Abstract
Objective:
The objective of this study was to evaluate angiotensin-converting enzyme (ACE) activity in dogs and with and without an ACE polymorphism in the canine ACE gene, before and after treatment with an ACE inhibitor.
Methods:
Thirty-one dogs (20 wild-type, 11 ACE polymorphism) with heart disease were evaluated with ACE activity measurement and systolic blood pressure before and after administration of an ACE inhibitor (enalapril).
Results:
Median pre-treatment ACE activity was significantly lower for ACE polymorphism dogs than for dogs with the wild-type sequence (P=0.007). After two weeks of an ACE inhibitor, ACE activity was significantly reduced for both genotypes (wild-type, P<0.0001; ACE polymorphism P=0.03); mean post-therapy ACE activity was no different between the groups.
Conclusion:
An ACE polymorphism is associated with lower levels of ACE activity. Dogs with the polymorphism still experience suppression of ACE activity in response to an ACE inhibitor. It is possible that the genetic status and ACE activity of dogs may impact the response of dogs with this variant to an ACE inhibitor.
Keywords: Polymorphism, canine, angiotensin-converting enzyme, enalapril
Introduction
Pharmacological manipulation of a highly activated renin–angiotensin–aldosterone system (RAAS) with an angiotensin-converting enzyme (ACE) inhibitor is a well-accepted component of the management of heart disease and congestive heart failure (CHF) in humans.1 Dogs are frequently used to study the management of heart disease and heart failure both as an animal model for human heart disease and for veterinarians managing natural heart disease in pet dogs with heart disease.2–4
In humans, an ACE gene insertion/deletion (I/D) in intron 16, has been shown to impact ACE activity with the deletion allele (D) associated with higher levels of ACE activity then the insertion allele (I).5 The polymorphism has been associated with a variable response to ACE inhibitors.6–8 Humans with this polymorphism have increased baseline ACE activity, increased levels of angiotensin II production and require higher doses of ACE inhibitors to achieve an adequate response to ACE inhibition. We have previously identified a single nucleotide polymorphism (SNP) in the same intron in the canine ACE gene.9 We hypothesized that this SNP in the canine ACE gene could be associated with a variable response to ACE inhibitor therapy in the dog. The use of ACE inhibitors in the pet dog with heart disease is a common component of medical management in veterinary medicine. The dog is also a very common model in which to study heart disease and the management of heart disease for humans, and pharmacogenetic variations that could impact the response to therapy may have important implications to cardiac research. In the study presented here we evaluated dogs with myxomatous mitral valve disease, the most common form of heart disease in the dog, with and without the ACE gene polymorphism and assessed ACE activity and systolic blood pressure before and after ACE inhibition with enalapril.
Materials and methods
This study was conducted in accordance with the guidelines of the North Carolina State University Institutional Animal Care and Use Committee.
Client-owned pet dogs with myxomatous mitral valve disease echocardiographically identified by board certified veterinary cardiologists were prospectively recruited from the patient population at North Carolina State University’s College of Veterinary Medicine between 1 September 2013 and 30 November 2015. All dogs were required to have a grade 4/6 left apical systolic murmur and radiographically evident cardiac enlargement (defined as a vertebral heart scale (VHS) ≥11).2,11
Physical examination, thoracic radiographs and indirect blood pressure measurements were performed. Dogs with ongoing cardiovascular (pulmonary hypertension, congenital heart disease, systemic hypertension, etc.) or systemic diseases (renal, endocrine, etc.) as evident from history, radiographs or routine serum biochemical profile were excluded.
Indirect systolic blood pressure measurement was performed using a Parks Medical Electronics Doppler, model 811-B (Parks Medical, Las Vegas, NV, USA). Cuff size was selected by measurement of the circumference of the area of cuff placement and choosing a cuff size with a width of 30–40% of the circumference of the area of placement.12 The coccygeal artery was the preferred location for measurement, but in some cases, the palmar or dorsal pedal artery was used. Measurements were performed until there were at least three consistent readings (differing no more than 10 mmHg) and the values were averaged.
A 3 ml blood sample was drawn from the jugular vein for ACE polymorphism genotyping as previously described.9 Briefly, polymerase chain reaction (PCR) amplification primers for the previously reported SNP at canine chromosome 9:11507816 (dbSNP rs#: 850683722) were designed using Primer 3 software (http://frodo.wi.mit.edu/) and the canine nucleotide sequences from the Ensemble genomic database (www.ensembl.org/index.html).9 The forward primer was 5′–TCAGCTCCATGCAATCCATA–3′, the reverse primer was 5′–CCCCTTGCCCTATCTGTAAA–3′.
Products were sequenced with both forward and reverse primers. PCR was carried out using a cocktail of water, 10X KCL Taq buffer, 1 mM MgCl2, 0.2 units/µl of reaction volume Taq DNA polymerase, 0.5 units/µl 0.4 mM dNTPs, 0.4 µM PCR amplification primers, and 100–200 µg DNA. The PCR protocol included 5 minutes at 95°C, 40 cycles of 94°C for 30 seconds, 57°C for 30 seconds, 72°C for 30 seconds and 72°C for 7 minutes. Products were sequenced with both forward and reverse primers and analyzed on an ABI Prism 377 Sequencer (Foster City, CA, USA). Nucleotide sequences were visually evaluated for sequence quality and aligned using SeqMan Pro (DNAStar; Madison, WI, USA) software to evaluate for DNA variants between the individual animals. The DNA sequence was compared to the normal canine sequence in this region as well as the previously reported polymorphism.9
Two milliliters of blood was placed into a glass tube without any anticoagulant and serum was separated and frozen at −80° for subsequent analysis of ACE activity using a radioenzymatic assay for direct determination of ACE activity (Mayo Clinic, Rochester, MN, USA). The variability of this assay had previously been determined by the laboratory with an intra-assay variation of 2.7% and an inter-assay variation of 8.1%.
Once dogs were genotyped, those dogs that were homozygous for the wild-type (canine reference sequence) or the ACE polymorphism were selected for further participation in the study.
All dogs were started on 0.5 mg/kg of enalapril orally every 12 hours and re-evaluated 10–14 days later. At the time of re-evaluation, the indirect blood pressure measurement was repeated, and a second blood sample was taken for post-enalapril ACE activity measurement.
Data were visually and statistically tested for normality using the D’Agostino Pearson omnibus normality test. Normally distributed data are reported as mean ± standard deviation (SD) while non-normally distributed data are reported as medians and upper and lower 95% confidence intervals.
A t-test was used to compare normally distributed data and a Mann–Whitney U test for non-normally distributed data between the two genotype groups.
A paired t-test was used to compare normally distributed data and a Wilcoxon signed-rank test was used to compare non-normally distributed data for each dog’s baseline ACE activity and systolic blood pressure to the same measurements taken 10–14 days after starting therapy.
Results
Forty-one dogs were genotyped over a 20-month period between 1 November 2013 and 1 July 2015. Thirty-one of the dogs (Table 1) were either wild-type (20 dogs) or homozygous (11 dogs) for the ACE polymorphism and were enrolled in the study.
Table 1.
Breeds and genotypes of study dogs.
| Breed | Number of dogs | Wild-type | ACE polymorphism |
|---|---|---|---|
| Boston terrier | 1 | 1 | 0 |
| Bull terrier | 1 | 1 | 0 |
| Cavalier King Charles spaniel | 10 | 4 | 6 |
| Chihuahua | 8 | 6 | 2 |
| Chinese crested | 1 | 1 | 0 |
| Dachshund | 1 | 0 | 1 |
| Italian greyhound | 1 | 1 | 0 |
| Maltese | 1 | 0 | 1 |
| Mixed breed | 2 | 1 | 1 |
| Schnauzer | 1 | 1 | 0 |
| Shih tzu | 2 | 2 | 0 |
| Yorkshire terrier | 2 | 2 | 0 |
The breeds and genotypes for the 31 dogs enrolled in the study. The number of dogs with the wild-type or angiotensin-converting enzyme (ACE) polymorphism sequence are provided for each breed.
The mean age of the wild-type dogs was 10 years ± 2.6 (SD), and the mean age of the ACE polymorphism dogs was 9 years ± 1.5. There was no significant difference between the groups (P=0.31).
There was no significant difference in cardiac enlargement between the two groups (P=0.17). The median VHS for the wild-type dogs was 11.3 (11.2 (lower 95% confidence interval)–12.2 (upper 95% confidence interval)), and the median VHS for the ACE polymorphism dogs was 11.8 (11.29–13.04).
Pre-treatment ACE activity was measured in all dogs. The mean pre-treatment ACE activity was significantly lower for dogs homozygous for the ACE polymorphism (16.4 U/L, ±9.3) than for dogs with the wild-type sequence (28.3 U/L, ±11.95) (P=0.008) (Figure 1).
Figure 1.
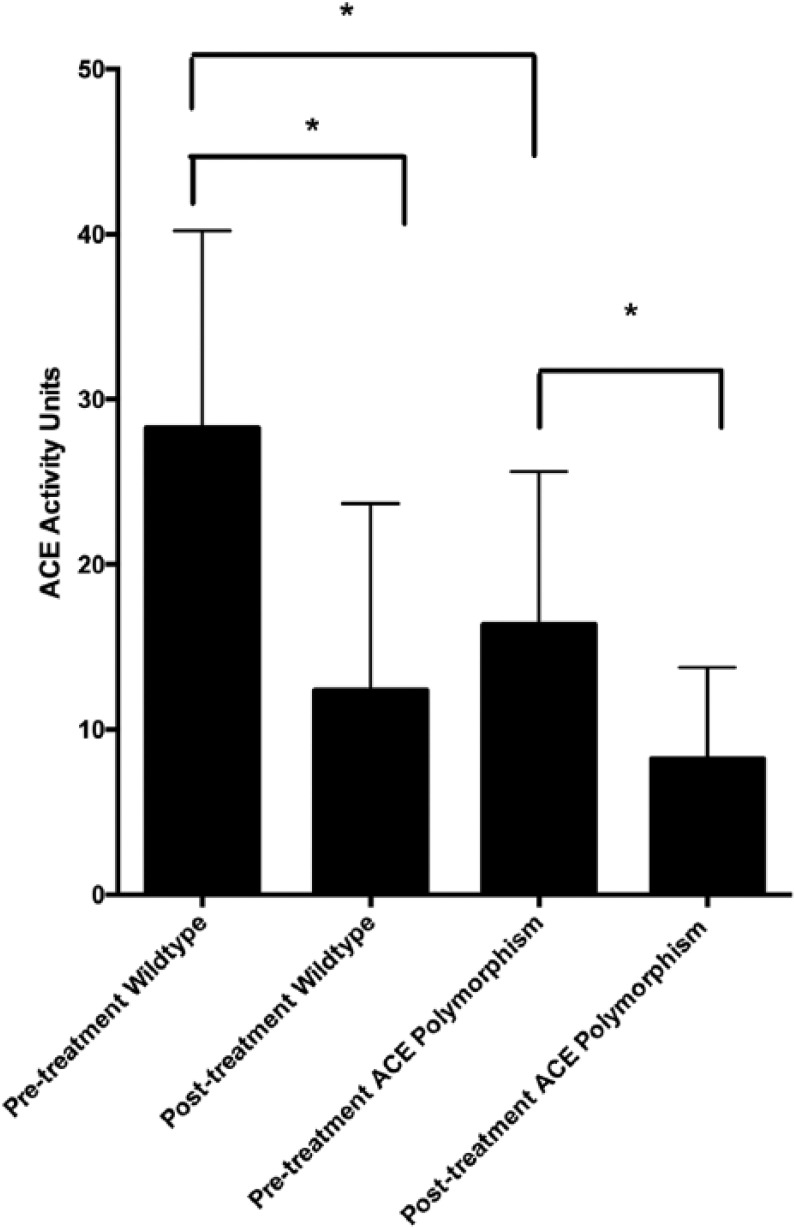
Mean and standard deviation (SD) angiotensin-converting enzyme (ACE) activity. The error whiskers indicate the standard deviation of the mean.
Post-treatment ACE activity was measured in 19 dogs with the wild-type sequence and nine dogs homozygous for the ACE polymorphism. Three dogs were not able to return for a re-evaluation as originally planned and were dropped from the study. After two weeks of enalapril therapy, ACE activity was significantly reduced for both genotype groups (wild-type, P<0.0001; ACE polymorphism, P=0.03). The median post-treatment ACE activity for dogs homozygous for the ACE polymorphism was 5.0 U/L (3.9–12.5) and for dogs with the wild-type sequence it was 5.0 U/L (7.0–17.8). The median post-therapy ACE activity was no different between the groups (P=0.60). Although the magnitude of change was significantly greater for wild-type dogs (median reduction of 17.5 U/L, 10.1–22.6) than those with the ACE polymorphism (median reduction of 4.7 U/L, 6.9–17.8) (P=0.047).
Pre-treatment blood pressure measurement was performed on 16 dogs with the wild-type sequence and 11 dogs with the ACE polymorphism. The mean pre-treatment systolic blood pressure was 138 mmHg (±23) for the wild-type group and 136 mmHg (±18) for the ACE polymorphism group and was not significantly different (P=0.80). The mean post-treatment blood pressure measurement was 131 mmHg (±17) for the wild-type group and 145 mmHg (±27) for the ACE polymorphism. This was also not significantly different (P=0.15). There was no significant difference in pre and post-treatment blood pressure measurements within each group (P=0.36; paired t-test, wild-type; P=0.43; paired t-test, variant).
Discussion
This study was designed to determine the impact of the ACE polymorphism on ACE activity and blood pressure in dogs with heart disease treated with an ACE inhibitor. Dogs homozygous for the ACE polymorphism had lower baseline levels of ACE activity compared to wild-type dogs, but still experienced suppression of ACE activity in response to enalapril therapy.
In this study, 12 different breeds of dogs were evaluated. The ACE polymorphism was identified in five of the 12 breeds, but was observed most commonly in the Cavalier King Charles spaniel (six of 10 dogs) (Table 1). In a previous study we identified this polymorphism in three of five boxers and four of five Doberman pinschers.9 This suggests that the ACE polymorphism may be observed more commonly in certain breeds of dogs, although the number of dogs studied from each breed was small and larger studies are warranted.
In humans, an ACE polymorphism of clinical importance is an I/D polymorphism located in the same intronic region of the gene as the single base change we have identified in the dog.7,9 Humans with cardiac disease and the insertion polymorphism have higher levels of ACE activity than those without the insertion.8 The differing impact of these polymorphisms on ACE activity (higher levels in humans with an insertion polymorphism, lower levels in dogs with a single base pair polymorphism) may suggest that this region of the intron has a role in gene expression that could vary depending on the location of the intron impacted and if a transcriptional regulator including a gene enhancer or silencer was in this location.12
This study has several limitations. We did identify a significant difference in ACE activity in dogs with the polymorphism; however, we did not assess the functional implications of this finding, such as a downstream measures of RAAS system activity (urine aldosterone to creatinine ratio). We did compare the indirect systolic blood pressure measurement between the two groups of dogs and did not detect a significant difference. However, we also did not detect a significant difference in blood pressure for either group between the pre and post-enalapril measurements. This may simply suggest that indirect blood pressure measurement is a fairly crude way to assess alterations in the RAAS.
Conclusions
In conclusion, we identified that the genetic polymorphism in the canine ACE gene was associated with lower levels of ACE activity. It is possible that the genetic status of the dog may impact the study of ACE activity in dogs with spontaneous heart disease as well as those being used as a model of human disease. Further study of the clinical significance of this finding is warranted.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by Grant D14CA-810 of the Morris Animal Foundation.
References
- 1. Borghi C, Rossi F. Role of the renin–angiotensin–aldosterone system and its pharmacological inhibitors in cardiovascular diseases: complex and critical issues. High Blood Press Cardiovasc Prev 2015; 22: 429–444. [DOI] [PubMed] [Google Scholar]
- 2. Kvart C, Haggstrom J, Pedersen HD, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med 2002; 16: 80–88. [PubMed] [Google Scholar]
- 3. Atkins CE, Keene BW, Brown WA. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral valve insufficiency. J Am Vet Med Assoc 2007; 231: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 4. Atkins CE, Haggstrom J. Pharmacologic management of myxomatous mitral valve disease in dogs. J Vet Cardiol 2012; 14: 165–184. [DOI] [PubMed] [Google Scholar]
- 5. Tiret L, Rigat B, Visvikis S, et al. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet 1992; 51: 197–205. [PMC free article] [PubMed] [Google Scholar]
- 6. Wu CK, Luo JL, Tsai CT, et al. Demonstrating the pharmacogenetic effects of angiotensin-converting enzyme inhibitors on long-term prognosis of diastolic heart failure. Pharmacogenomics J 2010; 10: 46–53. [DOI] [PubMed] [Google Scholar]
- 7. McNamara DM, Holubkov R, Postova L, et al. Pharmacogenetic interactions between angiotensin-converting enzyme inhibitor therapy and the angiotensin-converting enzyme deletion polymorphism in patients with congestive heart failure. J Am Coll Cardiol 2004; 44: 2019–2026. [DOI] [PubMed] [Google Scholar]
- 8. McNamara DM. Genomic variation and neurohormonal intervention in heart failure. Heart Fail Clin 2010; 6: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meurs KM, Chdid L, Reina-Doreste Y, et al. Polymorphisms in the canine and feline renin–angiotensin–aldosterone system genes. Anim Genet 2015; 46: 224–226. [DOI] [PubMed] [Google Scholar]
- 10. Buchanan JW. Vertebral scale system to measure heart size in radiographs. Vet Clin North Am Small Anim Pract 2000; 30: 379–393. [PubMed] [Google Scholar]
- 11. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation and management of systemic hypertension in dogs and cats; 2007 ACVIM Forum Consensus Statement. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 12. Chorev M, Carmel L. The function of introns. Front Genet 2012; 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


