Abstract
Objective:
We aimed at assessing the molecular adaptation of the renin-angiotensin system (RAS) after successful kidney transplantation (KTX).
Materials and methods:
In this prospective, exploratory study we analyzed 12 hemodialysis (HD) patients, who received a KTX and had excellent graft function six to 12 months thereafter. The concentrations of plasma Angiotensin (Ang) peptides (Ang I, Ang II, Ang-(1–7), Ang-(1–5), Ang-(2–8), Ang-(3–8)) were simultaneously quantified with a novel mass spectrometry-based method. Further, renin and aldosterone concentrations were determined by standard immunoassays.
Results:
Ang values showed a strong inter-individual variability among HD patients. Yet, despite a continued broad dispersion of Ang values after KTX, a substantial improvement of the renin/Ang II correlation was observed in patients without RAS blockade or on angiotensin receptor blocker (HD: renin/Ang II R2 = 0.660, KTX: renin/Ang II R2 = 0.918). Ang-(1–7) representing the alternative RAS axis was only marginally detectable both on HD and after KTX.
Conclusions:
Following KTX, renin-dependent Ang II formation adapts in non-ACE inhibitor-treated patients. Thus, a largely normal RAS regulation is reconstituted after successful KTX. However, individual Ang concentration variations and a lack of potentially beneficial alternative peptides after KTX call for individualized treatment. The long-term post-transplant RAS regulation remains to be determined.
Keywords: Renin-angiotensin system, blood pressure, hemodialysis, kidney transplantation, angiotensin
Introduction
Therapeutic blockade of the renin-angiotensin system (RAS) with either angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) has proven safety and effectiveness in chronic kidney disease (CKD) patients. Yet, despite their widespread prescription, RAS blockers remain controversial in hemodialysis (HD) patients and kidney transplant (KTX) recipients.1–9
There is strong evidence that the kidney crucially determines RAS regulation.10 Being responsible for the secretion of renin, the rate-limiting enzyme of the RAS, this organ is the central mediator of angiotensin II (Ang II) generation representing the “classical” RAS axis as well as formation of Ang-(1–7) as the central mediator of the “alternative” RAS axis that opposes the biological effects of Ang II.11,12
Upon KTX, the allograft is transferred into the recipient’s molecular and hemodynamic environment and becomes crucial for blood pressure regulation including salt and fluid homeostasis. During this process, the donor kidney needs to adapt its regulation of renin secretion to the recipient RAS. Unfortunately, accurate information on the molecular RAS regulation dynamics after KTX remains scarce to date.
In the 1990s and early 2000s, small animal studies suggested that KTX has a profound and long-term effect on the recipient’s systemic as well as local RAS.13,14 So far, human RAS analyses after KTX have mainly focused on clinical outcomes with conflicting findings regarding beneficial versus detrimental effects of RAS blockade.15–17 Recently, plasma renin activity and aldosterone concentration were measured serially in kidney transplant recipients for five years.17 Here, relatively normal values were found in the majority of patients. Despite these data, no analysis has simultaneously compared renin, angiotensin and aldosterone levels in humans before and after KTX.
In recent years, the RAS has regained momentum by further insights into the so-called “alternative” RAS axis, composed of angiotensin-converting enzyme 2 (ACE2), Ang-(1–7), the cascade’s end-product Ang-(1–5) and the MAS receptor, which counteracts the vaso-constrictive, pro-inflammatory and pro-fibrotic effects of the “classical” RAS axis comprising ACE/Ang II/AT1 receptor. Currently, promising new drugs interfering with mediators of both axes are entering the clinical application phase.18 Additionally, recently developed analytical methods allow novel insights because of higher specificity, accuracy and sensitivity.19–22
Various forms of RAS blockade are routinely used in the majority of HD and KTX patients.23 Hence, an in-depth analysis of the RAS activity should yield critical information about the effects of KTX on renal hemodynamics along with insights about potentially beneficial effects conferred by the RAS.
Here, we present a detailed analysis of the individual molecular RAS regulation in maintenance HD patients who successfully received kidney allografts. The implications of these findings are discussed.
Materials and methods
Patients
The present analysis was carried out as a prospective single-center, exploratory study according to good clinical practice guidelines. Approval of the local Ethics Committee of the Medical University of Vienna was obtained (EC#022/2012) and all participants provided their written informed consent prior to participation. We recruited 12 consecutive patients who were in stable clinical condition on maintenance HD three times weekly and actively listed for KTX. Patients were not affected by any sort of infection at the time of blood collection and all were clinically judged as normovolemic. All patients had previously received dietary counseling and were advised to follow a low-salt diet. All participants received a deceased-donor renal allograft within the year following their recruitment. After stabilization of renal function (six to 12 months after KTX) and after conditions secondary to end-stage renal disease (i.e. secondary hyperparathyroidism and volume overload) as well as transplant-associated complications (i.e. post-transplant diabetes mellitus) had subsided, we conducted a follow-up analysis. This comprised a clinical examination with blood pressure measurement, laboratory analysis including renal parameters (estimated glomerular filtration rate (eGFR) according to the Modification of Diet in Renal Disease (MDRD) formula), update of medication as well as the molecular analysis of the RAS.
Laboratory methods
Blood sample collection for the quantification of angiotensin metabolites was conducted at the beginning of the HD session after the long interdialytic interval at the pre-filter bloodline site and via cubital venous blood collection after KTX. Six milliliters of heparinized peripheral blood were collected from each patient and chilled on ice immediately. Plasma was obtained by centrifugation (4°C) at 2000 g for 10 minutes and 2 ml of plasma were stored at −80°C until analysis. Previous results of our research group have shown similar qualitative outcomes using protease inhibitor-stabilized and equilibrated patient samples for angiotensin peptide quantification.24 Thus, plasma was ex vivo incubated at 37°C for one hour and stabilized by addition of an enzyme inhibitor cocktail (Attoquant Diagnostics). Stable isotope-labeled internal standards for each angiotensin metabolite (Ang I, Ang II, Ang-(1–7), Ang-(1–5), Ang-(2–8), Ang-(3–8), Ang-(2–10), Ang-(2–7), Ang-(1–9) and Ang-(3–7)) were added to stabilized plasma samples at a concentration of 200 pg/ml and subjected to liquid chromatography tandem-mass spectrometry (LC-MS/MS)-based angiotensin quantification by Attoquant Diagnostics (Vienna, Austria) as described previously.19,25,26
Renin and aldosterone concentration were measured with a chemiluminescence immunoassay (DiaSorin LIAISON analyzer).
Statistical analysis
Normally distributed data are presented as mean values with standard deviation (SD) and paired Student’s t-test was performed to determine differences following KTX. Non-normally distributed values are described as medians (interquartile range (IQR)) and Kruskal-Wallis test was applied. For the relationship analysis between renin and angiotensin concentrations, linear regression analysis was carried out. The IBM SPSS System for Mac version 22.0.0 (SPSS Inc, 2010, Chicago, IL, USA) and GraphPad Prism Version 6 were used for all analyses.
Results
Patient characteristics
The average time on maintenance HD at the time of the first RAS analysis was 27 months; the median amount of residual urinary output was 600 ml/24 hours (IQR 0–1950). Underlying renal diseases were heterogeneous: One patient had diabetic nephropathy, another was affected by vascular nephropathy, one had polycystic kidney disease, two had glomerular disease and seven patients had atrophic kidneys without known causes.
In general, patients attained good renal function after KTX with an average serum creatinine of 1.4 mg/dl (±0.4) and eGFR values between 26 and 77 ml/min/1.73m2 at the time of follow-up analysis (Table 1). As expected, a statistically significant difference of electrolytes and parameters of mineral bone disorder before and after KTX was found on paired analysis. Weight and blood pressure remained largely unchanged. Cold ischemia time was 12 hours on average (Table 2). Two patients suffered from diabetes mellitus while on hemodialysis; both remained on oral antidiabetic medication after KTX. Overall, four other patients suffered from post-transplant diabetes mellitus. At the time of the post-KTX analysis, none of them were still dependent on antidiabetic agents. From the time of transplantation to follow-up (mean 13±8 months), only one patient was affected by an episode of acute biopsy-proven cellular rejection (borderline according to the Banff 2013 criteria).
Table 1.
Clinical and laboratory data before and after kidney transplantation.
| HD (n = 12) | KTX (n = 12) | p value | |
|---|---|---|---|
| Weight (kg) | 83 (19) | 77 (16) | 0.115 |
| RRsys (mmHg) | 143 (10) | 134 (16) | 0.189 |
| RRdiast (mmHg) | 77 (13) | 79 (8) | 0.625 |
| Sodium (mmol/l) | 138 (3) | 141 (3) | 0.015 |
| Potassium (mmol/l) | 5.0 (0.6) | 4.2 (0.6) | 0.013 |
| PTH (pg/ml) | 373 (261) | 154 (84) | 0.024 |
| OH-Vitamin D (nmol/l) | 38 (33) | 35 (17) | 0.714 |
| (OH)2-Vitamin D (pg/ml) | 22 (10) | 49 (17) | 0.001 |
| Albumin/creatinine ratio (mg/g) | NA | 221 (219) | |
| Albumin (g/l) | 38 (3) | 42 (4) | 0.031 |
| Hemoglobin (g/dl) | 10.5 (1.3) | 11.9 (2.5) | 0.050 |
| C-reactive protein (mg/dl) | 0.5 (0.6) | 0.3 (0.3) | 0.368 |
| Creatinine (mg/dl) | 10.1 (3.2) | 1.4 (0.4) | <0.001 |
| eGFR (ml/min) | 5.7 (1.7) | 52.4 (15.4) | <0.001 |
| Phosphate (mmol/l) | 2.0 (0.5) | 0.9 (0.2) | <0.001 |
| Calcium (mmol/l) | 2.2 (0.2) | 2.4 (0.2) | 0.006 |
HD: hemodialysis; KTX: kidney transplantation; RR: blood pressure; sys: systolic; diast: diastolic; PTH: parathyroid hormone; eGFR: estimated glomerular filtration rate; NA: not available. Data are shown as means (standard deviation).
Table 2.
Comorbidities, concomitant antihypertensive therapy and transplant data.
| Characteristics | HD | KTX | p value |
|---|---|---|---|
| LVH, n | 10 (83%) | NA | |
| CAD, n | 4 (33%) | NA | |
| Atrial fibrillation, n | 1 (8%) | NA | |
| COPD, n | 1 (8%) | NA | |
| DM, n | 2 (17%) | NA | |
| PAD, n | 2 (17%) | NA | |
| CVD, n | 2 (17%) | NA | |
| Calcium blocker, n | 6 (50%) | 7 (58%) | 0.655 |
| Beta blocker, n | 11 (92%) | 7 (58%) | 0.046 |
| Alpha blocker, n | 4 (33%) | 4 (33%) | 1.000 |
| Other antihypertensive | 8 (67%) | 1 (8%) | 0.020 |
| CIT, h | NA | 12 ± 6 | |
| HLA serotype mismatch (A, B, C, DR, DQ loci) | NA | 4 [2–5] | |
| CMV constellation | NA | ||
| Low risk, n | NA | 4 (33%) | |
| Intermediate risk, n | NA | 7 (58%) | |
| High risk, n | NA | 1 (8%) |
LVH: left ventricular hypertrophy; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; PAD: peripheral artery disease; CVD: cerebro-vascular disease; CIT: cold ischemia time; HLA: human leukocyte antigen; CMV: cytomegalovirus (low risk = donor immunoglobulin (Ig)G negative, recipient IgG negative; intermediate risk = donor IgG negative, recipient IgG positive OR donor IgG positive, recipient IgG positive; high risk = donor IgG positive, recipient IgG negative); NA: not available.
Immunosuppression consisted of standard triple therapy regimen with prednisone, tacrolimus and mycophenolate mofetil (MMF) in all patients but one, who ingested azathioprine instead of MMF. None of the analyzed patients ingested aldosterone antagonists. Yet, we were able to recruit a cohort with all forms of RAS blockade both on HD and after KTX (Table 3).
Table 3.
Patient-wise RAS medication.
| ID | RASi on HD | RASi after KTX |
|---|---|---|
| 1 | Ø | Ø |
| 2 | Ø | Ø |
| 3 | Ø | Ø |
| 4 | Ø | Ø |
| 5 | Ø | Ø |
| 6 | ARB | ARB |
| 7 | ARB | ARB |
| 8 | ARB | ARB |
| 9 | Ø | ARB |
| 10 | ACEi | Ø |
| 11 | Dual | Ø |
| 12 | Dual | ACEi |
RASi: renin-angiotensin system inhibitor; HD: hemodialysis; KTX: kidney transplantation; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
RAS reconstitution after KTX
Absolute plasma renin concentrations (PRC) displayed a wide distribution range in HD patients (0.8–567 μIU/ml). Following KTX, PRC levels were distributed within much narrower margins than before (2.9–113.7 μIU/ml).
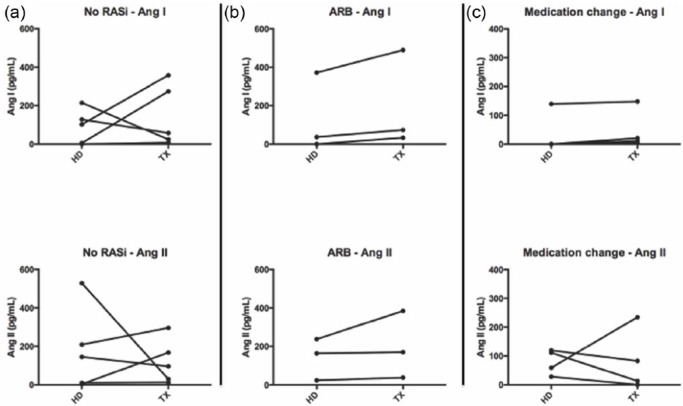
The principal RAS metabolites detected both on HD and after KTX were Ang I and Ang II, which is in line with previous findings.24 Other angiotensins were either present at very low concentrations or not detectable at all, including Ang-(1–9), Ang-(2–7) and Ang-(3–7). On a patient-specific level, Ang I and Ang II levels showed varying behavioral patterns (increase, decrease or no change) after KTX (Figure 1).
Figure 1.
Patient-wise Angiotensin (Ang) I and Angiotensin II course.
(a) No RAS blocker before and after KTX; (b) Angiotensin receptor blocker before and after KTX; (c) RAS blocker change after KTX. RASi: renin-angiotensin system inhibitor; KTX: kidney transplantation.
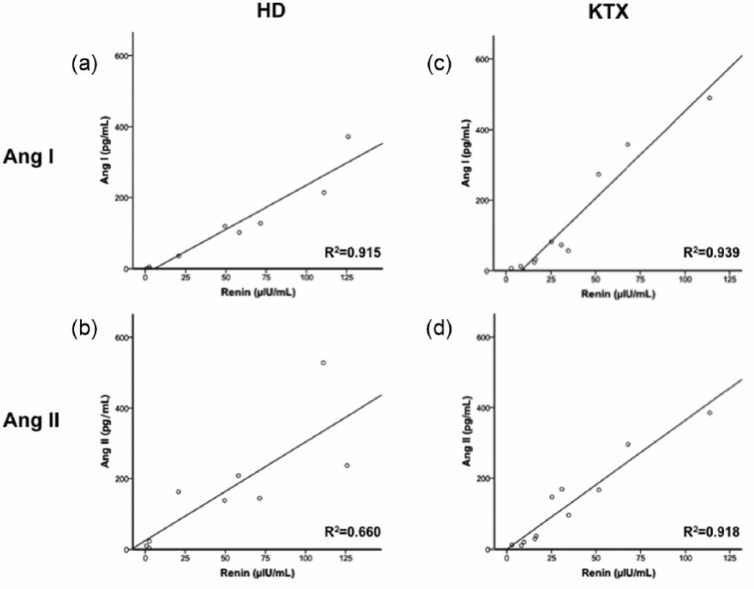
Despite these inter-individual Ang variations, a strong correlation between renin and Ang I was found in non-ACEi-treated patients on HD (Figure 2(a), R2 = 0.915, p < 0.001), while the renin/Ang II correlation was weaker (Figure 2(b), R2 = 0.660, p = 0.008). After renal function had been restored through KTX, the correlation persisted for Ang I (Figure 2(c), renin/Ang I R2 = 0.939, p < 0.001), while it profoundly improved for Ang II (Figure 2(d), renin/Ang II R2 = 0.918, p < 0.001). Neither before nor after KTX, a significant correlation pattern could be found between RAS parameters (renin, Ang I and Ang II) and aldosterone concentrations.
Figure 2.
Renin/Angiotensin (Ang) correlations.
(a) Renin/Ang I correlation on HD; (b) Renin/Ang II correlation on HD; (c) Renin/Ang I correlation after KTX; (d) Renin/Ang II correlation after KTX. Patients on ACEi or on dual RAS blockade were excluded from the analysis. HD: hemodialysis; KTX: kidney transplantation; ACEi: angiotensin-converting enzyme inhibitors; RAS: renin-angiotensin system.
RAS patterns according to medication groups
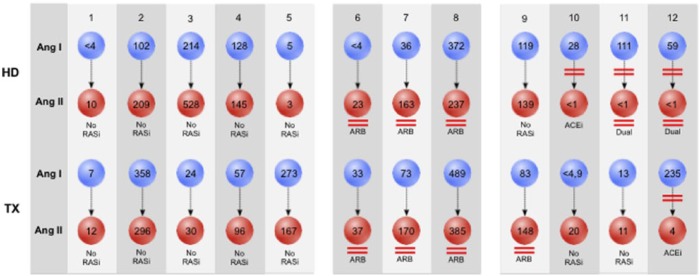
Both on HD and after KTX none of the patients on ACEi alone or on dual RAS blockade had detectable Ang II levels, demonstrating effectiveness of this enzymatic blockade (Figure 3). In contrast to this, each ARB patient displayed distinct Ang II levels. We further separately analyzed patients who did not undergo RAS blocker change between the two analyses (Table 4). Here, we found that KTX led to an increase of all analyzed RAS components in ARB-treated patients, while a decrease of systemic RAS activity was observed in patients without RAS blockers.
Figure 3.
Patient-wise Angiotensin concentrations on hemodialysis and after kidney transplantation.
Blue spheres represent Ang I, red spheres represent Ang II concentrations. Concentrations are given in pg/ml. RASi: renin-angiotensin system inhibitor; ARB: angiotensin receptor blockers; ACEi: angiotensin-converting enzyme inhibitors; Ang I: Angiotensin I; Ang II: Angiotensin II.
Table 4.
RAS concentrations before and after kidney transplantation according to medication group in patients without medication change.
| No RASi |
ARB |
|||
|---|---|---|---|---|
| HD | KTX | HD | KTX | |
| Renin (µIU/ml) | 58.2 (110.4) | 34.7 (65.0) | 20.7 (123.4) | 30.8 (97.3) |
| Ang 1–10 (pg/ml) | 102.0 (214.0) | 57.0 (350.3) | 36.0 (371.9) | 73.4 (456.6) |
| Ang 1–9 (pg/ml) | <4 | <4 | <4 | <4 |
| Ang 1–8 (pg/ml) | 144.8 (524.6) | 96.1 (283.2) | 163.0 (214.2) | 169.7 (347.4) |
| Ang 1–7 (pg/ml) | <2 | <2 (8.5) | <2 | <2 |
| Ang 1–5 (pg/ml) | 3.2 (17.0) | 2.5 (6.0) | 3.9 (6.0) | <1 (9.1) |
| Ang 2–8 (pg/ml) | 4.7 (18.0) | 3.6 (5.6) | <3 (18.3) | 5.7 (25.0) |
| Ang 3–7 (pg/ml) | <1 | <1 | <1 | <1 |
| Ang 3–8 (pg/ml) | 5.4 (15.0) | 3.6 (7.3\) | 3.0 (9.3) | 6.5 (20.5) |
| Ang 2–7 (pg/ml) | <4 | <4 | <4 | <4 |
| Ang 2–10 (pg/ml) | <5 (11.0) | <5 (30.1) | <5 (34.0) | <5 (20.1) |
| Aldosterone (pg/ml) | 332.0 (873.0) | 206.0 (459.0) | 33.0 (37.6) | 110.0 (135.0) |
| Ang II/Ang I ratio | 1.59 (1.80) | 1.02 (1.07) | 2.58 (3.89) | 1.55 (1.53) |
HD: hemodialysis; KTX: kidney transplantation; RASi: renin-angiotensin system inhibitor; ARB: angiotensin receptor blocker; Ang: angiotensin. Data are shown as medians (interquartile range).
In ARB-treated patients the median Ang II/Ang I ratio, which directly reflects systemic ACE activity, was found to be 2.6 (IQR 3.9) during HD and decreased to 1.6 (1.5) after KTX. In line with these observations, patients without RAS inhibition also exhibited higher Ang II/Ang I ratios on HD (1.6 (1.8)) than after KTX (1.0 (1.1)).
Analyzing the alternative RAS, it is noteworthy that none of the analyzed HD patients exhibited measurable Ang-(1–7) concentrations and only one KTX patient (who was not taking any RAS blockade at the time of analysis) displayed detectable Ang-(1–7) concentrations. Yet, in almost all patients without RAS inhibition or with an ARB, Ang-(1–5) was detectable at low concentrations indicating an activation of the alternative RAS (Table 4).
The concentrations of both smaller molecules Ang-(2–8) and Ang-(3–8) increased after KTX in ARB-treated patients, while they decreased in those without RAS blockade.
Discussion
Here, we provide the first detailed assessment of systemic angiotensin regulation in maintenance hemodialysis patients who underwent successful KTX in the presence and absence of different RAS blockers.
RAS blockade has shown contradictory results with regard to clinical outcomes such as overall and graft survival after KTX.8,27,28 However, little is known about underlying molecular effects that might explain the clinical effects taking place with this form of treatment.
By employing a highly sensitive mass spectrometry-based angiotensin quantification method, new insights into the molecular angiotensin regulation are now available: In line with a recent analysis by Issa et al., who analyzed renin and aldosterone regulation in KTX recipients,17 our patients also exhibited renin and aldosterone concentrations in a normal range after KTX. Intriguingly, renin concentration varied much less after transplantation, possibly due to a reinstated juxtaglomerular feedback mechanism.
Correspondingly, the first important finding of our study is that while angiotensin concentrations still vary after KTX, the correlation between renin and Ang II substantially improves after restoration of renal function. This implies the reconstitution of a direct dependency of Ang II concentrations on a renin-dependent mechanism, which may be explained by recovered stability of the intermediary product Ang I, the predominant substrate of ACE for Ang II formation under physiological conditions (Figure 4). It has been shown previously that the kidney can respond quickly to environmental stimuli under healthy conditions.29 However, in states of renal failure the kidney’s secretory and reabsorbing qualities are disturbed.24 Hence, it may be hypothesized that the demonstrated amelioration of a regulatory renin feedback mechanism might depend on the organ donor RAS that is engrafted into the highly distorted recipient RAS. Correspondingly, angiotensin values were up-regulated after KTX in patients with ARB therapy, reflecting a healthy reaction to this mode of RAS blockade.
Figure 4.
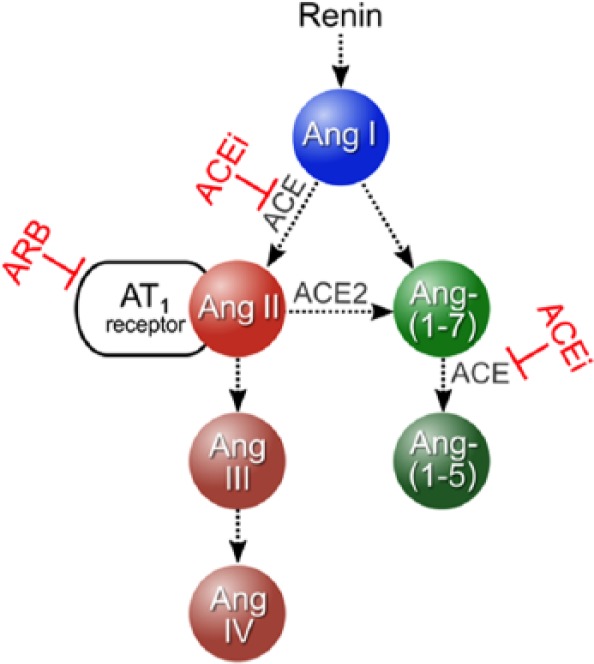
Basic model of the RAS, its main enzymes and interfering substances used in the study.
RAS: renin-angiotensin system; Ang: Angiotensin; ACE: angiotensin-converting enzyme; ACEi: ACE inhibitor; ARB: angiotensin receptor blocker.
Secondly, molecules representing the alternative RAS were present only at very low concentrations both before and after KTX. Roberts et al. showed in their 2013 analysis of HD and KTX patients that plasma ACE2 activity was lowest in HD and found slightly higher values of this essential alternative RAS enzyme after KTX.30 Several other studies have shown that Ang-(1–7) concentrations are often very low or immeasurable in states of health and disease.31,32 While we did not measure ACE2 activity, we are the first to quantify the effector molecules of the alternative RAS in these patients. Our results suggest that Ang-(1–7) might be quickly metabolized to Ang-(1–5) in patients without ACE inhibitors as most patients without RAS blockade or on ARB had detectable Ang-(1–5) levels, indicating an ongoing activity of the alternative RAS axis. ACE blockade was effective in our study, as all patients with ACEi therapy had neither significant Ang II nor Ang-(1–5) levels. Even though clinical effects of Ang-(1–7) and Ang-(1–5) remain to be fully elucidated at this point, several analyses have shown promising results with regard to vasodilation, anti-inflammatory effects and secretion of atrial natriuretic peptide.12,33,34
Thirdly, smaller RAS molecules, such as Ang-(2–8), which exerts functions similar to Ang II,35 and Ang-(3–8), which is supposed to be involved in learning and memory,36 were also analyzed. While values were undetectable in ARB patients and low in non-RASi patients on HD, a restoration of a physiological reaction (in particular higher concentrations in ARB-treated patients) occurred after KTX. This supports the notion that an adequately functioning RAS is strictly present in normal to only moderately reduced renal function and is distorted in end-stage renal disease.
We did not detect any correlations between the analyzed RAS components and aldosterone concentrations. This finding might signify that aldosterone regulation remains largely dependent on other factors than angiotensins in patients with renal failure, such as potassium-driven feedback regulation and/or blood volume.37–39 This concept has been suggested in an earlier work by Kokot et al., who previously described that a “dissociation of the physiological relationship between aldosterone synthesis and function of the renin-angiotensin system” occurs following KTX.40
Some limitations of our study need to be mentioned: The analysis is primarily descriptive as the number of included patients was small. Our intention was to gain comprehensive insight into the RAS regulation occurring with KTX through an exploratory study design; therefore, we intentionally included patients from different medication groups. Further, since our study only included patients early after renal transplantation (<1 year post-transplant), it will be highly interesting to analyze long-term transplanted patients. Chronic rejection and allograft dysfunction with progressive interstitial fibrosis might alter the relative distribution of RAS-processing enzymes including an increase of chymase expression due to increased mast cell infiltration, leading to a distortion of the RAS.10,41 On the other hand, an enhancement of beneficial RAS effects might become possible by selectively blocking classical and enforcing non-classical components in a reinstated, functioning system.42
In conclusion, our study is the first analysis to demonstrate that the RAS undergoes distinct changes on a molecular lever after KTX. Although angiotensin concentrations vary considerably between patients, an intra-individual restoration of physiological RAS regulation takes place. Thus, the RAS becomes a relevant and customizable target for antihypertensive medication at least in the early period after KTX.43 Further studies will be essential to study the regulation of the RAS also at later phases after transplantation and to translate our findings into clinically meaningful forms of optimized RAS blockade in renal transplant patients.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Opelz G, Zeier M, Laux G, et al. No improvement of patient or graft survival in transplant recipients treated with angiotensin-converting enzyme inhibitors or angiotensin II type 1 receptor blockers: A collaborative transplant study report. J Am Soc Nephrol 2006; 17: 3257–3262. [DOI] [PubMed] [Google Scholar]
- 2. Heinze G, Collins S, Benedict MA, et al. The association between angiotensin converting enzyme inhibitor or angiotensin receptor blocker use during postischemic acute transplant failure and renal allograft survival. Transplantation 2006; 82: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 3. Hernández D, Muriel A, Abraira V, et al. Renin-angiotensin system blockade and kidney transplantation: A longitudinal cohort study. Nephrol Dial Transplant 2012; 27: 417–422. [DOI] [PubMed] [Google Scholar]
- 4. Stigant CE, Cohen J, Vivera M, et al. ACE inhibitors and angiotensin II antagonists in renal transplantation: An analysis of safety and efficacy. Am J Kidney Dis 2000; 35: 58–63. [DOI] [PubMed] [Google Scholar]
- 5. Glicklich D, Gordillo R, Supe K, et al. Angiotensin converting enzyme inhibitor use soon after renal transplantation: A randomized, double-blinded placebo-controlled safety study. Clin Transplant 2011; 25: 843–848. [DOI] [PubMed] [Google Scholar]
- 6. Opelz G, Döhler B. Treatment of kidney transplant recipients with ACEi/ARB and risk of respiratory tract cancer: A collaborative transplant study report. Am J Transplant 2011; 11: 2483–2489. [DOI] [PubMed] [Google Scholar]
- 7. Opelz G, Döhler B. Cardiovascular death in kidney recipients treated with renin-angiotensin system blockers. Transplantation 2014; 97: 310–315. [DOI] [PubMed] [Google Scholar]
- 8. Knoll GA, Fergusson D, Chassé M, et al. Ramipril versus placebo in kidney transplant patients with proteinuria: A multicentre, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4: 318–326. [DOI] [PubMed] [Google Scholar]
- 9. Toto RD. Transplantation: The role of RAAS blockade in kidney transplantation. Nat Rev Nephrol 2016; 12: 129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251–287. [DOI] [PubMed] [Google Scholar]
- 11. Sasaki S, Higashi Y, Nakagawa K, et al. Effects of angiotensin-(1–7) on forearm circulation in normotensive subjects and patients with essential hypertension. Hypertension 2001; 38: 90–94. [DOI] [PubMed] [Google Scholar]
- 12. Carver KA, Smith TL, Gallagher PE, et al. Angiotensin-(1–7) prevents angiotensin II-induced fibrosis in cremaster microvessels. Microcirculation 2015; 22: 19–27. [DOI] [PubMed] [Google Scholar]
- 13. Rettig R, Büch M, Gerstberger R, et al. Effects of kidney transplantation on the renin-angiotensin systems of the recipients. Kidney Int 1994; 46: 1536–1538. [DOI] [PubMed] [Google Scholar]
- 14. Grisk O, Heukäufer M, Steinbach A, et al. Analysis of arterial pressure regulating systems in renal post-transplantation hypertension. J Hypertens 2004; 22: 199–207. [DOI] [PubMed] [Google Scholar]
- 15. Salzberg DJ. Is RAS blockade routinely indicated in hypertensive kidney transplant patients? Curr Hypertens Rep 2007; 9: 422–429. [DOI] [PubMed] [Google Scholar]
- 16. Hiremath S, Fergusson D, Doucette S, et al. Renin angiotensin system blockade in kidney transplantation: A systematic review of the evidence. Am J Transplant 2007; 7: 2350–2360. [DOI] [PubMed] [Google Scholar]
- 17. Issa N, Ortiz F, Reule SA, et al. The renin-aldosterone axis in kidney transplant recipients and its association with allograft function and structure. Kidney Int 2014; 85: 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 19. Poglitsch M, Domenig O, Schwager C, et al. Recombinant expression and characterization of human and murine ACE2: Species-specific activation of the alternative renin-angiotensin-system. Int J Hypertens 2012; 2012: 428950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haschke M, Schuster M, Poglitsch M, et al. Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin Pharmacokinet 2013; 52: 783–792. [DOI] [PubMed] [Google Scholar]
- 21. Savergnini SQ, Ianzer D, Carvalho MB, et al. The novel Mas agonist, CGEN-856S, attenuates isoproterenol-induced cardiac remodeling and myocardial infarction injury in rats. PLoS One 2013; 8: e57757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jessup M, Fox KA, Komajda M, et al. PARADIGM-HF—the experts’ discussion. N Engl J Med 2014; 371: e15. [DOI] [PubMed] [Google Scholar]
- 23. Prasad GV, Ruzicka M, Burns KD, et al. Hypertension in dialysis and kidney transplant patients. Can J Cardiol 2009; 25: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovarik JJ, Antlanger M, Domenig O, et al. Molecular regulation of the renin-angiotensin system in haemodialysis patients. Nephrol Dial Transplant 2015; 30: 115–123. [DOI] [PubMed] [Google Scholar]
- 25. Ye M, Wysocki J, Gonzalez-Pacheco FR, et al. Murine recombinant angiotensin-converting enzyme 2: Effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension 2012; 60: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haber PK, Ye M, Wysocki J, et al. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: Studies in vivo, ex vivo, and in vitro. Hypertension 2014; 63: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chatzikyrkou C, Eichler J, Karch A, et al. Short- and long-term effects of the use of RAAS blockers immediately after renal transplantation. Blood Press 2017; 26: 30–38. [DOI] [PubMed] [Google Scholar]
- 28. Shin JI, Palta M, Djamali A, et al. The association between renin-angiotensin system blockade and long-term outcomes in renal transplant recipients: The Wisconsin Allograft Recipient Database (WisARD). Transplantation 2016; 100: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 29. Belova N, Tzaneva N, Avramova T, et al. Renal excretory function, atrial natriuretic peptide (ANP) and plasma renin activity (PRA) during long-term sodium loading in rats. Acta Physiol Pharmacol Bulg 1991; 17: 104–110. [PubMed] [Google Scholar]
- 30. Roberts MA, Velkoska E, Ierino FL, et al. Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrol Dial Transplant 2013; 28: 2287–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Rooyen JM, Poglitsch M, Huisman HW, et al. Quantification of systemic renin-angiotensin system peptides of hypertensive black and white African men established from the RAS-Fingerprint®. J Renin Angiotensin Aldosterone Syst 2016; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hisatake S, Kiuchi S, Kabuki T, et al. Serum angiotensin-converting enzyme 2 concentration and angiotensin-(1–7) concentration in patients with acute heart failure patients requiring emergency hospitalization. Heart Vessels 2017; 32: 303–308. [DOI] [PubMed] [Google Scholar]
- 33. Yu L, Yuan K, Phuong HT, et al. Angiotensin-(1–5), an active mediator of renin-angiotensin system, stimulates ANP secretion via Mas receptor. Peptides 2016; 86: 33–41. [DOI] [PubMed] [Google Scholar]
- 34. Oudit GY, Liu GC, Zhong J, et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 2010; 59: 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yugandhar VG, Clark MA. Angiotensin III: A physiological relevant peptide of the renin angiotensin system. Peptides 2013; 46: 26–32. [DOI] [PubMed] [Google Scholar]
- 36. Gard PR. Cognitive-enhancing effects of angiotensin IV. BMC Neurosci 2008; (9 Suppl 2): S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bollag WB. Regulation of aldosterone synthesis and secretion. Compr Physiol 2014; 4: 1017–1055. [DOI] [PubMed] [Google Scholar]
- 38. Barber JK, Bartter FC, Delea C, et al. The regulation of aldosterone secretion in man: The role of fluid volume. J Clin Invest 1956; 35: 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bomback AS, Kshirsagar AV, Ferris ME, et al. Disordered aldosterone-volume relationship in end-stage kidney disease. J Renin Angiotensin Aldosterone Syst 2009; 10: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kokot F, Grzeszczak W, Zukowska-Szczechowska E, et al. Endocrine alterations in kidney transplant patients. Blood Purif 1990; 8: 76–86. [DOI] [PubMed] [Google Scholar]
- 41. Halloran PF, de Freitas DG, Einecke G, et al. The molecular phenotype of kidney transplants. Am J Transplant 2010; 10: 2215–2222. [DOI] [PubMed] [Google Scholar]
- 42. Gromotowicz-Poplawska A, Szoka P, Kolodziejczyk P, et al. New agents modulating the renin-angiotensin-aldosterone system—Will there be a new therapeutic option? Exp Biol Med (Maywood) 2016; 241: 1888–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lorenz M, Billensteiner E, Bodingbauer M, et al. The effect of ACE inhibitor and angiotensin II blocker therapy on early posttransplant kidney graft function. Am J Kidney Dis 2004; 43: 1065–1070. [DOI] [PubMed] [Google Scholar]