Abstract
Background and objective:
Currently, there is no effective therapy available for liver fibrosis. This study aims to evaluate the efficacy of renin angiotensin system inhibitors on liver fibrosis.
Method:
Full-text randomized controlled trials in patients with liver fibrosis were identified and included in the meta-analysis. The primary outcome measure was the histological fibrosis score of the liver. Secondary outcome measures included fibrosis area of the liver, serological levels of fibrosis markers, adverse events, and withdrawals.
Results:
From 6973 non-duplicated entries by systematic search, four randomized controlled trials with 210 patients were identified. The renin angiotensin system inhibitors therapy resulted in a marginally significant reduction in liver fibrosis score (MD = -0.30; 95% CI: -0.62–0.02, p = 0.05) and a significant reduction in liver fibrosis area (MD = -2.36%; 95% CI: -4.22%–-0.50%, p = 0.01) as compared with control. The therapy was well tolerated and there was no significant difference in withdrawals between treatment and control groups (RD = 0.00; 95% CI: -0.06–0.06, p = 0.97).
Conclusions:
Renin angiotensin system inhibitor therapy results in a reduction in liver fibrosis score and liver fibrosis area in patients with hepatic fibrosis with good safety profile. However, randomized controlled trials of high-quality will clarify the effectiveness of renin angiotensin system inhibitors on liver fibrosis.
Keywords: Angiotensin-converting-enzyme inhibitors, angiotensin receptor blocker, liver fibrosis, treatment
Introduction
Chronic liver diseases, including chronic viral hepatitis, non-alcoholic fatty liver disease, chronic alcoholic liver disease and others, if untreated, are generally characterized by progressive inflammation and liver fibrosis, which may lead to liver cirrhosis and hepatocellular carcinoma (HCC).1–3 The efficacy of current therapies for liver diseases is limited and there are no effective therapies currently available for liver fibrosis.
Liver fibrosis is characterized by the excess production and deposition of extracellular matrix (ECM). A major source of ECM includes hepatic satellite cells (HSCs).4 HSCs are stimulated by fibrogenic cytokines, one of which is angiotensin II,5 an effector hormone of the renin angiotensin system (RAS). The profibrogenic effect of angiotensin II is associated with an increased concentration of transforming growth factor β1 (TGF-β1).6 Previous research has showed that angiotensin II increased the TGF-β1 mRNA expression in the activated HSCs, and this effect was completely blocked by one of the RAS inhibitors, angiotensin II receptor blocker (ARB) candesartan.7 RAS inhibition causes a decrease in connective tissue growth factor and angiotensin II type-1 (AT1) receptor expression, and is associated with decreased TGF-β1 expression in the injured liver.8,9 In vitro experiments demonstrated that telmisartan, another ARB, and AT1 receptor knockdown following exposure of long chain fatty acids reduced cellular lipid accumulation,10 suggesting that AT1 receptor and its blocker may play a key biological role in the regulation of hepatic lipid metabolism.10 In another study, RAS was suggested to be involved in the transition of steatosis to steatohepatitis.11 Steatosis has been shown to be associated with fibrosis severity in chronic hepatitis caused by HBV or HCV infection12 and non-alcoholic fatty liver disease.13 Angiotensin II induces contraction and proliferation of HSCs by activating AT1 receptors, which are considered principal effectors of hepatic fibrosis.14 Studies in various animal models with liver fibrosis showed that angiotensin-converting-enzyme inhibitors (ACEIs)/ARBs may play an important role in anti-liver fibrosis.15–20 ACEIs are key negative regulators of the RAS, and function to limit fibrosis through the degradation of angiotensin II, and administration of recombinant ACEIs showed therapeutic potential in liver fibrosis.21 The ARB losartan was also shown to significantly inhibit the progression of liver fibrosis in a hepatic fibrosis rat model.22
In humans, many studies have shown the role of RAS in liver diseases. One study found that the circulating RAS components, such as plasma renin and angiotensin II, were markedly elevated in patients with advanced liver disease as compared with healthy controls.23 Another study demonstrated that elevated circulating angiotensin-converting enzyme (ACE) level may be used as a marker of fibrosis in patients with chronic hepatitis B.24 A recent study also showed that serum ACE levels may offer an easy, accurate and inexpensive noninvasive method for differentiating significant from nonsignificant liver fibrosis in autoimmune hepatitis.25 Treatment with losartan resulted in a significant decrease in hepatic fibrosis marker, plasma TGF-β1.26 Two retrospective studies found that hypertensive patients receiving ACEIs or ARBs had less fibrosis than hypertensive patients who did not receive these drugs.27,28 A pilot study showed that losartan could improve the liver fibrosis stage.29 Two prospective studies found that, in early stage cirrhosis and nonalcoholic steatohepatitis patients, ARBs could improve aminotransferases and decrease TGF-β1 levels.26,30 However, the effectiveness of ACEIs/ARBs on liver fibrosis is conflicting. A 48-month follow-up revealed that single treatment with ACEI did not exert inhibitory effects on hepatic fibrosis.31 In a hepatitis C long-term treatment against cirrhosis trial, continuous ACEIs/ARBs use for 3.5 years did not retard the progression of hepatic fibrosis.32 Several randomized controlled trials (RCTs) investigated the role of ACEI/ARBs in liver fibrosis with conflicting findings.23,28–30 The aim of this study was to conduct a systematic review in relation to the role of ACEI/ARBs in the treatment of liver fibrosis and a meta-analysis of RCTs assessing the efficacy and safety of using ACEI/ARBs for liver fibrosis.
Methods
Search strategy
Eligible trials were identified up to 30 April 2014 through electronic searches of The Cochrane Library, PubMed, Medline (Ovid), Web of Knowledge, Elsevier (ScienceDirect OnLine, SDOL), SpringerLink, and Wiley InterScience. The references of identified trials were hand-searched. Search terms were: “renin angiotensin aldosterone system”, “renin angiotensin system”, “angiotensin converting enzyme inhibitors”, “angiotensin receptor blockers”, “RAAS”, “RAS”, “ACEI”, “ARB”, and “liver fibrosis” and “hepatic fibrosis”.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were determined by two researchers (QZ and NL). Studies were considered to be included in this review if they met the following inclusion criteria: (i) English language; (ii) describing a pharmacological intervention for liver fibrosis or hepatic fibrosis; (iii) using ACEIs/ARBs therapy; (iv) liver fibrosis score and area or blood liver fibrosis marker undertaken at baseline and study end; (v) the participants without infection with HIV. The RCT would be considered to be included in meta-analysis. All other studies not meeting the inclusion criteria were excluded.
Data extraction and outcome measures
Data were extracted independently by two reviewers (QZ and NL) and validated by a third reviewer (ZL). The following data were extracted: primary author, year, and study design, numbers of patients randomized and lost during follow-up, and dosage and duration of intervention.
The primary outcome measure was histological fibrosis score of the liver. Secondary outcome measures included fibrosis area of the liver, serological levels of fibrosis markers, adverse events, and withdrawals.
Methodological quality score of the included studies
The quality of the RCTs was assessed by the Jadad score system.33 The Jadad score system is the only known 5-item scale developed with standard scale development techniques and has been used to assess the quality of RCTs for almost two decades. It is a simple, short, and reliable approach. The Jadad scores would be higher and more consistent if quality assessment was blinded; the inadequate allocation concealment would exaggerate treatment efficacy in RCTs.34,35 Therefore, the Jadad score system was used to assess the quality of the RCTs.
Data synthesis and statistical analysis
Continuous data were presented as mean difference (MD) with a 95% confidence interval (CI). Inter-study heterogeneity was assessed with χ2 tests and the I2 measure.36 I2 value < 25% was regarded as no heterogeneity. Meta-analysis was performed in Review Manager 5 (The Cochrane Collaboration, Oxford, England). Random effects model was used when a significant heterogeneity exists among the studies analyzed. Adverse events and withdrawals were reported as a risk difference (RD, 95% CI).
Results
Search
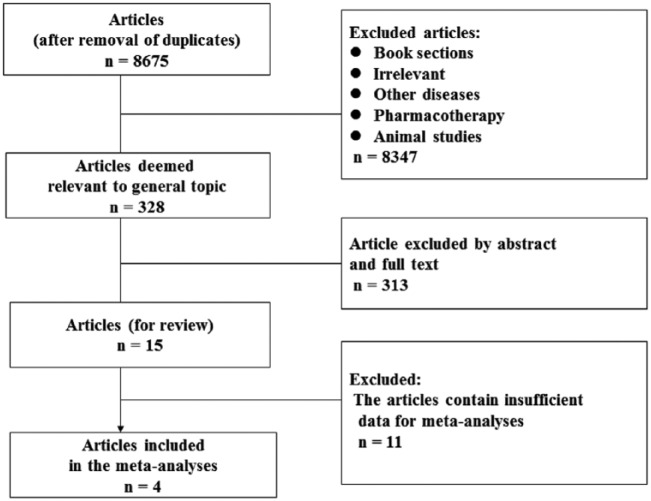
A total of 6973 non-duplicated entries were identified by the systematic search, and titles were reviewed. Abstracts and full texts were obtained for articles to determine eligibility for inclusion. Fifteen papers were included in this review27–32,37–45 (Table 1) and four RCTs30,40,43,44 (Table 2) were identified and included in the meta-analysis (Figure 1). The reasons for excluding the 11 studies from meta-analysis are listed in Table 3.
Table 1.
Patient characteristics of the review.
| First author, year | Participants | Duration | Liver biopsies | Noninvasive fibrosis markers | The effects of Angiotensin blockade on fibrosis | |
|---|---|---|---|---|---|---|
| Terui Y, 200230 | 30 patients with early stages of chronic hepatitis C | RCT | Not mentioned | Yes | Yes | Promising beneficial effects |
| Rimola A, 200427 | 128 recurrent hepatitis C after LT | Retrospective cohort study | 41 months (median) | Yes | No | Beneficial effects |
| Rincon D, 200537 | 123 HCV-patients who received LT | Retrospective cohort study | > 6 months | Yes | No | No beneficial effects |
| Sookoian S, 200529 | 23 patients with chronic hepatitis C non-responders |
Prospective cohort study | 14 months | Yes | No | Beneficial effects |
| Yoshiji H, 200538 | 20 patients with chronic hepatitis C | RCT | 12 months | No | Yes | Promising beneficial effects |
| Yoshiji H, 200639 | 40 patients with chronic hepatitis C |
RCT | 12 months | No | Yes | Beneficial effects |
| Debernardi-Venon W, 200740 | 47 selected cirrhotic patients | RCT | 12 months | No | Yes | Promising beneficial effects |
| Corey KE, 200928 | 234 patients with hepatitis C | Retrospective cohort study | 5.08 years | Yes | No | Beneficial effects |
| Colmenero J, 200941 | 14 patients with CHC with liver fibrosis | Uncontrolled open-label study | 18 months | Yes | No | Promising beneficial effects |
| Cholongitas E, 201042 | 102 recurrent hepatitis C after LT | Retrospective cohort study | 13 months (median) | Yes | No | No beneficial effects |
| Abu Dayyeh BK, 201132 | 192 patients with hepatitis C | RCT | 42 months | Yes | No | No beneficial effects |
| Hidaka H, 201143 | 48 selected cirrhotic patients | RCT | 12 months | No | Yes | Promising beneficial effects |
| Kim MY, 201244 | 85 patients with compensated alcoholic liver fibrosis | RCT | 6 months | Yes | No | Promising beneficial effects |
| Yoshiji H, 201231 | 110 patients with cirrhosis associated with hepatocellular carcinoma | RCT | 48 months | No | Yes | Beneficial effects |
| Guillaud O, 201345 | 109 recurrent hepatitis C after LT | Retrospective cohort study | 23 months (median) | Yes | No | No beneficial effects |
RCT, randomized controlled trial; LT, liver transplantation.
Table 2.
Characteristics and quality analysis of studies included in the meta-analysis.
| Author, year | No. of patients (total) | Etiology of liver fibrosis | Pharmacological intervention | Control | Duration | Design | Jadad score |
|---|---|---|---|---|---|---|---|
| Terui Y, 200230 | 30 | 30 viral liver disease | Losartan 50 mg/d plus UDCA 600 mg/d | UDCA 600 mg/d | N/A | RCT | 2 |
| Debernardi-Venon W, 200740 | 47 | 40 viral and 7 alcohol liver disease | Candesartan 8mg/d | No treatment | 12 mo | RCT | 5 |
| Hidaka H, 201143 | 48 | 31 viral, 9 alcohol liver disease and 8 others | Olmesartan 10–40 mg/d (10 to 20 then to 40 mg) | No treatment | 12 mo | RCT | 5 |
| Kim MY, 201244 | 85 | 85 alcohol liver disease | Candesartan 8mg/d plus UDCA 600mg/d | UDCA 600 mg/d | 6 mo | RCT | 5 |
N/A, data not available; UDCA, ursodeoxycholic acid.
Figure 1.
Flowchart showing the process of literature searching and selection.
Table 3.
Details of exclusion reasons.
| First author, year | The exclusion reasons |
|---|---|
| Rimola A, 200427 | Not RCT. The results were demonstrated by percentage of cirrhosis or mean and range of cirrhosis but not mean ± SD which could not be calculated with other studies in the meta-analysis. |
| Rincon D, 200537 | Not RCT. The results were demonstrated by percentage of fibrosis stage 2–4 but not mean ± SD which could not be calculated with other studies in the meta-analysis. |
| Sookoian S, 200529 | Not RCT. The basic fibrosis was not comparable between the two groups. |
| Yoshiji H, 200538 | The treated group was given both IFN and perindopril while the controlled group treated nothing. |
| Yoshiji H, 200639 | The results were demonstrated by changed percentage of fibrosis score which could not be calculated with other studies in the meta-analysis. |
| Corey KE, 200928 | Not RCT. The results were demonstrated by mean of cirrhosis without SD which could not be calculated with other studies in the meta-analysis. |
| Colmenero J, 200941 | Not RCT. No control group. |
| Cholongitas E, 201042 | Not RCT. The results were demonstrated by changed mean fibrosis score but not mean ± SD which could not be calculated with other studies in the meta-analysis. |
| Abu Dayyeh BK, 201132 | The results were demonstrated by percentage of 2-point increases in fibrosis which could not be calculated with other studies in the meta-analysis. |
| Yoshiji H, 201231 | The treated group was given both IFN and perindopril while the controlled group treated nothing. |
| Guillaud O, 201345 | Not RCT. The results were demonstrated by change of median fibrosis score which could not be calculated with other studies in the meta-analysis. |
RCT, randomized controlled trial; SD, standard deviation; IFN, interferon.
Patient characteristics
Two hundred and ten patients, including 148 male and 62 female, with liver fibrosis of various etiologies, participating in the four studies met the inclusion criteria. The mean age was 55.5 (18–75) years. Of the 210 patients, the etiologies of liver fibrosis were: 101 alcoholic, 101 viral and 8 other causes.
Interventions
For the intervention, all the studies used ARBs: one study used olmesartan for treatment and no treatment as control,43 one used candesartan for treatment and no treatment as control,40 one used losartan plus ursodeoxycholic acid (UDCA) for treatment and UDCA as control,30 and one used candesartan plus UDCA for treatment and UDCA as control.44 Details of treatment and control medication and duration are shown in Table 2.
Fibrosis score of the liver
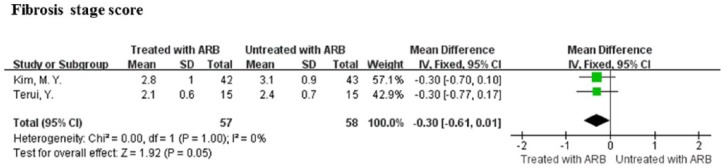
Two of the four studies underwent liver biopsy before study entry and at the end of the study and provided sufficient data for the calculation of mean differences and 95% CI of histological fibrosis score evaluated according to the METAVIR scoring system.30,44 The ARB therapy showed an insignificant but marginally positive effect on fibrosis score (MD = -0.30, 95% CI: -0.62–0.02, P = 0.05; Figure 2). No significant heterogeneity between these studies was observed (I2 = 0%, P = 1.00; Figure 2).
Figure 2.
Meta-analysis of fibrosis score of the liver in angiotensin receptor blockers treatment and control patients.
Fibrosis area of the liver
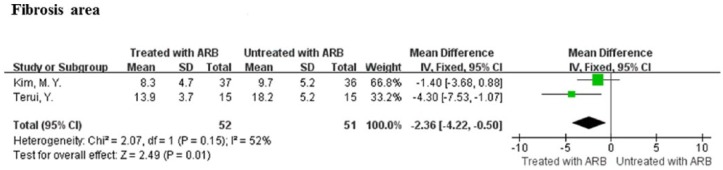
Two of the four studies provided sufficient data for the calculation of mean differences and 95% CI of fibrosis area of the liver (%),30,44 which was expressed as the percentage of the total area measured using an image analysis system.30,44 The ARB therapy resulted in a significant reduction in fibrosis area in comparison with control (MD = -2.36, 95% CI: -4.22–-0.50, P = 0.01; Figure 3). There was a modest heterogeneity among these studies (I2 = 52%, P = 0.15; Figure 3).
Figure 3.
Meta-analysis of fibrosis area of the liver in angiotensin receptor blockers treatment and control patients.
Fibrosis markers of the liver
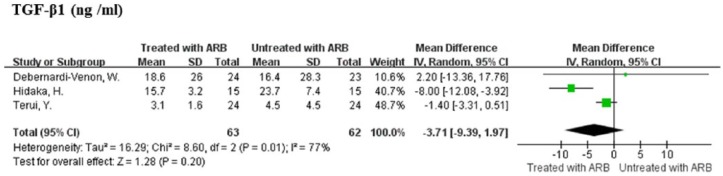
The serological levels of fibrosis markers assessed with sufficient data for the calculation of mean differences and 95% CI were type IV collagen in two studies30,43 and TGF-β1 in three studies.30,40,43 ARB therapy was not associated with decreased levels of type IV collagen as compared with control (MD = -0.64, 95% CI: -1.63–0.36, P = 0.21). There was a modest heterogeneity between the studies (I2 = 51%, P = 0.15). The ARB therapy was not significantly associated with reduction in TGF-β1 levels as compared with control (MD = -3.71, 95% CI: -9.39–1.97, P = 0.20; Figure 4). A significant heterogeneity existed among the studies (I2 = 77%, P = 0.01; Figure 4).
Figure 4.
Meta-analysis of fibrosis markers (serological levels of transforming growth factor beta 1 (TGF-β1)) in angiotensin receptor blockers treatment and control patients.
Adverse events and withdrawals
ARBs were well tolerated by all patients in all of the studies,30,40,43,44 except one patient in one study did not complete the study because of symptomatic hypotension.43 Overall, there was no significant difference in withdrawals in the treatment and control groups (RD = 0.00; 95% CI -0.06–0.06, P = 0.97). Details regarding adverse events and withdrawals are provided in Table 4.
Table 4.
Adverse events and withdrawals of studies included in meta-analyses.
| Author, year | Intervention group | Control group |
|---|---|---|
| Terui Y, 200230 | No adverse events or withdrawals | No adverse events or withdrawals |
| Debernardi-Venon W, 200740 | No adverse events or withdrawals | No adverse events or withdrawals |
| Hidaka H, 201143 | 2 withdrawals (1 for symptomatic hypotension, 1 for patient choice) | 1 withdrawal (patient choice) |
| Kim MY, 201244 | 5 withdrawals (2 for ingestion of alcohol, 1 for low medical compliance, 2 loss to follow-up) | 7 withdrawals (3 for ingestion of alcohol, 2 for low medical compliance, 2 loss to follow-up) |
Methodological quality of the included studies
The quality assessment for each study is shown in Table 2. No study was double-blinded. No study had > 5% loss to follow-up.
Discussion
In many animal models for liver fibrosis, ACEI/ARBs could retard the progression of liver fibrosis and/or decrease the serum TGF-β1.15,17,46–49 In human retrospective studies, ACEI/ARBs could decrease liver fibrosis in patients with chronic liver disease.27,28 In patients with recurrent hepatitis C virus infection after liver transplantation, the fibrosis stage and the fibrosis progression index in the liver biopsy obtained at the end of follow-up was significantly lower in patients who were treated with ACEI/ARBs than in those without the use of ACEI/ARBs.27 However, the findings pertinent to the effect of ACEI/ARBs on hepatic fibrosis are still conflicting. For example, in the studies of disease with the same etiology, a pilot study showed that losartan improved liver fibrosis stage in patients with chronic hepatitis C,29 but continuous ACEI/ARB use for 3.5 years in a hepatitis C long-term treatment against cirrhosis cohort was not shown to retard the progression of hepatic fibrosis.32 Of note, these studies are mainly retrospective or pilot, which may limit the validity of the studies and partly contribute to the inconsistencies.
The present study provides the first meta-analysis of RCTs investigating the effect of ARBs on the liver fibrosis in humans. Despite heterogeneity among the studies, the analyses show that ARB therapy was marginally associated with the improvement in histological liver score and significantly associated with the improvement in fibrosis area of the liver. The therapy was well tolerated by patients and there were no significant withdrawals.
Hepatic histopathology remains the gold standard of liver fibrosis. Two studies in our analysis evaluated histological fibrosis score of the liver.30,44 Our analysis showed that ARB therapy has a trend of positive effect on liver fibrosis assessed by METAVIR system. These two studies are well-designed RCTs with no significant heterogeneity. This may confer more authenticity on the results. One study also evaluated liver fibrosis using Laennec fibrosis scoring system which divided cirrhosis into three subclasses and was believed to have a more detailed estimation of fibrosis,30,44 showing that, although no significance in fibrosis according to the METAVIR system, candesartan therapy significantly increased the improvement rate in fibrosis according to the Laennec system evaluated by either intention-to-treat (ITT) (33.3% vs. 11.6%, P = 0.020) or per-protocol (PP) analysis (37.8% vs. 13.9%, P = 0.032).30,44 As the modification of METAVIR scoring system, the Laennec scoring system subdivides the liver cirrhosis stage F4 according to METAVIR scoring system into three (4A, 4B, 4C) stages based on fibrosis thickness and nodule size. Studies showed that Laennec scoring system had a significant correlation to the clinical stages of cirrhosis, Child-Pugh and MELD scores, severity of portal hypertension, and the Laennec scoring system might have the potential in predicting the liver-related events because of the sub-classification of cirrhosis.50–52
The present analysis also shows that ARB therapy has a significant positive effect on liver fibrosis assessed by the fibrosis area of the liver. With regard to serological fibrosis markers, ARB therapy has no significant effect on type IV collagen and TGF-β1. ARB therapy has a significant positive effect on levels of hyaluronic acid (HA) in two studies40,43 although pooled analysis was not performed because of the different measurements of this parameter in the studies.40,43 Serum HA measurement is indicated to be a sensitive, specific and reliable marker for assessing the degree of liver fibrosis in chronic liver diseases53–59 and monitoring the progressiveness of liver fibrosis and the histological response of hepatic fibrosis to treatment.53,60 Comparatively, the serum levels of HA were suggested to be correlated with the degree of hepatic fibrosis more closely and specifically than type IV collagen.60,61 The level of plasma TGF-β1 was not suggested to be a sensitive variable for the evaluation of hepatic fibrosis in both adult and child patients with chronic liver disease.62–64 Furthermore, it should be noted that a well-designed study included in our analysis assessed the relative expression of TGF-β1 in liver tissue by real-time reverse transcriptase–polymerase chain reaction, showing a significant decrease of the relative expression of TGF-β1 in liver tissue in the candesartan group.44 Collectively, it appears that ARB therapy is beneficial for the improvement of liver fibrosis, especially when evaluated by more sensitive, specific, and accurate measures such as Laennec fibrosis scoring system and serum HA level.
In all of the studies included in our analysis, the RAS inhibitors used were ARBs. Whether there are some distinctions between ARBs and ACEIs remains unclear. ARBs were shown to be superior to ACEIs in the suppression of hepatic fibrosis in an animal study.65 Another study, however, showed that ARBs improved only the necroinflammation in paired biopsies but not the fibrosis progression in human.42 Methodologically, all of the studies included in our analysis are RCTs, but only patients in two studies underwent liver biopsy.30,44 Moreover, though the random effects model was used in our analysis when there was significant heterogeneity among the studies, this may still lead to bias and decrease the authenticity and reliability of the analysis. Therefore, more prospective randomized controlled trials, including studies focusing on ACEIs and ARBs to distinguish their possible difference on liver fibrosis, are deserved to elucidate the role of RAS inhibitors in treating liver fibrosis.
In conclusion, this study shows that ARB therapy was associated with a trend in the improvement of histological liver fibrosis score and a significant improvement in fibrosis area of the liver in patients with liver fibrosis. More RCTs of high-quality using more precise evaluation parameters are needed to clarify the effectiveness of ACEI/ARBs on hepatic fibrosis in human liver disease.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant no. 81371798). We thank Dr Patrick C. Kellish from the Department of Anatomy and Cell Biology, University of Florida College of Medicine for the careful and expert editing of this paper.
References
- 1. Matsuzaki K, Murata M, Yoshida K, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology 2007; 46: 48–57. [DOI] [PubMed] [Google Scholar]
- 2. Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol 2012; 56: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berasain C, Castillo J, Perugorria MJ, et al. Inflammation and liver cancer: New molecular links. Ann N Y Acad Sci 2009; 1155: 206–221. [DOI] [PubMed] [Google Scholar]
- 4. Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: New insights and prospects for therapy. J Gastroenterol Hepatol 1999; 14: 618–633. [DOI] [PubMed] [Google Scholar]
- 5. Bataller R, Sancho-Bru P, Gines P, et al. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology 2003; 125: 117–125. [DOI] [PubMed] [Google Scholar]
- 6. Gaedeke J, Peters H, Noble NA, et al. Angiotensin II, TGF-beta and renal fibrosis. Contrib Nephrol 2001: 153–160. [DOI] [PubMed] [Google Scholar]
- 7. Yoshiji H, Kuriyama S, Yoshii J, et al. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 2001; 34: 745–750. [DOI] [PubMed] [Google Scholar]
- 8. Bataller R, Sancho-Bru P, Gines P, et al. Liver fibrogenesis: A new role for the renin-angiotensin system. Antioxid Redox Signal 2005; 7: 1346–1355. [DOI] [PubMed] [Google Scholar]
- 9. Kurikawa N, Suga M, Kuroda S, et al. An angiotensin II type 1 receptor antagonist, olmesartan medoxomil, improves experimental liver fibrosis by suppression of proliferation and collagen synthesis in activated hepatic stellate cells. Br J Pharmacol 2003; 139: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nabeshima Y, Tazuma S, Kanno K, et al. Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol 2009; 50: 1226–1235. [DOI] [PubMed] [Google Scholar]
- 11. Vergniol J, Barbu V, Lemoine M, et al. Progression of NAFLD in humans is associated with the activation of the renin-angiotensin system. J Hepatol 2011; 54: S348. [Google Scholar]
- 12. Cacopardo B, Camma C, Petta S, et al. Diagnostic and therapeutical role of vitamin D in chronic hepatitis C virus infection. Front Biosci (Elite Ed) 2012; 4: 1276–1286. [DOI] [PubMed] [Google Scholar]
- 13. Albano E, Mottaran E, Vidali M, et al. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut 2005; 54: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005; 115: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li X, Meng Y, Yang X, et al. Perindopril attenuates the progression of CCl4-inducing rat hepatic fibrosis. Chin J Hepatol 2004; 12: 32–34. [PubMed] [Google Scholar]
- 16. Park DH, Baik SK, Choi YH, et al. Inhibitory effect of angiotensin blockade on hepatic fibrosis in common bile duct-ligated rats. Korean J Hepatol 2007; 13: 61–69. [PubMed] [Google Scholar]
- 17. Toblli JE, Munoz MC, Cao G, et al. ACE inhibition and AT1 receptor blockade prevent fatty liver and fibrosis in obese Zucker rats. Obesity 2008; 16: 770–776. [DOI] [PubMed] [Google Scholar]
- 18. Ohishi T, Saito H, Tsusaka K, et al. Anti-fibrogenic effect of an angiotensin converting enzyme inhibitor on chronic carbon tetrachloride-induced hepatic fibrosis in rats. Hepatol Res 2001; 21: 147–158. [DOI] [PubMed] [Google Scholar]
- 19. Xu W, Song S, Huang Y, et al. Effects of perindopril and valsartan on expression of transforming growth factor-beta-Smads in experimental hepatic fibrosis in rats. J Gastroenterol Hepatol 2006; 21: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 20. Martini S, Alessandria C, Debernardi Venon W, et al. Angiotensin II type 1 receptor inhibition in early stage cirrhotic patients: Effects on portal pressure and liver fibrosis. J Hepatol 2004; 40: 70. [DOI] [PubMed] [Google Scholar]
- 21. Oesterreicher CH, Taura K, De Minicis S, et al. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology 2009; 50: 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei HS, Li DG, Lu HM, et al. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl(4). World J Gastroenterol 2000; 6: 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vilas-Boas WW, Ribeiro-Oliveira A, Jr., Pereira RM, et al. Relationship between angiotensin-(1–7) and angiotensin II correlates with hemodynamic changes in human liver cirrhosis. World J Gastroenterol 2009; 15: 2512–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Purnak T, Beyazit Y, Oztas E, et al. Serum angiotensin-converting enzyme level as a marker of fibrosis in patients with chronic hepatitis B. J Renin Angiotensin Aldosterone Syst 2012; 13: 244–249. [DOI] [PubMed] [Google Scholar]
- 25. Efe C, Cengiz M, Kahramanoglu-Aksoy E, et al. Angiotensin-converting enzyme for noninvasive assessment of liver fibrosis in autoimmune hepatitis. Eur J Gastroenterol Hepatol 2015; 27: 649–654. [DOI] [PubMed] [Google Scholar]
- 26. Yokohama S, Yoneda M, Haneda M, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 2004; 40: 1222–1225. [DOI] [PubMed] [Google Scholar]
- 27. Rimola A, Londono MC, Guevara G, et al. Beneficial effect of angiotensin-blocking agents on graft fibrosis in hepatitis C recurrence after liver transplantation. Transplantation 2004; 78: 686–691. [DOI] [PubMed] [Google Scholar]
- 28. Corey KE, Shah N, Misdraji J, et al. The effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Liver Int 2009; 29: 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sookoian S, Fernandez MA, Castano G. Effects of six months losartan administration on liver fibrosis in chronic hepatitis C patients: A pilot study. World J Gastroenterol 2005; 11: 7560–7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terui Y, Saito T, Watanabe H, et al. Effect of angiotensin receptor antagonist on liver fibrosis in early stages of chronic hepatitis C. Hepatology 2002; 36: 1022. [DOI] [PubMed] [Google Scholar]
- 31. Yoshiji H, Noguchi R, Ikenaka Y, et al. Combination of branched-chain amino acid and angiotensin-converting enzyme inhibitor improves liver fibrosis progression in patients with cirrhosis. Mol Med Report 2012; 5: 539–544. [DOI] [PubMed] [Google Scholar]
- 32. Abu Dayyeh BK, Yang M, Dienstag JL, et al. The effects of angiotensin blocking agents on the progression of liver fibrosis in the HALT-C trial cohort. Dig Dis Sci 2011; 56: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 34. Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995; 273: 408–412. [DOI] [PubMed] [Google Scholar]
- 35. Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998; 352: 609–613. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 37. Rincon D, Nogales O, Salcedo M, et al. Influence of ace-inhibitors (ACEI) in the evolution of fibrosis after liver transplantation (LT) in HCV-patients. J Hepatol 2005; 42: 58. [Google Scholar]
- 38. Yoshiji H, Noguchi R, Fukui H. Combined effect of an ACE inhibitor, perindopril, and interferon on liver fibrosis markers in patients with chronic hepatitis C. J Gastroenterol 2005; 40: 215–216. [DOI] [PubMed] [Google Scholar]
- 39. Yoshiji H, Noguchi R, Kojima H, et al. Interferon augments the anti-fibrotic activity of an angiotensin-converting enzyme inhibitor in patients with refractory chronic hepatitis C. World J Gastroenterol 2006; 14: 6786-6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Debernardi-Venon W, Martini S, Biasi F, et al. AT1 receptor antagonist Candesartan in selected cirrhotic patients: Effect on portal pressure and liver fibrosis markers. J Hepatol 2007; 46: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 41. Colmenero J, Bataller R, Sancho-Bru P, et al. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol 2009; 297: G726–G734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cholongitas E, Vibhakorn S, Lodato F, et al. Angiotensin II antagonists in patients with recurrent hepatitis C virus infection after liver transplantation. Liver Int 2010; 30: 334–335. [DOI] [PubMed] [Google Scholar]
- 43. Hidaka H, Nakazawa T, Shibuya A, et al. Effects of 1-year administration of olmesartan on portal pressure and TGF-beta1 in selected patients with cirrhosis: A randomized controlled trial. J Gastroenterol 2011; 46: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 44. Kim MY, Cho MY, Baik SK, et al. Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis – a randomized open-label controlled study. Liver Int 2012; 32: 977–987. [DOI] [PubMed] [Google Scholar]
- 45. Guillaud O, Gurram KC, Puglia M, et al. Angiotensin blockade does not affect fibrosis progression in recurrent hepatitis C after liver transplantation. Transplant Proc 2013; 45: 2331–2336. [DOI] [PubMed] [Google Scholar]
- 46. Turkay C, Yonem O, Arici S, et al. Effect of angiotensin-converting enzyme inhibition on experimental hepatic fibrogenesis. Dig Dis Sci 2008; 53: 789–793. [DOI] [PubMed] [Google Scholar]
- 47. Subeq Y-M, Ke C-Y, Lin N-T, et al. Valsartan decreases TGF-beta 1 production and protects against chlorhexidine digluconate-induced liver peritoneal fibrosis in rats. Cytokine 2011; 53: 223–230. [DOI] [PubMed] [Google Scholar]
- 48. Tuncer I, Ozbek H, Ugras S, et al. Anti-fibrogenic effects of captopril and candesartan cilexetil on the hepatic fibrosis development in rat. The effect of AT1-R blocker on the hepatic fibrosis. Exp Toxicol Pathol 2003; 55: 159–166. [DOI] [PubMed] [Google Scholar]
- 49. Shah N, Zheng H, Misdraji J, et al. Beneficial effect of angiotensin-blocking agents on liver fibrosis in patients with hepatitis C. Hepatology 2007; 46: 703A–704A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim MY, Cho MY, Baik SK, et al. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol 2011; 55: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 51. Kim SU, Oh HJ, Wanless IR, et al. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol 2012; 57: 556–563. [DOI] [PubMed] [Google Scholar]
- 52. Rastogi A, Maiwall R, Bihari C, et al. Cirrhosis histology and Laennec staging system correlate with high portal pressure. Histopathology 2013; 62: 731–741. [DOI] [PubMed] [Google Scholar]
- 53. Wong VS, Hughes V, Trull A, et al. Serum hyaluronic acid is a useful marker of liver fibrosis in chronic hepatitis C virus infection. J Viral Hepat 1998; 5: 187–192. [DOI] [PubMed] [Google Scholar]
- 54. McHutchison JG, Blatt LM, de Medina M, et al. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. Consensus Interferon Study Group. J Gastroenterol Hepatol 2000; 15: 945–951. [DOI] [PubMed] [Google Scholar]
- 55. Patel K, Gordon SC, Jacobson I, et al. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol 2004; 41: 935–942. [DOI] [PubMed] [Google Scholar]
- 56. Mehta P, Ploutz-Snyder R, Nandi J, et al. Diagnostic accuracy of serum hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating fibrosis stages in chronic hepatitis C. Am J Gastroenterol 2008; 103: 928–936. [DOI] [PubMed] [Google Scholar]
- 57. Kaneda H, Hashimoto E, Yatsuji S, et al. Hyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2006; 21: 1459–1465. [DOI] [PubMed] [Google Scholar]
- 58. Suzuki A, Angulo P, Lymp J, et al. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int 2005; 25: 779–786. [DOI] [PubMed] [Google Scholar]
- 59. Pares A, Deulofeu R, Gimenez A, et al. Serum hyaluronate reflects hepatic fibrogenesis in alcoholic liver disease and is useful as a marker of fibrosis. Hepatology 1996; 24: 1399–1403. [DOI] [PubMed] [Google Scholar]
- 60. Yamada M, Fukuda Y, Koyama Y, et al. Serum hyaluronic acid reflects the effect of interferon treatment on hepatic fibrosis in patients with chronic hepatitis C. J Gastroenterol Hepatol 1996; 11: 646–651. [DOI] [PubMed] [Google Scholar]
- 61. Crawford DH, Murphy TL, Ramm LE, et al. Serum hyaluronic acid with serum ferritin accurately predicts cirrhosis and reduces the need for liver biopsy in C282Y hemochromatosis. Hepatology 2009; 49: 418–425. [DOI] [PubMed] [Google Scholar]
- 62. Oberti F, Pilette C, Rifflet H, et al. Effects of simvastatin, pentoxifylline and spironolactone on hepatic fibrosis and portal hypertension in rats with bile duct ligation. J Hepatol 1997; 26: 1363–1371. [DOI] [PubMed] [Google Scholar]
- 63. Zhou X, Li D, Li X, et al. A combination of Ang II and carbon tetrachloride accelerates process of hepatic fibrosis. Chin Med J (Engl) 2003; 116: 62–65. [PubMed] [Google Scholar]
- 64. Lebensztejn DM, Sobaniec-Lotowska M, Kaczmarski M, et al. Serum concentration of transforming growth factor (TGF)-beta 1 does not predict advanced liver fibrosis in children with chronic hepatitis B. Hepatogastroenterology 2004; 51: 229–233. [PubMed] [Google Scholar]
- 65. Kim MY, Baik SK, Park DH, et al. Angiotensin receptor blockers are superior to angiotensin-converting enzyme inhibitors in the suppression of hepatic fibrosis in a bile duct-ligated rat model. J Gastroenterol 2008; 43: 889–896. [DOI] [PubMed] [Google Scholar]