Abstract
Introduction:
The renal renin–angiotensin system (RAS) and the ultrasensitive energy sensor AMP-activated protein kinase (AMPK) have been implicated in normal and aberrant states of the kidney, but interaction between the RAS and AMPK remains unknown.
Methods:
Ninety-six rats were stratified into four groups: sham, uninephrectomised, uninephrectomised rats treated with the angiotensin-converting enzyme inhibitor lisinopril or the angiotensin receptor blocker losartan. Histopathological examination at 9 months post-operation and biochemical measurements at 3, 6 and 9 months were performed for changes in renal structure and function. The expression of AMPK and angiotensin II at 9 months was detected by immunofluorescence microscopy and western blot.
Results:
Compared with sham rats, uninephrectomised rats demonstrated progressive glomerulosclerosis, tubular atrophy with cast formation and chronic inflammatory infiltration, in parallel to elevated serum urea, creatinine, urine total protein to creatinine ratio and reduced serum albumin. Overexpression of angiotensin II coexisted with a 85.6% reduction of phosphorylated to total AMPK ratio in the remnant kidney of uninephrectomised rats. RAS blockade by the angiotensin-converting enzyme inhibitor or angiotensin receptor blocker substantially normalised AMPK expression, morphological and functional changes of the remnant kidney.
Conclusions:
Uninephrectomy-induced RAS activation and AMPK inhibition in the remnant kidney could be substantially corrected by RAS blockade, suggesting a cross-talk between AMPK and RAS components in uninephrectomised rats.
Keywords: Renin–angiotensin system, AMP-activated protein kinase, uninephrectomy, renal impairment, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker
Introduction
The kidney is one of the major organs devoted to maintaining whole body homeostasis mainly with its functions being the excretion of waste metabolism and regulation of blood pressure.1 Due to the changes in social environment and people’s concept and lifestyle, as well as improvements in medical and health services, the high prevalence of chronic kidney disease (CKD) around the world has caught all the researchers and medical personnel off guard, especially for CKD. According to a survey in 2012, the overall prevalence of CKD in China is 10.8%, therefore the number of patients with CKD is estimated to be about 119.5 million.2 Kidney transplantation is the last chance of survival for uraemic patients after dialysis.3 On the other hand, the kidney disease outcomes quality initiative has declared that stage 3 CKD or worse occurs in 24.4% of living kidney donors, suggesting that long-term prospective studies of donors are needed to identify the complications.4 Similar to living kidney donors, persons with severe renal impairment are also subjected to cardiovascular disease and cerebrovascular accident in addition to the damaged kidney. The negative effects of a single kidney in the broad sense have inspired us to explore the mechanisms underlying renal pathophysiology.
Renin–angiotensin system (RAS) activation has been recognised to be responsible for the occurrence of kidney disease.5 Both in non-diabetic and diabetic models of renal disease, angiotensin II (Ang II) can perpetuate podocyte injury and promote progression to end-stage kidney disease.6 The implication of RAS activation in renal impairment and hypertension has been well established. Moreover, persistent RAS activation is also involved in energy generation and energy consumption, which is closely related to metabolic disturbance7 and cardiovascular disease.8 In addition, the kidney may be considered the second metabolic organ after the liver to regulate lipid and glucose metabolism9,10 with the positive regulation of AMP-activated protein kinase (AMPK) on renal structure and function.11,12 Therefore, we hypothesise that intra-renal RAS and AMPK may both be associated with renal damage after uninephrectomy. In our previous studies, uninephrectomised (UNX) rats developed renal impairments and metabolic disorders that could be attenuated by angiotensin-converting enzyme inhibitor (ACEI).13,14 Validation of pathogenic RAS activation in uninephrectomised rats has been carried out through direct detection and ACEI correction.15 ACEI, inhibiting the transformation from angiotensin I (Ang I) to Ang II, only verifies RAS activation from the viewpoint of the main effector generation. In order to demonstrate fully the mechanism of RAS activation in uninephrectomised rats, we have used rats treated with angiotensin receptor blocker (ARB) on the basis of a previous experimental design. Finally, we have detected AMPK expression in untreated and RAS blockade-treated uninephrectomised rats, to explore the interaction between RAS and AMPK after uninephrectomy.
Materials and methods
Animals
Three-month-old male Sprague–Dawley rats (300–350 g) were housed at the Laboratory of Animal Services Centre of Guilin Medical University. The animals were caged in pairs, housed at 23±1°C with a 12-hour dark/light cycle, having free access to water and fed on a standard laboratory rat diet (5001 Rodent Diet; LabDiet, St Louis, MO, USA). The total duration of studies was 9 months.
Ninety-six rats were randomly assigned into sham operation (n=24), left uninephrectomy (n=24), uninephrectomised rats treated with the ACEI lisnopril (ACEI, n=24) and with the ARB losartan (ARB, n=24). Ethical approval for animal studies was according to the animal experimentation ethics committee of the institution.
Uninephrectomy procedures
Rats were anaesthetised with ketamine (75 mg/kg; Alfasan, Woerden, Holland) and xylazine (10 mg/kg; Alfasan) and were subjected to sham operation, left nephrectomy, or uninephrectomised rats treated with ACEI and ARB. Lisinopril and losartan were dissolved in sterile distilled water, with a once daily dosage of 4 mg per kg body weight. All the sham and uninephrectomised rats were also gavaged with distilled water (3 ml) as placebo control. The left kidney was exposed via a flank 1–1.5 cm length incision and was removed, leaving the adrenal gland intact. After testing baseline data, six rats from each group were killed for biochemical assessments at 3, 6 and 9 months post-operation. The rest of the rats were killed for histopathological examination, immunofluorescence and western blot.
Biochemical studies
Fasting blood samples were taken for measurements of albumin and renal function reflected by fasting serum urea and creatinine. Serum urea (enzymatic method), serum albumin and urine total protein (immunoturbidimetry), serum/urine creatinine (Jaffe kinetic method) with urine samples collected using metabolic cages (Su-Zhou, China) were measured using a Modular Analytics analyser (C501; Roche Diagnostics GmbH, Mannheim, Germany), and reagent kits were supplied by the same manufacturer. All reagents were used according to the manufacturer’s instructions and the analytical performance of these methods was within the manufacturer’s specifications.
Examinations for histopathology and immunofluorescence
Microscopic examination of the remnant kidney was performed to assess renal structure and in situ expression of RAS components and AMPK in the remnant kidneys and right kidney of sham rats. Specimens were fixed in 10% neutral formaldehyde for light microscopy or stored in liquid nitrogen for protein assessment. After 24 h fixation, tissues were embedded in paraffin and dissected with 4 μm cross-sections. Tissue slides were then stained with periodic acid Schiff for light microscopy and with goat anti-Ang I/II (sc-7419; dilution 1:200; Santa Cruz Biotechnology, Inc. 2145 Delaware Ave Santa Cruz, CA. 95060, USA) and rabbit anti-phosphorylated AMPKα1/α2 (p-AMPKα1/α2) (Thr 172: sc-33524; 1:100; Santa Cruz Biotechnology) for immunofluorescence. Thereafter, tissue slides were blocked with 1% bovine serum albumin (BSA) for 30 minutes before the treatment of the primary antibodies overnight at 4°C. Sections of human kidney were used as positive controls, while goat and rabbit serum were used to replace the primary antibodies as negative controls. Immunofluorescence was detected with donkey anti-goat or anti-rabbit secondary antibodies (Molecular Probes, Eugene, OR, USA) conjugated with Alexa 488 (green) at a dilution of 1:400. Slides were mounted with a ProLong (Molecular Probes) anti-fading reagent, stored in the dark at 4°C, and examined within 1–3 days. All stained slides were examined with a light and fluorescence microscope (AX10; Carl Zeiss, Hamburg, Germany) and anti-p-AMPKα1/α2 using a confocal laser microscope (LSM710; Carl Zeiss), and representative images were automatically captured using a digital spot camera. Semiquantitative analysis of immunofluorescence images was performed by selecting 20 representative fields at ×400 magnification, as described in our previous reports.
Western blot
Tissue extracted from the renal cortex was homogenised in a buffer containing 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L EDTA, 1% sodium deoxycholate, 1% Triton X-100, 1% sodium dodecyl sulfate (SDS) and 5% protein enzyme inhibitor cocktail (cat. no. P2714; Sigma, St Louis, MO, USA). The homogenate was centrifuged at 13,000 rpm for 10 minutes at 4°C. The resulting supernatant was removed, and protein concentrations in the supernatant were determined by the BCA Protein Assay Kit (cat. no. 23225; ThermoFisher Scientific, Waltham, MA, USA) using BSA as the standard. Tissue lysates (100 mg) and prestained molecular weight markers (Bio-Rad, Hercules, CA, USA) were loaded onto SDS-polyacrylamide electrophoresis gels (4% acrylamide stacking gel and 8% running gel). The resolved proteins were then transferred onto nitrocellulose membranes. The membranes were blocked for 1 hour at room temperature with 5% skimmed milk, incubated with rabbit anti-p-AMPKα1/α2 (Ser 496: sc-101631; 1:200; Santa Cruz Biotechnology), goat anti-total AMPKα1 (t-AMPKα1) (C-20: sc-19128; 1:200; Santa Cruz Biotechnology) in TBS containing 0.05% Tween 20 (TBS-T) with 5% skimmed milk overnight at 4°C. After washing with TBS-T, membranes were incubated with secondary antibody conjugated to horseradish peroxidase (Upstate, Temecula, MA, USA) with dilution of 1:2000. Proteins were detected by enhanced chemiluminescence (Amersham, Piscataway, NJ, USA) on Hyperfilm. To ensure equal loading of proteins, membranes were incubated and probed with a rabbit anti-β-actin antibody (1:10,000, Abcam), which recognises the β-actin protein at approximately 43 kD, just as important, the major protein bands with approximately 63 kD for p-AMPKα1/α2, 74 kD for t-AMPKα1 were detected. Signals were quantitated by densitometry and corrected for the β-actin signal, using the Kodak Digital Image station 440CF and the ID Image Analysis software program.
Statistical analysis
Data were mean±SD unless specified. The statistical significance of differences noted in the biochemical parameters was evaluated using one-way analysis of variance, followed by post-hoc comparison with Bonferroni analysis. A two-tailed P value of less than 0.05 was taken as a criterion for a statistically significant difference.
Results
Overexpression of RAS induced by uninephrectomy
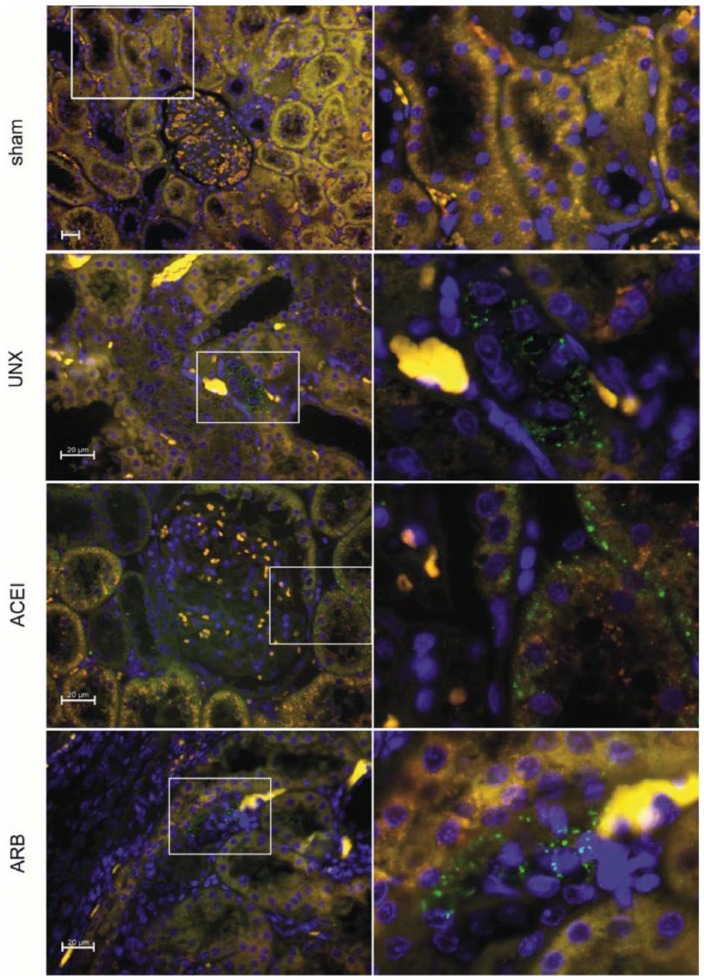
Firstly, Ang I/II from the renal cortex of the rats was examined by immunofluorescence at 9 months post-operation to validate the persistent RAS activation induced by uninephrectomy. In uninephrectomised rats, a positive reaction was mainly located in the cytoplasm of tubular epithelial cells characterising the loop of Henle (Figure 1. UNX), contrasting the rare immunoreactivity in sham rats (Figure 1. sham). The number of positive loop of Henle epithelial cells in the ARB group was slightly less than that in uninephrectomised rats (Figure 1. ARB). Interestingly, uninephrectomised rats treated with ACEI showed the immunoreactivity in proximal tubular epithelial cells and glomerular Bowman’s capsule cells (Figure 1. ACEI). According to Figure 2, expressions of Ang I/II in uninephrectomised rats were 1.1 times higher than that of sham rats (sham versus uninephrectomised rats: 1.3±0.483 versus 2.7±0.476, P<0.001). Intriguingly, the immunoreactivity of AngI/II was reduced 28.6% by ACEI (uninephrectomised rats versus ACEI: 2.7±0.476 versus 1.9±0.316, P<0.001) and 23.8% by ARB (uninephrectomised rats versus ARB: 2.7±0.476 versus 2.1±0.738, P=0.045).
Figure 1.
Immunofluorescence of Ang I/II reactivity in right kidneys. Renal sections were obtained from sham, uninephrectomised (UNX), angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) rats at 9 months after uninephrectomy and stained with anti-Ang I/II (green). Immunofluorescent microscopy showed an increased expression of Ang I/II in renal tubules of uninephrectomised rats than that of sham rats. Treatment with ACEI or ARB reduced the immunoreactivity slightly less than that in the uninephrectomised group. Original magnification for negative control, sham ×200; original magnification for uninephrectomised rats, ACEI, ARB, ×400.
Figure 2.
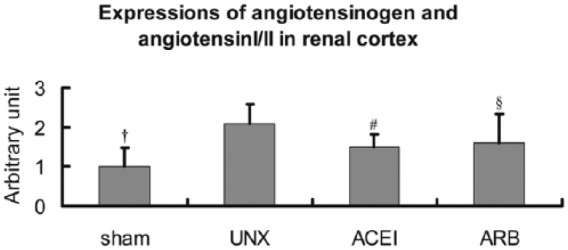
Semi-quantitative analysis of Ang I/II expressions in right kidneys. Compared with sham rats, expressions of Ang I/II in uninephrectomised rats (UNX) increased about 1.1 times, and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) reduced the high expressions about 28.6% or 23.8%. Data are mean±SD. †P<0.001, uninephrectomised rats versus sham rats; #P<0.001, uninephrectomised rats versus ACEI rats; §P<0.05, uninephrectomised rats versus ARB rats.
Renal impairment after uninephrectomy
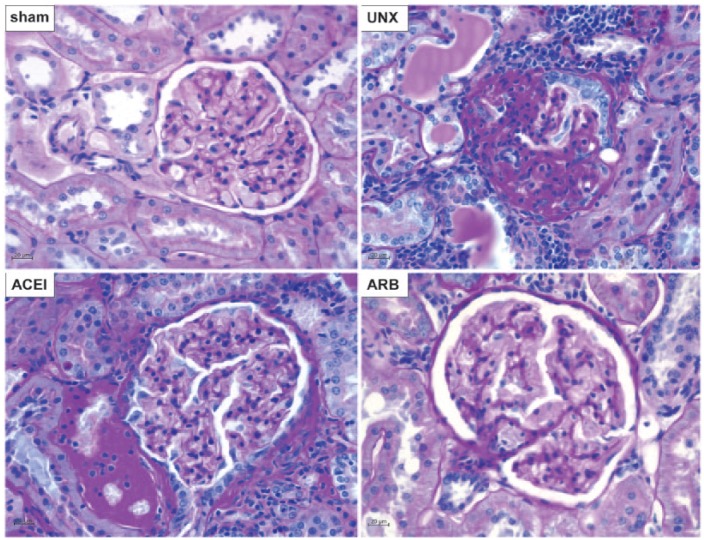
We also conducted a series of morphological examinations to test the histological changes in the remnant kidneys of treated and untreated uninephrectomised rats and the corresponding right kidney in sham rats at 9 months after operation. Uninephrectomised rats showed progressive glomerulosclerosis, podocyte damage, basement membrane thickening, tubular atrophy with cast formation and chronic inflammation in interstitial tissues adjacent to glomeruli (Figure 3. UNX). These pathological changes were not found in sham rats despite the fact that the rats were rather old at the end of observation (12 months of age) (Figure 3. sham). The ACEI-treated uninephrectomised rats showed mild to moderate glomerular hypertrophy and basement membrane thickening with mild inflammatory cells infiltration (Figure 3. ACEI). The renal histopathology of ARB-treated rats was similar to that of sham rats (Figure 3. ARB).
Figure 3.
Histopathological changes of right kidneys. Periodic acid Schiff stain was performed on kidney tissue sections of sham, uninephrectomised (UNX), angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) rats at 9 months after uninephrectomy. Compared with sham rats, uninephrectomised rats showed diffuse glomerulosclerosis, basement membrane thickening, tubular atrophy with cast formation, and chronic inflammatory infiltration in the remnant kidney. Treatments with ACEI and ARB largely ameliorated these renal structural damages. Original magnification ×400.
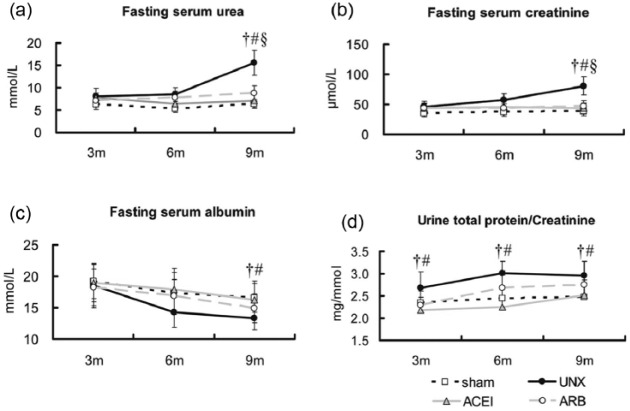
In parallel to the histological damages of the remnant kidney, uninephrectomised rats demonstrated progressive chronic kidney dysfunction comparing with the sham group by 3, 6 and 9 months post-operation, as reflected by elevated fasting serum urea (Figure 4(a): sham versus uninephrectomised, 7.0±1.0 versus 19.6±2.8 mmol/L, P=0.002), creatinine (Figure 4(b): sham versus uninephrectomised, 42.9±7.4 versus 98.5±15.1 µmol/L, P=0.0005), but decreased albumin (Figure 4(c): sham versus uninephrectomised, 15.6±2.3 versus 12.2±1.8 g/L, P=0.034) at 9 months. The urine total protein to creatinine ratio between the sham and uninephrectomised groups showed a significant difference early at 3 months (Figure 4(d): sham versus uninephrectomised, 2.3±0.2 versus 2.7±0.5 mg/mmol, P=0.027). Both ACEI and ARB statistically attenuated the elevated urea (ACEI versus uninephrectomised: 7.3±1.5 versus 19.6±2.8 mmol/L, P=0.004; ARB versus uninephrectomised: 9.1±1.7 versus 19.6±2.8 mmol/L, P=0.009) and creatinine (ACEI versus uninephrectomised: 45.6±8.7 versus 98.5±15.1 µmol/L, P=0.002; ARB versus uninephrectomised: 48.7±9.2 versus 98.5±15.1 µmol/L, P<0.0001) levels in uninephrectomised rats. In addition, the administration of ACEI completely normalised the albumin level (ACEI versus uninephrectomised: 14.6±3.0 versus 12.2±1.8 g/L, P=0.010) at 9 months and the ratio of urine total protein to creatinine.
Figure 4.
Longitudinal changes of renal function. Fasting serum urea, albumin, creatinine and urine total protein to creatinine were measured in sham, uninephrectomised (UNX), angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) rats at 3, 6 and 9 months after uninephrectomy. There were no statistical differences in fasting serum urea, albumin and creatinine among the four groups at 3 and 6 months, but in the ratio of total protein to creatinine. Uninephrectomised rats had higher serum urea (a), creatinine (b) and urine total protein to creatinine ratio (d), but lower albumin (c) than that of the sham group, and treatments with ACEI and ARB corrected the abnormal fasting serum levels close to the sham group at 9 months and attenuated the urine ratio during the whole detection. Data are mean±SD. †P<0.05, uninephrectomised rats versus sham rats; #P<0.05, uninephrectomised rats versus ACEI rats; §P<0.05, uninephrectomised rats versus ARB rats.
Activation of AMPK diminished by uninephrectomy
The expression of t-AMPK and p-AMPK in renal cortex was detected by western blot at 9 months post-operation. The expression of t-AMPK was similar in four groups, but uninephrectomised rats showed significantly decreased expression of p-AMPK compared with that of the other three groups (Figure 5(a)). According to the histogram based on the bands, the ratio of p-AMPK to t-AMPK in the uninephrectomised group decreased by 85.6% compared with that of sham rats. The p-AMPK/t-AMPK ratio in the treated uninephrectomised rats was increased 7.5 times by ACEI and 6.9 times by ARB (Figure 5(b)).
Figure 5.
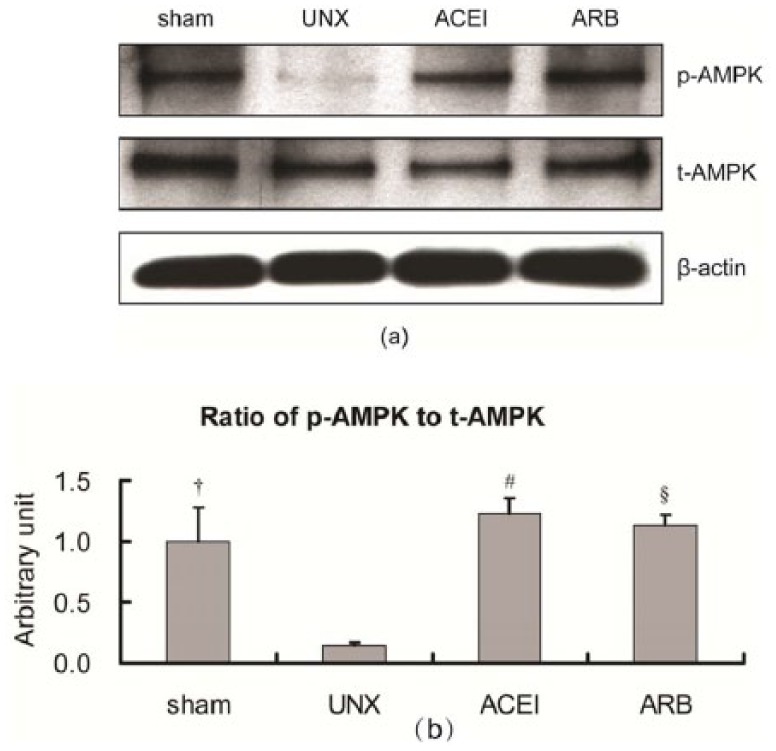
Expressions of t-AMPK and p-AMPK in the renal cortex of right kidneys by western blot. Fresh renal cortex samples were obtained from sham, uninephrectomised (UNX), angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) rats at 9 months after uninephrectomy. Proteins were subjected to immunoblot for p-AMPK and t-AMPK. Expressions of t-APMK in the four groups were nearly the same, but p-AMPK of uninephrectomised rats was significantly decreased compared with those of the other three groups (a). Histogram showed that the ratio of p-AMPK to t-AMPK in the uninephrectomised group also decreased and treatment with ACEI or ARB corrected the ratio higher than that of sham rats (b). Data are mean±SD. †P<0.001, uninephrectomised rats versus sham rats; #P<0.001, uninephrectomised rats versus ACEI rats; §P<0.001, uninephrectomised rats versus ARB rats.
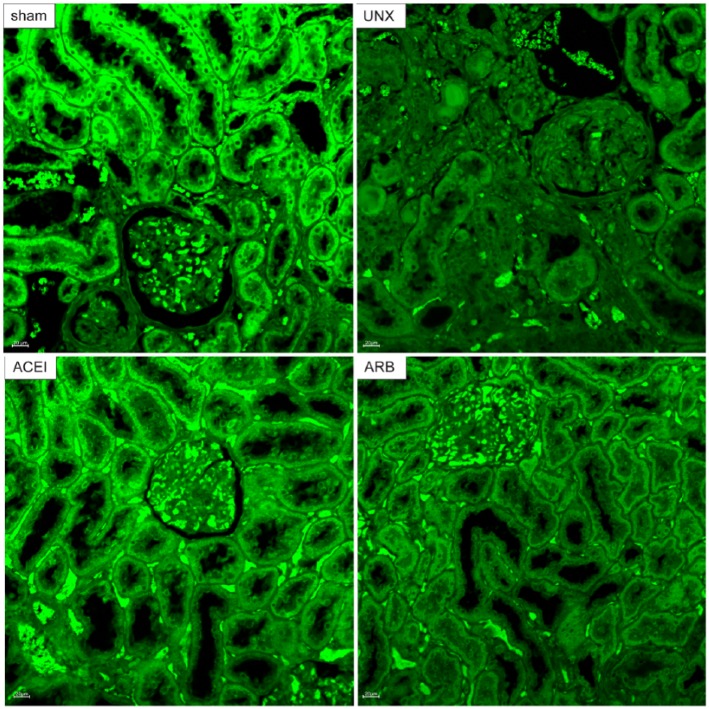
Furthermore, confocal laser microscopy demonstrated AMPK immunoreactivity predominantly located in the normal-looking renal tubular epithelial cells. Consistent with the kidney histological changes and western blot results, immunoreactivity of AMPK was extensive and intensive in sham rats and rats treated with either ACEI or ARB. In contrast, uninephrectomised rats showed positive AMPK immunostain in normal renal tubular cells, but not in the atrophic epithelial cells (Figure 6). Figure 7 shows the diminished expression of AMPK in uninephrectomised rats when compared with the other three groups. AMPK expressions decreased 50% in uninephrectomised rats (sham versus uninephrectomised: 2.8±0.109 versus 1.4±0.021, P<0.001). Treatment with ACEI recovered the expression by 85.7% (uninephrectomised versus ACEI: 1.4±0.021 versus 2.6±0.210, P<0.001). ARB also improved the AMPK immunoreactivity by 57.1% (uninephrectomised versus ARB: 1.4±0.021 versus 2.2±0.119, P=0.006).
Figure 6.
Expression of p-AMPK by confocal laser miscroscopy. Renal tissue sections were obtained from sham, uninephrectomised (UNX), angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) rats at 9 months after uninephrectomy and stained with anti-p-AMPK (green). AMPK-positive renal tubular epithelial cells were extensive and intensive in sham rats and rats treated with either ACEI or ARB. In uninephrectomised rats, few tubular epithelial cells with normal histology showed immunoreactivity of p-AMPK. Original magnification, ×200.
Figure 7.
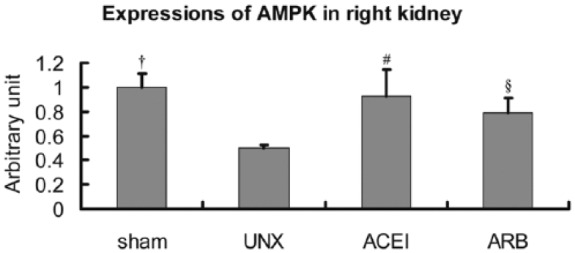
Semi-quantitative analysis of p-AMPK expressions in right kidneys. Compared with sham rats, expressions of AMPK in uninephrectomised (UNX) rats decreased about 50%, and angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) corrected the low expressions about 85.7% or 57.1%. Data are mean±SD. †P<0.001, uninephrectomised rats versus sham rats; #P<0.001, uninephrectomised rats versus ACEI rats; §P<0.05, uninephrectomised rats versus ARB rats.
Discussion
In this study, we report that uninephrectomy-induced renal impairment with RAS activation and AMPK inhibition could be largely prevented by treatment with ACEI and ARB. Our findings support potential cross-talk between renal RAS and AMPK in uninephrectomised rats.
In this study, we used a uninephrectomised rat model with 9-month observation instead of 5/6 or 90% nephrectomy that may cause acute and severe renal failure.16 Therefore, our chronic model is closer to the real changes in individuals living with a single kidney.
Our previous studies discovered that uninephrectomised rats developed metabolic disorders,14,16 including hyperglycaemia, hyperlipidaemia and insulin resistance. AMPK, defined as activated AMPK or phosphorylated AMPK here in this study, is a cellular energy sensor activated by metabolic stresses that inhibit catabolic adenosine triphosphate (ATP) production or accelerate ATP consumption.17 Previously, we have shown that uninephrectomy-induced RAS activation followed by renal impairments and metabolic abnormalities could be normalised by RAS blockade.14,16 In this study, we have consistently confirmed our previous findings and disclosed that uninephrectomy-induced AMPK inhibition could also be recovered by treatment with ACEI and ARB. Therefore, we hypothesise that RAS blockade may correct metabolic disturbances through restoring AMPK expression in the remnant kidney. Indeed, AMPK maintains direct energy homeostasis.17 In recent years, metabolic regulations by Ang II are partly reflected by diminished AMPK phosphorylation as described in the metabolic syndrome,18,19 especially in glucose metabolism.20,21 In addition, persistent renal RAS activation may lead to lipid dysmetabolism and renal impairment in uninephrectomised rats.13 Both uninephrectomy-induced RAS activation and AMPK inhibition could generally be improved by RAS blockade. Taken together, there might exist a cross-talk of renal RAS and AMPK in both normal and abnormal states of the kidney.
AMPK action is important in regulating renal tubular transport12,22 and podocyte maintenance.23-25 When the kidney is undergoing ischaemia, energy depletion or fibrosis, activating AMPK may represent a novel therapeutic approach in clinical practice.26-28 In this study, none of the uninephrectomised rats were treated with any AMPK activator such as metformin. Therefore, we do not know whether there are therapeutic effects of metformin on uninephrectomy-induced renal impairments. Although the uninephrectomy-induced AMPK inhibition could largely be recovered by RAS blockade, the causal relationship between RAS activation and AMPK inhibition remains open to exploration. In subtotal nephrectomised rats, early remnant kidney damages coexisted with decreased AMPK activity.29 Interestingly, AMPK activator remarkably corrected the early renal damage in this acute rat model.29 Possible links between RAS and the AMPK pathway include oxidative stress. It is known that oxidative stress can be reduced by AMPK.30 On the other hand, Ang II is known to promote apoptosis of renal mesangial cells and proximal tubular cells through increased oxidative stress.31,32 The effects of Ang II are mediated by downstream signalling involving transforming growth factor-β (TGF-β) and heme oxygenase (HO)-1 expression.31 Inversely, the activation of AMPK and AMPK activators has the ability to inhibit TGF-β and promote HO-1 expression in the kidney,28,33 contributing to reduced oxidative stress and renal cell apoptosis. Nevertheless, the cross-talk between RAS and AMPK warrant future investigation.
In conclusion, uninephrectomy induces chronic renal impairments accompanied by persistent renal RAS activation and AMPK inhibition. The uninephrectomy-induced aberrant RAS and AMPK expression could generally be improved by RAS blockade with ACEI and ARB, suggesting a potential cross-talk between RAS and AMPK pathways. Whether combined treatment with AMPK activator and RAS blocker adds synergistic benefits for people with renal damage requires future clinical trials.
Acknowledgments
The authors would like to thank Yu-Zhong Ouyang for his technical assistance.
Footnotes
Author contributions: The authors’ contributions were as follows: the study was designed by KKY, YS and HLZ; KKY and HLZ were responsible for critical revision of the article for important intellectual content, and drafted the manuscript; HRZ, NT and YHP conducted the collection and assembly of the data; JS, YMH and HRZ performed the statistical analyses; JS, XXZ, YS and HLZ interpreted the data; KKY and YS drafted the manuscript; HLZ had primary responsibility for the final content.
All authors agreed on the final version of the manuscript.
Declaration of conflicting interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of China (81270934, 81471054).
References
- 1. Stumvoll M, Chintalapudi U, Perriello G, et al. Uptake and release of glucose by the human kidney. Postabsorptive rates and responses to epinephrine. J Clin Invest 1995; 96: 2528–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012; 379: 815–822. [DOI] [PubMed] [Google Scholar]
- 3. Fujiwara T, Tanaka S, Okada K, et al. Impact of recipient aging on kidney allograft in living donor transplantation. Transplant Proc 2014; 46: 454–456. [DOI] [PubMed] [Google Scholar]
- 4. Tan L, Tai BC, Wu F, et al. Impact of Kidney Disease Outcomes Quality Initiative guidelines on the prevalence of chronic kidney disease after living donor nephrectomy. J Urol 2012; 185: 1820–1825. [DOI] [PubMed] [Google Scholar]
- 5. Sarafidis PA, Bakris GL. Renin-angiotensin blockade and kidney disease. Lancet 2008; 372: 511–512. [DOI] [PubMed] [Google Scholar]
- 6. Gagliardini E, Perico N, Rizzo P, et al. Angiotensin II contributes to diabetic renal dysfunction in rodents and humans via Notch1/Snail pathway. Am J Pathol 2013; 183: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lo CS, Liu F, Shi Y, et al. Dual RAS blockade normalizes angiotensin-converting enzyme-2 expression and prevents hypertension and tubular apoptosis in Akita angiotensinogen-transgenic mice. Am J Physiol Renal Physiol 2011; 302: F840–F852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia G, Sowers JR. Endothelial dysfunction potentially interacts with impaired glucose metabolism to increase cardiovascular risk. Hypertension 2014; 64: 1192–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim HJ, Moradi H, Yuan J, et al. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am J Physiol Renal Physiol 2009; 296: F1297–F1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Underwood AH, Newsholme EA. Some properties of phosphofructokinase from kidney cortex and their relation to glucose metabolism. Biochem J 1967; 104: 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Svensson RU, Shaw RJ. Cancer metabolism: tumour friend or foe. Nature 2012; 485: 590–591. [DOI] [PubMed] [Google Scholar]
- 12. Fraser SA, Gimenez I, Cook N, et al. Regulation of the renal-specific Na+-K+-2Cl- co-transporter NKCC2 by AMP-activated protein kinase (AMPK). Biochem J 2007; 405: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao HL, Sui Y, Guan J, et al. Fat redistribution and adipocyte transformation in uninephrectomized rats. Kidney Int 2008; 74: 467–477. [DOI] [PubMed] [Google Scholar]
- 14. Zhao HL, Sui Y, He L, et al. Lipid partitioning after uninephrectomy. Acta Diabetol 2011; 48: 317–328. [DOI] [PubMed] [Google Scholar]
- 15. Sui Y, Zhao HL, Fan RR, et al. Renin-angiotensin system activation in renal adipogenesis. Am J Physiol Renal Physiol 2009; 298: F391–F400. [DOI] [PubMed] [Google Scholar]
- 16. Sui Y, Zhao HL, Ma RC, et al. Pancreatic islet beta-cell deficit and glucose intolerance in rats with uninephrectomy. Cell Mol Life Sci 2007; 64: 3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obes (Lond) 2008; 32 (Suppl 4): S36–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamazaki T, Tanimoto M, Gohda T, et al. Combination effects of enalapril and losartan on lipid peroxidation in the kidneys of KK-Ay/Ta mice. Nephron Exp Nephrol 2009; 113: e66–e76. [DOI] [PubMed] [Google Scholar]
- 19. Deji N, Kume S, Araki S, et al. Role of angiotensin II-mediated AMPK inactivation on obesity-related salt-sensitive hypertension. Biochem Biophys Res Commun 2012; 418: 559–564. [DOI] [PubMed] [Google Scholar]
- 20. Yoshida D, Higashiura K, Shinshi Y, et al. Effects of angiotensin II receptor blockade on glucose metabolism via AMP-activated protein kinase in insulin-resistant hypertensive rats. J Am Soc Hypertens 2009; 3: 3–8. [DOI] [PubMed] [Google Scholar]
- 21. Shinshi Y, Higashiura K, Yoshida D, et al. Angiotensin II inhibits glucose uptake of skeletal muscle via the adenosine monophosphate-activated protein kinase pathway. J Am Soc Hypertens 2007; 1: 251–255. [DOI] [PubMed] [Google Scholar]
- 22. Pastor-Soler NM, Hallows KR. AMP-activated protein kinase regulation of kidney tubular transport. Curr Opin Nephrol Hypertens 2012; 21: 523–533. [DOI] [PubMed] [Google Scholar]
- 23. Alzamora R, Al-Bataineh MM, Liu W, et al. AMP-activated protein kinase regulates the vacuolar H+-ATPase via direct phosphorylation of the A subunit (ATP6V1A) in the kidney. Am J Physiol Renal Physiol 2013; 305: F943–F956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gong F, Alzamora R, Smolak C, et al. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am J Physiol Renal Physiol 2010; 298: F1162–F1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li H, Thali RF, Smolak C, et al. Regulation of the creatine transporter by AMP-activated protein kinase in kidney epithelial cells. Am J Physiol Renal Physiol 2010; 299: F167–F177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Seo-Mayer PW, Thulin G, Zhang L, et al. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am J Physiol Renal Physiol 2011; 301: F1346–F1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lempiainen J, Finckenberg P, Levijoki J, et al. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br J Pharmacol 2012; 166: 1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen KH, Hsu HH, Lee CC, et al. The AMPK agonist AICAR inhibits TGF-beta1 induced activation of kidney myofibroblasts. PLoS One 2014; 9: e106554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Satriano J, Sharma K, Blantz RC, et al. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol Renal Physiol 2013; 305: F727–F733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 2008; 118: 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lodha S, Dani D, Mehta R, et al. Angiotensin II-induced mesangial cell apoptosis: role of oxidative stress. Mol Med 2002; 8: 830–840. [PMC free article] [PubMed] [Google Scholar]
- 32. Bhaskaran M, Reddy K, Radhakrishanan N, et al. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol 2003; 284: F955–F965. [DOI] [PubMed] [Google Scholar]
- 33. Liu XM, Peyton KJ, Shebib AR, et al. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol 2010; 300: H84–H93. [DOI] [PMC free article] [PubMed] [Google Scholar]