Abstract
Introduction:
The main purpose of the present study was to investigate whether I/D polymorphism of the ACE gene might affect metabolic changes related to the metabolic syndrome through a long-term interdisciplinary therapy in obese adolescents.
Methods:
In total, 125 obese adolescents who entered the interdisciplinary obesity programme were assigned to the following two subgroups: metabolic syndrome or non-metabolic syndrome. They were evaluated at baseline and after 1 year. Genomic DNA was extracted from circulating leukocytes.
Results:
Subjects with the II genotype in the non-metabolic syndrome group were only to increase their fat-free mass after therapy. Regarding lipid profile, subjects with ID and DD genotypes from both groups reduced their low-density lipoprotein cholesterol levels significantly. The metabolic parameters from the ID and DD genotypes of the non-metabolic syndrome group showed a significantly improved insulin response.
Conclusion:
In the present study, we showed that the ACE polymorphism was able to influence the fat-free mass in the I-carry allele in the non-metabolic syndrome group positively. In addition, the I-carry allele was able to improve the insulin resistance of the metabolic syndrome group significantly. These results suggest that the ACE I/D genotypes can influence, in different ways, the specific parameters of metabolism among obese adolescents submitted for long-term interdisciplinary therapy.
Keywords: Interdisciplinary therapy, metabolic syndrome, obesity, angiotensin-converting enzyme, polymorphism
Introduction
Obesity is characterised as a condition in which adiposity is abnormally high, which represents a substantial health risk.1 This increase in fat mass has also been related to the sub-chronic inflammation state of obesity, favouring the development of several diseases, such as type 2 diabetes, hypertension, dyslipidaemias, non-alcoholic fatty liver disease and metabolic syndrome (MS).2
It is well known that childhood obesity is a well-recognised risk factor for developing MS in adulthood.3 MS has a complex pathogenesis in which interactions are involved between certain environmental factors, the lack of physical activity, stress and high-fat food. However, in the last few years, genome-wide association studies have identified numerous candidate polymorphisms that are unequivocally associated with metabolic-related traits, including obesity, diabetes, hypertension and dyslipidaemia.4 These studies have shown a range of 10–30% of participation in the heritability of MS.5–8
Among the candidate genes related to metabolic pathways, the genes for the B2 and B3-adrenergic receptors, lipoprotein lipase, hormone-sensitive lipase, peroxisome proliferator-activated receptor-γ, insulin receptor substrate-1 (IRS1) and glycogen synthase,4,9 among others,10,11 are involved in the development of MS.
Several studies have recently concentrated on genetic variants in the renin–angiotensin system (RAS) in association with the components of MS. The RAS plays an essential role in the regulation of blood pressure levels and renal homeostasis, and it is involved in clinical alterations in organ damage through the variation of gene expression as well as growth, fibrosis and inflammatory response.12–14
Angiotensin-converting enzyme (ACE) is recognised as one of the main effector enzymes of this system; ACE (EC 3.4.15.1) is a zinc metallopeptidase that converts the inactive decapeptide angiotensin I (Ang I) to the potent vasopressor angiotensin II (Ang II) in the RAS and inactivates the vasodilator bradykinin in the kallikrein–kinin system (KKS).15,16 The KKS is involved in glycaemic metabolism mainly via B2 receptor activation, which is able to mediate glucose uptake and improve insulin sensitivity. Thus the ACE enzyme concentration and activity could regulate the energetic homeostasis through modulation of this system.17
The ACE gene is localised on the long arm of chromosome 17 (17q23) and contains 26 exons. The gene is known to contain polymorphisms consisting of an insertion (I)/deletion (D) polymorphism of a 287-bp Alu-repeat sequence in intron 16 of the ACE gene that was identified and for which three different genotypes (II, ID, DD) have been characterised. These three genotypes display distinct ACE plasma activities of which the DD genotype is responsible for the high activity of the enzyme.18,19 Studies have demonstrated that ACE I/D polymorphism is associated with hypertension,20 nephropathy,21 elevated glucose,22 coronary artery disease,13 plasma triglyceride, total cholesterol levels, abdominal fat accumulation, central obesity and weight gain.23
An ample number of studies has recently focused on the ACE I/D polymorphism in association with MS; however, some results have been inconsistent.11,14,24–27 The effects of ACE polymorphism on the improvement of components of the MS profile in response to a multifaceted obesity-management programme, addressed using a multidisciplinary team, has not been clarified in obese adolescents. Thus the aim of the present study was to investigate whether I/D polymorphism of the ACE gene might affect metabolic changes related to MS through long-term interdisciplinary therapy in obese adolescents.
Methods
Subjects
A total of 198 obese adolescents (77 boys and 121 girls) aged between 15 and 19 years who entered the interdisciplinary obesity programme of the Federal University of São Paulo – Escola Paulista de Medicina were assigned to the following two subgroups: MS or non-metabolic syndrome (n-MS). Those who were considered to have MS presented with three or more criteria of the International Diabetes Federation.28 Both groups were submitted for weight loss therapy. The evaluations were performed at baseline and after 1 year of an interdisciplinary approach. The ages of the participants ranged from 15 to 19 years, and they all presented with simple obesity (body mass index (BMI) >95th percentile).29 The inclusion criteria for the post-pubertal stage were based on the Tanner scale stage 5,30 for both boys and girls. Non-inclusion criteria were as follows: other metabolic or endocrine diseases, such as hypothyroidism and Cushing syndrome; chronic alcohol consumption; previous use of drugs, such as anabolic androgenic steroids or psychotropics, which may affect appetite regulation; and pregnancy. The study was carried out in accordance with the principles of the Declaration of Helsinki and was approved by the ethical committee of the Federal University of São Paulo. Written informed consent was obtained from all subjects and/or their parents. The clinical trial registration number was NCT01358773, and this study has been archived in ClinicalTrials.gov.
Serum analysis
Blood samples were collected in the outpatient clinic approximately 8 hours after an overnight fast. Insulin resistance was assessed by the homeostasis model assessment–insulin resistance (HOMA–IR) index, and it was calculated using fasting blood glucose (FBG) and immunoreactive insulin (I), as follows: (FBG (mg/dL)×I (mU/L))/405. The concentrations of total cholesterol, glucose (commercial Beckman Coulter glucose kit, ref: OSR6221), insulin (commercial Architect Insulin reagent kit, ref: 8K41-27), triglycerides, high-density lipoprotein (HDL), low density lipoprotein (LDL)-cholesterol and very low density lipoprotein (VLDL)-cholesterol were determined and analysed by an automatic Beckman Coulter (Brea, California, USA) with a enzymatic colorimetric method (CELM, Barueri, Brazil). The HOMA–IR data were analysed according to reference values reported by Schwimmer et al.,31 and the cut-off adopted for adolescents was 3.16.32
ACE genotyping
Genomic DNA was extracted from circulating leukocytes using a blood DNA extraction kit (ChargeSwitch gDNA blood kits, Invitrogen). The ACE I/D polymorphism was genotyped as described in Almeida et al.18 in 198 subjects with obesity. The sense primer was ECAS 5′-CTGGAGACCACTCCCATCCTTTCT-3′, and the antisense primer was ECAR 5′-GATGTGGCCATCACATTCGTCAGAT-3′. Each subject gave informed consent before collection of blood for DNA extraction at the beginning of the experimental procedure.
Anthropometric variables and body composition
Body mass (kg), height (m), BMI (kg/m2) and waist circumference were measured according to previously adopted procedures.33 Body composition was measured by plethysmography in a BOD POD body composition system (version 1.69; Life Measurement Instruments, Concord, CA, USA).34 Visceral and subcutaneous fat were estimated using abdominal ultrasonography as described in Masquio et al.33 Cut-off points to define visceral obesity by ultrasonography parameters were based on previous methodological descriptions by Ribeiro-Filho et al.35
Multidisciplinary intervention
The multidisciplinary intervention consisted of medical follow-up, psychological therapy, nutritional and exercise programmes. Details of all procedures can be found in de Carvalho-Ferreira et al.36
Statistical analysis
The SPSS statistical package version 22.0 for Mac (SPSS, Chicago, IL, USA) was used for statistical evaluation, and graphics were produced using GraphPad version 6.0 (GraphPad Software, San Diego, CA, USA). The Gaussian distribution of variables (including ∆ values) was verified with a Kolmogorov–Smirnov test. Variables were expressed as the mean±SEM. Comparisons between measures at baseline, after the weight loss intervention and between groups were made using the univariate general linear model followed by a Bonferroni post-test. Genotype frequencies observed in our cohort were in Hardy–Weinberg equilibrium. Differences were considered significant at P<0.05. Statistical data are provided in the figures.
Results
Baseline
A total of 198 obese adolescents were enrolled in the programme; however, 125 obese adolescents (45 boys and 80 girls) completed 1 year of the interdisciplinary obesity programme and more than 75% of the treatment sessions of the Federal University of São Paulo – Escola Paulista de Medicina. In the present study, the patients were analysed as two groups according to MS diagnosis, as follows: MS or non-MS; 40% fit the criteria for MS. The genotype and allele frequency of the ACE I/D polymorphism are listed in Table 1. The genotype frequency distributions of this polymorphism in the study did not differ between n-MS and MS and were all in accordance with Hardy–Weinberg equilibrium.
Table 1.
Distribution of ACE I/D genotype and allele frequencies in n-MS and MS groups.
| Genotypes | n-MS (n=75) | MS (n=50) | Total | HW | P value |
|---|---|---|---|---|---|
| II | 13% (n=10) | 12% (n=7) | 14% (17) | 17.3 | 0.84 |
| ID | 50% (n=37) | 45% (n=22) | 47% (59) | 58.4 | |
| DD | 37% (n=28) | 43% (n=21) | 39% (49) | 49.3 | |
| Allele frequencies | |||||
| I | 0.38 | 0.36 | 0.37 | 0.75 | |
| D | 0.62 | 0.64 | 0.63 |
ACE: angiotensin-converting enzyme; n-MS: non-metabolic syndrome; MS: metabolic syndrome; II: insertion/insertion; ID: insertion/deletion; DD: deletion/deletion; HW: Hardy–Weinberg expected frequencies for total number of subjects; P>0.05.
When analysing the prevalence of the alterations between groups, it was observed that HDL was the most frequently altered parameter. Figure 1 illustrates the different percentages among the alterations between the genotypes.
Figure 1.
The percentage frequencies of the risk determinants of metabolic syndrome in non-metabolic syndrome (n-MS) and metabolic syndrome (MS) groups are divided for angiotensin-converting enzyme (ACE) genotypes. The prevalence altered parameters were obtained according to parameters established by the International Diabetes Federation criteria. HDL: high-density lipoprotein; TG: triglyceride; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Body composition
When analysing the difference between groups (MS vs. n-MS), it was observed that the genotypes ID and DD in the MS group presented a higher body mass (kg) and waist circumference when compared with the same genotypes in the n-MS group. The body composition data are summarised in Supplementary Table 1.
Metabolic parameters
When analysing the difference between groups (MS vs. n-MS), it was observed that the three genotypes in the MS group presented a lower HDL level than that of the same genotype of n-MS. Considering carriers of the D allele from the MS group, higher values of systolic blood pressure (SBP), diastolic blood pressure (DBP) and triglycerides were observed. In analysing insulin levels, the HOMA index and LDL levels, the genotype ID presented higher values in the MS group when compared with the same genotypes in the n-MS group. All metabolic data are presented in Supplementary Table 2.
Comparison within the groups
When analysing the differences within the group, the subjects with the DD genotype showed significantly higher LDL than the subjects with the II genotype in the n-MS group. This genotype also showed higher VLDL when compared with the ID genotype in the same group.
After therapy
Body composition
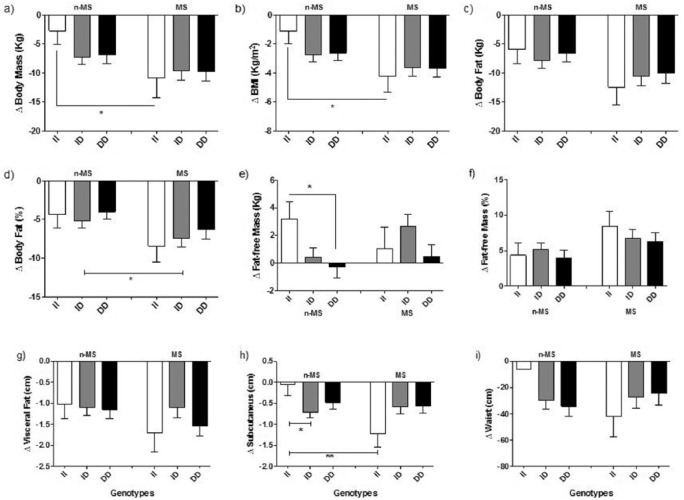
Regarding body composition, both groups (n-MS and MS) showed significant improvements in the body mass (kg), BMI, body fat (kg and %), fat-free mass (kg and %), visceral fat, subcutaneous fat and waist circumference measurements (Figure 2; Supplementary Table 1).
Figure 2.
Delta values of body composition in the non-metabolic syndrome (n-MS) and metabolic syndrome (MS) groups by angiotensin-converting enzyme (ACE) I/D genotype. Data are mean±SEM. BMI: body mass index. *P<0.05; **P<0.01.
When analysing the difference between groups (n-MS vs. MS) after therapy, it was observed that the ID and DD genotypes in the MS group presented an increase in fat-free mass (kg) when compared with the same genotypes in the n-MS group. The II genotype in the MS group showed a greater decrease in subcutaneous fat when compared with the same genotype in the n-MS group. We observed a reduction in the visceral–subcutaneous ratio in the ID and DD genotypes of n-MS, while this reduction was observed only in the DD genotype in the MS group. These data are presented in Supplementary Table 1.
Metabolic parameters
Both groups presented significant improvements in the insulin levels in ID and DD genotypes (n-MS) as well in II and ID genotypes (MS). In the MS group, the DD genotype remained unchanged after therapy. Considering the difference between the two groups (n-MS vs. MS), the ID genotype in the MS group had a greater delta value than the same genotype in the n-MS group (Figure 3).
Figure 3.
Delta values of insulin and homeostasis model assessment–insulin resistance in the non-metabolic syndrome (n-MS) and metabolic syndrome (MS) groups by angiotensin-converting enzyme (ACE) I/D genotype. Data are mean±SEM. *P<0.05; **P<0.01.
Regarding the lipid profile, the ID and DD genotypes from both groups (n-MS and MS) exhibited significantly reduced LDL-cholesterol, while VLDL-cholesterol was reduced only in the n-MS group with the DD genotype. On the other hand, all MS genotypes were able to reduce triglyceride levels significantly. Comparing the same genotypes between the two groups, we observed that subjects with ID and DD genotypes in the MS group had significantly lower HDL levels when compared with the same genotypes in the n-MS group. In addition, both ID and DD genotypes presented higher VLDL and triglycerides than subjects from the n-MS group. In the analysis of the LDL, we observed significant differences in the ID genotype compared with the same genotype in the n-MS group. Although the MS group exhibited a significantly improved LDL–HDL ratio, this group still presented with higher values in all three genotypes in relation to n-MS. All metabolic data are presented in Supplementary Table 2.
Concerning SBP, the MS group showed a significant reduction in ID and DD genotypes, and it was observed that none of the genotypes had a significant reduction in the n-MS group. We observed that the ID genotype in the MS group maintained differences with regard to the same genotype in the n-MS group after therapy. Only the DD genotype in the n-MS group and ID from the MS group presented significant reductions in DBP after therapy. We observed a significant difference between the groups only for the DD genotype. All metabolic data are shown in Supplementary Table 2.
Comparison within the groups
Body composition
Subjects with the II genotype in the n-MS group presented a lower significant difference in body composition components and did not show a significance difference in body mass (kg), BMI and subcutaneous fat. However, only the II carriers of the n-MS group showed increases in fat-free mass, while individuals with ID and DD genotypes did not show changes in their fat-free mass (Figure 2). Carriers of the DD genotype also did not present differences in waist circumference in the n-MS group. However, analysing the visceral–subcutaneous ratio, we observed a reduction in ID and DD in the n-MS group and only in the DD genotype in the MS group (Supplementary Table 1).
Metabolic parameters
Analysing the metabolic parameters of the ID and DD genotypes in the n-MS group, both showed a significantly improved insulin response, while the II and ID genotypes in the MS group also showed a significantly improved insulin response. This result is the opposite of that of the subjects with the DD genotype, who did not show changes in their insulin levels after therapy. In synergy with these results, while the genotypes II and ID improved HOMA–IR, the DD genotype increased significantly (Figure 3).
Considering lipid metabolism, in the MS group only carriers of the D allele improved LDL, while HDL and VLDL did not respond to intervention. Regarding HDL-cholesterol and LDL–HDL ratios, we observed a significant reduction only in the ID and DD genotypes in the n-MS group. Moreover, the carriers of the D allele in the MS group showed increased triglyceride–HDL ratio values. Finally, the MS group showed a significant reduction of SBP in the ID and DD genotypes (Supplementary Table 2).
Discussion
The main purpose of the present study was to investigate whether the I/D polymorphism of the ACE gene might affect metabolic changes related to MS through a long-term interdisciplinary therapy in obese adolescents. The most important finding is that we have shown that genetic alteration in the ACE gene may influence the benefits of an interdisciplinary therapy in obese adolescents with and without MS.
It is important to emphasise that, for the first time, we have demonstrated that the II genotype might have a favourable effect on the improvement of body composition in obese adolescents without MS when compared with the DD genotype. In agreement with this, the II genotype presented better results regarding glucose metabolism when compared with the ID and DD genotypes in the MS group. Finally, the II genotype seemed to impair improvement in lipid metabolism when compared with ID and DD in both groups.
As previously demonstrated by our group,37 improvement in body composition was observed in both groups after an interdisciplinary weight loss therapy. However, when we stratified the groups by ACE genotype, the result was different; the II genotype showed significant improvement in fat-free mass in the n-MS group when compared with ID and DD within the same group.
As mentioned before, the I-carry allele is associated with higher levels of bradykinin, which could partly justify these results. Bradykinin stimulates protein synthesis pathways, including the Pi3K/AKT/m-TOR pathway. The proposed mechanism is based on the role of bradykinin in increasing the IRS receptor phosphorylation, which activates the AKT–mTOR pathway, favouring protein synthesis.17,38 Corroborating results obtained by the group of Popadic Gacesa39 provided evidence of a positive relationship between another polymorphism that can result in an increase in bradykinin signaling and a better response to an exercise intervention for which the goal was improving muscle mass in young men (Figure 4).
Figure 4.
Diagram showing the possible mechanism involved in the difference between the angiotensin-converting enzyme (ACE) I/D polymorphisms affecting the generation of kinin peptide agonists for B2R and B1R in the downstream variation signaling effects. (a) Schematic representation of II/ID genotypes that presented lower ACE concentration, which leads to a decrease of bradykinin degradation and consequently an increase of downstream signaling effects via B2 and B1 receptors. (b) Molecular signaling pathway via B2 and B1 receptor affected by the higher concentration of ACE in DD subjects, which increase the bradykinin degradation consequently reducing the downstream signaling. The pathways highlighted in black in each genotype are supposed to be the more active. BK-(1-5), FFA: fat-free acid; CPN: soluble plasma carboxypeptidase N; CPM: membrane-bound carboxypeptidase M; IR: insulin receptor; B2R: bradykinin B2 receptor; B1R: bradykinin B1 receptor; P: phosphate; IRS1: insulin receptor substrate 1; PI3K: phosphoinositide 3-kinase; AKT: protein kinase B; mTOR: mechanistic target of rapamycin; GLUT4: MAP kinase: mitogen activated protein kinases; iNOS: inducible nitric oxide synthases; l-Arg: l-arginine; NO: oxide nitric; ROS: reactive oxygen species. We have provided references to the boxes of each of the pathways shown.
Interestingly, it has recently been shown that an increase in free-fat mass, in response to exercise training, may enhance some myokines, including increased secretion of FGF-21 in muscle, mediation in white adipose tissue and brown adipose tissue (BAT), the browning phenomenon and an increase in thermogenesis in humans. Moreover, there is consensus on the presence of BAT in humans and its potential consequences on energy metabolism, glucose regulation and blood lipid balance.40
In addition, it has been suggested that exercise could exert a key role in BAT metabolism. Exercise training might activate and recruit human BAT through activation of the sympathetic nervous system, heart and skeletal muscle. However, more investigations for understanding which type of exercise, the different effects of intensity, and how much time is needed to induce an effective BAT activation and recruitment in humans with obesity and MS is needed.41
All together, these results may suggest the importance of increases in free-fat mass after weight loss, as shown in the present study. In order to ameliorate both factors, the energy balance and whole body metabolism avoid undesirable yo-yo effects and the inflammatory state related to obesity, which may lead to the development of MS and cardiometabolic risks.
Another interesting result observed in the present investigation was that only I-carry alleles presented an improvement in insulin and HOMA–IR in the MS group, demonstrating that the DD genotype presented impairment of glucose metabolism. It is well established in the literature that bradykinin is capable of positively influencing glucose uptake.17 Bradykinin acts via a synergistic mechanism with the pathway of insulin and by translocation of GLUT-4 in an independent manner.42 Thus, considering that the literature has strongly demonstrated that DD genotypes present lower values of bradykinin, this could partly explain the difference between the genotypes after interdisciplinary therapy. A possible mechanism involved in I/D polymorphism and glucose homeostasis is also shown in Figure 4.
Insulin resistance is one of the strongest predictors of many chronic diseases in adolescents, including MS, non-alcoholic fatty liver disease (NAFLD), diabetes, dyslipidaemia and others. Therefore, the improvement of insulin sensitivity in the presence of MS in obese adolescents may benefit obesity control as well as its above-mentioned comorbidities. In addition, it was previously shown that after weight loss therapy, an increase in adiponectin mediates the improvements in insulin resistance in obese adolescents, leading to a reduction in the prevalence of MS and NAFLD.43,44
However, regarding lipid metabolism, the framework seems to be different in the present study. The II genotype in the MS group presented a worse lipid profile at baseline that did not improve after treatment. This result indicates that, besides the unfavourable lipid profile at baseline, this genotype also presents difficulty in responding positively to the intervention because it is expected that lifestyle changes are able to modulate the lipid profile. D-carrier allele genotypes seemed to respond better, demonstrated by an increase in HDL-cholesterol and decreases in LDL-cholesterol and triglycerides in the MS group, while the II genotype was able to improve triglyceride levels only (Figure 4).
These effects can be modulated partially by the conversion of bradykinin in Des-Arg-9-BK, which is able to bind the B1 kinin receptor. It is well known that these receptors are expressed only in pro-inflammatory conditions, which are observed in the present population, especially in the MS group.45 Experimental studies showed that B1 receptor knockout mice, which were submitted to a high-fat diet, presented a more favourable lipid profile when compared with wild-type mice.46 Thus, considering that the I-carry allele has higher bradykinin levels, more substrate may be available for the conversion of bradykinin in Des-Arg-9-BK. However, clinical studies to confirm this are recommended in the analysed population.
In addition, research in which experimental models of obesity and insulin resistance were evaluated shows that B1 kinin expression, especially in the liver, was involved with disturbances in lipid metabolism, through mechanisms related to oxidative stress.47 It is important to highlight that, despite the temporary blockade of this receptor being able to restore lipid homeostasis, this intervention does not provide improvements in insulin resistance, showing the influences of these receptors in lipid metabolism, without modifying glucose uptake, corroborating our data.45
Finally, the control of lipid profiles in both obesity and MS groups is desirable because MS is present in 32% of Brazilian adolescents with obesity. In eutrophic American children (NHANES III), this prevalence was only approximately 0.1%. In addition, we were able to show that 50% of obese adolescents have a diagnosis of NAFLD. Unfortunately, after weight loss therapy, some of them continue with MS and NAFLD, showing the importance of controlling lipid profiles in this population in order to prevent cardiovascular diseases.2,48–50
Currently, identifying obesity-resistant genotypes and how they respond to different therapies is becoming extremely interesting, as obesity has reached epidemic proportions globally. In the present study, genetic variation in the ACE gene influences the response to interdisciplinary treatment in different ways, acting positively in glucose metabolism and protein synthesis and negatively on lipid profiles. Once the role of different polymorphisms is established, a more efficient and personalised treatment can be designed.
Nevertheless, to our knowledge, the current study is the first investigation showing the influence of these specific polymorphisms in response to a long-term interdisciplinary therapy in obese adolescents, and is an important issue for clinical practice. The limitations of the present study include the sample size and the lack of the quantification of kinin peptides. These issues may be explored in future studies.
Conclusion
The multicomponent intervention was able to improve body composition and metabolism among obese adolescents. In the present study, we showed that the ACE polymorphism was able to influence positively fat-free mass in I-carry allele genotypes in the n-MS group. In addition, the I-carry allele was able to improve significantly insulin resistance in the MS group. Conversely, I-carry allele genotypes seemed to hamper lipid profile improvement in the MS group. The results suggest that the genotypes can influence, in different ways and in specific parameters, the metabolism of Brazilian obese adolescents.
Supplementary Material
Acknowledgments
The authors would like to thank the volunteers who participated in the study.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study had the financial support of CAPES (AUX-PE-PNPD 256/2011), FAPESP (2015/20082-7, 2013/041364, 2011/50356-0 and 2011/50414-0), AFIP, CNPq (141533/2012-9) and UNIFESP-EPM.
References
- 1. World Health Organization. Obesity and overweight. 2015. http://www.who.int/mediacentre/factsheets/fs311/en/.
- 2. Damaso AR, de Piano A, Campos RM, et al. Multidisciplinary approach to the treatment of obese adolescents: effects on cardiovascular risk factors, inflammatory profile, and neuroendocrine regulation of energy balance. Int J Endocrinol 2013; 2013: 541032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magnussen CG, Koskinen J, Juonala M, et al. A diagnosis of the metabolic syndrome in youth that resolves by adult life is associated with a normalization of high carotid intima-media thickness and type 2 diabetes mellitus risk: the Bogalusa Heart and Cardiovascular Risk in Young Finns studies. J Am Coll Cardiol 2012; 60: 1631–1639. [DOI] [PubMed] [Google Scholar]
- 4. Yang J, Liu J, Liu J, et al. Genetic association study with metabolic syndrome and metabolic-related traits in a cross-sectional sample and a 10-year longitudinal sample of chinese elderly population. PloS One 2014; 9: e100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bosy-Westphal A, Onur S, Geisler C, et al. Common familial influences on clustering of metabolic syndrome traits with central obesity and insulin resistance: the Kiel obesity prevention study. Int J Obes (Lond) 2007; 31: 784–790. [DOI] [PubMed] [Google Scholar]
- 6. Henneman P, Aulchenko YS, Frants RR, et al. Prevalence and heritability of the metabolic syndrome and its individual components in a Dutch isolate: the Erasmus Rucphen Family study. J Med Genet 2008; 45: 572–577. [DOI] [PubMed] [Google Scholar]
- 7. Bellia A, Giardina E, Lauro D, et al. "The Linosa Study": epidemiological and heritability data of the metabolic syndrome in a Caucasian genetic isolate. Nutr, Metab, Cardiovasc Dis: NMCD 2009; 19: 455–461. [DOI] [PubMed] [Google Scholar]
- 8. Lin HF, Boden-Albala B, Juo SH, et al. Heritabilities of the metabolic syndrome and its components in the Northern Manhattan Family Study. Diabetologia 2005; 48: 2006–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Groop L. Genetics of the metabolic syndrome. Br J Nutr 2000; 83 (Suppl 1): S39–S48. [DOI] [PubMed] [Google Scholar]
- 10. Hamid YH, Rose CS, Urhammer SA, et al. Variations of the interleukin-6 promoter are associated with features of the metabolic syndrome in Caucasian Danes. Diabetologia 2005; 48: 251–260. [DOI] [PubMed] [Google Scholar]
- 11. Lee YJ, Tsai JC. ACE gene insertion/deletion polymorphism associated with 1998 World Health Organization definition of metabolic syndrome in Chinese type 2 diabetic patients. Diabetes Care 2002; 25: 1002–1008. [DOI] [PubMed] [Google Scholar]
- 12. Matsusaka T, Hymes J, Ichikawa I. Angiotensin in progressive renal diseases: theory and practice. J Am Soc Nephrol: JASN 1996; 7: 2025–2043. [DOI] [PubMed] [Google Scholar]
- 13. Nicholls MG, Richards AM, Agarwal M. The importance of the renin–angiotensin system in cardiovascular disease. J Hum Hypertens 1998; 12: 295–299. [DOI] [PubMed] [Google Scholar]
- 14. Motawi TK, Shaker OG, Shahin NN, et al. Angiotensin-converting enzyme insertion/deletion polymorphism association with obesity and some related disorders in Egyptian females: a case–control observational study. Nutr Metab 2016; 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorer FE, Kahn JR, Lentz KE, et al. Purification and properties of angiotensin-converting enzyme from hog lung. Circ Res 1972; 31: 356–366. [DOI] [PubMed] [Google Scholar]
- 16. Yang HY, Erdos EG, Levin Y. Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme). J Pharmacol Exp Therapeut 1971; 177: 291–300. [PubMed] [Google Scholar]
- 17. Carvalho CR, Thirone AC, Gontijo JA, et al. Effect of captopril, losartan, and bradykinin on early steps of insulin action. Diabetes 1997; 46: 1950–1957. [DOI] [PubMed] [Google Scholar]
- 18. Almeida SS, Barros CC, Moraes MR, et al. Plasma kallikrein and angiotensin I-converting enzyme N- and C-terminal domain activities are modulated by the insertion/deletion polymorphism. Neuropeptides 2010; 44: 139–143. [DOI] [PubMed] [Google Scholar]
- 19. Almeida SS, Naffah-Mazzacoratti MG, Guimaraes PB, et al. Carbamazepine inhibits angiotensin I-converting enzyme, linking it to the pathogenesis of temporal lobe epilepsy. Translat Psychiatry 2012; 2: e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ji LD, Zhang LN, Shen P, et al. Association of angiotensinogen gene M235T and angiotensin-converting enzyme gene I/D polymorphisms with essential hypertension in Han Chinese population: a meta-analysis. J Hypertens 2010; 28: 419–428. [DOI] [PubMed] [Google Scholar]
- 21. Amorim CE, Nogueira E, Almeida SS, et al. Clinical impact of an angiotensin I-converting enzyme insertion/deletion and kinin B2 receptor +9/−9 polymorphisms in the prognosis of renal transplantation. Biol Chem 2013; 394: 369–377. [DOI] [PubMed] [Google Scholar]
- 22. Irvin MR, Lynch AI, Kabagambe EK, et al. Pharmacogenetic association of hypertension candidate genes with fasting glucose in the GenHAT Study. J Hypertens 2010; 28: 2076–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strazzullo P, Iacone R, Iacoviello L, et al. Genetic variation in the renin-angiotensin system and abdominal adiposity in men: the Olivetti Prospective Heart Study. Ann Intern Med 2003; 138: 17–23. [DOI] [PubMed] [Google Scholar]
- 24. Shaikh R, Shahid SM, Mansoor Q, et al. Genetic variants of ACE (insertion/deletion) and AGT (M268T) genes in patients with diabetes and nephropathy. J Renin–Angiotens–Aldost Syst: JRAAS 2014; 15: 124–130. [DOI] [PubMed] [Google Scholar]
- 25. Milionis HJ, Kostapanos MS, Vakalis K, et al. Impact of renin–angiotensin–aldosterone system genes on the treatment response of patients with hypertension and metabolic syndrome. J Renin–Angiotens–Aldost Syst: JRAAS 2007; 8: 181–189. [DOI] [PubMed] [Google Scholar]
- 26. Fiatal S, Szigethy E, Szeles G, et al. Insertion/deletion polymorphism of angiotensin-1 converting enzyme is associated with metabolic syndrome in Hungarian adults. J Renin–Angiotens–Aldost Syst: JRAAS 2011; 12: 531–538. [DOI] [PubMed] [Google Scholar]
- 27. Mao S, Huang S. A meta-analysis of the association between angiotensin-converting enzyme insertion/deletion gene polymorphism and the risk of overweight/obesity. J Renin–Angiotens–Aldost Syst: JRAAS 2015; 16: 687–694. [DOI] [PubMed] [Google Scholar]
- 28. Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr Diabet 2007; 8: 299–306. [DOI] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention. Prevalence of Overweight Among Children and Adolescents: United States 1999–2002. Atlanta: CDC; 2002. [Google Scholar]
- 30. Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Childhood 1976; 51: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwimmer JB, Deutsch R, Rauch JB, et al. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatrics 2003; 143: 500–505. [DOI] [PubMed] [Google Scholar]
- 32. Keskin M, Kurtoglu S, Kendirci M, et al. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005; 115: e500–e503. [DOI] [PubMed] [Google Scholar]
- 33. Masquio DC, de Piano A, Campos RM, et al. The role of multicomponent therapy in the metabolic syndrome, inflammation and cardiovascular risk in obese adolescents. Br J Nutr 2015; 113: 1920–1930. [DOI] [PubMed] [Google Scholar]
- 34. Fields DA, Higgins PB, Radley D. Air-displacement plethysmography: here to stay. Curr Opin Clin Nutr Metab Care 2005; 8: 624–629. [DOI] [PubMed] [Google Scholar]
- 35. Ribeiro-Filho FF, Faria AN, Azjen S, et al. Methods of estimation of visceral fat: advantages of ultrasonography. Obes Res 2003; 11: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 36. de Carvalho-Ferreira JP, Masquio DC, da Silveira, Campos RM, et al. Is there a role for leptin in the reduction of depression symptoms during weight loss therapy in obese adolescent girls and boys? Peptides 2015; 65: 20–28. [DOI] [PubMed] [Google Scholar]
- 37. Masquio DC, Ganen Ade P, Campos RM, et al. Cut-off values of waist circumference to predict metabolic syndrome in obese adolescents. Nutricion Hospitalaria 2015; 31: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 38. Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc 2006; 38: 1950–1957. [DOI] [PubMed] [Google Scholar]
- 39. Popadic Gacesa JZ, Momcilovic M, Veselinovic I, et al. Bradykinin type 2 receptor −9/–9 genotype is associated with triceps brachii muscle hypertrophy following strength training in young healthy men. BMC Musculoskel Disord 2012; 13: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanchez-Delgado G, Martinez-Tellez B, Olza J, et al. Activating brown adipose tissue through exercise (ACTIBATE) in young adults: rationale, design and methodology. Contemp Clin Trials 2015; 45: 416–425. [DOI] [PubMed] [Google Scholar]
- 41. Sepa-Kishi DM, Ceddia RB. Exercise-mediated effects on white and brown adipose tissue plasticity and metabolism. Exerc Sport Sci Rev 2016; 44: 37–44. [DOI] [PubMed] [Google Scholar]
- 42. Kishi K, Muromoto N, Nakaya Y, et al. Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes 1998; 47: 550–558. [DOI] [PubMed] [Google Scholar]
- 43. de Piano A, de Mello MT, Sanches Pde L, et al. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur J Gastroenterol Hepatol 2012; 24: 1313–1324. [DOI] [PubMed] [Google Scholar]
- 44. Sanches PL, de Mello MT, Elias N, et al. Hyperleptinemia: implications on the inflammatory state and vascular protection in obese adolescents submitted to an interdisciplinary therapy. Inflammation 2014; 37: 35–43. [DOI] [PubMed] [Google Scholar]
- 45. Talbot S, Dias JP, El Midaoui A, et al. Beneficial effects of kinin B1 receptor antagonism on plasma fatty acid alterations and obesity in Zucker diabetic fatty rats. Can J Physiol Pharmacol 2016; 94: 752–757. [DOI] [PubMed] [Google Scholar]
- 46. Mori MA, Araujo RC, Reis FC, et al. Kinin B1 receptor deficiency leads to leptin hypersensitivity and resistance to obesity. Diabetes 2008; 57: 1491–1500. [DOI] [PubMed] [Google Scholar]
- 47. Midaoui AE, Talbot S, Lahjouji K, et al. Effects of alpha-lipoic acid on oxidative stress and kinin receptor expression in obese Zucker diabetic fatty rats. J Diabet Metab 2015; 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Caranti DA, Tock L, Prado WL, et al. Long-term multidisciplinary therapy decreases predictors and prevalence of metabolic syndrome in obese adolescents. Nutr, Metab, Cardiovasc Dis: NMCD 2007; 17: e11–e13. [DOI] [PubMed] [Google Scholar]
- 49. Tock L, Prado WL, Caranti DA, et al. Nonalcoholic fatty liver disease decrease in obese adolescents after multidisciplinary therapy. Eur J Gastroenterol Hepatol 2006; 18: 1241–1245. [DOI] [PubMed] [Google Scholar]
- 50. Damiani DDD, Kuba V, Cominato L. Metabolic syndrome in the child and teenager. Pediatria Moderna 2015; 51: 156–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.