Abstract
Hypothesis/objectives:
Early prevention of diabetic nephropathy by way of blocking the renin-angiotensin system (RAS) in patients with normoalbuminuria seems rational, but trials have so far shown conflicting results. The present meta-analysis was undertaken to investigate if such treatment can prevent development of microalbuminuria.
Materials and methods:
We searched MEDLINE, EMBASE and the Cochrane Library (2 June 2014) for randomised controlled trials, with a population of patients with type 2 diabetes and normoalbuminuria, comparing angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) to placebo. Studies had to have at least 50 participants in each arm and one year of follow-up. Random and fixed effect models were performed as well as trial sequential analysis.
Results:
Six trials were included in the analysis (n=16,921). Overall risk of bias was variable. In a fixed model analysis ACE or ARB treatment was superior to placebo in relation to prevention of development of microalbuminuria, risk ratio 0.84 (95% confidence interval (CI) 0.79–0.88) p<0.001, I2=23%, similar to random model results. Treatment also showed a trend towards a reduction in all-cause mortality(p=0.07).
Conclusions:
We conclude that in patients with type 2 diabetes and normoalbuminuria, early intervention with ACEis or ARBs reduces the risk for development of microalbuminuria.
Keywords: Type 2 diabetes, microalbuminuria, renin angiotensin system, diabetic nephropathy, review
Introduction
Of the global population of patients with type 2 diabetes, approximately half have signs of chronic kidney disease (CKD).1 Any successful early intervention that reduces or delays progression in diabetic kidney disease is therefore expected to have a major impact on life expectancy and quality of life, as well as chronic care health economic costs. Primary prevention of microalbuminuria could be such an early intervention, that might truly prevent complications. This intervention would contrast to what is most frequently provided: treatment after the first signs of damage is present.
A major part of the chronic care of patients with type 2 diabetes is focused on the prevention of complications such as diabetic kidney disease. Specifically, the use of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin II receptor blockers (ARBs) is recommended2 in the presence of microalbuminuria/moderately increased (urinary albumin creatinine ratio (UACR)>30 mg/g and <300 mg/g) or macroalbuminuria/severely increased (UACR>300 mg/g). The intervention leads to decreased albuminuria, prevents progression from microalbuminuria to macroalbuminuria and results in a reduced risk of cardiovascular disease (CVD), end stage renal disease (ESRD) and death.
Only a few studies have been conducted with primary prevention of albuminuria as outcome, and with conflicting results.3–5 Currently there is no recommendation2 for the use of renin-angiotensin system (RAS) inhibitors for primary prevention of the occurrence of albuminuria in patients with type 2 diabetes. Previous attempts to summarise studies, including a Cochrane analysis6 have combined type 1 and type 2 diabetes, which may not be appropriate given the heterogeneity in kidney pathology. Diabetic nephropathy in type 1 diabetes is considered to be strictly a glomerular pathology with microalbuminuria as an early herald of molecular and cellular changes. In proteinuric type 2 diabetes, however, the causes of nephropathy are heterogenous.7–9 This warrants a separate analysis in type 2 diabetes. In addition, the recent Cochrane analysis investigated antihypertensive treatment in general and not exclusively studies using ACEi or ARBs.6
The aim of our analysis was therefore not only to specifically assess the effect on development of microalbuminuria with intervention with an ACEi or ARB in patients with type 2 diabetes and normoalbuminuria, but also to try to assess whether further studies are needed. By presenting such a review of findings, we hope to assist clinicians in order to be better informed regarding treatment decisions.
Materials and methods
We searched MEDLINE, EMBASE and the Cochrane Library (2 June 2014), and search strings are included in the Supplementary Material. The protocol with details for this meta-analysis was published on the PROSPERO website (http://www.crd.york.ac.uk/PROSPERO/) (PROSPERO CRD42014009983) ahead of the initiation of the literature search. In summary, in order to be included in our analysis studies had to be double-masked randomised controlled trials, with a population of patients with type 2 diabetes and normoalbuminuria (UACR<30 mg/g) or urinary albumin excretion rate (UAER)<30 mg/24 h). In order to assess the effect of RAS inhibition, we only included studies comparing ACEi or ARB to placebo. At least one year of follow-up was considered necessary for evaluation of the effect on development of micro- or macroalbuminuria, and studies had to have at least 50 participants in each arm. The primary outcome for our analysis was development of micro/macroalbuminuria defined as UACR>30 mg/g or UAER>30 mg/24 h or corresponding converted units.
Our intention was also to investigate secondary outcomes including all-cause mortality, total CVD mortality (death from myocardial infarction, stroke and peripheral vascular disease) and CVD morbidity (non-fatal myocardial infarction, non-fatal stroke, amputation of lower extremity and coronary or peripheral revascularisation). In addition renal outcomes defined as doubling of baseline serum creatinine or progression to ESRD was investigated.
Selection of studies
An independent experienced librarian performed the initial literature search. Studies were included in the meta-analysis after full agreement between two authors (FP and ML).
Assessment of risk of bias in included studies
Two authors (FP and ML) independently assessed the risk of bias in each trial by means of the Cochrane Collaboration’s risk of bias tool.
Measures of treatment effect
Data on dichotomous outcomes were statistically summarised as relative risks (RRs) with 95% confidence intervals (CIs).
Assessment of heterogeneity
A priori the authors evaluated clinical heterogeneity of the included trials. Heterogeneity was identified by visual inspection of the forest plots, by using a standard chi2 test with a significance level of α=0.1. Heterogeneity was specifically examined with the I2 statistic, where I2 values of 0–40% indicate heterogeneity might not be important; 30–60% may represent moderate heterogeneity; 50–90% may represent substantial heterogeneity; and 75–100% may represent considerable heterogeneity.10
Assessment of overall effect on outcome
We used both a random effects model11 and a fixed effect model12 with no a priori consideration regarding which model to consider as primary model.
Data extraction and management
Two authors (FP and ML) independently extracted information on each trial using standard data extraction forms. The forms included data concerning trial design, participants, interventions and outcomes as detailed in the selection criteria described above.
Data synthesis
Statistical analysis was performed according to the statistical guidelines in the newest version of The Cochrane handbook for systematic reviews of interventions.10 Calculations and modelling were performed using the RevMan software version 5.3. The effect of treatment is calculated as risk ratios (RRs) and reported with 95% CIs, with a p-value <0.05 considered statistically significant.
Trial sequential analysis (TSA)
TSA is a methodology that combines an information size calculation (cumulated sample sizes of included trials) for meta-analysis with the threshold of statistical significance. TSA is a tool for quantifying the statistical reliability of data in a cumulative meta-analysis adjusting p-values for repetitive testing on accumulating data. TSA was conducted on the outcomes showing statistical significance in both random effects and fixed effect model.13,14
We applied TSA since it prevents an increase of the risk of type I error (less than 5%) due to potential multiple updating in a cumulative meta-analysis and provides important information in order to estimate the level of evidence of the experimental intervention. We applied trial sequential monitoring boundaries according to an information size suggested by the intervention effect estimated based on an a priori 10% relative risk reduction (RRR) employing α=0.05 and ß=0.20.
We used TSA version 0.9 beta for these analyses (Copenhagen Trial Unit, Trial Sequential Analysis (computer program), version 0.9 beta, Centre for Clinical Intervention Research, 2011, www.ctu.dk/tsa).
Subgroup analyses
Subgroup analyses according to hypertension status were initially planned for the primary outcome, but this approach was abandoned during the data analysis process owing to different and missing definitions of hypertension threshold in the included studies.
Results
A flow diagram of the numbers of studies identified and rejected at each stage in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement15 can be seen as Figure 1. In brief, an experienced academic librarian searched and found 64 abstracts from MEDLINE, EMBASE and the Cochrane Library, and these underwent thorough evaluation in relation to inclusion criteria for this analysis. In addition, 1035 abstracts that were not captured in the initial search were screened, but no further studies fulfilling inclusion criteria were identified. Authors were not contacted for further data from any of the included studies.
Figure 1.
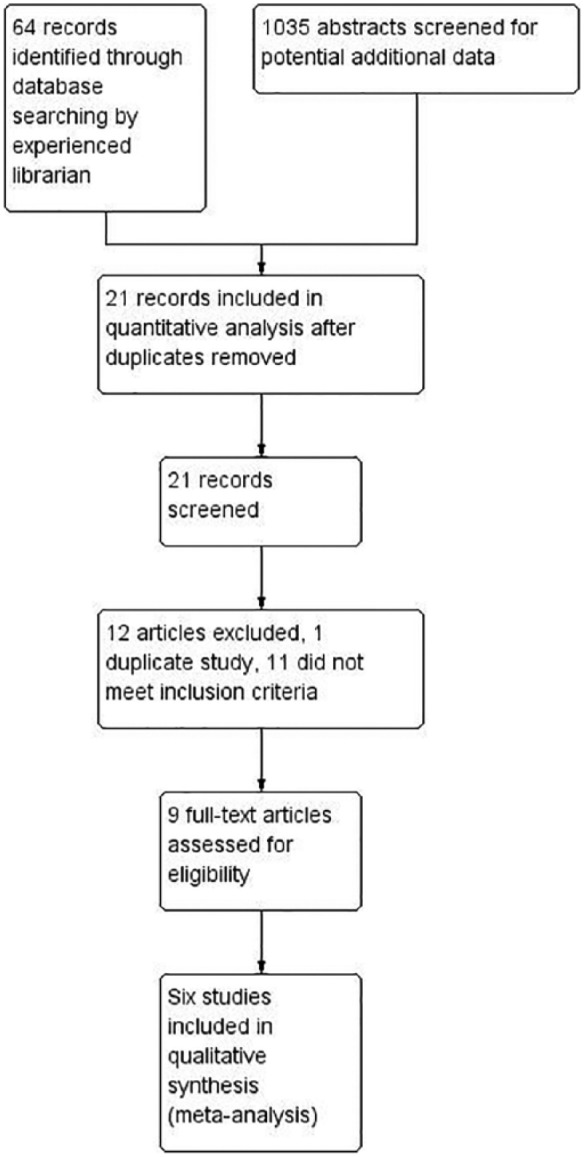
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) chart.
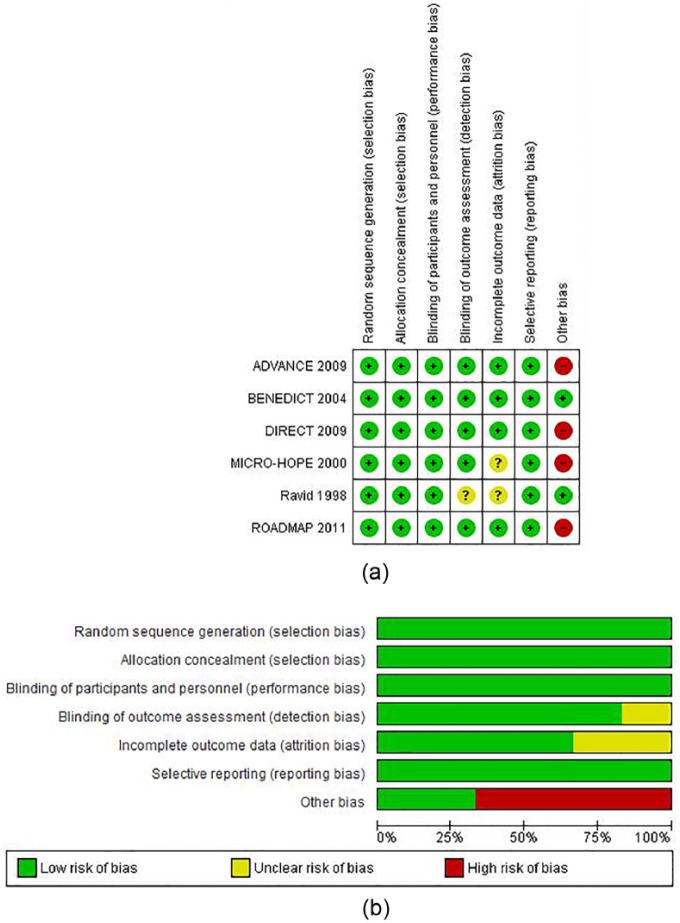
We included six randomised controlled clinical trials3–5,16–18 in our meta-analysis, and their characteristics can be seen in Table 1 (n=16,921 patients). Based on the inclusion criteria chosen for this analysis, the number of trials were considerably lower than a previous Cochrane metanalysis on the subject.6 The overall risk of bias was variable (Figure 2).
Table 1.
Characteristics of the included studies.
| Study | Year | Patients (n) | Follow-up (years) | RAS blocking agent | Daily dose (mg) | Hard renal outcome reported (yes/no) |
|---|---|---|---|---|---|---|
| Ravid et al.16 | 1998 | 156 | 6 | Enalapril | 10 | No |
| MICROHOPE17 | 2000 | 3577 a | 4.5 | Ramipril | 10 | Yes |
| BENEDICT3 | 2004 | 1204 | 3.6 | Trandolapril | 2 | No |
| DIRECT 24 | 2009 | 1905 | 4.7 | Candesartan | 32 | No |
| ADVANCE18 | 2009 | 11,140 | 4.3 | Perindopril | 2 | Yes |
| ROADMAP5 | 2011 | 4447 | 3.2 | Olmesartan | 40 | Yes |
RAS: renin-angiotensin system.
Total no. of included patients in the study. Approximately 30% had microalbuminuria at baseline and were excluded from this analysis.
Figure 2.
Risk of bias as evaluated by the authors. (a) risk of bias item for each included study. (b) risk of bias item presented as percentages across all included studies.
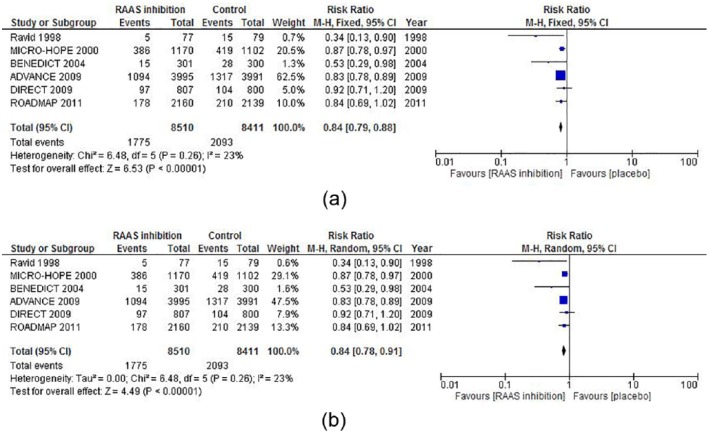
In fixed model analysis, ACEi or ARB treatment was superior to placebo in order to prevent development of microalbuminuria, with a risk ratio of 0.84 (95% CI 0.79–0.88) p<0.001. In the random model the results showed a risk ratio of 0.84 (0.78–0.91) p<0.001. There was no sign of heterogeneity in the risk ratio outcome with a I 2=23%, p=0.26. Forest plots are presented in Figure 3(a) and (b).
Figure 3.
(a) Fixed and (b) random model forest plots of the effect of angiotensin-converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB) therapy vs placebo to prevent microalbuminuria in type 2 diabetes.
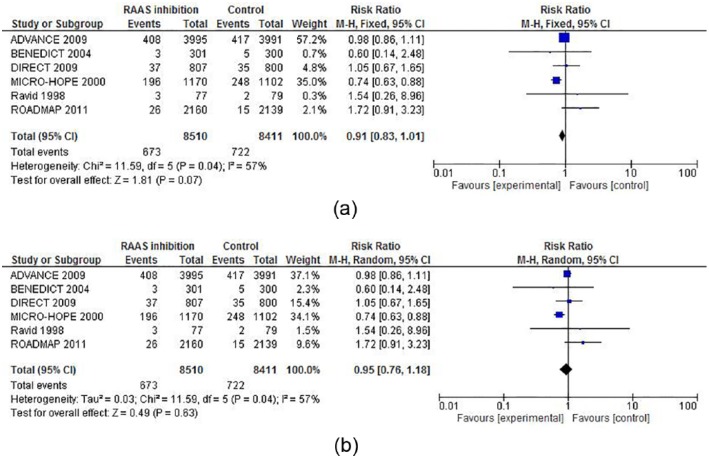
In the trials included as well as in the abstracts screened there were insufficient data available for the originally intended calculation of secondary outcomes, except for all-cause mortality shown in Figure 4, in which ACEi/ARB treated tended to reduce mortality, RR 0.91(0.83–1.01) p=0.07.
Figure 4.
(a) Fixed and (b) random model forest plots of the effect of angiotensin-converting enzyme inhibitor (ACEi)/ angiotensin receptor blocker (ARB) therapy vs placebo on mortality in patients with type 2 diabetes and normoalbuminuria.
The populations included in the six trials were a mix of normotensive and hypertensive, with differing definitions or thresholds for hypertension. The fraction of hypertensive subjects ranged from 0–100 %, see Table 2 for an overview of hypertension status in the six trials. It was not possible to conduct subgroup analysis of effect of the intervention according to baseline normotension vs hypertension, but approximately 38% of included patients were normotensive, or at least 31% if all subjects from the MICRO-HOPE study17 are considered hypertensive.
Table 2.
Blood pressure and hypertension status at baseline as reported in the six included trials (values are mean (SD)).
| Study | n | Baseline SBP |
Baseline DBP |
Baseline MAP |
Hypertensive subjects (%) |
Hypertension threshold (SD) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Active |
Placebo |
Active |
Placebo |
Active |
Placebo |
Active |
Placebo |
mm Hg | ||
| mm Hg | mm Hg | mm Hg | mm Hg | mm Hg | mm Hg | |||||
| Ravid et al.16 | 156 | 98 ± 4 (84–105) | 96 ± 4 (84–104) | 0 | 0 | 140/90 (MAP 107) | ||||
| ROADMAP5 | 4299 | 137 ± 16 | 136 ± 15 | 81 ± 10 | 80 ± 9 | 51 | 48 | 135/80 | ||
| ADVANCE18 | 7986 | 145 ± 22 | 145 ± 21 | 81 ± 11 | 81 ± 11 | 68 | 69 | 140/90 | ||
| DIRECT 24 | 1607 | 123 ± 9 | 123 ± 9 | 75 ± 6 | 76 ± 7 | 62 | 62 | 130/85 | ||
| BENEDICT3 | 601 | 151 ± 15 | 152 ± 15 | 87 ± 8 | 88 ± 7 | 109 ± 9 | 109 ± 9 | 100 | 100 | 130/85 |
| MICROHOPEa,17 | 2272 | 142 ± 20 | 142 ± 20 | 80 ± 11 | 79 ± 11 | 58 | 54 | 160/90 | ||
BP: blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure; SBP: systolic blood pressure; SD: standard deviation.
Mean BP values from full study population including approximately 30% with baseline microalbuminuria.
Trial sequential analysis revealed a Z-value of 6.53 and an information size of 4163 patients (O’Brien two-sided 5% boundaries), indicating that the available studies and the available data in our analysis are sufficient to make these investigations and conclusions (Supplementary Material, Figure 1). For all secondary outcomes except all-cause mortality, numbers in the included studies were too small to be analysed in our review.
Discussion
In our meta-analysis of studies testing whether ACEi/ARB treatment, compared to placebo, can prevent development of microalbuminuria in type 2 diabetic patients, we identified six trials including 16,921 normoalbuminuric patients with type 2 diabetes. We found a 16% RR reduction for development of microalbuminuria in the ACE/ARB treatment group as compared to placebo groups. The findings were significant both in random effects and fixed effect models, although given the clinical heterogeneity of the studies, the random model probably can be considered the most robust. The risk ratio of 0.84 corresponds to a number needed to treat (NNT) of 25, meaning that for every 25 normoalbuminuric patients treated with ACEi/ARB, one case of microalbuminuria will be prevented or delayed (although the required treatment time needed cannot be determined from this analysis). From a recent paper from Schievink et al.19 it is simulated that early RAS blockade intervention, as in the present analysis, can postpone ESRD by 4.2 years, compared to later stage interventions (3.6 and 1.1 years, for micro-and macroalbuminuria, respectively). Based on the TSA the available information size was found sufficient to support the inclusion, which means that it could be considered unethical to do further trials investigating this treatment compared to placebo.
The importance of change in albuminuria is evident from analyses of the IRMA2 study20 and the Steno 2 study,21 where reduction in albuminuria, and regression of micro- to normoalbuminuria translated into preserved kidney function. The primary outcome in our review is a surrogate outcome, and not a hard renal endpoint as i.e. ESRD, but the studies available do not have enough follow-up time to allow for hard renal endpoint evaluation. Our primary outcome is however frequently used clinically, and is a simple way to assess not only renal but also cardiovascular risk.
In type 2 diabetes the RAS is activated and has long been thought to play a major part in development of chronic vascular complications, including diabetic nephropathy and diabetic retinopathy. Following landmark studies like the IRMA2, RENAAL and IDNT trials,22–24 RAS inhibition is widely accepted and recommended for patients with established micro- and macroalbuminuria, but the remaining issue of primary prevention is not yet solved. As evident from our literature search, only a few trials have reported this outcome, and not all were primarily designed with this aim, which could be part of the explanation for the diverging results.
The biochemical aim for inhibition of the RAS is to reduce the deleterious effects of the angiotensin II peptide, most importantly exerting its effects by binding to the angiotensin II receptor subtype 1 (AT1R). The ACEi will inhibit the angiotensin-converting enzyme in converting angiotensin I to angiotensin II, thereby lowering substrate for the AT1R. The ARB binds directly to the AT1R, thereby preventing angiotensin II from activating the receptor. Reduced glomerular capillary hypertension has been demonstrated experimentally to minimise structural injury with mesangial expansion and fibrosis in the glomeruli leading to sclerosis, thereby slowing the development of glomerular leakage.25
Since Strippoli et al. published a systematic review on primary prevention of diabetic nephropathy in 2005,26 several additional studies have been published, adding to the amount of evidence. Since larger studies are now available, we excluded smaller studies (n<100) in an attempt to minimise publication and reporting bias. The first large Cochrane review by Strippoli et al. in 2006 did not investigate primary prevention of diabetic kidney disease, but recently the Cochrane report was updated by Lv et al.6 All antihypertensive treatment was analysed, despite the fact that most evidence and functional understanding regarding beneficial treatment come from RAS blocking drugs. Palmer et al.27 published a network meta-analysis investigating blood pressure lowering agents in patients with diabetes and CKD. The overall conclusion was that ACEis and ARBs offer the best protection against progression to ESRD. The study included several relevant secondary outcomes, i.e. regression of albuminuria, but did not evaluate first occurrence of microalbuminuria, and thus we cannot compare to the findings in our analysis. Catalá-López et al.28 performed a similar but more specific network meta-analysis on the effect on cardiorenal outcome of RAS blocking treatment in patients with diabetes, but incidence of microalbuminuria was not included as outcome. Recently, Bangalore et al.29 published a somewhat different analysis comparing RAS blocking treatment with other types of antihypertensive treatment in diabetes. It was concluded that RAS offers no advantage compared other antihypertensive drugs in relation to all-cause mortality, CVD events and renal risk. However, placebo-controlled trials were excluded, and renal outcome only consisted of ESRD, leaving out doubling of baseline creatinine which is a generally accepted endpoint and frequently used in renal trials. No data on occurrence of microalbuminuria was included. Importantly, for all the abovementioned analyses, the authors made no distinction between nephropathy in type 1 and type 2 diabetes, thereby mixing conditions with different pathologies.7–9
We therefore performed the present systematic review only in patients with type 2 diabetes and normoalbuminuria, only comparing ACEis and ARBs to placebo, in order to get a cleaner picture of both patient populations and treatment. We hope this provides clinicians with more coherent information to use in a clinical setting.
Far from all patients with type 2 diabetes progress to diabetic nephropathy, and a future approach may be to select patients for early intervention based on risk markers. One such approach, using an urinary proteomic multimarker risk pattern, is ongoing (www.EU-PRIORITY.org), and may bring forth a more individualised preventive treatment in normoalbuminuric patients with type 2 diabetes.30
Our findings could have implications for future clinical practice. Side effects of RAS inhibitors are relatively mild and few in low-risk populations such as the study populations included, i.e. partly normotensive and well preserved kidney function. For instance, in the DIRECT study,4,31 evaluating 5224 type 1 and type 2 diabetic patients, the occurrence of any serious adverse event, discontinuation due to adverse events or death were similar in the normotensive and hypertensive patients treated with RAS inhibition or placebo. Many hypertensive patients with type 2 diabetes will already be on RAS inhibitors, but extension of this to normotensive normoalbuminuric patients can now be contemplated. In addition, recent evidence from another meta-analysis suggests that RAS inhibitors can prevent and delay diabetic retinopathy in normotensive patients, further adding a reason for early initiation of treatment.32 Current guidelines2 do not recommend RAS inhibition in patients with normoalbuminuria, but our findings alongside the meta-analysis by Wang et al.32,33 in diabetic retinopathy suggest that guidelines should be revised in order to further improve prevention of two major microvascular diabetic complications.
Owing to different definitions of hypertension, and since the included papers did not stratify participants or results according to hypertensive status, we did not compare normotensive and hypertensive subjects in our analysis, and this may not be relevant if most patients with elevated blood pressure will have this treatment applied anyway. Although it may well be that the major part of the effect found in our analysis is reserved to hypertensive patients, we cannot rule out a similar effect in normotensive subjects, making up approximately 38% of the analysed population. In fact many of the important renoprotective effects from RAS blocking treatment may well be related to other effects than those on systemic blood pressure, as seen in the IRMA 2, RENAAL and IDNT trials.22–24
Also we did not compare ACEi vs ARB due to data size, judging by the numbers needed in the comparative network meta-analysis by Palmer et al.27 and the doses used in the included studies were not investigated in detail. However, the ONTARGET study,34,35 the largest study so far, demonstrated that clinical effects are alike when compared head-to-head. There may be differences between optimal antiproteinuric doses between drugs that inhibit the RAS, and sometimes the optimal antiproteinuric dose is different from widely recommended antihypertensive doses.36–38
The included six trials used different endpoint definitions and methodology in relation to occurrence of microalbuminuria, but a recent paper looking at different endpoint definitions concludes that this has only minor impact on the robustness of the outcome.39 The authors performed a post-hoc analysis of four randomised controlled clinical trials and conclude that single urine collections at a study visit are sufficient to define a transition in albuminuria as endpoint in clinical trials. In addition, different ACEi/ARB treatments used may also add a certain degree of pharmacological heterogeneity, as seen in some of the few studies comparing different treatments in similar populations.40,41 However, in terms of effect on development of microalbuminuria, there seems to be an effect of similar magnitude across different ACEi/ARBs.
We did not have the opportunity to analyse further secondary endpoints or safety and side effects along with the analysis of effect of ACEi/ARB treatment. This is an important limitation to our meta-analysis, especially if we want to promote this as standard early treatment of normotensive, normoalbuminuric patients in future guidelines. However all-cause mortality was insignificantly reduced, which at least speaks for the overall safety of early intervention. In addition, there are no clear signs from the reporting of adverse events in the included trials nor from a large trial in type 1 diabetes (the RASS trial)42 or in prehypertension (the TROPHY study)43 that there is a clinically significant increased frequency of hypotension and related side effects in normotensive populations treated with ACEi/ARBs. Depending on the definition of hypertension, a large proportion of patients with type 2 diabetes will be hypertensive anyway, as seen during follow-up in the DIRECT 2 trial, so treating normotension may not a frequent occurrence. Rather, it may be important to ensure that patients with type 2 diabetes receive treatment with ACEi or ARB as the first drug, whether the indication is hypertension or primary or secondary prevention of microvascular complications. It is however also important to point out that not all patients with type 2 diabetes will develop microalbuminuria, and not all patients diagnosed with nephropathy have true glomerular disease.7–9 In addition, it could have been relevant to include to include studies using renin inhibitors, another RAS blocking treatment, but as no studies using renin inhibition in primary prevention have been performed or initiated, we did not include this in our review.
A future collaborative meta-analysis with individual patient-level data from the included six trials seems feasible and would allow for more detailed safety information, as well as time-to-event analyses for primary outcome. However, a comparison between one-stage and two-stage meta-analyses approach of clinical trials by Stewart et al.,44 concluded that the two methods produce similar results.
Conclusions
In conclusion, we demonstrate that there is sufficient data available to conclude that inhibition of RAS using ACEis or ARBs can reduce the incidence of microalbuminuria, a risk marker for both renal disease and CVD, in patients with type 2 diabetes. This conclusion is, however, given with reservations since the populations included are heterogenous and a full safety analysis was not available.
Supplementary Material
Acknowledgments
This study was presented at the European Association for the Study of Diabetes (EASD) Annual Meeting, 2015, and at the American Society of Nephrology (ASN) Kidney Week, 2015. The authors wish to thank Bianca Hemmingensen for helpful comments on methodology and reporting. FP contributed to: development of protocol, undertaking of searches, selection of trials, data extraction, quality assessment of trials, data analysis, contact person, development of final review. ML: development of protocol, selection of trials, data extraction, quality assessment of trials, development of final review. PR: development of protocol, development of final review. H-HP: developed the initial idea for the review, development of protocol, development of final review.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: FP reports having received research grants from Novartis and lecture fees from Novartis, Eli Lilly and Boehringer Ingelheim and having served as a consultant for Astra Zeneca and MSD. PR reports having received lecture fees from Novartis and Boehringer Ingelheim, and research grants from Novartis, has served as a consultant for Merck, and having equity interest in Novo Nordisk. H-HP reports having served as a consultant for Novartis, Merck, Pfizer and Sanofi-Aventis, having equity interest in Merck and Novo Nordisk and having received lecture fees from Novartis, Merck, Pfizer and Sanofi-Aventis. H-HP has received grant support from Novartis, AstraZeneca and Sanofi-Aventis. FP, ML and PR are employed at Steno Diabetes Center, Gentofte, Denmark. Steno Diabetes Center is an independent academic institution owned by Novo Nordisk A/S and The Novo Nordisk Foundation.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Parving H-H, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: A global perspective. Kidney Int 2006; 69: 2057–2063. [DOI] [PubMed] [Google Scholar]
- 2. Professional Practice Committee for the Standards of Medical Care in Diabetes-2016. Diabetes Care 2016; 39: S107–S108. [DOI] [PubMed] [Google Scholar]
- 3. Ruggenenti P, Fassi A, Ilieva AP, et al. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004; 351: 1941–1951. [DOI] [PubMed] [Google Scholar]
- 4. Sjolie AK, Klein R, Porta M, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): A randomised placebo-controlled trial. Lancet 2008; 372: 1385–1393. [DOI] [PubMed] [Google Scholar]
- 5. Haller H, Ito S, Izzo JL, Jr, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 2011; 364: 907–917. [DOI] [PubMed] [Google Scholar]
- 6. Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: A systematic review and meta-analysis. CMAJ 2013; 185: 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parving HH, Gall MA, Skott P, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 1992; 41: 758–762. [DOI] [PubMed] [Google Scholar]
- 8. Christensen PK, Larsen S, Horn T, et al. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int 2000; 58: 1719–1731. [DOI] [PubMed] [Google Scholar]
- 9. Rossing P, Feldt-Rasmussen B, Parving H-H. Diabetic nephropathy. Brenner and Rector’s The kidney. 10th ed. Elsevier 2015, p. 1283. [Google Scholar]
- 10. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 12. Demets DL. Methods for combining randomized clinical trials: Strengths and limitations. Stat Med 1987; 6: 341–350. [DOI] [PubMed] [Google Scholar]
- 13. Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008; 61: 64–75. [DOI] [PubMed] [Google Scholar]
- 14. Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009; 38: 276–286. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 2009; 62: e1–e34. [DOI] [PubMed] [Google Scholar]
- 16. Ravid M, Brosh D, Levi Z, et al. Use of enalapril to attennuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. Ann Intern Med 1998; 128: 982–988. [DOI] [PubMed] [Google Scholar]
- 17. HOPE trial investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: Results of the HOPE study and MICRO-HOPE substudy. Lancet 2000; 355: 253–259. [PubMed] [Google Scholar]
- 18. Patel A. ADVANCE Collaborative Group, MacMahon S, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised controlled trial. Lancet 2007; 370: 829–840. [DOI] [PubMed] [Google Scholar]
- 19. Schievink B, Kropelin T, Mulder S, et al. Early renin-angiotensin-system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes Metab 2016; 18: 64–71. [DOI] [PubMed] [Google Scholar]
- 20. Hellemons ME, Persson F, Bakker SJL, et al. Initial angiotensin receptor blockade-induced decrease in albuminuria is associated with long-term renal outcome in type 2 diabetic patients with microalbuminuria. Diabetes Care 2011; 34: 2078–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaede P, Tarnow L, Vedel P, et al. Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 2004; 19: 2784–2788. [DOI] [PubMed] [Google Scholar]
- 22. Parving H-H, Lehnert H, Bröchner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 Diabetes. N Engl J Med 2001; 345: 870–878. [DOI] [PubMed] [Google Scholar]
- 23. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 24. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860. [DOI] [PubMed] [Google Scholar]
- 25. Zatz R, Dunn BR, Meyer TW, et al. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest 1986; 77: 1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strippoli GF, Craig M, Schena FP, et al. Antihypertensive agents for primary prevention of diabetic nephropathy. J Am Soc Nephrol 2005; 16: 3081–3091. [DOI] [PubMed] [Google Scholar]
- 27. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: A network meta-analysis. Lancet 2015; 385: 2047–2056. [DOI] [PubMed] [Google Scholar]
- 28. Catala-Lopez F, Macias Saint-Gerons D, Gonzalez-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: A systematic review with network meta-analyses. PLoS Med 2016; 13: e1001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bangalore S, Fakheri R, Toklu B, et al. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: Systematic review and meta-analysis of randomized trials. Br Med J 2016; 352: i438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindhardt M, Persson F, Currie G, et al. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): Essential study design and rationale of a randomised clinical multicentre trial. BMJ Open 2016; 6: e010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chaturvedi N, Porta M, Klein R, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: Randomised, placebo-controlled trials. Lancet 2008; 372: 1394–1402. [DOI] [PubMed] [Google Scholar]
- 32. Wang B, Wang F, Zhang Y, et al. Effects of RAS inhibitors on diabetic retinopathy: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015; 3: 263–274. [DOI] [PubMed] [Google Scholar]
- 33. Parving HH, Persson F. Effects of RAS inhibitors on diabetic retinopathy. Lancet Diabetes Endocrinol 2015; 3: 315–316. [DOI] [PubMed] [Google Scholar]
- 34. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559. [DOI] [PubMed] [Google Scholar]
- 35. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372: 547–553. [DOI] [PubMed] [Google Scholar]
- 36. Schjoedt KJ, Astrup AS, Persson F, et al. Optimal dose of lisinopril for renoprotection in type 1 diabetic patients with diabetic nephropathy: A randomised crossover trial. Diabetologia 2009; 52: 46–49. [DOI] [PubMed] [Google Scholar]
- 37. Rossing K, Schjoedt KJ, Jensen BR, et al. Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type 2 diabetes and microalbuminuria. Kidney Int 2005; 68: 1190–1198. [DOI] [PubMed] [Google Scholar]
- 38. Hollenberg NK, Parving H-H, Viberti G, et al. Albuminuria response to very high-dose valsartan in type 2 diabetes mellitus. J Hypertens 2007; 25: 1921–1926. [DOI] [PubMed] [Google Scholar]
- 39. Kropelin TF, de Zeeuw D, Remuzzi G, et al. Determining the optimal protocol for measuring an albuminuria class transition in clinical trials in diabetic kidney disease. J Am Soc Nephrol. Epub ahead of print 7 April 2016. doi: 10.1681/ASN.2015101150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bakris GL, Ruilope L, Locatelli F, et al. Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk: Results of the IMPROVE trial. Kidney Int 2007; 72: 879–885. [DOI] [PubMed] [Google Scholar]
- 41. Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351: 1952–1961. [DOI] [PubMed] [Google Scholar]
- 42. Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009; 361: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354: 1685–1697. [DOI] [PubMed] [Google Scholar]
- 44. Stewart GB, Altman DG, Askie LM, et al. Statistical analysis of individual participant data meta-analyses: A comparison of methods and recommendations for practice. PloS One 2012; 7: e46042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.