Abstract
Objective:
The results of studies on the association between tumor necrosis factor-a -308G/A (TNF-a -308G/A) polymorphism, and susceptibility to essential hypertension are controversial. To derive a more precise estimation, we conducted a meta-analysis of all similar articles.
Methods:
The summary effect odds ratios and 95% confidence intervals were obtained. Funnel plots and Egger’s test were used to estimate publication bias, and heterogeneity was assessed by the chi-square-based Q-test and I2 test.
Results:
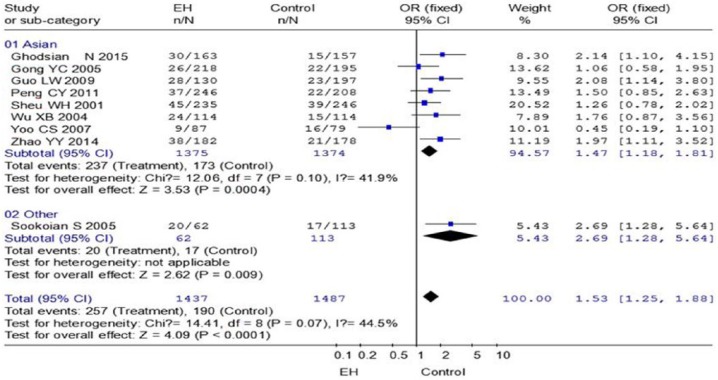
Nine studies (with 1437 cases and 1487 controls) were included. In the overall analysis, the combined results showed that there were significant differences in genotype distribution between essential hypertension cases and controls, AA+GA versus GG (OR = 1.53, 95% CI: 1.25–1.88, p < 0.00001). In the stratified analysis by country, we found that essential hypertension cases had a significantly higher frequency of AA+GA versus GG (OR = 1.47, 95% CI: 1.18–1.81, p = 0.0004) than control in the Asian population.
Conclusions:
This meta-analysis supports previous findings that TNF-a -308G/A polymorphism may increase the risk of essential hypertension, at least in the Asian population.
Keywords: Essential hypertension, gene, polymorphism, TNF-a -308G/A, meta-analysis
Introduction
Hypertension is thought to be a multifactorial disorder and is the major risk factor for cardiovascular disease.1 According to the large epidemiological investigation, patients with essential hypertension (EH) accounted for 90% of patients with hypertension.2 Although the etiology of EH has not been determined, genetic factors have a certain impact on it.3,4
In recent years, several studies have indicated that inflammation plays an important role in the development of EH.5,6 One study in 2015 showed that the levels of pro-inflammatory cytokines increased in EH patients, which suggested that an immune function disorder was associated with EH.7 Tumor necrosis factor-a (TNF-a) stimulates cytokine production, which enhances the expression of adhesion molecules and increases neutrophil activation. Navarro-González et al. in 2008 found that the serum level of TNF-a was higher in hypertensive patients than in healthy controls.8 A similar phenomenon was found in other studies.9-11 TNF-a protein levels were predominantly under genetic control.12 The TNF gene is located on chromosome 6, within the class III region of the human leukocyte antigen (HLA).13 Of these polymorphisms, G-to-A substitutions at position -308 (rs1800629) have been widely studied.14
Several studies have examined the relationship between TNF-a -308G/A polymorphism and EH, but the conclusions are often inconsistent. It may be difficult to achieve comprehensive and reliable results in an individual study due to sample size. In 2012, Li et al. performed a meta-analysis in order to clarify the effect of TNF-a -308G/A polymorphism on the risk of EH, which contained seven studies.14 Since 2012, an expanding body of literature assessing the association between the TNF-a -308G/A polymorphism and hypertension has been published. It is widely accepted that the same polymorphism plays a different role in different ethnic populations. To derive a more precise estimation, we performed an updated meta-analysis of published studies to pool results between studies.
Materials and methods
Literature search strategy
The electronic databases of PubMed, the Chinese Biomedical Database (CBM), and the Chinese National Knowledge Infrastructure (CNKI) were searched up to April 2016 for all genetic association human studies evaluating TNF-a -308G/A polymorphism and EH. The following index terms were used: “hypertension” or “blood pressure,” “tumor necrosis factor-alpha,” “polymorphism or variant.” Chinese and English articles were included. In addition, we read the titles of references of the selected articles.
Inclusion criteria and data extraction
We read all the titles and abstracts of the relevant articles. The following criteria were used to include published studies: (a) the publication was a case-control design; (b) the papers had available genotype frequency or had sufficient data to estimate an odds ratio (OR); and (c) the genotype distribution in the control group was in line with the Hardy Weinberg equilibrium (HWE).
According to the above criteria, the following information was extracted by two of the researchers country of origin from all eligible studies: the first author, the date of publication, ethnicity, number of genotypes, and genotyping information and frequencies of alleles. China, Malaysia, and Korea were classified into the Asian subgroup, and Argentina into the American subgroup.
Statistical analysis
The pooled ORs and 95% CIs (confidence intervals) were calculated to assess the risk of EH and TNF-a -308G/A polymorphism. We calculated pooled ORs under A-allele comparison (A vs. G) and dominant model (AA+GA vs. GG). The Z-test was used to calculate the combined OR value; p ≤ 0.05 was statistically significant.
The Q and I2 statistics were used to assess statistical heterogeneity.15 I2 values of 25%, 50%, and 75% represented low, moderate, and high heterogeneity, respectively. Heterogeneity p < 0.10 was considered significant as a significant heterogeneity. The random-effects model (if p < 0.10) or the fixed-effects model (if p ≥ 0.10) was used to summarize the combined OR. The HWE test (p < 0.05) among the control groups in each study was checked by using an online HWE calculator (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). Funnel plot and Egger’s test were used to evaluate the publication bias. A p-value < 0.1 indicated a potential publication bias.16 Sensitivity analysis was performed to assess the stability of the results. Analyses were performed using Stata software, version 10 (StataCorp LP, College Station, Texas, USA) and Review Manager 4.2 (Cochrane Collaboration).
Results
Study selection and subject characteristics
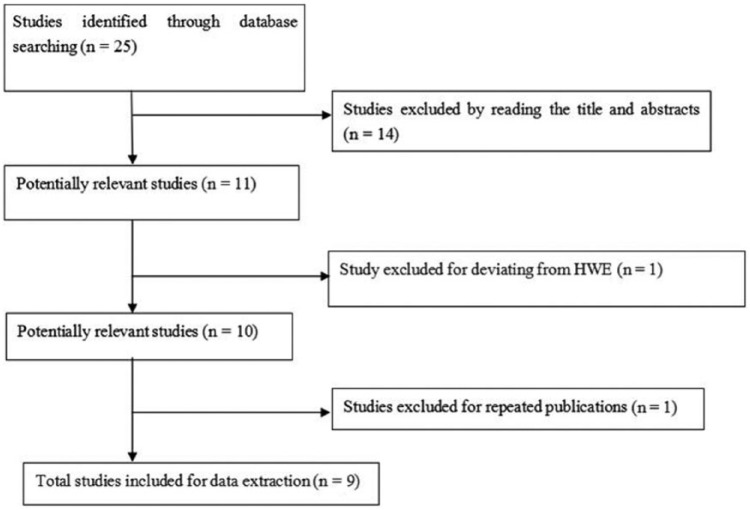
The flow diagram in Figure 1 summarizes the selection process of this literature review. Based on the inclusion criteria, a total of 10 relevant studies concerning TNF-a -308G/A polymorphism and EH were initially identified. Among the 10 eligible articles, one study was excluded because it was a duplicate.17 Finally, a total of nine case-control studies were identified, including 1437 cases and 1487 controls.18-26 These nine studies encompassed one Caucasian and eight Asian studies. The characteristics of the selected studies and the numbers of subjects are shown in Table 1. The included studies were published between 2001 and 2015. The sample sizes range from 166 to 481. Eight studies were conducted in Asian populations and one in an American population. Furthermore, the genotype distributions in the controls of all the included studies were consistent with HWE (p > 0.05).
Figure 1.
Flow diagram of paper screening.
Table 1.
Characteristics of studies included in the meta-analysis.
| Author (Reference) |
Year | Country(Ethnicity) | Sample size(Case/Con ) | Distribution of genotype |
HWE p |
||
|---|---|---|---|---|---|---|---|
| GG |
GA |
AA |
|||||
| Case/Con | Case/Con | Case/Con | |||||
| Sheu WH | 2001 | China(Asian) | 235/246 | 190/207 | 42/38 | 3/1 | 0.593 |
| Wu XB | 2004 | China(Asian) | 114/114 | 90/99 | 24/15 | 0/0 | 0.452 |
| Peng CY | 2011 | China(Asian) | 246/208 | 209/186 | 39/22 | 8/0 | 0.421 |
| Gong YC | 2005 | China(Asian) | 218/195 | 192/173 | 23/22 | 3/0 | 0.404 |
| Yoo CS | 2007 | Korea(Asian) | 87/79 | 78/63 | 9/16 | 0/0 | 0.317 |
| Sookoian | 2005 | Argentina(American) | 62/113 | 42/96 | 18/15 | 2/2 | 0.142 |
| Guo LW | 2009 | China(Asian) | 130/197 | 102/174 | 28/21 | 0/2 | 0.148 |
| Zhao YY | 2014 | China(Asian) | 182/178 | 144/157 | 37/19 | 1/2 | 0.119 |
| Ghodsian | 2015 | Malaysia(Asian) | 163/157 | 133/142 | 28/15 | 2/0 | 0.530 |
Case = Essential hypertension; Con = Control.
Meta-analysis results and sensitivity analysis
A meta-analysis of all subjects and a subgroup analysis by ethnicity was performed. For the dominant model, heterogeneity was observed (I2 = 44.5%, p = 0.07) and the original data were combined by using a random-effects model. The summary OR was 1.53 (GG+GA vs. AA: P<0.00001, 95% CI: 1.25-1.88) by the fixed effects model and 1.54 (p = 0.003, 95% CI: 1.16–2.04) by the random-effects model (Figure 2 TNF-a -308G’). It was indicated that there was an intensively positive association between allele and EH risk. In the subgroup analysis by ethnicity, significant risk was also found in the Asian population for GG+GA versus AA (p = 0.0004, OR = 1.47, 95% CI: 1.18–1.81, pheterogeneity = 0.10) under the fixed effects model. The allele results (Asian: p = 0.0001, OR = 1.48, 95% CI: 1.21–1.80, pheterogeneity = 0.17) were consistent with the dominant model.
Figure 2.
Meta-analysis with a random-effects model for the association between EH risk and the TNF-a -308G/A polymorphism (AA+GA vs. GG).
The OR estimate for each study is marked with a solid black square. The size of the square represents the weight that the corresponding study exerts in the meta-analysis. The CIs of the pooled estimates are displayed as a horizontal line through the diamond; this entire line may be inside the diamond if the CI is narrow.
A selected article in the included studies was deleted each time to reflect the influence of the individual data setting on the pooled ORs; no obvious change was observed in any of the pooled ORs following deletion.
Publication bias
Following confirmation that there was no obvious asymmetry in the funnel plot (Figure 3) (AA+AG vs. GG), an Egger test was carried out to verify the symmetry of the funnel plot. The results suggested that there was no publication bias in this study (data not shown).
Figure 3.
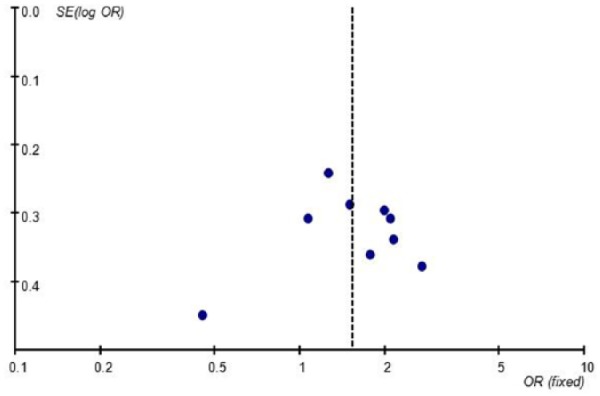
Funnel plot for publication bias in selection of studies on the TNF-a -308G/A polymorphism (AA+GA vs. GG).
Discussion
In recent years, increasing evidence has shown that inflammation plays an important role in the occurrence and development of EH. Based on the different role in the inflammatory process, inflammatory factors can be divided into pro-inflammatory cytokines and anti-inflammatory cytokines. TNF-a is a cytokine produced by the multinuclear giant cells, with a wide range of biological activities, which not only has the function of regulating the body’s immune function, but can also mediate the inflammatory reaction process. TNF-a, as a pro-inflammatory cytokine, can secrete potent contractile vasoactive substance endothelin 1, which can lead to increased blood pressure.27 Increased TNF-a can accelerate lipid peroxidation, which can destroy the structural integrity and function of endothelial cells, causing an imbalance in endothelial cells that secrete active substances. Increased secretion of endothelin by vasodilators and vasoconstriction accelerates vasoconstriction and improves peripheral resistance and blood pressure.
A cross-sectional study of the relationship between TNF-a, interleukin6, C-reactive protein, and other indicators of inflammation and hypertension found that, after adjusting for age, gender, Body mass index, and family history index, TNF-a might be an independent risk factor for EH.28 Another report studied by Kwang-II Kim in 2008 found that the TNF-a level correlated with the fluctuation of blood pressure.29 The TNF-a -308G/A polymorphism could increase TNF-a expression, which is associated with various inflammatory diseases.30,31 Increasing numbers of scholars are exploring scholars the relationship between TNF-a -308G/A polymorphism and EH, but the conclusions are not consistent.18-26 A single study may not draw a comprehensive and reliable conclusion due to the low effectiveness of the tests used. In addition, because of the different frequencies (TNF-a -308G/A) of the different regions and ethnic groups, the extrapolation of the foreign research is limited.
In 2012, Li et al. performed a meta-analysis that only contained seven studies on the relationship between TNF-a -308G/A polymorphism and EH. After that, other researchers published several new case-control studies on this topic in 2014 and 2015.25,26 The same polymorphism was found to play a different role in different ethnic populations. Therefore, for clarification, an updated meta-analysis and subgroup analysis by ethnicity including all the published manuscripts was needed.
Based on our meta-analysis, the results showed that the TNF-a -308G/A polymorphism may be an EH susceptibility gene across populations. In the subgroup analysis, there was an association between the TNF-a -308G/A polymorphism and EH in the Asian population. In our meta-analysis, there was only one study based on a Caucasian population, in Argentina, that found unless the AA+GA gene is responsible for carrying genotype increased the risk of EH. Heterogeneity was the main factor that affected the reliability of the results. Two genetic models had no obvious heterogeneity in our study. Sensitivity analysis and subgroup analysis were used in this study. The ORs and 95% CIs were calculated, and the results were consistent. The overall sensitivity and stability of the study were high.
One of the advantages of this study is that a number of comparable studies have been combined to increase sample size and statistical power, which helps to draw a more convincing result. In our meta-analysis, we established strict literature inclusion and exclusion criteria, and excluded duplicates, incomplete data and non-case-control trials. Furthermore, selection bias was controlled, and to a minimum, which, we believe, supports the objectivity and credibility of the conclusion. Primary hypertension is a multifactorial disease in the genetic background. This study only carried out a single factor analysis of TNF-a gene polymorphism, and had several limitations. First, the literature only included Chinese- and English-language articles, which might have led to a language bias. Second, the time span of each study and the specific detection technology were different, thus, there may have been bias within the study. In addition, although several new studies were included in this meta-analysis, the sample size was still small.
In conclusion, the results of the meta-analysis based on nine studies showed that TNF-a -308G/A polymorphism might increase the risk of EH, at least in the Asian population. Due to the number and level of current clinical trials, the impact of factors such as the level of the author and objective conditions, the above conclusions are yet to be further confirmed by further prospective studies.
Footnotes
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant numbers 81541071 and 81673266). We would like to express our gratitude to the physicians participating in this study. The authors would also like to thank the editors of this manuscript.
Author contributions: Analyzed the data: YYS, CWW, and JYL. Contributed reagents/materials/analysis tools: JYL and CWW. Wrote the paper: YYS and CWW.
References
- 1. Nguyen K-DH, Pihur V, Ganesh SK, et al. Effects of rare and common blood pressure gene variants on essential hypertension results from the Family Blood Pressure Program, CLUE, and Atherosclerosis Risk in Communities Studies. Circ Res 2013; 112: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghosh R, Bhattacharya M, Khan G, et al. Diagnosis of essential hypertension in humans by the determination of plasma renal cortexin using enzyme-linked immunosorbent assay. Clin Lab 2013; 59: 475–481. [PubMed] [Google Scholar]
- 3. Briet M, Schiffrin EL.Treatment of arterial remodeling in essential hypertension. Curr Hypertension Rep 2013; 15: 3–9. [DOI] [PubMed] [Google Scholar]
- 4. Singh M, Singh AK, Pandey P, et al. Molecular genetics of essential hypertension. Clin Exp Hypertens 2016; 38: 268–277. [DOI] [PubMed] [Google Scholar]
- 5. Krishnan SM, Dowling JK, Ling YH, et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br J Pharmacol 2016; 173: 752–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solak Y, Afsar B, Vaziri ND, et al. Hypertension as an autoimmune and inflammatory disease. Hypertens Res 2016; 39: 567–573. [DOI] [PubMed] [Google Scholar]
- 7. Dange RB, Agarwal D, Teruyama R, et al. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation 2015; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Navarro-González JF, Mora C, Muros M, et al. Association of tumor necrosis factor-alpha with early target organ damage in newly diagnosed patients with essential hypertension. J Hypertens 2008; 26: 2168–2175. [DOI] [PubMed] [Google Scholar]
- 9. Zhong MH, Gu J, Zhang EY. Clinical significances of plasma interleukin-6, C-reaction protein and tumor necrosis factor-alphain patients with aortic dissection. Sichuan Da Xue Xue Bao Yi Xue Ban 2015; 46: 234–237. [PubMed] [Google Scholar]
- 10. Bespalova ID, Riazantseva NV, Kaliuzhin VV, et al. Effect of atorvastatin on pro-inflammatory status (in vivo and in vitro) in patients with essential hypertension and metabolic syndrome. Kardiologiia 2014; 54:37–43. [DOI] [PubMed] [Google Scholar]
- 11. Okura T, Jotoku M, Irita J, et al. Association between cystatin C and inflammation in patients with essential hypertension. Clin Exp Nephrol 2010;14: 584–588. [DOI] [PubMed] [Google Scholar]
- 12. Wilson AG, Symons JA, McDowell TL, et al. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A 1997; 94: 3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen RD. Polymorphism of the human TNF-alpha promoter – random variation or functional diversity? Mol Immunol 1999; 36: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 14. Li YY. Tumor necrosis factor-alpha g308α gene polymorphism and essential hypertension: a meta-analysis involving 2244 participants. PLoS One 2012; 7: e35408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 16. Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo LW, Li D, Huang YM, et al. Correlation between TNF and gene polymorphisms and essential hypertension in HAN Chinese of Henan Province. Chinese Journal of Prevention and Control of Chronic Diseases 2011; 19: 571–573. (In Chinese) [Google Scholar]
- 18. Guo LW, Wu YM, Huang YM, et al. Correlation between tumor necrosis factor α and β gene polymorphisms and essential hypertension. Journal of Xinxiang Medical College 2009; 26: 352–354. (In Chinese) [Google Scholar]
- 19. Sheu WH, Lee WJ, Lin LY, et al. Tumor necrosis factor alpha 2238 and 2308 polymorphisms do not associate with insulin resistance in hypertensive subjects. Metabolism 2001; 50: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 20. Wu XB, Zhou SH. Association of the tumor necrosis factor-alpha gene G308A promoter polymorphism with essential hypertension. Chin J Hyper 2004; 12: 415–418. (In Chinese) [Google Scholar]
- 21. Yoo CS, Hwang WJ, Hong SH, et al. Relationship between iris constitution analysis and TNF-alpha gene polymorphism in hypertensives. Am J Chin Med 2007; 35: 621–629. [DOI] [PubMed] [Google Scholar]
- 22. Peng CY, Zhou CL, He QZ. Association of TNF-a gene 2308G/A polymorphism with essential hypertension in Han racial origin in Hunan. Chin J Clin Pharmacol Ther 2011; 16: 57–60. (In Chinese) [Google Scholar]
- 23. Sookoian S, García SI, Gianotti TF, et al. The G-308A promoter variant of the tumor necrosis factor-alpha gene is associated with hypertension in adolescents harboring the metabolic syndrome. Am J Hyperten 2005; 18: 1271–1275. [DOI] [PubMed] [Google Scholar]
- 24. Gong YC, Li H, Shen Y, et al. Tumor necrosis factor-α gene polymorphism in hypertensive obesities. Chin J Hy pretension 2005;13: 19–23. (In Chinese) [Google Scholar]
- 25. Ghodsian N, Akhlaghi M, Ramachandran V, et al. Association of TNF-α G308A gene polymorphism in essential hypertensive patients without type 2 diabetes mellitus. Genet Mol Res 2015; 14: 18974–18979. [DOI] [PubMed] [Google Scholar]
- 26. Zhao YY. Research on the polymorphism and relevance of the TNF gene for Guanxi Zhuang People’s essential hypertension. Guangxi: Guangxi Medical University Press. (In Chinese) [Google Scholar]
- 27. Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-α promoter polymorphism effects transcription. Mol Immunol 1997; 34: 391–399. [DOI] [PubMed] [Google Scholar]
- 28. Bautista LE, Vera LM, Arenas IA, et al. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 2005; 19: 149–154. [DOI] [PubMed] [Google Scholar]
- 29. Kim KI, Lee JH, Chang HJ, et al. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circ J 2008; 72: 293–298. [DOI] [PubMed] [Google Scholar]
- 30. Ulger M, Emekdaş G, Aslan G, et al. Determination of the cytokine gene polymorphism and genetic susceptibility in tuberculosis patients. Mikrobiyol Bul 2013; 47: 250–264. [DOI] [PubMed] [Google Scholar]
- 31. Gu L, Wu G, Su L, et al. TNF-a (-238G/A and -308G/A) gene polymorphisms may not contribute to the risk of ischemic stroke. Int J Neurosci 2016; 126: 219–226. [DOI] [PubMed] [Google Scholar]