Sill A, Rakhmanina N. Evaluating adherence to medication in children and adolescents with HIV. Arch Dis Child. 2009;94(12):970–3. doi: 10.1136/adc.2008.156232. [DOI] [PubMed] [Google Scholar]
- 94.Preininger L, Cantwell-McNelis K, James C, Sullivan MC, Szabo S, Bincsik A. Long-term medication adherence in patients receiving antiretroviral drug therapy. Curr HIV Res. 2011;9(4):253–5. doi: 10.2174/157016211796320351. [DOI] [PubMed] [Google Scholar]
- 95.Belzer ME, Kolmodin MacDonell K, Clark LF, et al. Acceptability and feasibility of a cell phone support intervention for youth living with HIV with nonadherence to antiretroviral therapy. AIDS Patient Care STDS. 2015;29(6):338–45. doi: 10.1089/apc.2014.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lewis MA, Uhrig JD, Bann CM, et al. Tailored text messaging intervention for HIV adherence: a proof-of-concept study. Health Psychol. 2013;32(3):248–53. doi: 10.1037/a0028109. [DOI] [PubMed] [Google Scholar]
- 97.Belzer ME, Naar-King S, Olson J, et al. The use of cell phone support for non-adherent HIV-infected youth and young adults: an initial randomized and controlled intervention trial. AIDS Behav. 2014;18(4):686–96. doi: 10.1007/s10461-013-0661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dowshen N, Kuhns LM, Johnson A, Holoyda BJ, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: a pilot study using personalized, interactive, daily text message reminders. J Med Internet Res. 2012;14(2):e51. doi: 10.2196/jmir.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hardy H, Kumar V, Doros G, et al. Randomized controlled trial of a personalized cellular phone reminder system to enhance adherence to antiretroviral therapy. AIDS Patient Care STDS. 2011;25(3):153–61. doi: 10.1089/apc.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moore DJ, Poquette A, Casaletto KB, et al. Individualized texting for adherence building (iTAB): improving antiretroviral dose timing among HIV-infected persons with co-occurring bipolar disorder. AIDS Behav. 2015;19(3):459–71. doi: 10.1007/s10461-014-0971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pellowski JA, Kalichman SC, White D, Amaral CM, Hoyt G, Kalichman MO. Real-time medication adherence monitoring intervention: test of concept in people living with HIV infection. J Assoc Nurses AIDS Care. 2014;25(6):646–51. doi: 10.1016/j.jana.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Simoni JM, Huh D, Frick PA, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52(4):465–73. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dieckhaus KD, Odesina V. Outcomes of a multifaceted medication adherence intervention for HIV-positive patients. AIDS Patient Care STDS. 2007;21(2):81–91. doi: 10.1089/apc.2006.0044. [DOI] [PubMed] [Google Scholar]
- 104.Nahvi S, Litwin AH, Heo M, Berg KM, Li X, Arnsten JH. Directly observed antiretroviral therapy eliminates adverse effects of active drug use on adherence. Drug Alcohol Depend. 2012;120(1–3):174–80. doi: 10.1016/j.drugalcdep.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petersen ML, Wang Y, van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45(7):908–15. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Page TF, Horvath KJ, Danilenko GP, Williams M. A cost analysis of an Internet-based medication adherence intervention for people living with HIV. J Acquir Immune Defic Syndr. 2012;60(1):1–4. doi: 10.1097/QAI.0b013e318250f011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blank MB, Hanrahan NP, Fishbein M, et al. A randomized trial of a nursing intervention for HIV disease management among persons with serious mental illness. Psychiatr Serv. 2011;62(11):1318–24. doi: 10.1176/ps.62.11.pss6211_1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Enriquez M, Cheng AL, Banderas J, et al. A peer-led HIV medication adherence intervention targeting adults linked to medical care but without a suppressed viral load. J Int Assoc Provid AIDS Care. 2014;14(5):441–8. doi: 10.1177/2325957414558301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horvath KJ, Oakes JM, Rosser BR, et al. Feasibility, acceptability and preliminary efficacy of an online peer-to-peer social support ART adherence intervention. AIDS Behav. 2013;17(6):2031–44. doi: 10.1007/s10461-013-0469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kenya S, Jones J, Arheart K, et al. Using community health workers to improve clinical outcomes among people living with HIV: a randomized controlled trial. AIDS Behav. 2013;17(9):2927–34. doi: 10.1007/s10461-013-0440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koenig LJ, Pals SL, Bush T, Pratt Palmore M, Stratford D, Ellerbrock TV. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27(2):159–69. doi: 10.1037/0278-6133.27.2.159. [DOI] [PubMed] [Google Scholar]
- 112.Simoni JM, Pantalone DW, Plummer MD, Huang B. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007;26(4):488–95. doi: 10.1037/0278-6133.26.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45(6):770–8. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berg KM, Litwin AH, Li X, Heo M, Arnsten JH. Lack of sustained improvement in adherence or viral load following a directly observed antiretroviral therapy intervention. Clin Infect Dis. 2011;53(9):936–43. doi: 10.1093/cid/cir537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: a randomized controlled trial. Drug Alcohol Depend. 2011;113(2–3):192–9. doi: 10.1016/j.drugalcdep.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaur AH, Belzer M, Britto P, et al. Directly observed therapy (DOT) for nonadherent HIV-infected youth: lessons learned, challenges ahead. AIDS Res Hum Retroviruses. 2010;26(9):947–53. doi: 10.1089/aid.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glikman D, Walsh L, Valkenburg J, Mangat PD, Marcinak JF. Hospital-based directly observed therapy for HIV-infected children and adolescents to assess adherence to antiretroviral medications. Pediatrics. 2007;119(5):e1142–8. doi: 10.1542/peds.2006-2614. [DOI] [PubMed] [Google Scholar]
- 118.Lucas GM, Mullen BA, Galai N, et al. Directly administered antiretroviral therapy for HIV-infected individuals in opioid treatment programs: results from a randomized clinical trial. PLoS ONE. 2013;8(7):e68286. doi: 10.1371/journal.pone.0068286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ma M, Brown BR, Coleman M, Kibler JL, Loewenthal H, Mitty JA. The feasibility of modified directly observed therapy for HIV-seropositive African American substance users. AIDS Patient Care STDS. 2008;22(2):139–46. doi: 10.1089/apc.2007.0063. [DOI] [PubMed] [Google Scholar]
- 120.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21(11):1473–7. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 121.Maru DS, Bruce RD, Walton M, Springer SA, Altice FL. Persistence of virological benefits following directly administered antiretroviral therapy among drug users: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;50(2):176–81. doi: 10.1097/QAI.0b013e3181938e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mitty JA, Huang D, Loewenthal HG, MacLeod C, Thompson L, Bazerman LB. Modified directly observed therapy: sustained self-reported adherence and HIV health status. AIDS Patient Care STDS. 2007;21(12):897–9. doi: 10.1089/apc.2007.0066. [DOI] [PubMed] [Google Scholar]
- 123.White BL, Golin CE, Grodensky CA, et al. Effect of directly observed antiretroviral therapy compared to self-administered antiretroviral therapy on adherence and virological outcomes among HIV-infected prisoners: a randomized controlled pilot study. AIDS Behav. 2015;19(1):128–36. doi: 10.1007/s10461-014-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giordano TP, Rodriguez S, Zhang H, et al. Effect of a clinic-wide social marketing campaign to improve adherence to antiretroviral therapy for HIV infection. AIDS Behav. 2013;17(1):104–12. doi: 10.1007/s10461-012-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Horberg MA, Hurley LB, Towner WJ, et al. Determination of optimized multidisciplinary care team for maximal antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2012;60(2):183–90. doi: 10.1097/QAI.0b013e31824bd605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keith McInnes D, Shimada SL, Rao SR, et al. Personal health record use and its association with antiretroviral adherence: survey and medical record data from 1871 US veterans infected with HIV. AIDS Behav. 2013;17(9):3091–100. doi: 10.1007/s10461-012-0399-3. [DOI] [PubMed] [Google Scholar]
- 127.Saberi P, Catz SL, Leyden WA, et al. Antiretroviral therapy adherence and use of an electronic shared medical record among people living with HIV. AIDS Behav. 2015;19(Suppl 2):177–85. doi: 10.1007/s10461-014-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wilson IB, Laws MB, Safren SA, et al. Provider-focused intervention increases adherence-related dialogue but does not improve antiretroviral therapy adherence in persons with HIV. J Acquir Immune Defic Syndr. 2010;53(3):338–47. doi: 10.1097/QAI.0b013e3181c7a245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rasu RS, Malewski DF, Banderas JW, Malomo Thomson D, Goggin K. Cost of behavioral interventions utilizing electronic drug monitoring for antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2013;63(1):e1–8. doi: 10.1097/QAI.0b013e318285d951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ingersoll KS, Farrell-Carnahan L, Cohen-Filipic J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV? crack cocaine users. Drug Alcohol Depend. 2011;116(1–3):177–87. doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007;46(5):574–80. doi: 10.1097/qai.0b013e318158a474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Johnson MO, Dilworth SE, Taylor JM, Neilands TB. Improving coping skills for self-management of treatment side effects can reduce antiretroviral medication nonadherence among people living with HIV. Ann Behav Med. 2011;41(1):83–91. doi: 10.1007/s12160-010-9230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones DL, McPherson-Baker S, Lydston D, et al. Efficacy of a group medication adherence intervention among HIV positive women: the SMART/EST Women’s Project. AIDS Behav. 2007;11(1):79–86. doi: 10.1007/s10461-006-9165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kalichman SC, Cherry C, Kalichman MO, et al. Integrated behavioral intervention to improve HIV/AIDS treatment adherence and reduce HIV transmission. Am J Public Health. 2011;101(3):531–8. doi: 10.2105/AJPH.2010.197608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kalichman SC, Kalichman MO, Cherry C, et al. Brief behavioral self-regulation counseling for HIV treatment adherence delivered by cell phone: an initial test of concept trial. AIDS Patient Care STDS. 2011;25(5):303–10. doi: 10.1089/apc.2010.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kalichman SC, Cherry C, Kalichman MO, et al. Randomized clinical trial of HIV treatment adherence counseling interventions for people living with HIV and limited health literacy. J Acquir Immune Defic Syndr. 2013;63(1):42–50. doi: 10.1097/QAI.0b013e318286ce49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Letourneau EJ, Ellis DA, Naar-King S, Chapman JE, Cunningham PB, Fowler S. Multisystemic therapy for poorly adherent youth with HIV: results from a pilot randomized controlled trial. AIDS Care. 2013;25(4):507–14. doi: 10.1080/09540121.2012.715134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Murphy DA, Marelich WD, Rappaport NB, Hoffman D, Farthing C. Results of an antiretroviral adherence intervention: STAR (Staying Healthy: Taking Antiretrovirals Regularly) J Int Assoc Physicians AIDS Care (Chic) 2007;6(2):113–24. doi: 10.1177/1545109707301243. [DOI] [PubMed] [Google Scholar]
- 139.Naar-King S, Parsons JT, Murphy DA, Chen X, Harris DR, Belzer ME. Improving health outcomes for youth living with the human immunodeficiency virus: a multisite randomized trial of a motivational intervention targeting multiple risk behaviors. Arch Pediatr Adolesc Med. 2009;163(12):1092–8. doi: 10.1001/archpediatrics.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parsons JT, Golub SA, Rosof E, Holder C. Motivational interviewing and cognitive-behavioral intervention to improve HIV medication adherence among hazardous drinkers: a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46(4):443–50. doi: 10.1097/qai.0b013e318158a461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reich WA. Medication adherence feedback intervention predicts improved human immunodeficiency virus clinical markers. Int J Nurs Pract. 2013;19(6):577–83. doi: 10.1111/ijn.12100. [DOI] [PubMed] [Google Scholar]
- 142.Reynolds NR, Testa MA, Su M, et al. Telephone support to improve antiretroviral medication adherence: a multisite, randomized controlled trial. J Acquir Immune Defic Syndr. 2008;47(1):62–8. doi: 10.1097/QAI.0b013e3181582d54. [DOI] [PubMed] [Google Scholar]
- 143.Robbins GK, Testa MA, Su M, et al. Site nurse-initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretroviral therapy, ACTG 5031: substudy of ACTG 384. HIV Clin Trials. 2013;14(5):235–53. doi: 10.1310/hct1405-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roth AM, Holmes AM, Stump TE, et al. Can lay health workers promote better medical self-management by persons living with HIV? An evaluation of the Positive Choices program. Patient Educ Couns. 2012;89(1):184–90. doi: 10.1016/j.pec.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 145.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: a randomized controlled trial. J Consult Clin Psychol. 2012;80(3):404–15. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Skrajner MJ, Camp CJ, Haberman JL, Heckman TG, Kochman A, Frentiu C. Use of videophone technology to address medication adherence issues in persons with HIV. HIV AIDS (Auckl) 2009;1:23–30. doi: 10.2147/HIV.S6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tetrault JM, Moore BA, Barry DT, et al. Brief versus extended counseling along with buprenorphine/naloxone for HIV-infected opioid dependent patients. J Subst Abuse Treat. 2012;43(4):433–9. doi: 10.1016/j.jsat.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 149.Wagner GJ, Lovely P, Schneider S. Pilot controlled trial of the adherence readiness program: an intervention to assess and sustain HIV antiretroviral adherence readiness. AIDS Behav. 2013;17(9):3059–65. doi: 10.1007/s10461-013-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Webel AR. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22(9):1029–40. doi: 10.1080/09540120903214389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cooperman NA, Heo M, Berg KM, et al. Impact of adherence counseling dose on antiretroviral adherence and HIV viral load among HIV-infected methadone maintained drug users. AIDS Care. 2012;24(7):828–35. doi: 10.1080/09540121.2011.644231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.DiIorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS care. 2008;20(3):273–83. doi: 10.1080/09540120701593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goggin K, Gerkovich MM, Williams KB, et al. A randomized controlled trial examining the efficacy of motivational counseling with observed therapy for antiretroviral therapy adherence. AIDS Behav. 2013;17(6):1992–2001. doi: 10.1007/s10461-013-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weiss SM, Tobin JN, Antoni M, et al. Enhancing the health of women living with HIV: the SMART/EST Women’s Project. Int J Womens Health. 2011;3:63–77. doi: 10.2147/IJWH.S5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bova C, Burwick TN, Quinones M. Improving women’s adjustment to HIV infection: results of the Positive Life Skills Workshop Project. J Assoc Nurses AIDS Care. 2008;19(1):58–65. doi: 10.1016/j.jana.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 156.Enriquez M, Cheng AL, McKinsey DS, Stanford J. Development and efficacy of an intervention to enhance readiness for adherence among adults who had previously failed HIV treatment. AIDS Patient Care STDS. 2009;23(3):177–84. doi: 10.1089/apc.2008.0170. [DOI] [PubMed] [Google Scholar]
- 157.Ownby RL, Waldrop-Valverde D, Jacobs RJ, Acevedo A, Caballero J. Cost effectiveness of a computer-delivered intervention to improve HIV medication adherence. BMC Med Inform Decis Mak. 2013;13:29. doi: 10.1186/1472-6947-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brock TP, Smith SR. Using digital videos displayed on personal digital assistants (PDAs) to enhance patient education in clinical settings. Int J Med Inform. 2007;76(11–12):829–35. doi: 10.1016/j.ijmedinf.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 159.Claborn KR, Leffingwell TR, Miller MB, Meier E, Stephens JR. Pilot study examining the efficacy of an electronic intervention to promote HIV medication adherence. AIDS Care. 2014;26(3):404–9. doi: 10.1080/09540121.2013.824534. [DOI] [PubMed] [Google Scholar]
- 160.Fisher JD, Amico KR, Fisher WA, et al. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows Project. AIDS Behav. 2011;15(8):1635–46. doi: 10.1007/s10461-011-9926-x. [DOI] [PubMed] [Google Scholar]
- 161.Garvie PA, Lensing S, Rai SN. Efficacy of a pill-swallowing training intervention to improve antiretroviral medication adherence in pediatric patients with HIV/AIDS. Pediatrics. 2007;119(4):e893–9. doi: 10.1542/peds.2006-1488. [DOI] [PubMed] [Google Scholar]
- 162.Holstad MM, Ofotokun I, Higgins M, Logwood S. The LIVE Network: a music-based messaging program to promote ART adherence self-management. AIDS Behav. 2013;17(9):2954–62. doi: 10.1007/s10461-013-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kurth AE, Spielberg F, Cleland CM, et al. Computerized counseling reduces HIV-1 viral load and sexual transmission risk: findings from a randomized controlled trial. J Acquir Immune Defic Syndr. 2014;65(5):611–20. doi: 10.1097/QAI.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lopez E, Jones DL, Ishii M, Tobin JN, Weiss SM. HIV medication adherence and substance use: the Smartest Women’s Project. Am J Infect Dis. 2007;3(4):240–7. doi: 10.3844/ajidsp.2007.240.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Naar-King S, Outlaw AY, Sarr M, et al. Motivational Enhancement System for Adherence (MESA): pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment. J Pediatr Psychol. 2013;38(6):638–48. doi: 10.1093/jpepsy/jss132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ownby RL, Waldrop-Valverde D, Caballero J, Jacobs RJ. Baseline medication adherence and response to an electronically delivered health literacy intervention targeting adherence. Neurobehav HIV Med. 2012;4:113–21. doi: 10.2147/NBHIV.S36549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):384–90. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 168.Pyne JM, Fortney JC, Curran GM, et al. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch Intern Med. 2011;171(1):23–31. doi: 10.1001/archinternmed.2010.395. [DOI] [PubMed] [Google Scholar]
- 169.Tsai AC, Karasic DH, Hammer GP, et al. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV-positive adults: a randomized controlled trial. Am J Public Health. 2013;103(2):308–15. doi: 10.2105/AJPH.2011.300422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Teixeira PA, Jordan AO, Zaller N, Shah D, Venters H. Health outcomes for HIV-infected persons released from the New York City jail system with a transitional care-coordination plan. Am J Public Health. 2015;105(2):351–7. doi: 10.2105/AJPH.2014.302234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kalichman SC, Amaral C, Swetsze C, et al. Monthly unannounced pill counts for monitoring HIV treatment adherence: tests for self-monitoring and reactivity effects. HIV Clin Trials. 2010;11(6):325–31. doi: 10.1310/hct1106-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Westling E, Garcia K, Mann T. Discovery of meaning and adherence to medications in HIV-infected women. J Health Psychol. 2007;12(4):627–35. doi: 10.1177/1359105307078169. [DOI] [PubMed] [Google Scholar]
- 173.Centers for Disease Control and Prevention. HIV Surveillance Report, 2012. 2014 http://www.cdc.gov/hiv/library/reports/surveillance/. Accessed 1 Feb 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


