Abstract
Invasive fungal infections caused by drug-resistant organisms are an emerging threat to heavily immunosuppressed patients with hematological malignancies. Modern early antifungal treatment strategies, such as prophylaxis and empirical and preemptive therapy, result in long-term exposure to antifungal agents, which is a major driving force for the development of resistance. The extended use of central venous catheters, the nonlinear pharmacokinetics of certain antifungal agents, neutropenia, other forms of intense immunosuppression, and drug toxicities are other contributing factors. The widespread use of agricultural and industrial fungicides with similar chemical structures and mechanisms of action has resulted in the development of environmental reservoirs for some drug-resistant fungi, especially azole-resistant Aspergillus species, which have been reported from four continents. The majority of resistant strains have the mutation TR34/L98H, a finding suggesting that the source of resistance is the environment. The global emergence of new fungal pathogens with inherent resistance, such as Candida auris, is a new public health threat. The most common mechanism of antifungal drug resistance is the induction of efflux pumps, which decrease intracellular drug concentrations. Overexpression, depletion, and alteration of the drug target are other mechanisms of resistance. Mutations in the ERG11 gene alter the protein structure of C-demethylase, reducing the efficacy of antifungal triazoles. Candida species become echinocandin-resistant by mutations in FKS genes. A shift in the epidemiology of Candida towards resistant non-albicans Candida spp. has emerged among patients with hematological malignancies. There is no definite association between antifungal resistance, as defined by elevated minimum inhibitory concentrations, and clinical outcomes in this population. Detection of genes or mutations conferring resistance with the use of molecular methods may offer better predictive values in certain cases. Treatment options for resistant fungal infections are limited and new drugs with novel mechanisms of actions are needed. Prevention of resistance through antifungal stewardship programs is of paramount importance.
Keywords: Invasive fungal infections, Antifungal resistance, Hematological malignancies, New antifungal agents
Abstract
İlaca dirençli organizmaların neden olduğu invaziv mantar enfeksiyonları, ağır immün baskılanma altındaki hematolojik kanserli hastalar için bir tehdittir. Profilaktik, ampirik ve önleyici tedaviler gibi güncel anti-fungal tedavi yaklaşımları, direnç gelişiminde büyük bir itici güç olan anti-fungal ajanlara uzun süreli maruz kalma ile sonuçlanmaktadır. Santral venöz kateterlerin uzun süreli kullanımı, bazı anti-fungal ajanların doğrusal olmayan farmakokinetiği, nötropeni, yoğun immün baskılamanın farklı formları ve ilaç toksisitesi direnç gelişimine katkıda bulunan diğer faktörlerdir. Benzer kimyasal yapılara ve etki mekanizmalarına sahip, tarımsal ve endüstriyel fungisitlerin yaygın kullanımı, dört kıtadan bildirilen bazı ilaca dirençli mantarlar, özellikle azole dayanıklı Aspergillus türleri için çevresel kaynakların gelişmesine neden olmaktadır. Dirençli suşların çoğunda bulunan TR34 / L98H mutasyonu, direncin çevresel kaynaklı olduğunu düşündürmektedir. Candida auris gibi doğal dirençli yeni fungal patojenlerin ortaya çıkması, yeni bir halk sağlığı tehdididir. Anti-fungal ilaç direncinin en yaygın mekanizması, hücre içi ilaç konsantrasyonlarını azaltan hücre dışına atım pompalarının uyarılmasıdır. Diğer direnç mekanizmaları arasında ilaç hedefinin aşırı ekspresyonu, tükenmesi ya da değişmesi bulunmaktadır. ERG11 genindeki mutasyonlar, antif-fungal triazollerin etkinliğini azaltarak C-demetilazın protein yapısını değiştirir. Candida türleri, FKS genlerindeki mutasyonlarla ekinokandine dirençli hale gelir. Candida epidemiyolojisinde dirençli albicans-dışı Candida spp. hematolojik kanseri olan hastalarda ön plana çıkmaktadır. Bu popülasyondaki hasta grubunda artmış minimum inhibitör konsantrasyonlarla tanımlanan anti-fungal direnç ile klinik sonuçlar arasında kesin bir ilişki yoktur. Moleküler yöntemlerin kullanımı ile dirence neden olan genlerin veya mutasyonların saptanması, bazı olgularda daha iyi klinik ön görü sağlayabilir. Dirençli fungal enfeksiyonlara yönelik tedavi seçenekleri sınırlıdır ve yeni mekanizmalara sahip ilaçlara ihtiyaç duyulmaktadır. Anti-fungal idame programlarıyla direncin önlenmesi büyük önem taşır.
Introduction
Invasive fungal infections (IFIs) are associated with increased morbidity and unacceptably high mortality among patients with hematological malignancies (HMs) [1,2]. However, treatment options are limited, including only four chemical classes: polyenes, triazoles, echinocandins, and flucytosine. The expansion of the use of antifungal agents over the last two decades not unexpectedly contributed to the development of antifungal resistance [3,4,5]. Another factor driving the emergence of resistance is the widespread use of agricultural and industrial fungicides with chemical structures and mechanisms of action similar to those of human antifungal agents, resulting in the development of environmental reservoirs for some drug-resistant fungi, especially triazole-resistant Aspergillus species [6,7]. Recently, researchers showed that even the household environment may serve as a potential source of triazole-resistant invasive aspergillosis [8].
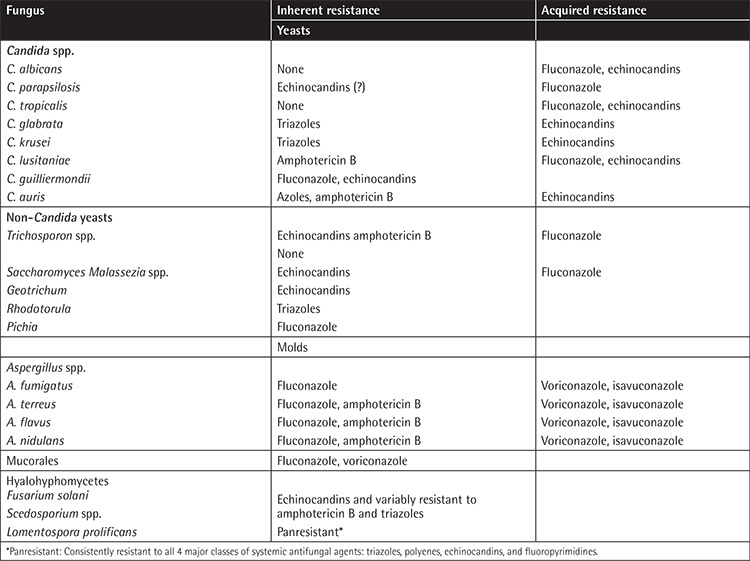
Antifungal resistance can be either intrinsic or acquired (Table 1) [9,10,11]. Intrinsic drug resistance can occur naturally among certain fungi without previous exposure to antifungal agents, such as fluconazole-resistant Candida krusei [9,12]. The emergence of new fungal species with intrinsic resistance to some or all antifungal agents is a new threat. The recent outbreaks of multidrug-resistant Candida auris [13] in many hematology centers around the world and the increasing reports of infections caused by panresistant Lomentospora prolificans [14,15] are characteristic examples.
Table 1. Inherited and acquired resistance reported among pathogenic fungi infecting patients with hematological malignancies.
Acquired or iatrogenic antifungal resistance is favored by specific risk factors in patients with HMs. Modern early treatment strategies, such as prophylaxis and empirical and preemptive therapy, result in long-term exposure to antifungal agents, which is a major driving force for the development of resistance [5]. Repeated cycles of chemotherapy and/or hematopoietic stem cell transplantation (HSCT) prolong even more the exposure to antifungal agents. Chemotherapy-induced neutropenia limits the pharmacodynamic response to antifungal agents and dictates prolonged therapeutic courses. Indwelling catheters, especially central venous catheters (CVCs), are a major factor for the development of resistance, as their surfaces are often infected by pathogenic fungi and the ensuing biofilm formation does not allow drug penetration, thus rendering the infection refractory to treatment [16,17,18,19]. Nonlinear pharmacokinetics of certain antifungal agents, especially certain triazoles, may result in suboptimal antifungal drug levels, favoring the development of resistance [20,21]. Intraabdominal fungal infections in patients with HMs, such as intraabdominal abscesses, can promote drug resistance because antifungal drug delivery in the abdomen is poor and fungi are exposed to possibly subtherapeutic drug concentrations [22].
The emergence of antifungal drug resistance has tremendous clinical implications, as it further restricts the already limited antifungal armamentarium, raising concerns among clinicians that we are close to the “post-antifungal” era, in parallel to the “post-antibiotic” era [4,10]. The outlook is similarly grim, as there is a paucity of new antifungal agents with novel mechanisms of action in development [23].
The focus of this review will be the emergence of fungal infections with innate or acquired resistance to antifungal agents among patients with HMs. We will visit the many different facets of this complex area, including mechanisms of resistance, epidemiology, clinical implications, and current treatment options. Finally, we will review new antifungal agents in development and the priorities for future research in the field.
Antifungal-Resistant Invasive Aspergillosis
Mechanisms of Resistance
Triazole-Resistant Aspergillus spp.: Triazoles with activity against Aspergillus spp. (i.e. itraconazole, voriconazole, posaconazole, and isavuconazole) are recommended for the treatment of invasive aspergillosis among patients with HMs. Antifungal triazoles act by inhibiting the cytochrome P450 enzyme sterol 14a-demethylase, which converts lanosterol to ergosterol, and is encoded by the gene CYP51 in filamentous fungi. Inhibition of 14a-demethylase by an azole results in the interruption of biosynthesis of ergosterol, which is fungicidal for molds, as it leads to intracellular accumulation of toxic 14a-methyl sterols and to alterations in cell membrane structure, impairing its permeability and stability and thus the viability of the fungus. Mutations in the CYP51A fungal gene alter the structure of the 14a-demethylase, leading to reduced azole binding and thus generating triazole-resistant phenotypes [24,25]. The two most common alterations in CYP51A offering resistance to triazoles are tandem repeats in the promoter region of the gene along with gene mutations and point mutations [5]. There are also other non-CYP51 mechanisms associated with azole resistance [24].
The most frequently identified mechanism of triazole resistance in Aspergillus fumigatus involves a 34-bp tandem repeat (TR34) in the promoter region of the CYP51A gene combined with a substitution of leucine 98 to histidine (TR34/L98H). These alterations cause overexpression of the gene [25,26]. Another mechanism of resistance involves a 46-bp tandem repeat in the CYP51A promoter region combined with two substitutions: tyrosine 121 for phenylalanine and threonine 289 for alanine (TR46/Y121F/T289A) [27]. This modification of the CYP51A gene makes Aspergillus fumigatus resistant to voriconazole [28]. Finally, a 53-bp tandem repeat in the promoter region of the CYP51A gene without any other substitution conferring azole resistance has been detected in environmental [29] and clinical triazole-resistant Aspergillus fumigatus strains [30].
Another mechanism of triazole resistance for Aspergillus spp.is nonsynonymous hot-spot mutations in the CYP51A gene. Numerous amino acid substitutions associated with reduced susceptibility for triazoles have been reported [24,31,32,33,34,35,36,37,38,39,40]. Recently, many azole-resistant Aspergillus isolates were found not to have point mutations in CYP51A or promoter duplications, suggesting that alternative mechanisms for azole resistance exist [40,41]. Researchers reported that 43% of 64 azole-resistant Aspergillus isolates did not carry a CYP51A mutation, indicating that other mechanisms must be responsible [42]. Potential mechanisms conferring resistance include activation of efflux pumps [43]; overexpression of transporter genes [44]; loss of the algA gene [45]; the point mutation P88L in HapE, an important transcription factor [46]; biofilm formation [43,47]; and cholesterol import by Aspergillus fumigatus to overcomeergosterol deprivation [48].
Cryptic Aspergillus spp. may be resistant to voriconazole. For example, Aspergillus calidoustus typically has elevated minimum inhibitory concentrations (MICs) for voriconazole that exceed CLSI and EUCAST interpretive breakpoints. Aspergillus lentulus, which may phenotypically resemble a slowly growing Aspergillus fumigatus, may also have elevated MICs for voriconazole [24].
Polyene-Resistant Aspergillus spp.:Polyeneantifungal agents bind to ergosterol on the cell membrane of the fungus and cause formation of intramembrane channels that kill the cell. Amphotericin B is a first-line treatment for invasive aspergillosis in patients with HMs. Although it has been used since 1957, emergence of resistance is usually not an issue and typically involves selection of inherently resistant strains. Development of acquired resistance during therapy is rare [5]. The most common amphotericin B-resistant species include Aspergillus terreus, Aspergillus flavus, Aspergillus nidulans, Aspergillus calidoustus, and Aspergillus lentulus [49,50,51]. The main mechanism of resistance is believed to be the modification of the cell membrane, by diminishing its ergosterol content [51].
Researchers have found that previous treatment with triazoles also may reduce the amount of membrane ergosterol in Candida spp. resistant to amphotericin B [52]. Reduction of membrane ergosterol renders Cryptococcus neoformans less susceptible to amphotericin B [53]. Whether this mechanism also confers polyene resistance to Aspergillus spp. is uncertain.
Epidemiology
Triazole-resistant Aspergillus fumigatus has been described in the Netherlands since 1999, with an estimated prevalence of 6.0%-12.8% of patients with invasive aspergillosis [6]. In 2007, infections caused by triazole-resistant Aspergillus fumigatus were reported in hematology patients from six different hospitals in the Netherlands [25]. One year later, another Dutch hospital noted that 28.1% of 32 patients with invasive aspergillosis had an azole-resistant isolate of Aspergillus fumigatus [54]. The predominant mechanism of resistance of clinical isolates from patients in different hospitals was TR34/L98H, a finding suggesting that the source of resistance was the environment [54,55]. Subsequent studies from the Netherlands [55] and the United Kingdom [56] showed that, from 1994 to 2009, the incidence of triazole-resistant aspergillosis rapidly increased to 20%. Recently, a prospective study on the prevalence and the mechanisms of azole-resistance was conducted among 22 centers in 19 European countries [25]. Triazole-resistant Aspergillus fumigatus isolates have been reported in 11 countries, although the prevalence ranged widely, from 0% to 26%, among the participating centers and even among centers from the same country. The overall triazole resistance prevalence was 3.2% [25]. To date, triazole-resistant clinical isolates of Aspergillus fumigatus have been reported in the majority of European counties [24], as well as Turkey [56].
Most reports of triazole-resistant Aspergillus spp. have originated from Europe, but recently researchers from four continents reported increasing numbers of infections caused by resistant Aspergillus strains [34,57,58,59,60,61], suggesting that azole resistance is a global threat.
Clinical Significance
Data on the clinical significance of triazole resistance are limited and contradictory. In vitro studies have shown that the presence of triazole resistance mechanisms is associated with reduced susceptibility of Aspergillus fumigatus to all azoles [62], including the recently licensed isavuconazole [63,64,65]. Several studies have shown that triazole resistance is associated with treatment failure, especially among patients with HMs [24,28,29,36,39]. In a study from India, invasive aspergillosis caused by a resistant isolate was associated with a significantly higher mortality rate (88%) compared with that of aspergillosis caused by wild-type isolates (30%-50%) [66]. On the contrary, in a retrospective study from the United States, higher azole MICs were not correlated with outcome of aspergillosis in patients with HMs or HSCT recipients [67]. Clearly, more data are needed to delineate the clinical significance of triazole resistance in Aspergillus spp.
Treatment
Due to the low worldwide prevalence of azole-resistant aspergillosis, there are no clinical studies on its treatment. In 2015, an expert panel published an opinion paper on how to treat azole-resistant aspergillosis [68]. They suggested that in areas with high (>10%) environmental resistance, first-line therapy should be liposomal amphotericin B or a combination of voriconazole and an echinocandin. These suggestions require meticulous surveillance studies to define areas of high resistance; such studies are not always feasible.
Antifungal-Resistant Invasive Candidiasis
Mechanisms of Resistance
Triazole-Resistant Candida spp.: Antifungal azoles act by inhibiting the enzyme sterol 14a-demethylase, resulting in the interruption of biosynthesis of ergosterol, which is an essential Candida cell membrane component. The inhibition of ergosterol synthesis may be fungicidal for molds, but only fungistatic for yeasts. Several mechanisms confer azole resistance to Candida spp. [18]. The most common mechanism is the induction of efflux pumps, which decrease the intracellular drug concentration. Efflux pumps are encoded by various genes belonging to the ATP-binding cassette superfamily or to the major facilitator superfamily [69]. The transcription of these genes is regulated by transcription factors, such as Tac1 and Mrr1 for Candida albicans and CgPdr1 for Candida glabrata [69]. Overexpression or alteration of the drug target, 14a-demethylase, is another mechanism of resistance. Numerous point mutations in the ERG11 gene, usually after exposure to fluconazole, can generate structural changes in the active site of the demethylase, causing reduced target affinity and thus triazole resistance [70]. Overexpression of ERG11 [71] and loss of function of the sterol Δ5,6-desaturase gene (ERG3) [72] also confer azole resistance. Loss of function of the sterol Δ5,6-desaturase gene in Candida glabrata may also result in resistance to amphotericin B. These mechanisms can occur either alone or concurrently in a single isolate and may lead to cross-resistance to many azoles.
Echinocandin-Resistant Candida spp.: The mechanism of action of the echinocandins is inhibition of (1,3)-b-D-glucan synthesis [73]. Beta-D-glucans are cross-linked to chitin and mannoproteins, providing structural integrity to cell walls of various fungi. Echinocandins are fungicidal for Candida spp., as b-D-glucan accounts for approximately 30%-60% of the cell wall mass in Candida species [73]. Conversely, among filamentous fungi, echinocandins have only fungistatic effects, as the cell wall contains less glucan, concentrated at the apical tips and branching points of hyphae.
Echinocandins exert their antifungal activity by binding to the enzyme FKS, which catalyzes the synthesis of (1,3)-b-D-glucans. Glucan synthase has two catalytic subunits, FKS1 and FKS2, encoded by their respective FKS genes. Candida species become echinocandin-resistant by genetic acquisition of mutations in FKS genes, which encode amino acid substitutions in two narrow hot-spot regions of FKS1 for all Candida species and FKS2 for C. glabrata [74]. The most common (>90%) FKS1 substitutions among echinocandin-resistant Candida albicans isolates occur at Ser-641 or Ser-645 [74]. In Candida glabrata, the most common amino acid substitutions occur in FKS2 [75].
Resistance to two or more classes of antifungal agents further augments the threat of Candida glabrata in patients with HMs. Candida glabrata bloodstream isolates from patients with HMs developed cross-resistance to both triazoles and echinocandins [76]. While the molecular events leading to triazole and echinocandin resistance may occur independently, one potential unifying mechanism is the development of DNA mismatch-repair gene mutations, which lead to “hypermutable” clinical strains [12].
Polyene-Resistant Candida spp.: Candida species with acquired resistance to polyenes are uncommon, although researchers have reported cases of Candida albicans, Candida krusei, Candida glabrata, Candida tropicalis, Candida rugosa, Candida lusitaniae, and Candida guilliermondii with high MICs to amphotericin B [5,18]. The main mechanism of resistance involves a reduction in cell membrane ergosterol, which is the biological target of amphotericin B. Reduction of ergosterol can be caused by previous treatment with triazoles, which lowers membrane sterol concentrations, or mutations affecting sterol biosynthesis, such as defects in ERG1, ERG2, ERG3, ERG4, ERG6, and ERG11 [18,77].
Biofilm Formation and Candida Resistance: Biofilm formation on artificial devices, especially CVCs, is an essential factor driving the development of drug-resistant Candida spp. in patients with HMs. Antifungal drugs do not achieve therapeutic levels within the biofilm because they are trapped in a glucan-rich matrix polymer. The hypoxic environment within biofilms results in a metabolic stress response that leads to increased MICs to triazoles. Moreover, once the Candida strain is embedded in the biofilm, it may not need to be resistant in order to grow despite adequate antifungal treatment and may cause breakthrough candidemia [19].
Epidemiology
Antifungal drug resistance has emerged through the development of acquired resistance and an epidemiological shift in the distribution of Candida species towards inherently less susceptible non-albicans species [16]. In large-scale surveillance studies of bloodstream isolates, the overall prevalence of Candida albicans resistance is less than 1% [78]. Resistance rates are higher among non-albicans Candida species, notably Candida glabrata, reaching 2%-4% in most epidemiological prevalence studies [79]. A trend towards increasing rates of Candida glabrata resistance has been noted, as the proportion of nonsusceptible isolates increased from 4.2% in 2008 to 7.8% in 2014 [80], while some institutions reported resistance rates close to 10% [75]. In hematology patients, a rise in Candida glabrata with echinocandin and azole resistance and cross-resistance to two or more antifungal classes (multidrug resistance) has been reported, mainly in the United States, but not in Europe [81]. In a European study of candidemia among hematology patients, in vitro resistance to at least one antifungal agent was observed for 27% of Candida isolates [17].
The problem of antifungal-resistant yeast infections has been aggravated by recent epidemiological changes. A shift in the distribution of candidemia-associated Candida species towards more resistant non-albicans species, such as Candida parapsilosis, Candida tropicalis, Candida glabrata, and Candida krusei, has been reported among patients with HMs in both the United States and Europe [16,17]. In addition, the recent emergence of Candida auris, an uncommon species that exhibits both multidrug resistance and strong potential for nosocomial transmission, raises concerns worldwide [82]. Cases and hospital outbreaks of Candida auris invasive infections have been reported from four continents, mainly among patients with HMs, with high mortality [82,83].
Clinical Significance
There are no clinical studies showing a definite association between in vitro susceptibility testing and outcomes of invasive candidiasis in neutropenic patients [4,18,19], with the exception of Candida glabrata, where clinical studies demonstrated that infection with an echinocandin-resistant strain was associated with worse outcomes [9,75]. Clinical failure was associated with the presence of the FKS mutation and not MIC values [9]. Finally, the recent epidemiological shift of Candida species distribution towards non-albicans species in patients with HMs [16] has an impact on outcomes as many non-albicans species, especially Candida glabrata and Candida krusei, exhibit higher resistance rates and higher mortality [16,17].
Treatment
There are no clinical studies on the optimal initial treatment of patients with or at risk for antifungal-resistant invasive Candida infections. Current guidelines for treatment of candidiasis recommend lipid formulation of amphotericin B (3-5 mg/kg daily) for patients with suspected azole- and echinocandin-resistant Candida infections [84]. This recommendation is characterized as “strong” but is based on “low-quality evidence”. Regarding the emerging problem of multidrug-resistant Candida glabrata infection, there are no good clinical data on the optimal treatment. The best strategy for the initial treatment of suspected or documented resistant Candida infection is to be tailored according to individual risk factors and the local epidemiology [18].
Antifungal Resistance in Fungal Infections Caused by Rare Molds and Non-Candida, Non-Cryptococcus Yeasts
The frequency of invasive fungal disease caused by resistant filamentous fungi other than Aspergillus is increasing. The majority of these rare molds are Mucorales, hyalohyphomycetes (Fusarium spp., Scedosporium spp.), and dematiaceous fungi and they occur mainly in heavily immunosuppressed patients with HMs [85]. The TRANSNET study reported that among 983 IFIs identified in 875 HSCT recipients, 8% were mucormycosis and 14% of infections were caused by other filamentous fungi [86]. The intrinsic resistance of many of these rare fungi to antifungal agents is of concern. Mucorales species are resistant to some triazoles, while multidrug resistance has been reported for Fusarium spp., Scedosporium spp., and dematiaceous fungi.
Although Candida infections comprise the vast majority of yeasts growing in blood cultures, clinicians should be aware that a substantial proportion of fungemia cases are caused by non-Candida, non-Cryptococcus yeasts [87], such as Trichosporon asahii, Magnusiomyces (Blastoschizomyces) capitatus, Saccharomyces cerevisiae, Malassezia spp., Saprochaete (Geotrichum)spp., and Rhodotorula spp. The majority of these rare yeasts are intrinsically resistant to one or more classes of antifungal agents, and infections occur frequently as breakthrough infections in hematology patients receiving antifungals and with a CVC in place [87,88]. For instance, Trichosporon spp. are resistant to echinocandins and to the fungicidal activity of polyenes, while Rhodotorula spp. are resistant to the triazoles [18]. In vitro susceptibility testing is not always useful in patients with infections caused by less frequent opportunistic yeast or mold infections. In these patients, breakpoints are not based on data derived from clinical responses or outcomes but only from epidemiological cut-off values and pharmacokinetic and pharmacodynamic data from animal models [89].
Diagnostic Tests for the Detection of Fungal Resistance
Isolation of the infecting fungus through conventional culture of biological fluids and tissues, identification to the species level, and in vitro testing to determine the susceptibility to antifungal agents is the current standard for the diagnosis of IFIs caused by resistant fungi and for decision making [90]. Species identification is time-consuming, prompting physicians to initiate empirical treatment until the results become available. Newer methods, including MALDI-TOF mass spectroscopy and T2 magnetic resonance assay, allow rapid species identification with excellent sensitivity and specificity [90,91]. Antifungal susceptibility testing is recommended for the triazoles against all bloodstream Candida isolates and for the echinocandins against resistant species, such as Candida glabrata and Candida parapsilosis isolates [84]. As mentioned previously, clinical breakpoints are only available for certain species of fungi and are not useful for the diagnosis of resistance, as they do not always correlate with clinical outcomes, especially in patients with HMs [18,19,90]. Thus, a low MIC value does not necessarily predict successful treatment and an elevated MIC does not automatically predict treatment failure.
Currently, only polymerase chain reaction (PCR) has the potential for early detection of resistance [92]. Even PCR, though, has its drawbacks, such as low sensitivity for detection of resistance markers and difficulty in differentiating colonization from invasive infection or a living from a dead organism [93]. Therefore, clinicians should be cautious as to how to interpret these non-culture-based diagnostic tests in everyday clinical practice and for decision making. New molecular detection methods, including HRMA/PCR, microarrays, and metagenomic shotgun sequencing, are under development and hold promise for the future [92].
New Antifungal Agents for Resistant Fungi
IFIs caused by drug-resistant organisms are an emerging threat to heavily immunosuppressed patients with HMs. Therefore, there is an urgent need for new antifungals with activity against resistant fungi. It should be underlined, though, that fungi are eukaryotes, just like human cells; thus, discovering new antifungal agents not interfering with human cells is challenging.
Recent developments in fungal functional genomics, proteomics, and gene mapping allowed the discovery of potential new drug targets that could offer additional options to treat resistant fungal infections [94]. Cellular and biochemical targets of investigational agents against drug-resistant fungal pathogens include metabolic pathways (such as the glyoxylate cycle, iron metabolism, and heme biosynthesis), cell wall and cell membrane components, signal transduction pathways (such as MAP kinase), and gene expression. However, there is a paucity of novel antifungal compounds in preclinical or clinical development, as the majority of these new antifungal agents are in the very early stages of development.
SCY-078 is the first orally bioavailable inhibitor of (1,3)-b-D-glucan synthesis of the fungal cell wall. A triterpene derivative, SCY-078 has demonstrated in vitro and in vivo activity against all tested Candida spp., including Candida auris, as well as triazole-resistant and echinocandin-resistant Candida spp. [94]. Its spectrum includes Aspergillus spp., where it may be particularly effective in combination with anti-mold triazoles. E1210 is a novel isoxazolyl-bis-pyridine wall-active antifungal compound that inhibits inositol acylation of mannosylated cell wall proteins, resulting in arrest of fungal growth [94]. The antifungal spectrum includes most yeast with the exception of Candida krusei and molds, including isolates resistant to triazoles and polyenes. Biafungin (CD101) is a novel, long-acting, semisynthetic echinocandin derivative of anidulafungin that is currently in phase III clinical studies. In vitro susceptibility testing showed that biafungin has activity against caspofungin-resistant Candida strains containing FKS mutations [95]. Other antifungal agents under development include F901318 (dihydroorotate dehydrogenase inhibitor), VT-1598 (metalloenzyme inhibitors of CYP51), and ASP2397 (hydroxamate siderophores-like agent) [94].
Future Research Directions in Fungal Resistance
Invasive infections caused by resistant fungi are emerging global problems of public health, associated with increased morbidity and mortality, particularly among patients with HMs. There are unanswered questions and unmet needs in all areas of knowledge of fungal resistance, including epidemiology, diagnostics, therapeutics, prevention, and education, that require expertise from many different disciplines to be addressed [96].
The emerging epidemiological data raise intriguing questions: why is the prevalence of azole resistance in Aspergillus so variable? The frequency of resistance may vary considerably, not only between continents and countries but also between hospitals within the same country, between departments, or between risk groups within the same hospital [97,98,99]. Is this under- or overreporting, suboptimal sampling, and/or technical issues in Aspergillus fumigatus isolation and resistance detection? Alternatively, are there any geoclimatic factors that create ecological niches favoring the spread of resistance? Obviously, general surveillance studies are not sufficient to capture the problem. In the future, meticulous well-funded epidemiological studies targeted to specific high-risk groups, especially patients with HMs, are necessary.
Development and implementation of laboratory diagnostic tools should be a priority for future research in the field of resistant fungal infections, as current technology does not allow rapid species identification and assessment of resistance. Development of interpretive breakpoints for fungal infections in neutropenic patients with HMs is an unmet need. New molecular technologies for the prompt and accurate detection of genes and mutations associated with fungal resistance are urgently needed.
The existing antifungal agents are not sufficient to confront the growing trend of resistance. The limited antifungal armamentarium should be enriched with agents with novel mechanisms of action to overcome resistance. A fascinating direction for future research is the development of new antifungal agents that do not kill or inhibit the growth of fungi but impair key virulence properties, such as invasion or adherence.
Prevention of fungal resistance should be at the core of future research. Antifungal stewardship programs should ensure that there is an indication for antifungal therapy, that the appropriate antifungal agent is selected, and that the dosage, route of administration, and duration are optimal and that de-escalation is implemented when feasible. A robust antifungal stewardship program might have beneficial effects on the prevention of resistance. Understanding the pathophysiology of biofilm formation and reducing the use of CVCs might also prevent the development of catheter-related resistant fungal infections.
Footnotes
Authorship Contributions
Concept: M.N.G.; Design: M.N.G., T.J.W., N.V.S.; Data Collection or Processing: M.N.G.; Analysis or Interpretation: M.N.G., T.J.W., N.V.S.; Literature Search: M.N.G.; Writing: M.N.G., T.J.W., N.V.S.
Conflict of Interest: MNG reports no conflict of interest for this specific work; TJW reports receiving research grants for experimental and clinical antifungal pharmacotherapeutics from Astellas, Novartis, Merck, and Pfizer; he has served as a consultant to Astellas, Drais, iCo, Novartis, Pfizer, Methylgene, and Sigma-Tau. NVS reports receiving consulting fees, grant support, lecture fees, and honoraria from Astellas Greece, Gilead Greece, MSD Greece, and Pfizer Greece.
Financial Disclosure: This work was supported by the Special Account for Research Grants (ELKE) of the National and Kapodistrian University of Athens (grant number 70/3/11724) and by a grant from the Save Our Sick Kids Foundation (http://soskidsfoundation.org).
References
- 1.Sipsas NV, Bodey GP, Kontoyiannis DP. Perspectives for the management of febrile neutropenic patients with cancer in the 21st century. Cancer. 2005;103:1103–1113. doi: 10.1002/cncr.20890. [DOI] [PubMed] [Google Scholar]
- 2.Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265–273. doi: 10.1086/595846. [DOI] [PubMed] [Google Scholar]
- 3.Kontoyiannis DP, Lewis RE. Antifungal drug resistance of pathogenic fungi. Lancet. 2002;359:1135–1144. doi: 10.1016/S0140-6736(02)08162-X. [DOI] [PubMed] [Google Scholar]
- 4.Kontoyiannis DP. Antifungal resistance: an emerging reality and a global challenge. J Infect Dis. 2017;216(Suppl 3):431–435. doi: 10.1093/infdis/jix179. [DOI] [PubMed] [Google Scholar]
- 5.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17:383–392. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 6.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. 2009;9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 7.Snelders E, Melchers WJ, Verweij PE. Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol. 2011;6:335–347. doi: 10.2217/fmb.11.4. [DOI] [PubMed] [Google Scholar]
- 8.Lavergne RA, Chouaki T, Hagen F, Toublanc B, Dupont H, Jounieaux V, Meis JF, Morio F, Le Pape P. Home environment as a source of life-threatening azole-resistant Aspergillus fumigatus in immunocompromised patients. Clin Infect Dis. 2017;64:76–78. doi: 10.1093/cid/ciw664. [DOI] [PubMed] [Google Scholar]
- 9.Shields RK, Nguyen MH, Press EG, Kwa AL, Cheng S, Du C, Clancy CJ. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother. 2012;56:4862–4869. doi: 10.1128/AAC.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. 2016;62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowdhary A, Sharma C, Meis JF. Azole-resistant aspergillosis: epidemiology, molecular mechanisms and treatment. J Infect Dis. 2017;216(Suppl 3):436–444. doi: 10.1093/infdis/jix210. [DOI] [PubMed] [Google Scholar]
- 12.Healey KR, Zhao Y, Perez WB, Lockhart SR, Sobel JD, Farmakiotis D, Kontoyiannis DP, Sanglard D, Taj-Aldeen SJ, Alexander BD, Jimenez-Ortigosa C, Shor E, Perlin DS. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat Commun. 2016;7:11128. doi: 10.1038/ncomms11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamaris GA, Chamilos G, Lewis RE, Safdar A, Raad II, Kontoyiannis DP. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989-2006. Clin Infect Dis. 2006;43:1580–1584. doi: 10.1086/509579. [DOI] [PubMed] [Google Scholar]
- 15.Park BJ, Pappas PG, Wannemuehler KA, Alexander BD, Anaissie EJ, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt L, Ito JI, Kauffman CA, Lyon GM, Marr KA, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wingard JR, Walsh TJ, Kontoyiannis DP. Invasive non-Aspergillus mold infections in transplant recipients, United States, 2001-2006. Emerg Infect Dis. 2011;17:1855–1864. doi: 10.3201/eid1710.110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sipsas NV, Lewis RE, Tarrand J, Hachem R, Rolston KV, Raad II, Kontoyiannis DP. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001-2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer. 2009;115:4745–4752. doi: 10.1002/cncr.24507. [DOI] [PubMed] [Google Scholar]
- 17.Gamaletsou MN, Walsh T, Zaoutis T, Pagoni M, Kotsopoulou M, Voulgarelis M, Panayiotidis P, Vassilakopoulos T, Angelopoulou MK, Marangos M, Spyridonidis A, Kofteridis D, Pouli A, Sotiropoulos D, Matsouka P, Argyropoulou A, Perloretzou S, Leckerman K, Manaka A, Oikonomopoulos P, Daikos G, Petrikkos G, Sipsas NV. A prospective, cohort, multicenter study of candidemia in hospitalized adult patients with hematological malignancies. Clin Microbiol Infect. 2014;20:50–57. doi: 10.1111/1469-0691.12312. [DOI] [PubMed] [Google Scholar]
- 18.Farmakiotis D, Kontoyiannis DP. Epidemiology of antifungal resistance in human pathogenic yeasts: current viewpoint and practical recommendations for management. Int J Antimicrob Agents. 2017;50:318–324. doi: 10.1016/j.ijantimicag.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Gamaletsou MN, Daikos GL, Walsh TJ, Perlin DS, Ortigosa CJ, Psaroulaki A, Pagoni M, Argyropoulou A, Nepka M, Perivolioti E, Kotsopoulou M, Perloretzou S, Marangos M, Kofteridis D, Grammatikou M, Goukos D, Petrikkos G, Sipsas NV. Breakthrough candidemia caused by phenotypically susceptible Candida spp. in patients with hematological malignancies does not correlate with established interpretive breakpoints. Int J Antimicrob Agents. 2014;44:248–255. doi: 10.1016/j.ijantimicag.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Siopi M, Neroutsos E, Zisaki K, Gamaletsou M, Piroynaki M, Tsirigotis P, Sipsas N, Dokoumetzidis A, Goussetis E, Zerva L, Valsami G, Meletiadis J. Bioassay for determining voriconazole serum levels in patients receiving combination therapy with echinocandins. Antimicrob Agents Chemother. 2015;60:632–636. doi: 10.1128/AAC.01688-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh TJ, Driscoll T, Milligan PA, Wood ND, Schlamm H, Groll AH, Jafri H, Arrieta AC, Klein NJ, Lutsar I. Pharmacokinetics, safety, and tolerability of voriconazole in hospitalized immunocompromised children. Antimicrob Agents Chemother. 2010;54:4116–4123. doi: 10.1128/AAC.00896-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shields RK, Nguyen MH, Press EG, Clancy CJ. Abdominal candidiasis is a hidden reservoir of echinocandin resistance. Antimicrob Agents Chemother. 2014;58:7601–7615. doi: 10.1128/AAC.04134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osherov N, Kontoyiannis DP. The anti-Aspergillus drug pipeline: is the glass half full or empty? Med Mycol. 2017;55:118–124. doi: 10.1093/mmy/myw060. [DOI] [PubMed] [Google Scholar]
- 24.Chowdhary A, Sharma C, Meis JF. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216(Suppl 3):436–444. doi: 10.1093/infdis/jix210. [DOI] [PubMed] [Google Scholar]
- 25.van der Linden JW, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJ, Verweij PE. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis. 2015;21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verweij PE, Mellado E, Melchers WJ. Multiple-triazole-resistant aspergillosis. N Engl J Med. 2007;356:1481–1483. doi: 10.1056/NEJMc061720. [DOI] [PubMed] [Google Scholar]
- 27.Mellado E, Garcia-Effron G, Alcázar-Fuoli L, Melchers WJ, Verweij PE, Cuenca-Estrella M, Rodríguez-Tudela JL. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother. 2007;51:1897–1904. doi: 10.1128/AAC.01092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis. 2013;57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 29.Le Pape P, Lavergne RA, Morio F, Alvarez-Moreno C. Multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus, Colombia, 2015. Emerg Infect Dis. 2016;22:156–157. doi: 10.3201/eid2201.150978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodiamont CJ, Dolman KM, Ten Berge IJ, Melchers WJ, Verweij PE, Pajkrt D. Multiple-azole-resistant Aspergillus fumigatus osteomyelitis in a patient with chronic granulomatous disease successfully treated with long-term oral posaconazole and surgery. Med Mycol. 2009;47:217–220. doi: 10.1080/13693780802545600. [DOI] [PubMed] [Google Scholar]
- 31.Howard SJ, Arendrup MC. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med Mycol. 2011;49 (Suppl 1):90–95. doi: 10.3109/13693786.2010.508469. [DOI] [PubMed] [Google Scholar]
- 32.Mann PA, Parmegiani RM, Wei SQ, Mendrick CA, Li X, Loebenberg D, DiDomenico B, Hare RS, Walker SS, McNicholas PM. Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome P450 14α-demethylase. Antimicrob Agents Chemother. 2003;47:577–581. doi: 10.1128/AAC.47.2.577-581.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. Substitutions at methionine 220 in the 14α-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob Agents Chemother. 2004;48:2747–2750. doi: 10.1128/AAC.48.7.2747-2750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Li H, Li R, Bu D, Wan Z. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J Antimicrob Chemother. 2005;55:31–37. doi: 10.1093/jac/dkh507. [DOI] [PubMed] [Google Scholar]
- 35.Bellete B, Raberin H, Morel J, Flori P, Hafid J, Manhsung RT. Acquired resistance to voriconazole and itraconazole in a patient with pulmonary aspergilloma. Med Mycol. 2010;48:197–200. doi: 10.3109/13693780902717018. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan Natesan S, Wu W, Cutright JL, Chandrasekar PH. In vitro-in vivo correlation of voriconazole resistance due to G448S mutation (cyp51A gene) in Aspergillus fumigatus. Diagn Microbiol Infect Dis. 2012;74:272–277. doi: 10.1016/j.diagmicrobio.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Paul RA, Rudramurthy SM, Meis JF, Mouton JW, Chakrabarti A. A novel Y319H substitution in CYP51C associated with azole resistance in Aspergillus flavus. Antimicrob Agents Chemother. 2015;59:6615–6619. doi: 10.1128/AAC.00637-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arendrup MC, Jensen RH, Grif K, Skov M, Pressler T, Johansen HK, Lass-Flörl C. In vivo emergence of Aspergillus terreus with reduced azole susceptibility and a Cyp51a M217I alteration. J Infect Dis. 2012;206:981–985. doi: 10.1093/infdis/jis442. [DOI] [PubMed] [Google Scholar]
- 39.Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009;15:1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albarrag AM, Anderson MJ, Howard SJ, Robson GD, Warn PA, Sanglard D, Denning DW. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51A promoter. Antimicrob Agents Chemother. 2011;55:5113–5121. doi: 10.1128/AAC.00517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moye-Rowley WS. Multiple mechanisms contribute to the development of clinically significant azole resistance in Aspergillus fumigatus. Front Microbiol. 2015;6:70. doi: 10.3389/fmicb.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bueid A, Howard SJ, Moore CB, Richardson MD, Harrison E, Bowyer P, Denning DW. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother. 2010;65:2116–2118. doi: 10.1093/jac/dkq279. [DOI] [PubMed] [Google Scholar]
- 43.Rajendran R, Mowat E, McCulloch E, Lappin DF, Jones B, Lang S, Majithiya JB, Warn P, Williams C, Ramage G. Azole resistance of Aspergillus fumigatus biofilms is partly associated with efflux pump activity. Antimicrob Agents Chemother. 2011;55:2092–2097. doi: 10.1128/AAC.01189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul S, Diekema D, Moye-Rowley WS. Contributions of both ATP-binding cassette transporter and Cyp51A proteins are essential for azole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 2017;61:e02748–16. doi: 10.1128/AAC.02748-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei X, Chen P, Gao R, Li Y, Zhang A, Liu F, Lu L. Screening and characterization of the non-cyp51A mutation Afcox10 conferring azole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 2017;61:e02101–16. doi: 10.1128/AAC.02101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camps SM, Dutilh BE, Arendrup MC, Rijs AJ, Snelders E, Huynen MA, Verweij PE, Melchers WJ. Discovery of a hapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One. 2012;7:e50034. doi: 10.1371/journal.pone.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35:340–355. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 48.Xiong Q, Hassan SA, Wilson WK, Han XY, May GS, Tarrand JJ, Matsuda SP. Cholesterol import by Aspergillus fumigatus and its influence on antifungal potency of sterol biosynthesis inhibitors. Antimicrob Agents Chemother. 2005;49:518–524. doi: 10.1128/AAC.49.2.518-524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot Cell. 2005;4:625–632. doi: 10.1128/EC.4.3.625-632.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alastruey-Izquierdo A, Cuesta I, Houbraken J, Cuenca-Estrella M, Monzon A, Rodriguez-Tudela JL. In vitro activity of nine antifungal agents against clinical isolates of Aspergillus calidoustus. Med Mycol. 2010;48:97–102. doi: 10.3109/13693780902803040. [DOI] [PubMed] [Google Scholar]
- 51.Walsh TJ, Petraitis V, Petraitiene R, Field-Ridley A, Sutton D, Ghannoum M, Sein T, Schaufele R, Peter J, Bacher J, Casler H, Armstrong D, Espinel-Ingroff A, Rinaldi MG, Lyman CA. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis. 2003;188:305–319. doi: 10.1086/377210. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez JA, Arganoza MT, Boikov D, Yoon S, Sobel JD, Akins RA. Stable phenotypic resistance of Candida species to amphotericin B conferred by preexposure to subinhibitory levels of azoles. J Clin Microbiol. 1998;36:2690–2695. doi: 10.1128/jcm.36.9.2690-2695.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perfect JR, Cox GM. Drug resistance in Cryptococcus neoformans. Drug Resist Updat. 1999;2:259–269. doi: 10.1054/drup.1999.0090. [DOI] [PubMed] [Google Scholar]
- 54.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snelders E, Huis In ‘t Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol. 2009;75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Özmerdiven GE, Ak S, Ener B, Ağca H, Cilo BD, Tunca B, Akalın H. First determination of azole resistance in Aspergillus fumigatus strains carrying the TR34/L98H mutations in Turkey. J Infect Chemother. 2015;21:581–586. doi: 10.1016/j.jiac.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Hsueh PR, Lau YJ, Chuang YC, Wan JH, Huang WK, Shyr JM, Yan JJ, Yu KW, Wu JJ, Ko WC, Yang YC, Liu YC, Teng LJ, Liu CY, Luh KT. Antifungal susceptibilities of clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus species from Taiwan: surveillance of multicenter antimicrobial resistance in Taiwan program data from 2003. Antimicrob Agents Chemother. 2005;49:512–517. doi: 10.1128/AAC.49.2.512-517.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother. 2011;55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiederhold NP, Gil VG, Gutierrez F, Lindner JR, Albataineh MT, McCarthy DI, Sanders C, Fan H, Fothergill AW, Sutton DA. First detection of TR34 L98H and TR46 Y121F T289A Cyp51 mutations in Aspergillus fumigatus isolates in the United States. J Clin Microbiol. 2016;54:168–171. doi: 10.1128/JCM.02478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonçalves SS. Global aspects of triazole resistance in Aspergillus fumigatus with focus on Latin American countries. J Fungi. 2017;3:5. doi: 10.3390/jof3010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses. 2015;58:350–355. doi: 10.1111/myc.12324. [DOI] [PubMed] [Google Scholar]
- 62.van Ingen J, van der Lee HA, Rijs TA, Zoll J, Leenstra T, Melchers WJ, Verweij PE. Azole, polyene and echinocandin MIC distributions for wild-type, TR34/L98H and TR46/Y121F/T289A Aspergillus fumigatus isolates in the Netherlands. J Antimicrob Chemother. 2015;70:178–181. doi: 10.1093/jac/dku364. [DOI] [PubMed] [Google Scholar]
- 63.Howard SJ, Lass-Flörl C, Cuenca-Estrella M, Gomez-Lopez A, Arendrup MC. Determination of isavuconazole susceptibility of Aspergillus and Candida species by the EUCAST method. Antimicrob Agents Chemother. 2013;57:5426–5431. doi: 10.1128/AAC.01111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gregson L, Goodwin J, Johnson A, McEntee L, Moore CB, Richardson M, Hope WW, Howard SJ. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: correlation with itraconazole, voriconazole, and posaconazole. Antimicrob Agents Chemother. 2013;57:5778–5780. doi: 10.1128/AAC.01141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 66.Chowdhary A, Kathuria S, Xu J, Sharma C, Sundar G, Singh PK, Gaur SN, Hagen F, Klaassen CH, Meis JF. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. PLoS One. 2012;7:e52871. doi: 10.1371/journal.pone.0052871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heo ST, Tatara AM, Jiménez-Ortigosa C, Jiang Y, Lewis RE, Tarrand J, Tverdek F, Albert ND, Verweij PE, Meis JF, Mikos AG, Perlin DS, Kontoyiannis DP. Changes in in vitro susceptibility patterns of Aspergillus to triazoles and correlation with aspergillosis outcome in a tertiary care cancer center, 1999-2015. Clin Infect Dis. 2017;65:216–225. doi: 10.1093/cid/cix297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat. 2015;21-22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. Mechanisms of antifungal drug resistance. Cold Spring Harb Perspect Med. 2015;5:a019752. doi: 10.1101/cshperspect.a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sagatova AA, Keniya MV, Wilson RK, Monk BC, Tyndall JD. Structural insights into binding of the antifungal drug fluconazole to Saccharomyces cerevisiae lanosterol 14α-demethylase. Antimicrob Agents Chemother. 2015;59:4982–4989. doi: 10.1128/AAC.00925-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flowers SA, Barker KS, Berkow EL, Toner G, Chadwick SG, Gygax SE, Morschhäuser J, Rogers PD. Gain-of-function mutations in UPC2 are a frequent cause of ERG11 upregulation in azole-resistant clinical Isolates of Candida albicans. Eukaryotic Cell. 2012;11:1289–1299. doi: 10.1128/EC.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vale-Silva LA, Coste AT, Ischer F, Parker JE, Kelly SL, Pinto E, Sanglard D. Azole resistance by loss of function of the sterol Δ5,6-desaturase gene (ERG3) in Candida albicans does not necessarily decrease virulence. Antimicrob Agents Chemother. 2012;56:1960–1968. doi: 10.1128/AAC.05720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Douglas CM. Fungal beta(1,3)-D-glucan synthesis. Med Mycol. 2001;39(Suppl 1):55–66. doi: 10.1080/mmy.39.1.55.66. [DOI] [PubMed] [Google Scholar]
- 74.Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis. 2014;27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bailly S, Maubon D, Fournier P, Pelloux H, Schwebel C, Chapuis C, Foroni L, Cornet M, Timsit JF. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.- trends over 10 years. J Infect. 2016;72:103–111. doi: 10.1016/j.jinf.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 77.Hull CM, Bader O, Parker JE, Weig M, Gross U, Warrilow AG, Kelly DE, Kelly SL. Two clinical isolates of Candida glabrata exhibiting reduced sensitivity to amphotericin B both harbor mutations in ERG2. Antimicrob Agents Chemother. 2012;56:6417–6421. doi: 10.1128/AAC.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol. 2013;51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, Schaffner W, Beldavs ZG, Chiller TM, Park BJ, Cleveland AA, Lockhart SR. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother. 2014;58:4690–4696. doi: 10.1128/AAC.03255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, Derado G, Pham CD, Lockhart SR, Smith RM. Epidemiology and risk factors for echinocandin non-susceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, 2008-2014. Open Forum Infect Dis. 2015;2:ofv163. doi: 10.1093/ofid/ofv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klotz U, Schmidt D, Willinger B, Steinmann E, Buer J, Rath PM, Steinmann J. Echinocandin resistance and population structure of invasive Candida glabrata isolates from two university hospitals in Germany and Austria. Mycoses. 2016;59:312–318. doi: 10.1111/myc.12472. [DOI] [PubMed] [Google Scholar]
- 82.McCarthy MW, Walsh TJ. Containment strategies to address the expanding threat of multidrug-resistant Candida auris. Expert Rev Anti Infect Ther. 2017;15:1095–1099. doi: 10.1080/14787210.2017.1402678. [DOI] [PubMed] [Google Scholar]
- 83.Calvo B, Melo AS, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Meis JF, Colombo AL. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect. 2016;73:369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 84.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Executive summary: Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- 85.Slavin M, van Hal S, Sorrell TC, Lee A, Marriott DJ, Daveson K, Kennedy K, Hajkowicz K, Halliday C, Athan E, Bak N, Cheong E, Heath CH, Orla Morrissey C, Kidd S, Beresford R, Blyth C, Korman TM, Owen Robinson J, Meyer W, Chen SC; Australia and New Zealand Mycoses Interest Group. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clin Microbiol Infect. 2015;21:490. doi: 10.1016/j.cmi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 86.Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- 87.Fernández-Ruiz M, Guinea J, Puig-Asensio M, Zaragoza Ó, Almirante B, Cuenca-Estrella M, Aguado JM; CANDIPOP Project; GEIH-GEMICOMED (SEIMC) and REIPI. Fungemia due to rare opportunistic yeasts: data from a population-based surveillance in Spain. Med Mycol. 2017;55:125–136. doi: 10.1093/mmy/myw055. [DOI] [PubMed] [Google Scholar]
- 88.Bretagne S, Renaudat C, Desnos-Ollivier M, Sitbon K, Lortholary O, Dromer F; French Mycosis Study Group. Predisposing factors and outcome of uncommon yeast species-related fungaemia based on an exhaustive surveillance programme (2002-14) J Antimicrob Chemother. 2017;72:1784–1793. doi: 10.1093/jac/dkx045. [DOI] [PubMed] [Google Scholar]
- 89.Espinel-Ingroff A, Turnidge J. The role of epidemiological cutoff values (ECVs/ECOFFs) in antifungal susceptibility testing and interpretation for uncommon yeasts and moulds. Rev Iberoam Micol. 2016;33:63–75. doi: 10.1016/j.riam.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Ostrosky-Zeichner L, Andes D. The role of in vitro susceptibility testing in management of fungal infections. J Infect Dis. 2017;216(Suppl 3):452–457. doi: 10.1093/infdis/jix239. [DOI] [PubMed] [Google Scholar]
- 91.Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, Garey KW, Alangaden GJ, Vazquez JA, Groeger JS, Judson MA, Vinagre YM, Heard SO, Zervou FN, Zacharioudakis IM, Kontoyiannis DP, Pappas PG. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis. 2015;60:892–899. doi: 10.1093/cid/ciu959. [DOI] [PubMed] [Google Scholar]
- 92.Perlin DS, Wiederhold NP. Culture-independent molecular methods for detection of antifungal resistance mechanisms and fungal identification. J Infect Dis. 2017;216(Suppl 3):458–465. doi: 10.1093/infdis/jix121. [DOI] [PubMed] [Google Scholar]
- 93.Alanio A, Bretagne S. Performance evaluation of multiplex PCR including Aspergillus-not so simple! Med Mycol. 2017;55:56–62. doi: 10.1093/mmy/myw080. [DOI] [PubMed] [Google Scholar]
- 94.McCarthy MW, Kontoyiannis DP, Perfect J, Cornely OS, Walsh TJ. Novel agents and drug targets to meet the challenges of resistant fungi. J Infect Dis. 2017;216(Suppl 3):474–483. doi: 10.1093/infdis/jix130. [DOI] [PubMed] [Google Scholar]
- 95.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother. 2016;71:2868–2873. doi: 10.1093/jac/dkw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCarthy MW, Denning DW, Walsh TJ. Future research priorities in fungal resistance. J Infect Dis. 2017;216(Suppl 3):484–492. doi: 10.1093/infdis/jix103. [DOI] [PubMed] [Google Scholar]
- 97.Verweij PE, Lestrade PP, Melchers WJ, Meis JF. Azole resistance surveillance in Aspergillus fumigatus: beneficial or biased? J Antimicrob Chemother. 2016;71:2079–2082. doi: 10.1093/jac/dkw259. [DOI] [PubMed] [Google Scholar]
- 98.Alanio A, Denis B, Hamane S, Raffoux E, Peffault de la Tour R, Touratier S, Bergeron A, Bretagne S. New therapeutic strategies for invasive aspergillosis in the era of azole resistance: how should the prevalence of azole resistance be defined? J Antimicrob Chemother. 2016;71:2075–2078. doi: 10.1093/jac/dkw036. [DOI] [PubMed] [Google Scholar]
- 99.Lestrade PP, Meis JF, Arends JP, van der Beek MT, de Brauwer E, van Dijk K, de Greeff SC, Haas PJ, Hodiamont CJ, Kuijper EJ, Leenstra T, Muller AE, Oude Lashof AM, Rijnders BJ, Roelofsen E, Rozemeijer W, Tersmette M, Terveer EM, Verduin CM, Wolfhagen MJ, Melchers WJ, Verweij PE. Diagnosis and management of aspergillosis in the Netherlands: a national survey. Mycoses. 2016;59:101–107. doi: 10.1111/myc.12440. [DOI] [PubMed] [Google Scholar]


