Abstract
Background
Recently, 44Sc (T1/2 = 3.97 h, Eβ+ av = 632 keV, I = 94.3 %) has emerged as an attractive radiometal candidate for PET imaging using DOTA-functionalized biomolecules. The aim of this study was to investigate the potential of using NODAGA for the coordination of 44Sc. Two pairs of DOTA/NODAGA-derivatized peptides were investigated in vitro and in vivo and the results obtained with 44Sc compared with its 68Ga-labeled counterparts.
DOTA-RGD and NODAGA-RGD, as well as DOTA-NOC and NODAGA-NOC, were labeled with 44Sc and 68Ga, respectively. The radiopeptides were investigated with regard to their stability in buffer solution and under metal challenge conditions using Fe3+ and Cu2+. Time-dependent biodistribution studies and PET/CT imaging were performed in U87MG and AR42J tumor-bearing mice.
Results
Both RGD- and NOC-based peptides with a DOTA chelator were readily labeled with 44Sc and 68Ga, respectively, and remained stable over at least 4 half-lives of the corresponding radionuclide. In contrast, the labeling of NODAGA-functionalized peptides with 44Sc was more challenging and the resulting radiopeptides were clearly less stable than the DOTA-derivatized matches. 44Sc-NODAGA peptides were clearly more susceptible to metal challenge than 44Sc-DOTA peptides under the same conditions. Instability of 68Ga-labeled peptides was only observed if they were coordinated with a DOTA in the presence of excess Cu2+. Biodistribution data of the 44Sc-labeled peptides were largely comparable with the data obtained with the 68Ga-labeled counterparts. It was only in the liver tissue that the uptake of 68Ga-labeled DOTA compounds was markedly higher than for the 44Sc-labeled version and this was also visible on PET/CT images. The 44Sc-labeled NODAGA-peptides showed a similar tissue distribution to those of the DOTA peptides without any obvious signs of in vivo instability.
Conclusions
Although DOTA revealed to be the preferred chelator for stable coordination of 44Sc, the data presented in this work indicate the possibility of using NODAGA in combination with 44Sc. In view of a clinical study, thorough investigations will be necessary regarding the labeling conditions and storage solutions in order to guarantee sufficient stability of 44Sc-labeled NODAGA compounds.
Electronic supplementary material
The online version of this article (doi:10.1186/s41181-016-0013-5) contains supplementary material, which is available to authorized users.
Keywords: 44Sc, PET, Imaging, Stability, DOTA-RGD, NODAGA-RGD, DOTA-NOC, NODAGA-NOC, 68Ga, AR42J, U87MG
Background
44Sc is a novel radiometal which is attractive for positron emission tomography (PET) imaging, due to the emission of positrons with a high branching ratio (Eβ+ av = 632 keV, I = 94.3 %) (Rösch 2012; Müller et al. 2013). Its physical half-life of 3.97 h enables the acquisition of PET images several hours after injection of the 44Sc-radiopharmaceutical and is of particular interest for application with biomolecules, providing slower kinetic profiles (Chakravarty et al. 2014). Importantly, application of 44Sc would allow a centralized production of radiopharmaceuticals, followed by transportation to more remote hospitals (van der Meulen et al. 2015).
44Sc is thought to be useful as a diagnostic match to therapeutic radionuclides with similar chemical properties, such as 90Y and 177Lu (Müller et al. 2013). Most interesting, however, would be to use 44Sc in combination with its therapeutic counterpart 47Sc, which provides excellent β−-decay properties for radionuclide therapy (Eβ− av = 162 keV, T1/2 = 3.35 d). The potential of 44Sc/47Sc as a theragnostic pair has been demonstrated recently in a preclinical pilot study with tumor-bearing mice (Müller et al. 2014).
A crucial requirement for the application of radiopharmaceuticals is the formation of a thermodynamically stable and kinetically inert complex of the radiometal with a suitable chelator, which is then linked to the targeting agent (Majkowska-Pilip & Bilewicz 2011). The coordination of Sc(III) and Ga(III) has previously been investigated with several macrocyclic polyaminocarboxylic chelators, including 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) (Majkowska-Pilip & Bilewicz 2011; Huclier-Markai et al. 2011; Notni et al. 2012). The studies were performed with natSc(III) stock solutions containing 46Sc as tracer and the sole chelator without an attached biomolecule. As a result of these investigations, it was found that Sc(III) forms complexes with both chelators, DOTA and NOTA, however, the stability of Sc-DOTA was superior to Sc-NOTA complexes (Majkowska-Pilip & Bilewicz 2011; Huclier-Markai et al. 2011). On the other hand, Ga(III) displays a reverse behavior, forming more stable complexes with NOTA than with DOTA (Majkowska-Pilip & Bilewicz 2011; Notni et al. 2012). The DOTA-chelator provides eight coordination sites, which are all coordinated by Sc(III), whereas NOTA can only form six coordinative bonds. Due to the higher denticity the Sc-DOTA complex is believed to be thermodynamically more stable than the Sc-NOTA complex (Huclier-Markai et al. 2011; Port et al. 2008). Due to the preference of Ga(III) for the coordination number six, all coordination sites of NOTA are used, while two sites of DOTA remain uncoordinated in a Ga-DOTA-complex (Majkowska-Pilip & Bilewicz 2011; Viola-Villegas & Doyle 2009).
Despite the reduced stability of a Ga-DOTA complex compared to a Ga-NOTA and Ga-1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid (NODAGA) complex, there are several examples of 68Ga-DOTA labeled peptides which showed promising in vivo properties. The 68Ga-labeled somatostatin receptor analogues 68Ga-DOTA-TOC, 68Ga-DOTA-TATE and 68Ga-DOTA-NOC represent the most prominently applied radiopharmaceuticals in clinical studies for imaging of neuroendocrine tumors (Kwekkeboom et al. 2010). Recent PET imaging studies in patients using the αvβ3 integrin-targeting radiotracer 68Ga-NOTA-RGD indicated promising results (Kim et al. 2012; Choi et al. 2013; Yoon et al. 2014). These reports demonstrate that 68Ga is successfully used in clinics with both DOTA- and NOTA/NODAGA-derivatized targeting agents.
The stable complexation of 44Sc with DOTA initiated a number of preclinical studies with a range of DOTA-derivatized biomolecules, including bombesin analogues (Koumarianou et al. 2012), puromycin (Eigner et al. 2013), folate-conjugates (Müller et al. 2013) and dimeric cyclic RGD peptides (Hernandez et al. 2014). To date, all research with regard to the in vivo and in vitro behavior of 44Sc-labeled radiopharmaceuticals was performed with DOTA chelators connected to the respective targeting agent. The only exception to our knowledge was a study in which an EGFR-targeted antibody was labeled with 44Sc using a CHX-A-DTPA (N-[(R)-2-amino-3-(para-isothiocyanato-phenyl)propyl]trans-(S,S)-cyclohexane-1,2-diamine N,N,N’,N”N”-pentaacetic acid) chelator (Chakravarty et al. 2014). The question on whether or not a NOTA or NODAGA chelator would be suited for 44Sc-labeling has remained unclear thus far. Using these chelators for coordination of 44Sc would be of interest in combination with radiopharmaceuticals where only the NOTA- or NODAGA-derivatized species are available for clinical studies, as is the case for NODAGA-RGD: a peptide which is currently employed in clinical trials when labeled with 68Ga. Since application of NODAGA-RGD at later time points after injection of the radioconjugate would be of interest for nuclear physicians, we set out to investigate whether 44Sc-labeling with NODAGA-derivatized biomolecules is possible and whether the in vitro and in vivo behavior of the radiolabeled peptides would be equal to the DOTA-derivatized matches.
The aim of this study was, therefore, to compare the in vitro and in vivo behavior of two pairs of peptides with a DOTA- and NODAGA-chelator (Fig. 1), respectively, after radiolabeling with 44Sc and 68Ga. Cyclic RGD peptides based on the Arg-Gly-Asp sequence and NOC, a somatostatin analogue ([Tyr3,1-NaI3]octreotide), were chosen as targeting agents. The in vitro stability of 44Sc- and 68Ga-labeled DOTA/NODAGA-RGD and DOTA/NODAGA-NOC was examined in saline in the presence and absence of competing metal cations. The in vivo behavior was evaluated by the performance of biodistribution studies and in vivo PET imaging of tumor-bearing mice.
Fig. 1.
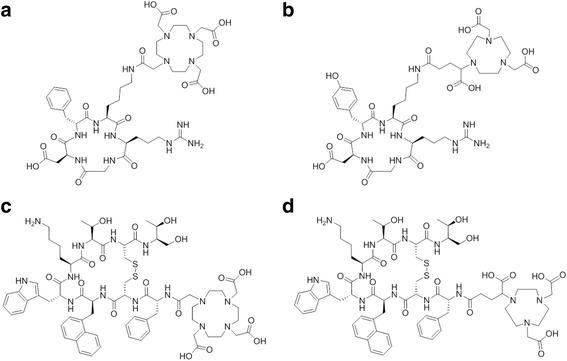
Chemical structures of DOTA-RGD (a), NODAGA-RGD (b), DOTA-NOC (c) and NODAGA-NOC (d)
Methods
Chemicals
44CaCO3, 97.0 % enriched (Trace Sciences International, USA) and graphite powder, 99.9999 % (Alfa Aesar, Germany) were used for target preparation. The N,N,N’,N’-tetra-n-octyldiglycolamide, non-branched resin (DGA, particle size 50–100 μm, TrisKem International, France) was used for the separation of Sc(III) from Ca(II). The chemical separations were performed using MilliQ water (resistance >18 MΩ) and hydrochloric acid (HCl, 30 % Suprapur, Merck KGaA, Germany). The recycling of the target material was performed with oxalic acid dihydrate, (Trace SELECT, ≥99.9999 % metals basis, Fluka Analytical, Germany) and 25 % ammonia solution (Suprapur, Merck KGaA, Germany).
68Ga was obtained from a 68Ge/68Ga generator IGG100 (Eckert & Ziegler, Berlin, Germany). The generator was eluted in fractions and the fraction of eluate containing the highest quantity of 68Ga (approximately 200–250 MBq in 700 μL 0.1 M HCl) was directly used for radiolabeling purposes without purification. DOTA-RGD (DOTA-cyclo(RGDfK) acetate, Cat-N° 9863), NODAGA-RGD (NODAGA-RGD trifluoroacetate, Cat-N° 9805), DOTA-NOC (DOTA-NOC acetate, Cat-N° 9712) and NODAGA-NOC (NODAGA-NOC acetate, Cat-N° 9718) were obtained from ABX GmbH, advanced biochemical compounds, Germany. Copper chloride dihydrate was purchased from Merck Millipore, while iron chloride hexahydrate was obtained from Sigma-Aldrich GmbH. Phosphate buffered saline (PBS) pH 7.4 was prepared in-house (Additional file 1).
Production of 44Sc
44Sc was prepared by proton irradiation of enriched 44Ca targets at the Injector 2 cyclotron at PSI, as previously reported (van der Meulen et al. 2015). The irradiation of targets with 11 MeV proton beam energy, and a beam current of 50 μA, lasted for 90 min. A column (1 mL cartridge fitted with 20 μm frit ISOLUTE SPE Accessories, UK) was filled with 50 – 70 mg DGA extraction chromatographic resin and a second column with 20–25 mg of the same resin. A 20 μm frit was placed on top of the resin in each column. DGA columns were preconditioned with 3.0 M HCl. The first step of the separation was performed as previously reported (van der Meulen et al. 2015). In brief, the target was dissolved in 2.5 mL 3.0 M HCl and loaded onto the first DGA column. The rinsing of the target container and the first DGA column with 3.0 M HCl ensured a complete transfer of the 44Sc radioactivity and complete removal of residual Ca(II), respectively. 44Sc was eluted from the first DGA column with 3.5 mL 0.1 M HCl. Subsequently, the solution was acidified with the addition of 3.3 mL 6.0 M HCl to yield a 3.0 M HCl solution, which was then passed through the second DGA column, to which the 44Sc activity was sorbed. The elution of 44Sc from the second column was performed with 700 μL 0.05 M HCl (pH 1.3) and was used directly for labeling reactions. The radionuclidic purity of the 44Sc- eluate was quantified by γ-spectrometry using an N-type high-purity germanium (HPGe) coaxial detector (EURISYS MESURES, France) and the Ortec InterWinner 7.1 software. The 44Ca-contained waste fraction from the first DGA column was collected and processed to recover the target material, as described previously (van der Meulen et al. 2015).
Radiolabeling of DOTA- and NODAGA-functionalized peptides
The total 44Sc and 68Ga activity in the obtained eluate was quantitatively determined with a dose calibrator (ISOMED 2010, Nuclear–Medizintechnik Dresden GmbH, Germany) so that the activity required for radiolabeling could be withdrawn. Sodium acetate solution (0.5 M, pH 8) was added at a ratio of 1:1 to the 44Sc eluate (0.05 M HCl, pH 0.4–0.6) and at a ratio of 1:2 to the 68Ga generator eluate (0.1 M HCl, pH 1) to give a pH of 4–4.5. The corresponding peptides (DOTA-RGD, NODAGA-RGD, DOTA-NOC and NODAGA-NOC, in a 1 mM stock solution) were added to obtain a specific activity of up to 10 MBq/nmol and the reaction mixture incubated at 95 °C for 10 min. High-performance liquid chromatography (HPLC) with a C-18 reversed–phase column (XterraTM MS, C18, 5 μm, 150 × 4.6 mm; Waters) was used for quality control. The mobile phase consisted of MilliQ water containing 0.1 % trifluoracetic acid (A) and acetonitrile (B) with a gradient of 95 % A and 5 % B to 20 % A and 80 % B over a period of 15 min at a flow rate of 1.0 mL/min.
In vitro stability of 44Sc- and 68Ga-labeled peptides
44Sc- and 68Ga-labeled peptides (radiochemical purity >95 %) were used for the investigation of stability in 0.9 % NaCl. An activity of 80–100 MBq of 44Sc or 68Ga labeling solution was diluted with 0.9 % NaCl solution to a volume of 800 μL and incubated for four half-lives of the corresponding radionuclide at 37 °C.
Aliquots were taken from the prepared solutions at different time points over at least four half-lives, respectively, of 44Sc and 68Ga and analyzed by TLC (TLC was used, as the high metal concentrations used in this study may impair the long-term performance of HPLC columns). If initial experiments suggested instability of a compound, aliquots were retrieved more frequently. The TLC plates (Silica-gel 60, Merck) were developed using 0.1 M sodium citrate (pH 4.7) as mobile phase. The quantitative distribution of radioactivity was determined with an autoradiography system (Cyclone Plus, Perkin Elmer) and its associated software (Optiquant, version 5.0). Rf values of 0.2 were observed for 44Sc- and 68Ga-labeled DOTA-RGD and NODAGA-RGD, and of 0.1 for DOTA-NOC and NODAGA-NOC, respectively, whereas for unlabeled 44Sc and 68Ga a Rf value of 0.9 was calculated.
The influence of high metal cation concentration on the stability of 44Sc and 68Ga labeled peptides was monitored in solutions containing 0.01 M Fe3+ or Cu2+. An aqueous metal cation solution (16 μL of a 0.5 M Fe3+/Cu2+ solution) was added to 80–100 MBq of 44Sc or 68Ga labeling solution and diluted with 0.9 % NaCl solution to a volume of 800 μL. The incubation conditions, time points of retrieving samples and analysis by TLC were kept the same, as described above.
Cell culture
U87MG cells (human glioblastoma cells; ACC® HTB-14TM) and AR42J cells (rat exocrine pancreatic tumor cells; ACC® CRL1492TM) were purchased from European Collection of Cell Cultures (ECACC, operated by Public Health England). U87MG cells were grown in MEM cell culture medium supplemented with 1 % non-essential amino acids (MEM NEAA solution 100×, Bioconcept), 1 mM sodium pyruvate (Bioconcept), 10 % fetal calf serum, L-glutamine and antibiotics. AR42J cells were grown in RPMI cell culture medium supplemented with 10 % fetal calf serum, L-glutamine and antibiotics. Routine cell culture was performed twice a week using trypsin (Gibco by life technologies 0.25 % trypsin-EDTA) for detachment of the cells.
Tumor mouse models
In vivo experiments were approved by the local veterinarian department and conducted in accordance with the Swiss law of animal protection. Female athymic nude mice (CD-1 nude), age 5–6 weeks, were obtained from Charles River Laboratories, Sulzfeld, Germany. U87MG cells and AR42J-cells were suspended in PBS (5 × 106 cells in 100 μL) and subcutaneously inoculated on each shoulder. Two to three weeks later, when the tumor reached a size of about 300–500 mm3, the mice were used for the in vivo studies.
Biodistribution studies
Biodistribution studies were performed with U87MG tumor and AR42J tumor-bearing mice 2 weeks after tumor cell inoculation. 44Sc- and 68Ga-labeled peptides (~5 MBq, ~1 nmol per mouse) were intravenously injected in a volume of 100–200 μL. Mice were sacrificed at 30 min, 2 h and 5 h after injection (p.i.) of the 44Sc-labeled peptides. Mice which were injected with 68Ga-labeled peptides were sacrificed at 30 min and 2 h p.i. Selected tissues and organs were collected, weighed, and counted for radioactivity using a γ-counter (Wallac Wizard 1480, Perkin Elmer). The results were listed as a percentage of the injected activity per gram of tissue mass (% IA/g), using counts of a defined volume of the original injection solution counted at the same time. The data were analyzed for significance using a two-way ANOVA test (Graph Pad Prism 6 software, version 6.05). A p-value of < 0.05 was considered statistically significant.
Preclinical PET imaging
A bench-top preclinical PET scanner (G8, Sofie Biosciences, California, U.S.A. and Perkin Elmer, Massachusetts, U.S.A.) was employed for the PET scans of the tumor-bearing mice. The energy window was set to 150–650 keV. Mice were injected intravenously with 44Sc- or 68Ga-labeled peptides (~10 MBq, ~1 nmol per mouse) in a volume of 100–200 μL. The PET scans were performed 3 h and 5 h after injection of the 44Sc-labeled peptides and 3 h after injection of the 68Ga-labeled peptides, using G8 acquisition software (version 2.0.0.10). All static PET scans lasted for 20 min. During the acquisition the mice were anesthetized by inhalation of a mixture of isoflurane and oxygen. The images were reconstructed with maximum-likelihood expectation maximization (MLEM). Gauss post-reconstruction filtering was performed using VivoQuant post-processing software (version 2.10, inviCRO Imaging Services and Software, Boston, U.S.A.).
Results
Production and separation of 44Sc
44Sc was quantitatively sorbed on DGA resin in 3.0 M HCl solution, whereas Ca was not retained. Rinsing the resin with additional 4 mL 3.0 M HCl ensured the complete removal of Ca, after which 44Sc was eluted with 3.5 mL 0.1 M HCl. The 44Sc eluate was further acidified with the addition of 6.0 M HCl to yield a 3.0 M HCl solution. The resultant solution was passed through a second, smaller DGA column at a flow rate of ~0.3 mL/min, retaining 97 % of the eluted 44Sc activity. 44Sc was eluted quantitatively (85 ± 2 %), at activities of ~2.0 GBq, with 700 μL 0.05 M HCl and was used directly for labeling experiments. The separation procedure was initially developed using trace activities of 46Sc, produced by the 45Sc(n,γ) nuclear reaction at the BR2 reactor at SCK.CEN, Mol, Belgium.
The 44Sc activity separated from proton irradiated targets containing recycled 44CaCO3 was of the same quality and quantity as using the originally-purchased 44CaCO3 target material.
Radiolabeling and stability of 44Sc- and 68Ga-labeled peptides
Radiolabeling with 44Sc was readily achieved with DOTA-compounds, but found to be more challenging for NODAGA-compounds, which did not allow reproducible labeling procedures at high specific activities. Radiolabeling with 68Ga was reproducibly achieved for both DOTA- and NODAGA-functionalized peptides, however. The radiochemical yield of the 44Sc and 68Ga radiosyntheses at the specific activity of 10 MBq/nmol was >95 %. Quantitative 68Ga-labeling of NODAGA-RGD and NODAGA-NOC was also possible at room temperature in less than 10 min. TLC and HPLC quality control were in good agreement. HPLC analysis demonstrated a peak of free 44Sc and 68Ga at a retention time of 2.2 ± 0.1 min, while the retention times of the radiopeptides were between 6 and 10 min (Table 1).
Table 1.
HPLC retention times of radiolabeled peptides
| Radiopeptide | 44Sc-/68Ga-DOTA-RGD | 44Sc-/68Ga-NODAGA-RGD |
| Retention time | 6.8 ± 0.1 min | 6.1 ± 0.1 min |
| Radiopeptide | 44Sc-/68Ga-DOTA-NOC | 44Sc-/68Ga-NODAGA-NOC |
| Retention time | 9.3 ± 0.1 min | 9.8 ± 0.1 min |
The stability of 44Sc- and 68Ga-labeled DOTA- and NODAGA-peptides was first investigated in 0.9 % NaCl over a period of four half-lives of the corresponding nuclide by means of TLC. 44Sc- and 68Ga-DOTA-RGD and DOTA-NOC exhibited a high stability. After four half-lives at 37 °C the amount of intact compound did not decrease below 98 %. 68Ga-labeled NODAGA-RGD and NODAGA-NOC remained stable, but the 44Sc-NODAGA peptides became more unstable over time. The amount of intact 44Sc-NODAGA-RGD dropped to 77 % and, in the case of 44Sc-NODAGA-NOC, a mere 37 % after more than 4 half-lives.
The presence of different metal cations can cause displacement of the radionuclide from the chelator and its release into solution (Pruszynski et al, 2012). The stability of 44Sc- and 68Ga-labeled DOTA- and NODAGA-peptides was investigated in the presence of Fe3+ and Cu2+ over four half-lives of the corresponding radionuclide (Fig. 2). The addition of solutions containing these metal cations to result in final metal concentrations as high as 10 mM did not induce any transmetalation of 44Sc-labeled DOTA- and 68Ga-labeled NODAGA-compounds. The integrity of 68Ga-DOTA-peptides was not impaired by the presence of Fe3+, however, the addition of 10 mM Cu2+ reduced the amount of intact 68Ga-DOTA-RGD to 10 % and 68Ga-DOTA-NOC to 50 %, respectively, after two half-lives. The presence of both metal cations further destabilized the already less stable 44Sc-labeled NODAGA-peptides.
Fig. 2.
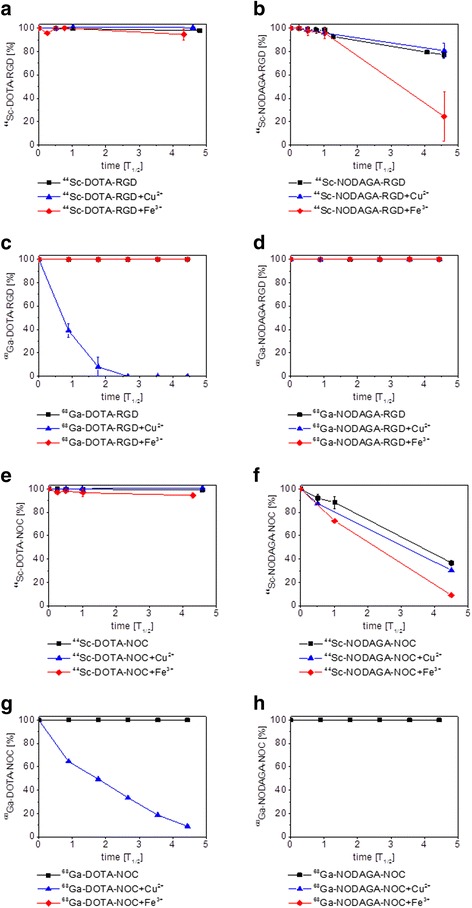
Stability of 44Sc-DOTA-RGD (a), 44Sc-NODAGA-RGD (b), 68Ga-DOTA-RGD (c), 68Ga-NODAGA-RGD (d), 44Sc-DOTA-NOC (e), 44Sc-NODAGA-NOC (f), 68Ga-DOTA-NOC (g), 68Ga-NODAGA-NOC (h) in saline with and without the presence of 10 mM Cu2+ and Fe3+, respectively
Biodistribution studies with 44Sc- and 68Ga-labeled peptides
Biodistribution studies were performed with 44Sc- and 68Ga-labeled DOTA/NODAGA-RGD and DOTA/NODAGA-NOC in mice bearing U87MG and AR42J tumor xenografts, respectively (Additional file 1: Table S2-S5).
Time-dependent distribution studies of 44Sc-DOTA-RGD and 44Sc-NODAGA-RGD revealed a similar pattern for both compounds, resulting in a tumor uptake of 4.88 ± 0.67 % IA/g and 4.50 ± 0.77 % IA/g, respectively, at 0.5 h after injection (Fig. 3a). The wash-out of radioactivity from the tumor tissue was somewhat faster for the 44Sc-DOTA-RGD than for the 44Sc-NODAGA-RGD, but at 5 h after injection the values were almost the same (3.00 ± 0.38 % IA/g vs 3.01 ± 0.55 % IA/g). Clearance from the blood was fast for both 44Sc-DOTA-RGD and 44Sc-NODAGA-RGD. This was also reflected by the high renal uptake shortly after injection (4.44 ± 0.51 % IA/g vs 3.89 ± 0.68 % IA/g, 0.5 h p.i.) which decreased with time, resulting in a retention of <2 % IA/g at 5 h p.i. Whereas the accumulation in non-targeted organs and tissues was generally comparable, the liver uptake of 44Sc-DOTA-RGD (5.16 ± 0.99 % IA/g, 0.5 h) was clearly higher than for 44Sc-NODAGA-RGD (1.49 ± 0.06 % IA/g, 0.5 h) at early time points. At 5 h after injection uptake in the liver was ~1 % IA/g for both radiopeptides (Fig. 3a).
Fig. 3.
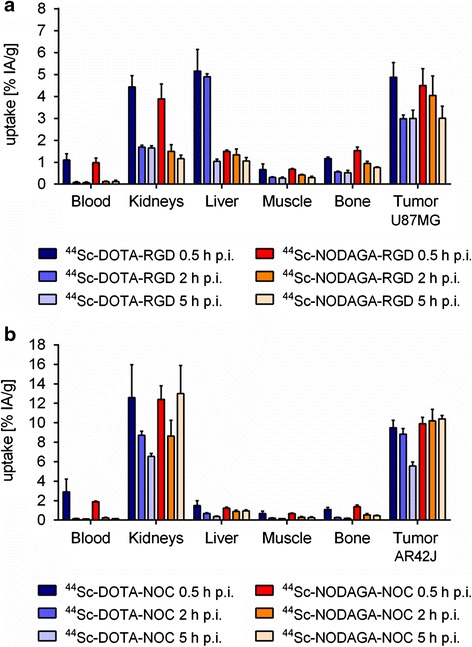
Biodistribution data obtained at different time points after injection of ~5 MBq (1 nmol) 44Sc-DOTA/NODAGA-RGD in U87MG tumor-bearing mice (a) and after injection of ~5 MBq (1 nmol) 44Sc-DOTA/NODAGA-NOC in AR42J tumor-bearing mice (b), respectively. Data bars represent the average ± SD of values obtained from n = 3 mice
The tissue distribution pattern of 44Sc-DOTA-NOC and 44Sc-NODAGA-NOC was comparable with regard to the uptake in AR42J tumors (9.49 ± 0.76 % IA/g vs. 9.90 ± 0.66 % IA/g), kidneys (12.6 ± 3.36 % IA/g vs. 12.4 ± 1.41 % IA/g) and liver (1.49 ± 0.49 % IA/g vs. 1.22 ± 0.11 % IA/g) at 0.5 h after injection (Fig. 3b). The tissue distribution profiles at 2 h after injection of the NOC-based radiopeptides were also comparable. At 5 h after injection, retention of 44Sc-DOTA-NOC in tumors (5.56 ± 0.40 % IA/g) was lower than for 44Sc-NODAGA-NOC (10.8 ± 0.37 % IA/g), which indicates a faster wash-out of 44Sc-DOTA-NOC. Renal retention of 44Sc-DOTA-NOC decreased further over the period of investigation (6.54 ± 0.30 % IA/g, 5 h p.i.) while the accumulation of activity in the kidneys (13.0 ± 2.98 % IA/g) was increased at 5 h after injection of 44Sc-NODAGA-NOC (Fig. 3b).
For selected organs and tissues, the uptake of the 44Sc-labeled peptides was compared with the uptake of the 68Ga-labeled peptides in mice at 2 h after injection (Fig. 4). The tissue distribution of DOTA-RGD was almost the same, independent of the radionuclide (44Sc vs 68Ga) used (Fig. 4a), although the tumor uptake was higher for 44Sc-DOTA-RGD (2.99 ± 0.16 % IA/g) than for 68Ga-DOTA-RGD (2.35 ± 0.27 % IA/g, p <0.05). The NODAGA-derivatized RGD-peptides revealed the same trend: the tumor uptake of 44Sc-NODAGA-RGD (4.05 ± 0.89 IA/g) was significantly higher (p <0.05) than the tumor uptake of 68Ga-NODAGA-RGD (3.13 ± 0.27 % IA/g). Undesired accumulation of 44Sc-NODAGA-RGD in the liver (1.34 ± 0.27 % IA/g, p <0.05) was significantly reduced compared to 68Ga-NODAGA-RGD (2.09 ± 0.08 % IA/g, Fig. 4b). Renal uptake of 44Sc-NODAGA-RGD was also slightly lower than for 68Ga-NODAGA-RGD. The DOTA-RGD accumulated to a higher extent in the liver than the NODAGA-RGD, irrespective of which radionuclide was used for labeling (Fig. 4a/b). In all other organs of interest, the distribution profile was roughly the same, irrespective of the chelator used for coordination of the radionuclide.
Fig. 4.
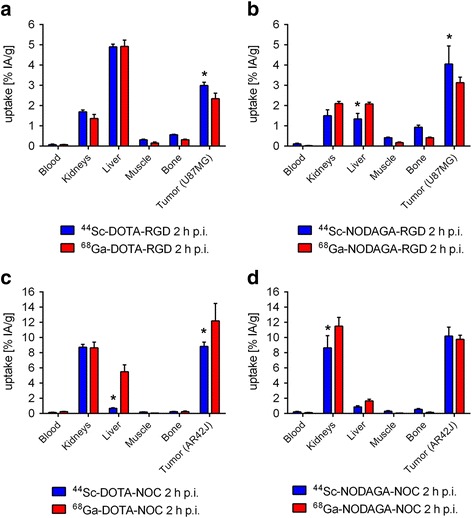
Biodistribution data obtained 2 h after injection of ~5 MBq (~1 nmol) 44Sc- and 68Ga-labeled DOTA-RGD (a) and NODAGA-RGD (b) and ~5 MBq (~1 nmol) DOTA-NOC (c) and NODAGA-NOC (d). Data bars represent the average ± SD of values obtained from n = 3 mice (* significantly different uptake of the 44Sc-labeled peptide compared to the 68Ga-labeled peptide, p <0.05)
Accumulation of 44Sc-DOTA-NOC in the tumor xenografts (8.83 ± 0.57 % IA/g) was significantly lower (p <0.05) when compared to the uptake of 68Ga-DOTA-NOC (12.2 ± 2.29 % IA/g) (Fig. 4c). This was also the case in the liver, where the uptake of 44Sc-DOTA-NOC (0.68 ± 0.07 % IA/g) was significantly (p <0.05) lower than for 68Ga-DOTA-NOC (5.52 ± 0.88 % IA/g). In all other organs and tissues the distribution of radioactivity was comparable among the radiopeptides, irrespective of whether they were labeled with 44Sc or 68Ga (Fig. 4c). The tissue distribution of 44Sc-NODAGA-NOC and 68Ga-NODAGA-NOC was also comparable (Fig. 4d). The only significant difference (p <0.05) were the kidneys, in which 44Sc-NODAGA-NOC was less retained (8.64 ± 1.62 % IA/g) than the 68Ga-NODAGA-NOC (11.5 ± 1.15 % IA/g). The liver uptake of 44Sc-NODAGA-NOC (0.88 ± 0.15 % IA/g) was lower than for 68Ga-NODAGA-NOC (1.68 ± 0.19 % IA/g) but the difference was not significant (Fig. 4c). Comparison of the DOTA-NOC and NODAGA-NOC revealed similar distribution profiles, irrespective of the radionuclide employed. As the only exception, it should be mentioned that 68Ga-DOTA-NOC showed a clearly higher retention in the liver than all other NOC-based radiopeptides (Fig. 4c/d).
As a result of the tissue distribution data reported above, the tumor-to-background ratios were mostly similar between 44Sc-labeled DOTA-RGD and NODAGA-RGD as well as between 44Sc-labeled DOTA-NOC and NODAGA-NOC, respectively (Tables 2 and 3). When comparing variation of tumor-to-background ratios between the 44Sc- and 68Ga-labeled versions of each of the four peptides, the ratios appeared more pronounced for the NOC-based radiopeptides over their RGD-based counterparts (Tables 2 and 3).
Table 2.
Tumor-to-background ratios at different time points after injection of 44Sc/68Ga-labeled DOTA-RGD and NODAGA-RGD
| 44Sc-DOTA-RGD | 68Ga-DOTA-RGD | ||||
| U87MG | 30 min p.i. | 2 h p.i. | 5 h p.i. | 30 min p.i. | 2 h p.i. |
| Tumor-to-blood | 4.82 ± 1.60 | 40.1 ± 15.6 | 47.2 ± 18.0 | 4.34 ± 0.57 | 28.0 ± 4.26 |
| Tumor-to-liver | 0.99 ± 0.14 | 0.61 ± 0.04 | 2.90 ± 0.38 | 0.55 ± 0.09 | 0.48 ± 0.07 |
| Tumor-to-kidney | 1.16 ± 0.29 | 1.77 ± 0.13 | 1.82 ± 0.21 | 0.88 ± 0.07 | 1.74 ± 0.19 |
| 44Sc-NODAGA-RGD | 68Ga-NODAGA-RGD | ||||
| U87MG | 30 min p.i. | 2 h p.i. | 5 h p.i. | 30 min p.i. | 2 h p.i. |
| Tumor-to-blood | 4.73 ± 1.24 | 34.1 ± 7.62 | 30.5 ± 9.93 | 4.17 ± 0.47 | 114 ± 35.0 |
| Tumor-to-liver | 3.02 ± 0.40 | 3.01 ± 0.13 | 2.82 ± 0.21 | 1.72 ± 0.16 | 1.50 ± 0.20 |
| Tumor-to-kidney | 1.18 ± 0.28 | 2.68 ± 0.14 | 2.58 ± 0.24 | 0.89 ± 0.03 | 1.49 ± 0.21 |
Table 3.
Tumor-to-background ratios at different time points after injection of 44Sc/68Ga-labeled DOTA-NOC and NODAGA-NOC
| 44Sc-DOTA-NOC | 68Ga-DOTA-NOC | ||||
| AR42J | 30 min p.i. | 2 h p.i. | 5 h p.i. | 30 min p.i. | 2 h p.i. |
| Tumor-to-blood | 3.69 ± 1.52 | 58.2 ± 6.64 | 52.0 ± 4.38 | 5.22 ± 1.07 | 46.2 ± 2.28 |
| Tumor-to-liver | 6.87 ± 2.38 | 13.1 ± 2.16 | 15.7 ± 2.82 | 2.44 ± 0.36 | 2.22 ± 0.22 |
| Tumor-to-kidney | 0.79 ± 0.19 | 1.01 ± 0.06 | 0.85 ± 0.04 | 1.20 ± 0.21 | 1.41 ± 0.18 |
| 44Sc-NODAGA-NOC | 68Ga-NODAGA-NOC | ||||
| AR42J | 30 min p.i. | 2 h p.i. | 5 h p.i. | 30 min p.i. | 2 h p.i. |
| Tumor-to-blood | 5.23 ± 0.22 | 50.3 ± 16.8 | 78.6 ± 6.95 | 4.32 ± 0.58 | 76.0 ± 4.31 |
| Tumor-to-liver | 8.13 ± 0.72 | 11.9 ± 3.26 | 11.6 ± 1.51 | 4.36 ± 0.91 | 5.88 ± 0.68 |
| Tumor-to-kidney | 0.80 ± 0.10 | 1.22 ± 0.34 | 0.87 ± 0.24 | 0.58 ± 0.10 | 0.85 ± 0.08 |
Preclinical PET imaging studies with 44Sc- and 68Ga-labeled peptides
PET/CT experiments were performed with one or two mice 3 h after injection of 44Sc- and 68Ga-labeled DOTA-RGD, NODAGA-RGD, DOTA-NOC and NODAGA-NOC, respectively (Figs. 5 and 6). 44Sc-DOTA-RGD showed clearly less accumulation of radioactivity in the liver than 68Ga-DOTA-RGD (Fig. 5a/b). 44Sc-NODAGA-RGD was comparable to 68Ga-NODAGA-RGD but showed somewhat more background activity in the abdominal tract (Fig. 5c/d). The uptake pattern of 44Sc-DOTA-RGD was slightly more favorable over the distribution of 44Sc-NODAGA-RGD (Fig. 5a/c).
Fig. 5.
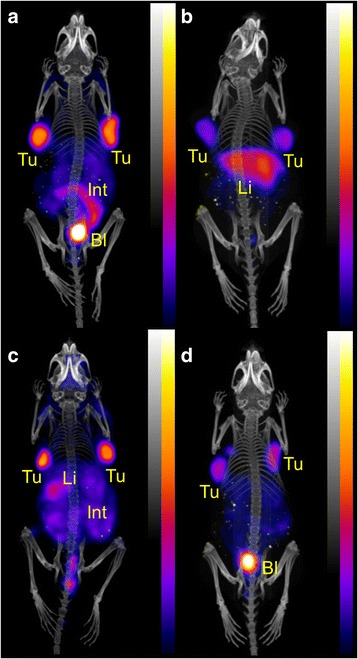
PET/CT scans of U87MG tumor-bearing mice 3 h after injection of ~10 MBq (~1 nmol) 44Sc-DOTA-RGD (a), (~10 MBq/~1 nmol) 68Ga-DOTA-RGD (b), ~10 MBq (~1 nmol) 44Sc-NODAGA-RGD (c) and ~10 MBq (~1 nmol) 68Ga-NODAGA-RGD (d). During the PET (20 min) and the CT (1.5 min) scans the mice were anesthetized with isoflurane/oxygen (Tu = U87MG tumor xenografts, Li = liver, Int = intestines, Bl = urinary bladder)
Fig. 6.
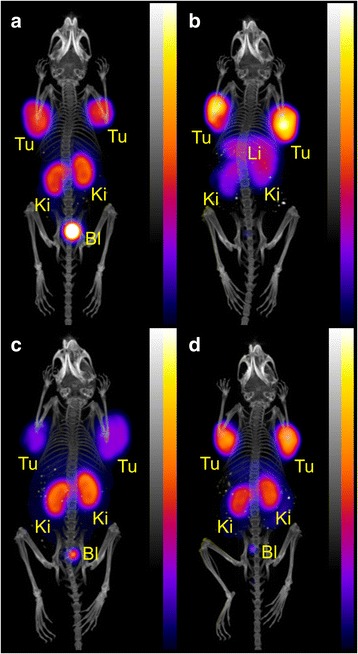
PET/CT scans of AR42J tumor-bearing mice 3 h after injection of ~10 MBq (~1 nmol) 44Sc-DOTA-NOC (a), ~10 MBq (~1 nmol) 68Ga-DOTA-NOC (b), ~10 MBq (~3 nmol) 44Sc-NODAGA-NOC (c) and ~10 MBq (~1 nmol) 68Ga-NODAGA-NOC (d). During the PET (20 min) and the CT (1.5 min) scans the mice were anesthetized with isoflurane/oxygen (Tu = U87MG tumor xenografts, Li = liver, Int = intestines, Bl = urinary bladder)
PET/CT scans obtained at 3 h after injection of 44Sc-DOTA-NOC revealed uptake of radioactivity only in the tumor xenografts and in the kidneys, while 68Ga-DOTA-NOC accumulated also to a significant extent in the liver (Fig. 6a/b). 44Sc-NODAGA-NOC and 68Ga-NODAGA-NOC accumulated solely in tumors and kidneys (Fig. 6c/d). The tumor uptake of 44Sc-NODAGA-NOC in the mouse, which was used for PET imaging, was reduced, resulting in lower tumor-to-kidney ratios compared to 68Ga-NODAGA-NOC. In this context, it has to be mentioned that 44Sc-NODAGA-NOC was prepared at a low specific activity for the PET scan (Fig. 6c) which implies that the injected molar amount of peptide was significantly increased compared to the peptide amount injected with 68Ga-NODAGA-NOC and, as a result, the binding sites in the tumor tissue may have been saturated.
An overview of the PET/CT scans of all four peptides labeled with 44Sc at 5 h after injection showed largely the same tissue distribution as was found at 3 h after injection (Fig. 7). While RGD-based peptides accumulated in the U87MG tumor xenografts and showed residual activity in the intestinal tract, NOC-based peptides accumulated in AR42J tumors xenografts and showed significant retention of radioactivity in the kidneys.
Fig. 7.
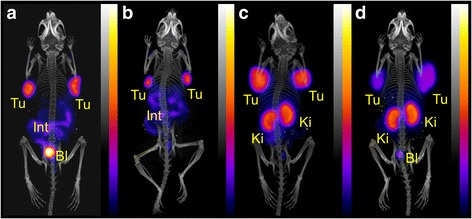
PET/CT scans of U87MG tumor-bearing mice 5 h after injection of ~10 MBq (~1 nmol) 44Sc-DOTA-RGD (a) and ~10 MBq (~1 nmol) 44Sc-NODAGA-RGD (b). PET/CT scans of AR42J tumor-bearing mice 5 h after injection of ~10 MBq (~1 nmol) 44Sc-DOTA-NOC (c) and ~10 MBq (~3 nmol) 44Sc-NODAGA-NOC (d). During the PET (20 min) and the CT (1.5 min) scans the mice were anesthetized with isoflurane/oxygen (Tu = U87MG (a/b) or AR42J tumor xenografts (c/d), Ki = kidney, Int = intestines, Bl = urinary bladder)
Discussion
A number of preclinical studies demonstrated the potential of 44Sc as an alternative PET radiometal to the currently-used 68Ga (Müller et al. 2013; Koumarianou et al. 2012; Hernandez et al. 2014; Miederer et al. 2011). With this in mind, the possibility to extend its applications to peptides of clinical relevance is of great interest for medical physicians. Herein, we reported on the first, to our knowledge, preclinical study concerning the 44Sc-labeling of peptides comprising a NODAGA-chelator, as well as their in vitro and in vivo behavior. Several authors proposed the DOTA-chelator as the most suitable ligand for binding Sc(III), whereas for Ga(III) it is known that NODAGA complexes provide higher thermodynamic stability than the DOTA complex (Huclier-Markai et al. 2011; Notni et al. 2012). Currently, the 68Ga-DOTA functionalized somatostatin receptor analogues are among the most prominent radiopharmaceuticals for clinical PET imaging (Banerjee & Pomper 2013). Since NODAGA derivatized biomolecules have not been used for labeling with 44Sc to date, the question arose on whether or not sufficient stability can be achieved for in vivo application of 44Sc-NODAGA compounds.
In order to perform the preclinical experiments effectively, it was necessary to obtain the 44Sc in a small solution volume, suitable for direct radiolabeling and subsequent in vivo application, without extensive dilution. Previously, we reported on the implementation of SCX resin to concentrate the 44Sc radioactivity in a small volume (van der Meulen et al. 2015). Although the use of this resin is already established for the concentration of the 68Ga eluate from the 68Ge generator, the high osmolarity of the eluate is not suitable for direct in vivo application. Herein, we report the use of DGA extraction chromatographic resin to effectively concentrate ~85 % of the 44Sc radioactivity in a small volume of 700 μL. The acidic solution containing the 44Sc was mixed with sodium acetate to obtain a pH of 4–4.5 for radiolabeling reactions. This procedure allowed in vivo application of the radiolabeled peptides without excessive dilution.
Reproducible labeling with 44Sc at high specific activities (10 MBq/nmol) was achieved for peptides functionalized with a DOTA-chelator. The same was possible for 68Ga with both DOTA- and NODAGA-functionalized peptides. Radiolabeling of NODAGA-compounds with 44Sc, however, proved to be more challenging and was not achieved reproducibly at high specific activity. It may be a result of potentially interfering metal contaminations to which NOTA/NODAGA is more susceptible than DOTA, as previously reported (Simecek et al. 2013). If NODAGA-functionalized peptides should be used with 44Sc for clinical studies, it will be important to determine the maximum concentration of metal contaminants which would still allow high specific and reproducible labeling with 44Sc. A potential optimization of the labeling may also be accessible by thorough investigation of different buffer systems and the use of microwave heating (Elander et al. 2000). Finally, even when the labeling was achieved successfully, the stability of 44Sc-labeled NODAGA-peptides was clearly inferior to the stability of 44Sc-labeled DOTA-compounds. It will be important, thus, to investigate the conditions which enhance the stability of 44Sc-NODAGA-peptides, potentially allowing an increased shelf-life which would be necessary in view of a clinical application.
In the initial in vitro test, the stability of 44Sc- and 68Ga-labeled peptides was investigated in saline with and without addition of excess and Fe3+ and Cu2+, respectively, to determine the possibility of metal challenge. None of the conditions impaired the integrity of 44Sc-labeled DOTA-RGD and DOTA-NOC, even after four half-lives of incubation at 37 °C. The obtained results are in agreement with those of Pruszynski et al., who reported an unchanged stability of 44Sc-DOTA-TOC in the presence of metal cations (Pruszynski et al. 2012). The amount of intact 68Ga-DOTA-RGD and 68Ga-DOTA-NOC was only decreased after the addition of Cu2+ (0.01 M) which was comparable with the time- and Cu2+-concentration dependent transmetalation of 68Ga-DOTA-TATE (Oehlke et al. 2013). 44Sc-labeled NODAGA-peptides were significantly less stable, indicating an onset of release of the radionuclide from the chelator only one half-life after labeling. This was in clear contrast to the 68Ga-NODAGA-peptides, which were completely stable over the whole period of investigation. The NODAGA-chelator revealed less stable coordination of 44Sc compared to the DOTA under the experimental conditions in this work. Distribution coefficients determined in n-octanol and PBS pH 7.4 revealed logD values in the same range (−4.70 to −4.26) for all RGD-based peptides, irrespective of the chelator and radionuclide which was employed. The logD values obtained with NOC-based peptides were slightly higher (−1.68 to −2.54) for all four radiopeptides (44Sc/68Ga-DOTA/NODAGA-NOC), indicating increased lipophilic properties compared to the RGD-peptides (Additional file 1: Table S1).
Biodistribution studies were performed with tumor-bearing mice at different time points after administration of the 44Sc-labeled peptides. Application of 44Sc-DOTA/NODAGA-RGD and 44Sc-DOTA/NODAGA-NOC, respectively, resulted in only small variations of the kinetics, independent of whether a DOTA or a NODAGA chelator was used (Fig. 3b). Comparing the tissue distribution profiles of 44Sc-labeled peptides with those of 68Ga-labeled peptides revealed that the differences between DOTA- and NODAGA-derivatized compounds were largely due to the different properties of these peptides, rather than the consequence of any kind of instability (Fig. 4). Indications of in vivo instability of the 44Sc-NODAGA-compounds were not apparent, with the exception of the increasing kidney uptake from 3 to 5 h after injection of 44Sc-NODAGA-NOC (Fig. 3b). It remained unclear, however, whether this was due to release of 44Sc from the NODAGA chelator since injection of free 44Sc resulted in unspecific retention of radioactivity in the liver and intestinal tract, rather than in the kidneys (Additional file 1: Figure S1). Generally, the instability of 44Sc-NODAGA compounds in solution was time-dependent and, as a consequence, it appeared not to be an issue for imaging purposes at relatively short time points (<1 half-life of 44Sc) after injection. The tissue distribution profiles with each peptide were similar, independent of whether it was labeled with 44Sc or 68Ga. One of the most conspicuous differences between 44Sc- and 68Ga-labeled peptides, however, was the increased liver uptake of 68Ga-DOTA-peptides, which was seen in PET images obtained with both DOTA-RGD and DOTA-NOC, respectively. Biodistribution studies confirmed these differences in liver uptake for DOTA-NOC (Fig. 4c), however, in the case of DOTA-RGD the liver uptake was relatively high for both 68Ga-DOTA-RGD and 44Sc-DOTA-RGD (Fig. 4a). This was in contrast to 44Sc- and 68Ga-labeled NODAGA-RGD peptides, which showed a clearly reduced liver uptake at 2 h p.i. in comparison (Fig. 4b). Since uncoordinated 68Ga may also accumulate to a significant extent in the bones as it was shown in a separate experiment (Additional file 1: Figure S1), it is unlikely that the described results are a consequence of released 68Ga from the DOTA-chelator.
Previously, it was reported that 68Ga-DOTA-RGD showed a higher blood pool activity than 68Ga-NODAGA-RGD (Knetsch et al. 2011; Decristoforo et al. 2008). As a result and in agreement with our studies, it was found that 68Ga-DOTA-RGD accumulates to a higher extent in the liver than 68Ga-NODAGA-RGD (Fig. 4a/b). In this context, it is also important to note that the peptide structure of the DOTA-RGD comprises a phenylalanine, whereas in the case of the NODAGA-RGD the phenylalanine was replaced with a tyrosine, which may have an influence on the pharmacokinetics of these peptides (Fig. 1). Overall, 68Ga-NODAGA-RGD revealed a more favorable tissue distribution profile than 68Ga-DOTA-RGD, shown in previous studies as well as in the experiments presented in this work (Knetsch et al. 2011; Pohle et al. 2012). A similar trend, albeit less pronounced, was seen for the 44Sc-labeled RGD peptides, showing lower background activity of 44Sc-NODAGA-RGD compared to that of 44Sc-DOTA-RGD (Fig. 4a/b).
The most striking difference between 44Sc- and 68Ga-labeled NOC-based peptides was the reduced liver uptake of 44Sc-DOTA-NOC as compared to 68Ga-DOTA-NOC, although 68Ga-DOTA-NOC accumulated to a higher extent in the tumor tissue than 44Sc-DOTA-NOC (Fig. 4c). When looking at the tissue distribution profiles of the 44Sc- and 68Ga-NODAGA-NOC peptides, they were found to be largely comparable with slightly higher retention of 68Ga-NODAGA-NOC in the kidneys (Fig. 4d). These results were also largely comparable to previously published data obtained with 68Ga-NODAGA-TOC (Eisenwiener et al. 2002). Even though the 44Sc-NODAGA-NOC revealed to be the least stable in vitro, its tissue distribution profile was largely comparable to 68Ga-labeled NODAGA-NOC indicating that the compound was stable in vivo.
Overall, it was found that 44Sc-DOTA-peptides were of significantly higher stability than the corresponding 44Sc-NODAGA-peptides, as expected, based on stability constants previously reported (Huclier-Markai et al. 2011; Port et al. 2008). Our experience also revealed that metal impurities would clearly interfere more distinctly with the stability of 44Sc-NODAGA-compounds than in the case of 44Sc-DOTA-compounds. Finally, it is important to note that the 44Sc-labeling of a NODAGA-functionalized biomolecule appears to be dependent on the overall chemical structure of the compound, as the 44Sc-NODAGA-RGD was found to be clearly more stable than the 44Sc-NODAGA-NOC. NODAGA-chelators are, thus, not excluded from use for labeling with 44Sc, but a thorough investigation of each case will be necessary in order to guarantee sufficient stability of the radiopharmaceutical.
Based on our results, which show clear differences in kinetics between labeled DOTA- and NODAGA-functionalized peptides, it is likely that 44Sc would be the preferred nuclide to be used with DOTA-functionalized biomolecules, in order to reflect the tissue distribution of 177Lu-labeled compounds more accurately, than if 68Ga was used for the same purpose.
Conclusions
In this work, it was demonstrated that 44Sc can be used for the labeling of biomolecules with both a DOTA and NODAGA chelator, although using a NODAGA-chelator proved to be more challenging. Other than with 68Ga, which shows clearly better results if coordinated with a NODAGA-chelator, 44Sc appears to be more stably complexed with DOTA. Based on these results, we conclude that even though 44Sc would be most favorably coordinated with a DOTA-chelator, coordination with a NODAGA-chelator is possible if the labeling conditions and storage buffers are validated. When using 44Sc and 47Sc for theragnostic application, however, DOTA is clearly the chelator of choice.
Compliance with Ethical Standards
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Additional file
Supplementary experimental data. (DOCX 777 kb)
Acknowledgements
The authors thank Dr. Christiaan Vermeulen, Klaudia Siwowska, Susan Cohrs, David Bölsterli, Walter Hirzel, Alexander Sommerhalder, Muhamet Djelili and André Isenschmid for technical assistance.
Funding information
The research was funded by the Swiss National Science Foundation (CR23I2_156852 and IZLIZ3_156800) and Paul Scherrer Institut (internal grant).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KD and RF performed the separation of 44Sc, did radiolabeling and stability experiments and drafted the manuscript. RMS performed in vitro and in vivo experiments and assisted in writing the manuscript. CM was responsible for the performance of the in vitro and in vivo studies and supervised these experiments. NvdM was responsible for the development of the production and separation process of 44Sc and supervised the whole study. NvdM and CM designed and wrote the final manuscript. RS and AT reviewed the manuscript. All authors read and approved the final manuscript.
Contributor Information
Katharina A. Domnanich, Email: katharina.domnanich@psi.ch
Cristina Müller, Email: cristina.mueller@psi.ch.
Renata Farkas, Email: renata.farkas@psi.ch.
Raffaella M. Schmid, Email: raffaella.schmid@psi.ch
Bernard Ponsard, Email: bernard.ponsard@sckcen.be.
Roger Schibli, Email: roger.schibli@psi.ch.
Andreas Türler, Email: andreas.tuerler@psi.ch.
Nicholas P. van der Meulen, Phone: +41-56-310 50 87, FAX: +41-56-310 28 49, Email: nick.vandermeulen@psi.ch
References
- Banerjee SR, Pomper MG. Clinical applications of gallium-68. Appl Radiat Isot. 2013;76:2–13. doi: 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty R, Goel S, Valdovinos HF, Hernandez R, Hong H, Nickles RJ, et al. Matching the decay half-life with the biological half-life: ImmunoPET imaging with 44Sc-labeled cetuximab Fab fragment. Bioconjug Chem. 2014;25(12):2197–204. doi: 10.1021/bc500415x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Phi JH, Paeng JC, Kim SK, Lee YS, Jeong JM, et al. Imaging of integrin alpha(V)beta(3) expression using 68Ga-RGD positron emission tomography in pediatric cerebral infarct. Mol Imaging. 2013;12(4):213–7. [PubMed] [Google Scholar]
- Decristoforo C, Hernandez Gonzalez I, Carlsen J, Rupprich M, Huisman M, Virgolini I, et al. 68Ga- and 111In-labelled DOTA-RGD peptides for imaging of avb3 integrin expression. Eur J Nucl Med Mol Imaging. 2008;35(8):1507–15. doi: 10.1007/s00259-008-0757-6. [DOI] [PubMed] [Google Scholar]
- Eigner S, Vera DR, Fellner M, Loktionova NS, Piel M, Lebeda O, et al. Imaging of protein synthesis: in vitro and in vivo evaluation of 44Sc-DOTA-puromycin. Mol Imaging Biol. 2013;15(1):79–86. doi: 10.1007/s11307-012-0561-3. [DOI] [PubMed] [Google Scholar]
- Eisenwiener KP, Prata MI, Buschmann I, Zhang HW, Santos AC, Wenger S, et al. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjug Chem. 2002;13(3):530–41. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]
- Elander N, Jones JR, Lu SY, Stone-Elander S. Microwave-enhanced radiochemistry. Chem Soc Rev. 2000;29(4):239–49. doi: 10.1039/a901713e. [DOI] [Google Scholar]
- Hernandez R, Valdovinos HF, Yang Y, Chakravarty R, Hong H, Barnhart TE, et al. 44Sc: an attractive isotope for peptide-based PET imaging. Mol Pharm. 2014;11(8):2954–61. doi: 10.1021/mp500343j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huclier-Markai S, Sabatie A, Ribet S, Kubicek V, Paris M, Vidaud C, et al. Chemical and biological evaluation of scandium(III)-polyaminopolycarboxylate complexes as potential PET agents and radiopharmaceuticals. Radiochim Acta. 2011;99:653–62. doi: 10.1524/ract.2011.1869. [DOI] [Google Scholar]
- Kim JH, Lee JS, Kang KW, Lee HY, Han SW, Kim TY, et al. Whole-body distribution and radiation dosimetry of 68Ga-NOTA-RGD, a positron emission tomography agent for angiogenesis imaging. Cancer Biother Radiopharm. 2012;27(1):65–71. doi: 10.1089/cbr.2011.1061. [DOI] [PubMed] [Google Scholar]
- Knetsch PA, Petrik M, Griessinger CM, Rangger C, Fani M, Kesenheimer C, et al. [68Ga]NODAGA-RGD for imaging avb3 integrin expression. Eur J Nucl Med Mol Imaging. 2011;38(7):1303–12. doi: 10.1007/s00259-011-1778-0. [DOI] [PubMed] [Google Scholar]
- Koumarianou E, Loktionova NS, Fellner M, Roesch F, Thews O, Pawlak D, et al. 44Sc-DOTA-BN[2–14]NH2 in comparison to 68Ga-DOTA-BN[2–14]NH2 in pre-clinical investigation. Is 44Sc a potential radionuclide for PET? Appl Radiat Isot. 2012;70(12):2669–76. doi: 10.1016/j.apradiso.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17(1):R53–73. doi: 10.1677/ERC-09-0078. [DOI] [PubMed] [Google Scholar]
- Majkowska-Pilip A, Bilewicz A. Macrocyclic complexes of scandium radionuclides as precursors for diagnostic and therapeutic radiopharmaceuticals. J Inorg Biochem. 2011;105(2):313–20. doi: 10.1016/j.jinorgbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Miederer M, Loktionova NS, Bausbacher N, Buchholz H, Piel M, Schreckenberger M, et al. Small Animal PET-Imaging with 44Sc-DOTATOC. Eur J Nucl Med Mol Imaging. 2011;38:S158-S. [Google Scholar]
- Müller C, Bunka M, Reber J, Fischer C, Zhernosekov K, Türler A, et al. Promises of cyclotron-produced 44Sc as a diagnostic match for trivalent b−-emitters: In vitro and in vivo study of a 44Sc-DOTA-folate conjugate. J Nucl Med. 2013;54(12):2168–74. doi: 10.2967/jnumed.113.123810. [DOI] [PubMed] [Google Scholar]
- Müller C, Bunka M, Haller S, Köster U, Groehn V, Bernhardt P, et al. Promising prospects for 44Sc-/47Sc-based theragnostics: application of 47Sc for radionuclide tumor therapy in mice. J Nucl Med. 2014;55(10):1658–64. doi: 10.2967/jnumed.114.141614. [DOI] [PubMed] [Google Scholar]
- Notni J, Pohle K, Wester HJ. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI Res. 2012;2(1):28. doi: 10.1186/2191-219X-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlke E, Le VS, Lengkeek N, Pellegrini P, Jackson T, Greguric I, et al. Influence of metal ions on the 68Ga-labeling of DOTATATE. Appl Radiat Isot. 2013;82:232–8. doi: 10.1016/j.apradiso.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ. 68Ga-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol. 2012;39(6):777–84. doi: 10.1016/j.nucmedbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Port M, Idee JM, Medina C, Robic C, Sabatou M, Corot C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals. 2008;21(4):469–90. doi: 10.1007/s10534-008-9135-x. [DOI] [PubMed] [Google Scholar]
- Pruszynski M, Majkowska-Pilip A, Loktionova NS, Eppard E, Rösch F. Radiolabeling of DOTATOC with the long-lived positron emitter 44Sc. Appl Radiat Isot. 2012;70(6):974–9. doi: 10.1016/j.apradiso.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Rösch F. Scandium-44: benefits of a long-lived PET radionuclide available from the 44Ti/44Sc generator system. Curr Radiopharm. 2012;5(3):187–201. doi: 10.2174/1874471011205030187. [DOI] [PubMed] [Google Scholar]
- Simecek J, Hermann P, Wester HJ, Notni J. How is 68Ga labeling of macrocyclic chelators influenced by metal ion contaminants in 68Ge/68Ga generator eluates? ChemMedChem. 2013;8(1):95–103. doi: 10.1002/cmdc.201200471. [DOI] [PubMed] [Google Scholar]
- van der Meulen NP, Bunka M, Domnanich KA, Müller C, Haller S, Vermeulen C, et al. Cyclotron production of 44Sc: From bench to bedside. Nucl Med Biol. 2015;42(9):745–51. doi: 10.1016/j.nucmedbio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Viola-Villegas N, Doyle R. The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N, N’, N”, N‴-tetraacetic acid (H4DOTA): Structural overview and analyses on structure-stability relationships. Coordin Chem Rev. 2009;253:1906–25. doi: 10.1016/j.ccr.2009.03.013. [DOI] [Google Scholar]
- Yoon HJ, Kang KW, Chun IK, Cho N, Im SA, Jeong S, et al. Correlation of breast cancer subtypes, based on estrogen receptor, progesterone receptor, and HER2, with functional imaging parameters from 68Ga-RGD PET/CT and 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2014;41(8):1534–43. doi: 10.1007/s00259-014-2744-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary experimental data. (DOCX 777 kb)


