Abstract
Objective:
Our previous studies found that angiotensin-(1–7) (Ang-(1–7)) is an endogenous counter-factor of angiotensin II (Ang-II). However, the balance between Ang-II and Ang-(1–7) in the development of human coronary atherosclerosis is not determined.
Methods and results:
The plasma levels of Ang-II and Ang-(1–7) were detected by enzyme-linked immunosorbent assay (ELISA) in 112 patients with known or suspected coronary artery disease (CAD) undergoing coronary angiography. Patients were divided into three groups based on the coronary angiography as follows: (1) normal (n = 13); (2) noncritical CAD (<50% stenosis, n = 17); and (3) critical CAD (⩾50% stenosis, n = 82). The plasma levels of Ang-II, Ang-(1–7) and the ratio of Ang-II and Ang-(1–7) (Ang-II/Ang-(1–7) were comparable between the normal and noncritical CAD groups. However, Ang-II, Ang-(1–7), and especially Ang-II/Ang-(1–7), were elevated in patients with critical CAD, compared with patients with normal or noncritical CAD. The level of Ang-II/Ang-(1–7) was positively associated with serious coronary stenosis, and correlated with tumor necrosis factor-alpha (TNF-α) level.
Conclusion:
Both Ang-II and Ang-(1–7) expression are significantly increased in patients with critical CAD. However, increased Ang-II/Ang-(1–7) ratios may lead to Ang-II over-activation and aggravate atherosclerosis progression.
Keywords: Atherosclerosis, coronary artery disease, angiotensin-(1–7), angiotensin II
Introduction
There is a growing body of evidence showing that the renin angiotensin system (RAS) contributes significantly to the pathogenesis of atherosclerosis. Recently, angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1–7) (Ang-(1–7)) have been proved to be protective components of the RAS against atherosclerosis.1 Our recent study found that chronic treatment with exogenous Ang-(1–7) dose-dependently inhibited Ang-II-induced atherosclerotic lesion formation and enhanced plaque stability in mice.2 In another study we demonstrated that the levels of both Ang-II and Ang-(1–7) were elevated in late atherosclerotic plaques in ApoE–/– mice models, and further experiments suggested that endogenous-activated Ang-(1–7) played a protective role against atherosclerotic plaque instability in mice.3
Although these animal studies point to a role of Ang-(1–7) in the pathogenesis of atherosclerosis, there have been no clinical studies to confirm a link between plasma Ang-(1–7) or Ang-II/Ang-(1–7) and coronary atherosclerosis in humans. This study aimed to determine whether Ang-(1–7) and the balance of Ang-II and Ang-(1–7) are associated with the presence or severity of coronary atherosclerotic stenosis defined by coronary angiography. The results may develop a novel predictor for diagnosis and therapeutic target of human coronary artery disease (CAD).
Materials and methods
Participants
This study’s groups consisted of 112 prospectively enrolled patients with known or suspected CAD undergoing diagnostic coronary angiography at Shandong University Qilu Hospital. Patients with active gastrointestinal bleeding or serious anemia (hemoglobin concentration <8.0 g/dl) were excluded from the study. Patients treated with angiotensin-converting enzyme inhibitors (ACEIs) were also excluded because ACEIs may affect the levels of Ang-(1–7) and Ang-II.4 This study was approved by the ethics committee of Shandong University Qilu hospital and all patients provided written informed consent before participation.
Anthropometric data and blood sampling
Anthropometric data, such as age, gender, height, body weight, heart rate, blood pressure and medical history, were obtained retrospectively from medical charts. The fasting blood samples tested for glucose, lipid profiles, urea nitrogen, and creatinine were taken from the antecubital vein after an overnight fast of at least 12 hours and sent to the biochemistry laboratory for estimation.
After an overnight fast of more than 12 hours, blood samples tested for Ang-II and Ang-(1–7) were taken from the arterial sheath at the time of angiography before the injection of contrast from all the enrolled patients. Blood samples were taken into protease inhibitors-containing tubes and centrifuged for 15 minutes at 3000 rpm at 4°C within one hour after collection. Then the samples were stored at −80°C until analysis. Plasma Ang-II and Ang-(1–7) levels were tested using commercial enzyme-linked immunosorbent assay (ELISA) kits (Uscnlife, Wuhan, China). Plasma tumor necrosis factor-alpha (TNF-α) level was tested using an ELISA kit from R&D Systems (London, UK).
Coronary angiography
Coronary angiographies were performed through the right femoral artery or the right radial artery of patients in the angiography room. Then, the left main trunk, the left anterior descending artery, left circumflex artery, and right coronary artery were examined to evaluate the stenosis of coronary arteries. The coronary angiography results were evaluated by two expert investigators independently. Fifty percent or more stenosis (zone vessel) in coronary vessels was defined as critical CAD, whereas less than 50% stenosis was defined as noncritical CAD.
Statistical analysis
All the statistical analyses were performed by SPSS 19.0. The study population was divided into three groups based on the coronary angiography as follows: (1) normal (no stenosis); (2) noncritical CAD (<50% stenosis); and (3) critical CAD (⩾50% stenosis). One-way analysis of variance (ANOVA) (least significant difference (LSD) test) was used to compare the differences among the three groups. The variables associated with critical CAD were identified with univariate linear regression. Multivariate linear regression was used to assess the association between Ang-II/Ang-(1–7) and critical CAD, using age, gender, smoking, alcohol consumption, heart rate, low-density lipoprotein cholesterol (LDL-C), hypertension, diabetes and medications as independent variables. The correlation between Ang-II/Ang-(1–7) and TNF-α was assessed with the Spearman rank correlation. A p value <0.05 was considered as statistically significant.
Results
Baseline clinical and biochemical characteristics
The characteristics of the groups are listed in Table 1. Patient parameters, including age, gender, smoking, alcohol consumption, heart rate, blood pressure, total cholesterol (TC), LDL-C, urea nitrogen, creatinine and coexistence of hypertension were comparable among the normal, noncritical CAD and critical CAD groups. The critical CAD group had a significantly higher prevalence of diabetes than those of the normal and noncritical CAD groups. The uses of medications, including aspirin, clopidogrel, statin, β-blocker, calcium channel blocker and nitrate, were not significantly different (Table 1).
Table 1.
Clinical characteristics grouped by CAD severity.
| Groups | Normal (n = 13) |
Noncritical CAD (n = 17) |
Critical CAD (n = 82) |
p value |
|---|---|---|---|---|
| Age (years) | 60 ± 8 | 62 ± 8 | 61 ± 9 | 0.674 |
| Gender (male/female) | 5/8 | 12/5 | 54/28 | 0.130 |
| Current smoking (%) | 0 | 11.8 | 8.5 | 0.476 |
| Alcohol consumption (%) | 23.1 | 52.9 | 30.5 | 0.145 |
| SBP (mmHg) | 130 ± 11 | 135 ± 13 | 139 ± 17 | 0.135 |
| DBP (mmHg) | 77 ± 13 | 86 ± 11 | 81 ± 12 | 0.090 |
| HR (per min) | 74 ± 9 | 68 ± 9 | 74 ± 11 | 0.076 |
| TC (mmol/l) | 4.7 ± 1.3 | 4.1 ± 0.8 | 4.6 ± 1.3 | 0.228 |
| LDL-C (mmol/l) | 2.7 ± 1.0 | 2.4 ± 0.6 | 2.7 ± 1.0 | 0.384 |
| FBG (mmol/l) | 5.9 ± 2.6 | 5.3 ± 1.2 | 5.9 ± 1.7 | 0.510 |
| Urea nitrogen (mmol/l) | 4.3 ± 0.7 | 4.8 ± 1.0 | 4.9 ± 1.5 | 0.362 |
| Creatinine (mol/l) | 65 ± 13 | 66 ± 11 | 74 ± 43 | 0.564 |
| Comorbid conditions (%) | ||||
| Hypertension | 30.8 | 35.3 | 34.1 | 0.964 |
| Diabetes | 23.1 | 47.1 | 67.1 | 0.006 |
| Medications (%) | ||||
| β-blocker | 76.9 | 70.6 | 76.8 | 0.857 |
| Aspirin | 100.0 | 94.1 | 100.0 | 0.460 |
| Clopidogrel | 100.0 | 94.1 | 97.6 | 0.593 |
| Statin | 100.0 | 88.2 | 92.7 | 0.353 |
| CCB | 46.2 | 47.1 | 54.9 | 0.742 |
| Nitrate | 61.5 | 70.6 | 80.5 | 0.260 |
CAD: coronary artery disease; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; FBG: fasting blood glucose; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CCB: calcium channel blocker.
Plasma levels of Ang-II and Ang-(1–7)
The levels of plasma Ang-II in the critical CAD group was significantly higher than those of the normal and noncritical CAD groups, while no significant differences were found between the normal and noncritical CAD groups (normal: 25.1±5.2 pg/ml; noncritical CAD: 23.3±4.8 pg/ml; critical CAD: 32.7±8.3 pg/ml). Similarly, the critical CAD group showed higher plasma Ang-(1–7) levels compared with the normal and noncritical CAD groups. However, the levels of Ang-(1–7) were comparable between the normal and noncritical CAD groups. (normal: 21.0±4.1 pg/ml; noncritical CAD:20.1±4.5 pg/ml; critical CAD: 24.1±5.1 pg/ml) (Table 2).
Table 2.
Plasma Ang-II, Ang-(1–7), ratio of Ang-II/Ang-(1–7) and TNF-α levels in three groups.
| Groups | Normal (n = 13) |
Noncritical CAD (n = 17) |
Critical CAD (n = 82) |
p value |
|---|---|---|---|---|
| Ang-II (pg/ml) | 25.1 ± 5.2 | 23.3 ± 4.8 | 32.7 ± 8.3 | 0.000 |
| Ang-(1–7) (pg/ml) | 21.0 ± 4.1 | 20.1 ± 4.5 | 24.1 ± 5.1 | 0.004 |
| Ang-II/Ang-(1–7) | 1.19 ± 0.08 | 1.17 ± 0.11 | 1.38 ± 0.30 | 0.006 |
| TNF-α | 145 ± 63 | 163 ± 43 | 233 ± 77 | 0.000 |
Ang-II: angiotensin II; Ang-(1–7): angiotensin-(1–7); TNF-α: tumor necrosis factor-α; CAD: coronary artery disease.
Ratio of Ang-II and Ang-(1–7)
The ratios of Ang-II/Ang-(1–7) increased in patients with critical CAD compared with patients with normal and noncritical CAD. However, the ratios of Ang-II/Ang-(1–7) were similar between patients with normal and noncritical CAD (Table 2).
Association of Ang-II, Ang-(1–7) and Ang-II/Ang-(1–7) with critical CAD
The univariate analysis showed that all the plasma Ang-II, Ang-(1–7) and Ang-II/Ang-(1–7) levels were positively associated with critical CAD. Of the above positive parameters, the association between Ang-II/Ang-(1–7) and critical CAD was the most significant. Thus, in the multivariate analysis, we assessed the association between Ang-II/Ang-(1–7) and critical CAD, and found that diabetes (p = 0.002) and Ang-II/Ang-(1–7) (p = 0.002) were significantly associated with critical CAD (Table 3).
Table 3.
Association of the ratio of Ang-II/Ang-(1–7) and critical CAD.
| Factors | B | p value |
|---|---|---|
| Univariate analysis | ||
| Ang-II | 0.024 | 0.000 |
| Ang-(1–7) | 0.027 | 0.001 |
| Ang-II/Ang-(1–7) | 0.452 | 0.001 |
| Multivariate analysis | ||
| Diabetes | 0.248 | 0.002 |
| Ang-II/Ang-(1–7) | 0.454 | 0.002 |
Ang-II: angiotensin II; Ang-(1–7): angiotensin-(1–7); CAD: coronary artery disease.
Correlation between Ang-II/Ang-(1–7) and TNF-α
The levels of plasma TNF-α in the critical CAD group was significantly higher than those of the normal and noncritical CAD groups (Table 2). Correlation analysis showed that the Ang-II/Ang-(1–7) level was positively associated with TNF-α (r = 0.765, p < 0.001), which is consistent with our previous basic laboratory observation that Ang-II increases while Ang-(1–7) decreases the expression of TNF-α in cultured macrophages2 (Figure 1).
Figure 1.
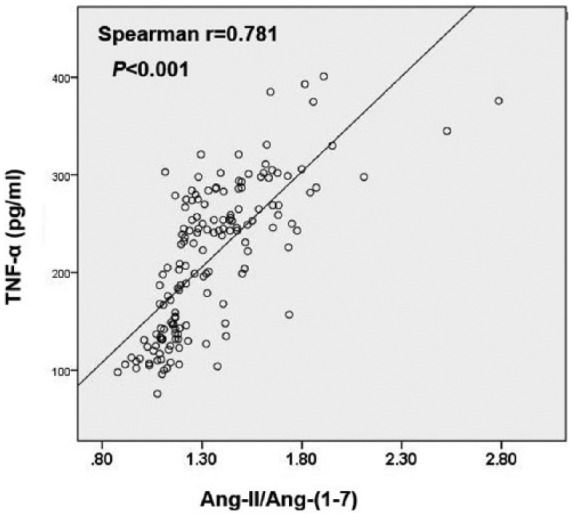
Correlation between plasma Ang-II/Ang-(1–7) and TNF-α levels.
Ang-II: angiotensin II; Ang-(1–7): angiotensin-(1–7); TNF-α: tumor necrosis factor-alpha.
Discussion
The RAS, especially Ang-II overexpression, plays an important role in atherosclerosis progression. Ang-(1–7), a newly discovered RAS component, has been proved as a protective factor against atherosclerosis because of its vasodilatory, antiproliferation, anti-inflammatory effects.1 Our previous animal experiments found that the levels of both Ang-II and Ang-(1–7) were elevated in late atherosclerotic plaques compared with those of early lesions.3 Further study demonstrated that blockage of endogenous Ang-(1–7) did not alter early atherosclerotic plaque formation, while accelerating late plaque vulnerability.3 Thus we speculated that both Ang-II and Ang-(1–7) are activated in patients with coronary atherosclerosis, but the imbalance of Ang-II and Ang-(1–7) may lead to further atherosclerosis deterioration. The major finding of the present study was that although both Ang-II and Ang-(1–7) were activated during human coronary atherosclerosis, the imbalance between Ang-II and Ang-(1–7) may be the critical factor determining atherosclerosis progression. To the best of our knowledge, our study has provided for the first time the role of balance between Ang-II and Ang-(1–7) in human atherosclerosis.
Ang-(1–7) can be produced from Ang-II under the hydrolyzing effect of ACE2. In addition to ACE2-dependent routes, Ang-(1–7) can also be generated from Ang I by prolyl endopeptidase and neutral endopeptidase. Ang-(1–7) is an active peptide of the RAS that has antihypertensive, anti-inflammatory, antiproliferative and vasodilatory properties.5 Thus, it has been found that it often counteracts the effects caused by Ang-II. Zhang et al. demonstrated that Ang-(1–7) decreases Ang-II-induced vascular cell adhesion molecule-1 (VCAM-1) expression on vascular endothelial cells.6 Another study found that Ang-(1–7) attenuates Ang-II-induced inflammation by inhibiting lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1) expression.7 Our laboratory also found that Ang-(1–7) protects endothelial cell function by inhibiting inflammatory response.8 A recent study found that Ang-(1–7) decreases Ang-II-induced intercellular adhesion molecule 1 (ICAM-1), VCAM-1, and monocyte chemoattractant protein-1 (MCP-1) expression through suppression of P38 and nuclear factor (NF)-κB pathways in human endothelial cells.9
These observations suggested that Ang-(1–7) might play a critical protective role in atherosclerosis progression. A series of animal experiments also confirmed its anti-atherosclerotic effect. For example, our previous study found that Ang-(1–7) dose-dependently inhibits Ang-II-induced atherosclerotic lesion formation and enhances plaque stability by targeting vascular cells.2 Further study showed that the anti-atherosclerosis effects of Ang-(1–7) and losartan in early lesion formation were equivalent.10 Tesanovic et al. also confirmed the vasoprotective and atheroprotective effects of Ang-(1–7) in apolipoprotein E (apoE)-deficient mice.11 Another study reported that treatment with Ang-(1–7) reduces inflammation in carotid atherosclerotic plaques.12 Additionally, the Ang-(1–7) receptor Mas agonist also ameliorates atherosclerotic progress in apoE-knockout mice.13 All the above results demonstrated the anti-atherosclerotic effect of exogenous Ang-(1–7) in animal models. However, little is known about the effect of endogenous Ang-(1–7) on atherosclerosis, especially for human coronary artery atherosclerosis.
In our previous study, we found that the levels of both Ang-II and Ang-(1–7) in late aortic lesions were higher than those in early aortic plaques.3 These results suggested that both Ang-II and Ang-(1–7) are gradually activated with the progression of atherosclerosis. Further study found that blockage of endogenous Ang-(1–7) attenuated late plaque stability but did not affect early atherosclerotic lesion formation.3 Thus, we speculated that the reason for the different effects of endogenous Ang-(1–7) on atherosclerosis is probably the different balance between Ang-II and Ang-(1–7) in the early and late atherosclerotic lesions.
ACE2, the critical regulator of Ang-II and Ang-(1–7), has been proved as an important factor in heart disease. Our previous study found that ACE2 over-expression enhanced plaque stability and attenuated early atherosclerosis formation in a rabbit model of atherosclerosis.14,15 However, the ACE2 protein expression and activity were diverse during different atherosclerosis stages in human atherosclerotic carotid arteries.16 Wang et al. found that increased ACE/ACE2 ratios may induce angiotensin II over-activation and accelerate cardiac remodeling in patients with heart failure.17 Because of the effects of ACE2 on Ang-II degradation and Ang-(1–7) production, we have ample evidence to speculate that the balance between Ang-II and Ang-(1–7) play an important role in heart disease.
Despite large number of animal studies, the role of Ang-(1–7) in the development of human atherosclerosis is not well elucidated. Different from our previous basic observation, our current study demonstrated that all the levels of plasma Ang-II, Ang-(1–7) and the ratio of Ang-II/Ang-(1–7) were not significantly changed in patients with noncritical coronary atherosclerosis. In agreement with our previous study,3 for patients with severe coronary atherosclerosis, although both Ang-II and Ang-(1–7) were elevated, the elevation of Ang-II is more obvious, leading to the remarkable increase of the ratio of Ang-II/Ang-(1–7) compared with those in patients with normal or noncritical CAD. The results revealed that Ang-(1–7) may act as a compensatory mechanism to prevent atherosclerosis during the early stages. However, for patients with severe atherosclerosis, Ang-II was over-activated and the balance between Ang-II and Ang-(1–7) was disrupted, which resulted in more vascular proliferation and inflammation, and expedited atherosclerosis. However, the changes of Ang-II and Ang-(1–7) were different from our study in patients with diabetes, suggesting the different role of Ang-II and Ang-(1–7) in different pathological progression.18
The role of TNF-α in atherosclerotic lesion formation and progression has been well established in basic and clinic observations.19 The functional crosstalk among Ang-II, Ang-(1–7) and TNF-α in vascular remodeling has also been assessed. Bihl et al. found that angiotensin-(1–7) counteracts the effects of Ang-II on vascular smooth muscle cells and vascular remodeling via decreased levels of TNF-α.20 The findings of the current study are also in accord with our previous observation that Ang-II increases the expression of TNF-α, while Ang-(1–7) counteracts the effect of Ang-II on TNF-α, leading to decreased inflammatory levels that can in turn reverse the promoting effects of Ang-II on inflammation and atherosclerosis.2 In summary, these findings establish the linkage between Ang-II/Ang-(1–7) and TNF-α expression, and the inflammation in human atherosclerosis.
Several limitations should be considered. First, the study is a cross-sectional study, which limited the conclusions about causality of our associations. Further prospective study may strengthen our conclusion. Second, the patients were consecutively enrolled and the patients with critical CAD had a higher percentage compared with those in the normal or noncritical CAD groups, which led to the great difference in the numbers in the critical group and other groups. However, to analyze the relationship between Ang-II, Ang-(1–7) or Ang-II/Ang-(1–7) and coronary stenosis severity, the groups of normal and noncritical CAD were merged, which may reduce the statistical bias. Third, we did not test the level and activity of ACE2, which may be the main limitation of our study. However, although ACE2 can degrade Ang-II and produce Ang-(1–7), it does not directly affect atherosclerosis progress.
In conclusion, our present study was the first to find the imbalance between Ang-II and Ang-(1–7) in patients with severe coronary atherosclerosis. Future clinical strategies to elevate plasma Ang-(1–7) and maintain the balance between Ang-II and Ang-(1–7) may provide a novel therapeutic approach for preventing and treating human coronary atherosclerosis.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National 973 Basic Research Program of China (No. 2011CB503906, No. 2012CB518603), the National High-tech Research and Development Program of China (No. 2012AA02A510), the Program of Introducing Talents of Discipline to Universities (No. B07035), the State Program of National Natural Science Foundation of China for Innovative Research Group (No. 81021001), and the grants of the National Natural Science Foundation of China (Nos. 81100207, 81570324, 81100102, 91339109, 81270350, 81425004,81530014), the Doctoral Fund of Shandong Province (No. BS2013SW009), the Key Research and Development Plan of Shandong Province (No. 2015GGE27109), and the sailing fund of Beijing Lisheng Cardiovascular Health Foundation (No. LHJJ20159728).
References
- 1. Jiang F, Yang J, Zhang Y, et al. Angiotensin-converting enzyme 2 and angiotensin 1–7: Novel therapeutic targets. Nat Rev Cardiol 2014; 11: 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang JM, Dong M, Meng X, et al. Angiotensin-(1–7) dose-dependently inhibits atherosclerotic lesion formation and enhances plaque stability by targeting vascular cells. Arterioscler Thromb Vasc Biol 2013; 33: 1978–1985. [DOI] [PubMed] [Google Scholar]
- 3. Yang J, Yang X, Meng X, et al. Endogenous activated angiotensin-(1–7) plays a protective effect against atherosclerotic plaques unstability in high fat diet fed ApoE knockout mice. Int J Cardiol 2015; 184: 645–652. [DOI] [PubMed] [Google Scholar]
- 4. Fraga-Silva RA, Da Silva DG, Montecucco F, et al. The angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas receptor axis: A potential target for treating thrombotic diseases. Thromb Haemost 2012; 108: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 5. McKinney CA, Fattah C, Loughrey CM, et al. Angiotensin-(1–7) and angiotensin-(1–9): Function in cardiac and vascular remodelling. Clin Sci (Lond) 2014; 126: 815–827. [DOI] [PubMed] [Google Scholar]
- 6. Zhang F, Ren J, Chan K, et al. Angiotensin-(1–7) regulates Angiotensin II-induced VCAM-1 expression on vascular endothelial cells. Biochem Biophys Res Commun 2013; 430: 642–646. [DOI] [PubMed] [Google Scholar]
- 7. Wang L, Hu X, Zhang W, et al. Angiotensin (1–7) ameliorates angiotensin II-induced inflammation by inhibiting LOX-1 expression. Inflamm Res 2013; 62: 219–228. [DOI] [PubMed] [Google Scholar]
- 8. Zhang YH, Zhang YH, Dong XF, et al. ACE2 and Ang-(1–7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm Res 2015; 64: 253–260. [DOI] [PubMed] [Google Scholar]
- 9. Liang B, Wang X, Zhang N, et al. Angiotensin-(1–7) attenuates angiotensin II-induced ICAM-1, VCAM-1, and MCP-1 expression via the MAS receptor through suppression of P38 and NF-kappaB pathways in HUVECs. Cell Physiol Biochem 2015; 35: 2472–2482. [DOI] [PubMed] [Google Scholar]
- 10. Yang J, Sun Y, Dong M, et al. Comparison of angiotensin-(1–7), losartan and their combination on atherosclerotic plaque formation in apolipoprotein E knockout mice. Atherosclerosis 2015; 240: 544–549. [DOI] [PubMed] [Google Scholar]
- 11. Tesanovic S, Vinh A, Gaspari TA, et al. Vasoprotective and atheroprotective effects of angiotensin (1–7) in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2010; 30: 1606–1613. [DOI] [PubMed] [Google Scholar]
- 12. Fraga-Silva RA, Savergnini SQ, Montecucco F, et al. Treatment with Angiotensin-(1–7) reduces inflammation in carotid atherosclerotic plaques. Thromb Haemost 2014; 111: 736–747. [DOI] [PubMed] [Google Scholar]
- 13. Jawien J, Toton-Zuranska J, Gajda M, et al. Angiotensin-(1–7) receptor Mas agonist ameliorates progress of atherosclerosis in apoE-knockout mice. J Physiol Pharmacol 2012; 63: 77–85. [PubMed] [Google Scholar]
- 14. Dong B, Zhang C, Feng JB, et al. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol 2008; 28: 1270–1276. [DOI] [PubMed] [Google Scholar]
- 15. Zhang C, Zhao YX, Zhang YH, et al. Angiotensin-converting enzyme 2 attenuates atherosclerotic lesions by targeting vascular cells. Proc Natl Acad Sci U S A 2010; 107: 15886–15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sluimer JC, Gasc JM, Hamming I, et al. Angiotensin-converting enzyme 2 (ACE2) expression and activity in human carotid atherosclerotic lesions. J Pathol 2008; 215: 273–279. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Li N, Gao F, et al. Balance between angiotensin converting enzyme and angiotensin converting enzyme 2 in patients with chronic heart failure. J Renin Angiotensin Aldosterone Syst 2015; 16: 553–558. [DOI] [PubMed] [Google Scholar]
- 18. Hao PP, Chen YG, Liu YP, et al. Association of plasma angiotensin-(1–7) level and left ventricular function in patients with type 2 diabetes mellitus. PLoS One 2013; 8: e62788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao X, Kong J, Zhao Y, et al. Gene silencing of TACE enhances plaque stability and improves vascular remodeling in a rabbit model of atherosclerosis. Sci Rep 2015; 5: 17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bihl JC, Zhang C, Zhao Y, et al. Angiotensin-(1–7) counteracts the effects of Ang II on vascular smooth muscle cells, vascular remodeling and hemorrhagic stroke: Role of the NFkB inflammatory pathway. Vascul Pharmacol 2015; 73: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]


