Abstract
Angiotensin converting enzyme inhibitors and propofol both exert hypotensive action and may affect hemostasis. We investigated the influence of quinapril and propofol on hemodynamics and hemostasis in renal-hypertensive rats with induced arterial thrombosis. Two-kidney, one clip hypertensive rats were treated with quinapril (3.0 mg/kg for 10 days), and then received propofol infusion (15 mg/kg/h) during ongoing arterial thrombosis. The hemodynamic and hemostatic parameters were assayed. Quinapril exerted a hypotensive effect increasing after propofol infusion. Quinapril showed an antithrombotic effect with the platelet adhesion reduction, fibrinolysis enhancement and oxidative stress reduction. Propofol did not influence thrombosis; however, it inhibited fibrinolysis and showed prooxidative action. The effect of propofol on fibrinolysis and oxidative stress was significantly lower in quinapril-pretreated rats. Mortality was increased among rats treated with both drugs together. Our study demonstrates that pretreatment with quinapril reduced the adverse effects of propofol on hemostasis. Unfortunately, co-administration of both drugs potentiated hypotension in rats, which corresponds to higher mortality.
Keywords: Quinapril, propofol, rat, thrombosis, hypertension
Introduction
Angiotensin-converting enzyme inhibitors (ACE-Is), widely used hypotensive drugs, reveal antithrombotic, endothelium-dependent activity even at non-hypotensive doses. We have previously shown antithrombotic action of ACE-Is in arterial and venous thrombosis in normotensive rats and this action was significantly greater in young rats than in adult and old rats.1–4 This antithrombotic activity of ACE-Is depends on the inhibition of angiotensin II (Ang II) formation, bradykinin (Bk) degradation, and potentiation of Ang-(1-7) formation.5 A decrease in the Ang II level supports functions of antithrombotic endothelium and decreases platelet activation.6 In turn, increased Bk levels, via type II G-protein-coupled Bk receptors, activate calmodulin and in consequence increase the production of such antithrombotic molecules as prostacyclin (PGI2) or tissue-type plasminogen activator (t-PA).7 Furthermore, ACE-Is increase Ang-(1-7) production, probably due to the activity of such enzymes as ACE 2 or endopeptidases, which are not susceptible to inhibition by ACE-Is.8 Ang-(1-7) activating its own G-protein coupled receptors, Mas1-7, increases the production of nitric oxide (NO) and PGI2 in endothelial cells.9 Moreover, ACE-Is affect hemostasis also through the inhibition of platelet and erythrocyte aggregation, and down regulation of glycoprotein IIb/IIIa complex on the platelet surface.9–11 ACE-Is also cause a decrease in serum and aortic ACE activity, PAI-1 protein level, and tissue factor (TF) activity.12,13 These favorable endothelial effects of ACE-Is have been confirmed in human studies and could explain reduced cardiovascular mortality caused by these drugs.14–17
Many patients treated with ACE-Is undergo general anesthesia with propofol (Pro), an intravenous anesthetic. Among its advantages directly in anesthesia, Pro also increases endothelium-dependent NO release.18 Mainly for this reason, Pro causes vasodilatation, resulting in clinically relevant hypotension.19 Indeed, our previous observations in hypertensive patients treated with ACE-Is showed that Pro given for induction of general anesthesia significantly decreased blood pressure (BP), but precise mechanism of this interaction is still unknown and needs further investigations.20
Pro also activates the kinin–kallikrein system in plasma and acts as an L-type calcium channel antagonist decreasing peripheral vascular resistance.21,22 In vitro and ex vivo data suggest that Pro exerts antiplatelet and anti-inflammatory effects.23–26 Moreover, Pro inhibits platelet thromboxane synthesis and NO production in leukocytes, stimulating the activity of guanyl cyclase in platelets.27 In our preliminary study, we also observed NO release from endothelial cells, which indicates the significant involvement of endothelium in the effect of Pro on hemostasis.28
Because of the structural similarity to vitamin E, Pro could also regulate oxidative stress.29 Moreover, since the chemical structure of the Pro molecule (a simple phenol substituted with two isopropyl groups in the ortho position) resembles the structure of acetylsalicylic acid it may potentially influence hemostasis.30
There are also some clinical studies about thrombotic complications of the deep brachial veins during Pro treatment, although the mechanism has not yet been examined.31,32 However, data concerning the effect of simultaneous administration of Pro and ACE-Is on hemostasis are lacking.
Therefore, the aim of the current study was to assess the influence of quinapril (Quin) and Pro on the process of arterial thrombosis, primary hemostasis, NO bioavailability, oxidative stress and hemodynamic parameters in two-kidney, one-clip (2K1C) hypertensive rats.
Materials and methods
Drugs and reagents
Pro (Plofed 1%, Polfa S.A., Poland), Quin (Accupro, Pfizer, Germany), lipofundin (MCT/LCT 10%, Braun, Germany), gum arabic (Pharma Cosmetic, Poland), pentobarbital (Vetbutal, Biovet, Poland), collagen type I (Collagen, Chronolog, USA), and heparin (Heparinum, Polfa, Poland) were used in the study. Bovine albumin, apyrase, HEPES (N-(2-hydroxyethyl)piperazine-N′-(2-ethanosulfonic acid) were delivered by Sigma-Aldrich (Poland). Calcium chloride, EDTA, glucose, magnesium chloride, sodium chloride, potassium chloride, sodium bicarbonate, sodium phosphate, Tris buffer, and trisodium citrate were provided by Polish Chemicals Reagents (Poland). Rabbit anti-rat TAFI monoclonal antibody (ImmunoKontact AMS Biotechnology, Germany), rat active PAI-1 ELISA kit (Innovative Research, USA), rat active t-PA ELISA kit (Innovative Research, USA), hydrogen peroxide colorimetric detection kit (Assay Designs, USA), correlate assay nitric oxide NO-2/NO-3 assay kit (Assay Designs, USA), MDA adducts ELISA kit (Cell Biolabs, USA), Trizol (Invitrogen Life Technologies, USA), qPCRTM Mastermix, SYBR Green I (Eurogentec Seraing, Belgium), Oligotex Kit (Qiagen, USA), and TaqMan reverse transcription reagents kit (Applied Biosystems, USA) were also used.
Animals
Male Wistar rats (180–250 g) were used in this study. The animals were housed in a room with a 12 h light/dark cycle, and were given tap water and fed standard rat chow. The animals’ health status was monitored throughout the experiments by a health surveillance program according to Federation of European Laboratory Animal Science Associations guidelines and Guidelines for the Care and Use of Animals in Biomedical Research.33 All the procedures involving animals and their care were approved by Local Ethical Committee on Animal Testing at the Medical University of Bialystok (Permit Number: 2004/27).
Induction of renovascular hypertension
Rats were anesthetized with pentobarbital (40 mg/kg, i.p.). Two-kidney, one-clip (2K1C) renovascular hypertension was induced by a partial, standardized clipping of the left renal artery.34 After 6 weeks, to verify hypertension development the systolic and diastolic BP (SBP/DBP) were measured using the “tail cuff” method (LE 5001 Non-invasive Blood Pressure System, Panlab, Spain) in conscious rats.35 Each value was the average of three consecutive readings. Only rats with BP higher than 140/90 mmHg were used in the further experiments evaluating the effect of Quin and Pro on hemostasis and hemodynamics.
Drug administration
Quin (3.0 mg/kg) or adequate volume of 5% aqueous gum arabic solution was administered per os for 10 days. The experiments were done on the 11th day after treatment initiation. During the study, the animals received intravenous infusion of Pro (15 mg/kg/h) or lipofundin (solvent for Pro) at the same dose. The animals were divided into four groups: Group I – 5% aqueous gum arabic solution + lipofundin (Veh); Group II – Quin + lipofundin (Quin); Group III – 5% aqueous gum arabic solution + Pro (Pro); Group IV – Quin + Pro (Quin+Pro).
Indirect BP measurement after 10 days of treatment
The SBP and DBP in conscious rats were measured after 10 days of treatment again, according to the method described above.35 Each value was the average of three consecutive readings.
Arterial thrombosis model
Arterial thrombosis induction was performed according to the method described previously.36,37 Rats were introduced into anesthesia with pentobarbital (40 mg/kg intraperitoneally) and then fixed on the operating table. Anesthesia was maintained with an additional dose of pentobarbital (20 mg/kg) intraperitoneally 40 min after the first dose, at a level such that painful stimuli and surgery did not evoke noticeable motor or cardiovascular responses. Anesthetic condition remained unchanged throughout the study.
Thrombosis was induced secondarily to electrical stimulation and endothelial injury with a current, as previously described.36,37 The left common carotid artery was then separated from the surrounding tissue along the length of at least 20 mm. A stainless, hook shaped, steel electrode was inserted under the left carotid artery. Under the electrode, a tiny piece of “M” parafilm (5 mm × 20 mm) was inserted for electrical isolation and the hook of the electrode was in contact with the artery. The second electrode was inserted subcutaneously in the abdominal region. Both electrodes were connected to a circuit with a constant current generator. A Doppler flow probe (1 mm-diameter, Hugo Sachs Elektronik – Harvard Apparatus GmbH, Germany) was placed in contact with the exposed artery downstream of the electrode and connected to a blood flowmeter (The HSE-TRANSONIC Transit Time Flowmeter, Germany). Blood flow was monitored continuously during thrombus formation.
After 5 min stabilization, the baseline blood flow was determined. Pro was administered into the femoral vein by a continuous infusion (Constant-Rate Infusion Pump, Kwapisz, Poland) 15 min before the induction of arterial thrombosis and was continued for 100 min, to the end of the experiment. The control animals obtained lipofundin in the same way and volume.
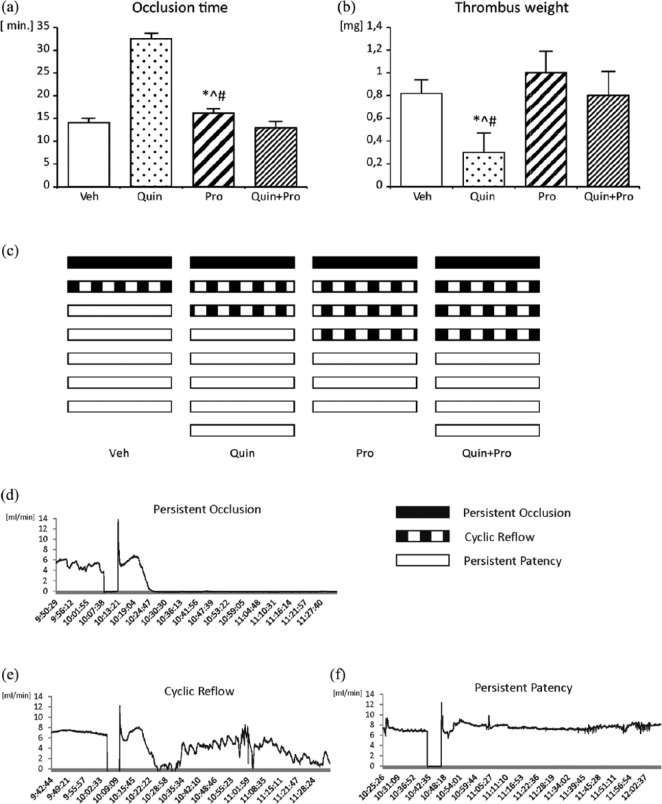
Fifteen minutes after the start of Pro infusion, thrombosis was induced by electrical stimulation (2 mA/5 min) and a hemostatic clamp delivered to the outer surface of the left common carotid artery (between 20 and 25 min). The hemostatic clamp was placed between the electrode and Doppler flow probe, and the degree of thrombotic occlusion was expressed by a decrease in carotid blood flow. Carotid patency status was monitored continuously for 80 min after the cessation of electrical stimulation and hemostatic clamp. The carotid patency status was expressed according to the following classifications: (1) persistent occlusion – no reflow; (2) cyclic reflow – cyclic reflow and reocclusion after initial reflow; (3) persistent patency – a persistent flow without reocclusion after the initial reflow. The patency status of the carotid artery for the 100 min observation was schematically represented for each animal (see Figure 2(c)). Representative examples of original recordings of blood flow from selected animals with persistent occlusion, cyclic reflow, and persistent patency are shown in Figure 2(d) to (f)
Figure 2.
The effect of Quin and Pro treatment on occlusion time (a) and dry thrombus weight (b). Schematic representation of the patency status of the carotid artery in individual animals for the 100 min observation (c). Representative examples of original recordings of blood flow from selected animals with persistent occlusion (d), cyclic reflow (e), and persistent patency (f). Data are expressed as mean ± SEM. *^#p <0.05 Pro vs Veh, Quin, Quin+Pro, respectively.
At the end of the study (100 min after Pro administration), blood samples were taken for hemostatic analysis, the aorta was taken for enzyme analysis, and the thrombus was collected at room temperature for 24 h and weighed. All animals were euthanized by exsanguination at the end of experiments under deep anesthesia.
Measurement of hemodynamic parameters
The SBP and DBP were measured in anesthetized rats receiving Pro or lipofundin directly through a cannula filled with heparin solution (150 IU/mL) placed in the left common carotid artery connected to a pressure transducer (Gabarith 1DT-XX, Poland) and an apparatus for pressure measurement (HSE-TRANSONIC, TAM-A, Germany). The carotid blood flow was measured by placing a flow probe on the right carotid artery connected to a blood flowmeter (HSE-TRANSONIC, TTFM, Germany). Both the upper limbs and the right lower limb were connected to electrodes for ECG measurement (HSE-TRANSONIC, ECGA, Germany). Heart rate was calculated from the ECG. In these animals, arterial thrombosis was not induced.
The blood flow was measured downstream from the site of electrical stimulation and monitored continuously from the beginning of the study until its end.
Primary hemostasis: template bleeding time
At the end of the experiment, prior to the removal of the arterial thrombus, the template bleeding time (BT) was measured, according to Dejana et al.38 A standardized device was applied longitudinally on the dorsal part of the tail between 6 and 9 cm from the tip, taking care to avoid the large veins. Immediately after injury, the tail was placed into a cylinder with isotonic saline at 37°C and bleeding time was measured from the moment the tail was surgically cut until bleeding completely stopped (i.e. lack of bleeding for <30 s).
Blood and aorta collection
At the end of the experiments, blood samples were drawn from the right ventricle of the heart. Whole blood and plasma were received and deep-frozen (−80°C) for further investigations. To obtain plasma, the blood was mixed with 3.13% trisodium citrate in a volume ratio of 9:1 and centrifuged for 20 min at 3500× g, at 4°C. At the end of the experiments (only arterial model of thrombosis), the aortas were obtained from rats and deep-frozen (−80°C).
Platelet adhesion to fibrillary collagen ex vivo
Platelet adhesion to fibrillary collagen was assayed ex vivo according to Mant et al.39 The washed platelet suspension was prepared from blood as previously described.40 The final concentration of platelets in washed platelet samples was 3 × 105 platelets/μL. The 250 µL of washed platelet samples were incubated in an Elvi 840 aggregometer at 37°C and stirred at 900 rpm with EDTA (13 µL; 100 mM) to prevent platelet aggregation. After 5 min pre-incubation, collagen (12 µL) was added and platelets were further incubated for 10 min. Samples of the suspension were obtained before and 15 min after adding the collagen, then platelets were counted in a hemocytometer after dilution with the Unopette system. The index of adhering platelets was calculated using the formula [(platelet count before adding the collagen − platelet count after adding the collagen)/platelet count before adding the collagen] × 100%.
Determination of fibrinolysis
To determine whether fibrinolysis could be changed after Quin and/or Pro administration, t-PA, PAI-1 and TAFI plasma levels were determined by enzyme immunoassays according to manufacturer’s directions. PAI-1 mRNA levels were measured in whole blood using the real-time polymerase chain reaction (PCR) technique, as previously described.40,41
Determination of plasma nitric oxide level
To establish the influence of Quin and Pro treatment on NO bioavailability, plasma NO metabolite levels were measured. Since NO is an extremely labile molecule and decomposes rapidly in biological solutions to nitrite (NO2−) and nitrate (NO3−), these stable metabolites of NO were analyzed in plasma as indirect markers for NO plasma bioavailability. In our study, the NO plasma level was measured colorimetrically as NO2/NO3 concentration with a commercially available kit.
Determination of aorta NOS mRNA level
To determine an influence of Quin and/or Pro administration on nitric oxide synthase (NOS) activity, eNOS and iNOS mRNA levels were measured in the aorta using the real-time PCR technique, as previously described.40,41 The following PCR primers were designed using software Primer Express (Applied Biosystems): 5′-CATCGGCGTGCTGCGGGATCAG-3′ and 5′-GGGCTGTTGGTGTCTGAGCCGG-3′ and 5′-CCAACAATGGCAACATCAGG-3′ and 5′-TCGTGCTTGCCATCACTCC-3′, specific for mRNA of eNOS and iNOS respectively. The reaction for each probe was conducted in the same conditions as a multiplex PCR with two pairs of primers (eNOS and iNOS) at the same time. The amount of eNOS and iNOS mRNA was quantified.
Oxidative stress parameters
To determine the influence of Quin and Pro treatment on the oxidative stress parameters, plasma concentrations of hydrogen peroxide (H2O2), malonyl dialdehyde (MDA), and enzymatic expression of superoxide dismutase (SOD) and oxidase NADPH in aortic rings were assayed.
H2O2 and MDA levels were assayed in plasma with commercially available kits. To evaluate the influence of Quin and/or Pro administration on oxidative stress in the aorta, amounts of mRNA of NADPH oxidase and SOD were measured in rat aortas using the real-time PCR technique, as previously described.40,41
Statistical analysis
The data are shown as mean ± SEM. The incidence of occlusion in carotid artery was calculated by Fisher’s exact test. Two-tail Mann–Whitney tests were used consistently throughout the study to test whether the mean of a variable differs between two groups. A p-value <0.05 was considered significant.
Comparisons between the experimental rat groups and the saline-treated placebo rat group were performed using one-way analysis of variance (ANOVA) or Kruskal–Wallis test, followed by post-hoc Dunnett’s or Dunn’s tests, depending on data distribution. A p-value <0.05 was considered statistically significant. Statistical analyses were performed with SPSS 16.0 (SPSS, Chicago, IL, USA)
Results
Hemodynamics
A 10-day Quin treatment (3 mg/kg) showed a significant (p < 0.05) decrease in BP (systolic and diastolic) as compared to Veh rats (Table 1). Pro alone and added to Quin treated rats showed a notable (p < 0.05) decrease in SBP and DBP at 100 min (T100) of infusion in comparison to the onset of the experiment (T0). Changes in BP did not result in the acceleration of the heart rate in all animal groups.
Table 1.
Hemodynamic parameters before and after 100 min Pro administration.
| Group | n | Heart rate (bpm) |
Systolic blood pressure (mmHg) |
Diastolic blood pressure (mmHg) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T 0 | T 100 | ΔT (T100 − T0) | T 0 | T 100 | ΔT (T100 − T0) | T 0 | T 100 | ΔT (T100 − T0) | ||
| Veh | 6 | 424 ± 17 | 418 ± 15 | −6 | 143 ± 8 | 137 ± 7 | −6 | 112 ± 6 | 102 ± 6 | −10 |
| Quin | 6 | 433 ± 14 | 393 ± 11Δ | −40 | 123 ± 5* | 113 ± 7* | −10 | 98 ± 4* | 88 ± 4* | −10 |
| Pro | 5 | 419 ± 19 | 400 ± 27 | −19 | 148 ± 5 | 118 ± 7Δ | −30 | 110 ± 5 | 90 ± 6Δ | −20 |
| Quin+Pro | 5 | 419 ± 23 | 409 ± 5 | −10 | 128 ± 9* | 104 ± 8*Δ | −24 | 99 ± 4* | 78 ± 3Δ* | −21 |
Veh – control with lipofundin infusion, Quin – rats treated with quinapril and lipofundin infusion, Pro – control with propofol infusion, Quin+Pro – rats treated with quinapril and propofol infusion,T0 – beginning of the experiment, T100 – end of the experiment, after 100 min flow registration. Data are expressed as mean ± SEM *p < 0.05 vs Veh; Δp < 0.05 T100 vs T0.
Arterial thrombosis
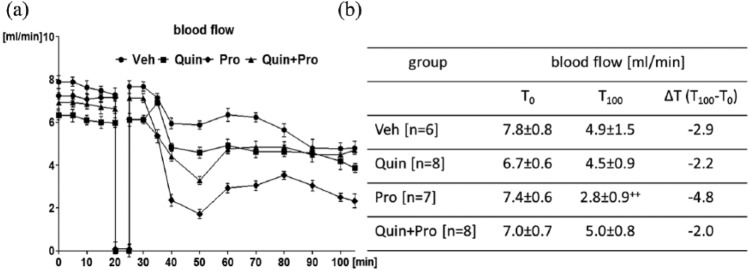
The initial blood flow was comparable in all study groups. A gradual decrease in the blood flow was observed in all the groups (Figure 1(a)). The difference between the final (T100) and the initial carotid blood flow (T0) assumed as ∆T was the highest in the Pro group (2.8±0.9 vs 7.4±0.6 mL/min, ∆T −4.8 mL/min; p < 0.01) (Figure 1(b)). Furthermore, the final blood flow after Pro infusion was the lowest in comparison to Veh and Quin treated rats (p < 0.05) (Figure 1(b)).
Figure 1.
Changes in the carotid blood flow (a) with the values of initial (T0) and final (T100) carotid blood flow (b) determined in Veh, Quin, Pro, and Quin+Pro rats subjected to electrical stimulation and clamping of the carotid artery between 20–25 min of the experiment. The lines represent the course of blood flow registered for 100 min after administration of Pro or Quin. T0 – beginning of the experiment, T100 – end of the experiment, after 100 min flow registration. Data are expressed as mean ± SEM. ++p <0.01 vs T0.
In the Quin group, the longest time to primary vessel occlusion (32±0.9 min, Figure 2(a)), the lowest thrombus weight (0.3±0.17 mg, p < 0.05, Figure 2(b)), and the highest persistent patency (62%, Figure 2(c)) were noted. The incidence of persistent occlusion or cyclic reflow in the Quin group was similar to the Veh-treated rats (37% vs 33%). The administration of Pro increased the incidence rates of cyclic reflow: Pro alone by 34% and added to Quin by 27%, when compared to Veh (Figure 2(c)).
Pro alone did not change significantly thrombus weight in comparison to Veh (1.0±0.3 mg vs 0.8±0.21 mg), and added to Quin reduced its benefit effect on thrombus weight (1.0±0.3 mg vs 0.3±0.17 mg, p < 0.05) (Figure 2(b)).
The primary hemostasis
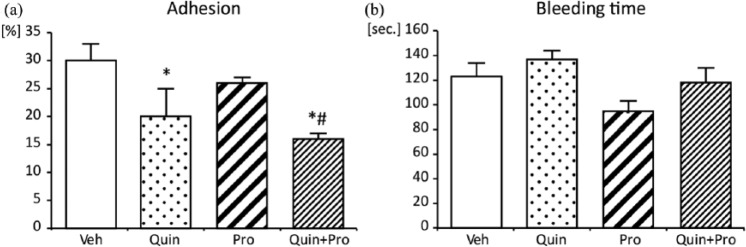
As shown in Figure 3(a), collagen-induced platelet adhesion was attenuated by Quin (20±4.4%, p < 0.05). Both Pro and Quin significantly decreased platelet adhesion to collagen in comparison to Veh and Pro alone (16±2.0% vs 30±2.5% and 26±2.0%, respectively, p < 0.05) (Figure 3(a)). Any changes in BT were observed; only Quin tended to prolong BT (Figure 3(b)).
Figure 3.
Effect of Quin and Pro treatment on platelet adhesion ex vivo (a) and bleeding time (b). Data are expressed as mean ± SEM. *p <0.05 vs Veh; #p<0.05 vs Pro.
Plasma fibrinolytic status
Quin greatly enhanced fibrinolysis through increased t-PA expression (p < 0.05) and decreased PAI-1 expression (p < 0.05), when administered alone (Table 2). The antifibrinolytic response exerted by Pro was partially abolished by Quin (p < 0.05 in t-PA, p < 0.01 in PAI-1) (Table 2).
Table 2.
Ex vivo plasma fibrinolytic parameters at the end of arterial thrombosis induction.
| t-PA (ng/mL) | PAI-1 (ng/mL) | TAFI (μg/mL) | |
|---|---|---|---|
| Veh (n = 6) | 7.02 ± 0.18 | 4.83 ± 0.15 | 4.34 ± 0.02 |
| Quin (n = 7) | 7.94 ± 0.14*## | 4.22 ± 0.05*## | 4.54 ± 0.07 |
| Pro (n = 6) | 5.68 ± 0.24**^^ | 7.13 ± 0.2**^^^ | 5.29 ± 0.03** |
| Quin+Pro (n = 8) | 6.74 ± 0.06#^^ | 5.41 ± 0.07*###^^^ | 4.54 ± 0.03## |
p < 0.05, **p < 0.01 vs Veh, #p<0.05, ##p<0.01, ###p<0.001 vs Pro, ^^p<0.01, ^^^p<0.001 vs Quin.
The strongest increase in TAFI was induced by Pro infusion in the rats that did not previously receive Quin (p < 0.01), as compared to Veh. Pretreatment with Quin decreased Pro-induced TAFI plasma concentration (p < 0.05) in comparison to Pro alone (Table 2).
NO bioavailability
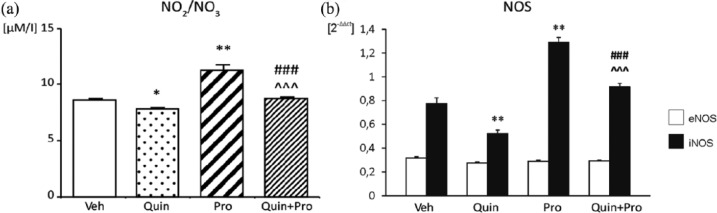
The 10-day Quin treatment caused a decrease in plasma NO2/NO3 concentration (Figure 4(a)) and reduction in aortic iNOS mRNA expression (Figure 4(b)), as compared to Veh (7.88±0.06 μM/L vs 8.76±0.08 μM/L, p < 0.05 and 0.52±0.03 2−ΔΔct vs 0.78±0.05 2−ΔΔct, p < 0.01; respectively). The increase in plasma NO metabolite level in the Pro group (11.35±0.43 μM/L vs 8.76±0.08 μM/L; p < 0.01 vs Veh) was paralleled by an increase of iNOS mRNA levels in the aortic rings (1.29±0.04 2−ΔΔct vs 0.78±0.05 2−ΔΔct, p < 0.01 vs Veh) (Figure 4(a) and (b)). During co-administration of Quin and Pro, we still observed an increase in iNOS activity and plasma NO2/NO3 concentration in comparison to Quin (p < 0.001) (Figure 4(a) and (b)). The expression of eNOS was comparable in all study groups.
Figure 4.
Effect of Quin and Pro treatment on NO2/NO3 plasma concentration (a) and eNOS and iNOS mRNA level in aorta (b). Data are expressed as mean ± SEM. *p <0.05; **p<0.01 vs Veh; # # # p<0.001 vs Pro; ^^^p<0.001 vs Quin.
Oxidative stress
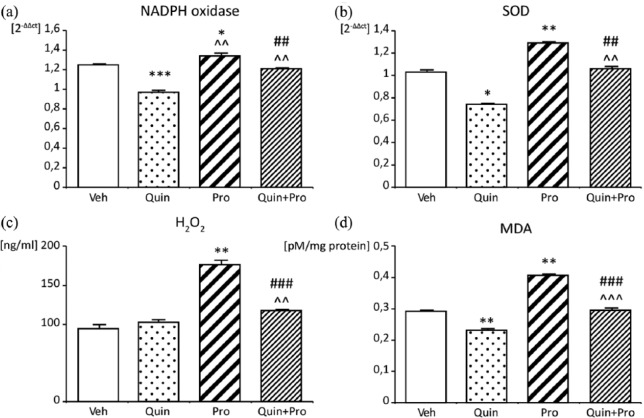
Quin and Pro co-administration did not change the expression of SOD and NADPH oxidase in comparison to Veh, whereas Quin alone significantly decreased SOD (p<0.05) and NADPH oxidase expression (p < 0.001) and prevented their increase caused by Pro (p < 0.01) (Figure 5(a) and (b)). Quin treatment did not change H2O2 concentration (102±3 ng/mL) (Figure 5(c)), while MDA concentration was decreased as compared to Veh (0.23±0.005 vs 0.29±0.004 pM/mg protein, p < 0.01) (Figure 5(d)). Pro infusion increased both H2O2 and MDA concentration (176.3±5.3, p < 0.01 vs Veh; 0.407±0.003, p < 0.01 vs Veh). Quin markedly attenuated prooxidative potency of Pro when administered jointly, in comparison to Pro alone (H2O2 117.2±1.9 mg/ml, MDA 0.295±0.007 pM/mg protein, p < 0.001) (Figure 5(c) and (d)).
Figure 5.
Effect of Quin and Pro treatment on NADPH oxidase in aorta (a), superoxide dismutase (SOD) in aorta (b), H2O2 plasma concentration (c), and malonyl dialdehyde (MDA) plasma concentration (d). Data are expressed as mean ± SEM. *p<0.05, **p <0.01 vs Veh; ##p<0.01, # # # p<0.001 vs Pro; ^^p<0.01, ^^^p<0.001 vs Quin.
Animal mortality
Interestingly, Pro added to Quin caused the highest mortality (38%, 3/8 animals) in rats. The animals died 52±9 min after the start of the experiment. We also registered one death (13%, 1/8) in the Quin group in minute 45 of the experiment.
Discussion
We showed previously that in hypertensive patients treated with ACE-Is, propofol given for induction of general anesthesia significantly decreased BP. This observation may indicate the additive effect of ACE-Is and intravenous anesthetic, but precise mechanism of this interaction is not clarified.20 Moreover, there are some data indicating antithrombotic effect of ACE-Is and very few suggesting the effect of Pro on hemostasis. However, data concerning the effect of simultaneous administration of Pro and ACE-Is on hemostasis are still lacking.
In the current study, the effects of Quin and Pro on hemostasis and hemodynamics were evaluated. We performed experiments in the model of renovascular hypertensive rats (2K1C). This model of hypertension is mediated by the increased activities of both systemic and tissues (heart, aorta, lung, and kidney) rennin–angiotensin–aldosterone system (RAAS) and is recommended for the evaluation of chemicals affecting RAAS such as ACE-Is, AT1-As or RAAS peptides.42,43 Bearing in mind that the arterial thrombosis is a dynamic and a very complex process we used a wide panel of tests evaluating both the role of platelets, as well as coagulation and fibrinolysis to find the mechanism(s) of Quin and Pro action in hemostasis in the arterial model of thrombosis.
Previously, we proved the antithrombotic activity of ACE-Is in venous and arterial thrombosis in normotensive rats and demonstrated the involvement of NO and PGI2 in this phenomenon.1–3 Presently, we observed the antithrombotic activity and hypotensive effect of Quin in renovascular hypertensive rats, developing hypertension on the basis of temporary over-activation of the RAAS accompanied by harmful biochemical changes and structural damages to the endothelium and vascular wall, involving changes in the activity of various enzymes, inflammation as well as platelet activation.44 Thus, the mechanisms involved seem to be at least partially different to those in normotensive rats.
Our results demonstrate that Quin was effective in decreasing platelet adhesion to collagen ex vivo. According to literature data, in Quin-treated animals platelet activation could be inhibited by NO, PGI2 as well as by CYP2C9-dependent endothelium-derived hyperpolarizing factor (EDHF).45–48 Interestingly, in our Quin-treated rats, the plasma NO level and aortic iNOS expression were reduced, but aortic eNOS expression was unchanged. Although it was documented in experimental and clinical studies that ACE-Is increase expression of eNOS consequently leading to NO bioavailability augmentation, we think that the antithrombotic and antiplatelet effect of Quin observed in our hypertensive rats is mediated via downregulated iNOS and oxidative stress reduction, than by eNOS up-regulation and NO bioavailability increase. Moreover, data concerning the regulation of eNOS expression in hypertensive rats are not consistent, since decreased, increased and unchanged eNOS expression have been reported.49–53 Therefore, the NO bioavailability in hypertension may not be directly affected by eNOS expression. Chou et al. showed that in spontaneously hypertensive rats (SHR) the activity and expression of eNOS is reduced, whereas the expression of iNOS is markedly increased (due to increased oxidative stress), thus the sum of NO production may be unchanged or even enhanced.54 The increased expression of iNOS in SHR was paralleled by augmented NO2/NO3 plasma level. Therefore, the higher basal NO2/NO3 levels in SHR were most likely mediated by iNOS rather than by eNOS. What is more, the exaggerated iNOS expression was attenuated with 2 weeks treatment of Quin. The authors suggested that the mechanism by which Quin reduced iNOS expression might be associated with the reduction of superoxide production. Similarly, Bachetti et al. reported that Quin downregulated iNOS with a possible TNF-α-mediated mechanism in normotensive rats.55 Our previous studies showed that the antithrombotic effect of Quin is not fully dependent on eNOS activity in hypertensive rats, while in normotensive rats this dependence seems to be the predominant mechanism of the NO level increase.1,3 Therefore, in our study NO seemed not to be the leading vasorelaxant factor during chronic Quin treatment. Similarly to EDHF-dependent platelet inhibition after ACE-I treatment, literature data confirm the involvement of EDHF-dependent relaxation in the improvement of endothelial function. Moreover, as expected, the prolongation of bleeding time was not significant since vasorelaxation and BP lowering after Quin led to bleeding time shortening due to decreased blood flow, but not due to the inhibition of thrombosis. The observed here Quin-induced reduction in aortic iNOS expression was accompanied by the reduction of superoxide production. Quin administration to 2K1C rats decreased the level of MDA in plasma and NADPH oxidase activity in aortic rings. It seems that the antioxidant function of Quin is modified through lowering Ang II formation or promoting Ang-(1-7) production, which reduces the increase in ROS and phosphorylation of c-Src kinase by Ang II in intact endothelial cells.56–58 This thesis is supported by our previous results in HUVECs, where oxidative stress and NO bioavailability reduction was observed after Quin.28
Our observations also support the concept that antithrombotic properties of Quin depend on its fibrinolytic activity, including a significant decrease in PAI-1 concentration and function and an increase in euglobulin clot lysis time in the mechanisms dependent on the inhibition of Ang II synthesis, as well as a Bk-dependent increase in t-PA concentration, which is a potent stimulus in the human and animal vasculature.2,59–65
For the first time, the influence of Pro on the coagulation process in hypertensive rats was evaluated. Pro alone did not affect thrombus weight. However, it decreased occlusion time as well as incidents of persistent vascular patency, probably by its antifibrinolytic and prooxidative action.
In the present study, Pro alone did not significantly influence platelet adhesion to collagen ex vivo, although it significantly increased the expression of aortic iNOS and NO metabolite levels in plasma. On the other hand, De La Cruz et al. showed that both erythrocytes and leukocytes increased the anti-aggregatory effect of Pro in the mechanism dependent on leukocyte production of NO.27 In our study, platelet adhesion was assessed separately in the platelet-poor plasma deprived of leukocytes and RBC. Therefore, in the absence of leukocyte-secreted NO, Pro did not affect platelet adhesion. On the other hand, Chung et al. observed, that Pro in concentrations required for sedation and general anesthesia had no inhibitory effect on platelet aggregation after 3 h of incubation.23
We used Pro in hypertensive rats with intensified oxidative stress. In face of increase in NADPH oxidase activity, NO as well may be converted into superoxide anions, e.g. peroxynitrite (ONOO−).66,67 Taken together, the upregulation in aortic iNOS expression, with the increase of NO plasma level may be a source of ROS, which is in line with H2O2 and MDA increase after Pro treatment. Pro also increased SOD activity in aortic rings and H2O2 production. On the other hand, higher SOD activity catalyzed dismutation of strongly toxic superoxide anion to less toxic H2O2. Thus, it seems that the rise in SOD activity protects NO from degradation by superoxide anion and increases the concentration of H2O2, which is believed to be a member of EDHF.68
For the first time we proved that Pro has a strong antifibrinolytic activity in the animal model ex vivo. This effect may considerably contribute to the increased incidence of thrombus formation despite elevated NO bioavailability after Pro. On the other hand, intensification of oxidative stress by Pro resulting in endothelial dysfunction may lead to further impairment of the fibrinolytic potential. This thesis is supported by our previous results in HUVEC cultures, where t-PA reduction, PAI-1, oxidative stress, and NO synthesis potentiation also occurred after Pro.28
The dose of Pro used in our study reached plasma concentration that allows rat anesthesia (2.8 µg/mL) and is clinically relevant, since it has been estimated that plasma concentration in humans ranges 2–10 µg/mL.20,69 Pro significantly reduced SBP and DBP. In our opinion, this is a combined effect of myocardial depression (SBP-dependent), decreased sympathetic activity, direct endothelium-derived vascular relaxation (DBP-dependent), activation of the kinin–kallikrein system, and increased NO bioavailability.18,70–72
We observed that the beneficial effect of Quin on thrombus weight was abolished after Pro infusion, but it was not higher than in the Veh group. This can be explained by an unfavorable influence of Pro on the mechanisms involved in thrombus formation (fibrinolysis and oxidative stress), and a cumulative antihypertensive effect of both drugs. Pro also decreased the number of rats with patent flow as compared to the Quin pre-treated animals, although this effect was still weaker than the one caused by Pro alone. Surprisingly, the two drugs administered jointly had a stronger inhibitory effect on platelet adhesion than Quin alone. The mechanism that potentiates platelet adhesion in the presence of both drugs should be further investigated. There was also no change in BT after Quin and Pro in comparison to Veh. We can explain this strong hypotension and vasodilatation after both drugs, because according to our observations these hemodynamic changes slowed down blood flow, which resulted in BT shortening.
The important finding in our study was an increase in animal mortality (by 38%) in the group treated with both drugs. Previously, we and others observed more episodes of hypotension in patients treated with Pro after therapy with ACE-Is that may be associated with the endothelium-derived EDHF release and additional NO production after Pro administration.20,70 Higher mortality of animals after Pro may be also the result of ventilation impairment with a considerable effect on central chemoreceptor sensitivity.73 Also a significant fall in hemoglobin concentration and hematocrit was observed 30 min after induction of anesthesia in patients who received increasing doses of Pro.74,75 Summing up, it seems that in our model the final effects of Quin, Pro and both drugs given together on arterial thrombosis comprise the influence of drugs on fibrinolysis, primary hemostasis and oxidative stress as well as systemic hemodynamic parameters.
Conclusion
The data presented here may offer an additional explanation of Quin antithrombotic activity in renal-hypertensive rats. Our study demonstrates beneficial effects of Quin on platelet adhesion and fibrinolysis. Pro alone did not influence the thrombotic process, although it showed strong antifibrinolytic and prooxidative actions. Interestingly, pretreatment with Quin decreased the unfavorable effects of Pro in hemostasis, but caused hemodynamic disorders.
Footnotes
Perspectives: Despite the fact that patients receiving both ACE-Is and Pro may suffer from hypotension, ACE-Is prevent hemostasis disorders caused by Pro. Considering all these problems, we suggest that the most beneficial action is not to eliminate the dose of Quin before surgery but to prevent the occurrence of intraoperative hypotension.
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research projects of the Medical University of Bialystok (grant numbers 143-26731 F and 143-26732 F).
References
- 1. Chabielska E, Pawlak R, Golatowski J, et al. The antithrombotic effect of captopril and losartan on experimental arterial thrombosis in rats. J Physiol Pharmacol 1998; 49: 251–260. [PubMed] [Google Scholar]
- 2. Wojewodzka-Zelezniakowicz M, Chabielska E, Mogielnicki A, et al. Antithrombotic effect of tissue and plasma type angiotensin converting enzyme inhibitors in experimental thrombosis in rats. J Physiol Pharmacol 2006; 57: 231–245. [PubMed] [Google Scholar]
- 3. Pawlak R, Chabielska E, Golatowski J, et al. Nitric oxide and prostacyclin are involved in antithrombotic activity of captopril in venous thrombosis in rats. Thromb Haemost 1998; 79: 1208–1212. [PubMed] [Google Scholar]
- 4. Chabielska E, Mogielnicki A, Kramkowski K, et al. Antithrombotic effect of captopril and enalapril in old rats. Pharmacol Rep 2005; 57: 135–137. [PubMed] [Google Scholar]
- 5. Kramkowski K, Mogielnicki A, Buczko W. The physiological significance of the alternative pathways of angiotensin II production. J Physiol Pharmacol 2006; 57: 529–539. [PubMed] [Google Scholar]
- 6. Buczko W, Kramkowski K, Mogielnicki A. Are the endothelial mechanisms of ACE-Is already established? Pharmacol Rep 2006; 58: 126–131. [PubMed] [Google Scholar]
- 7. Chlopicki S, Gryglewski RJ. Angiotensin converting enzyme (ACE) and HydroxyMethylGlutaryl-CoA (HMG-CoA) reductase inhibitors in the forefront of pharmacology of endothelium. Pharmacol Rep 2005; 57: 86–96. [PubMed] [Google Scholar]
- 8. Flores-Monroy J, Valencia-Hernández I, Martínez-Aguilar L. Ang (1-7) is a modulator of the vasoconstrictor actions of Ang I and Ang II. J Renin Angiotensin Aldosterone Syst 2015; 16: 254–259. [DOI] [PubMed] [Google Scholar]
- 9. Korbut RA, Madej J, Adamek-Guzik T, et al. Secretory dysfunction of vascular endothelium limits the effect of angiotensin converting enzyme inhibitor quinapril on aggregation of erythrocytes in experimental hypertension. J Physiol Pharmacol 2003; 54: 397–408. [PubMed] [Google Scholar]
- 10. Islim IF, Bareford D, Beevers DG. A single (investigator)-blind randomised control trial comparing the effects of quinapril and nifedipine on platelet function in patients with mild to moderate hypertension. Platelets 2001; 12: 274–288. [DOI] [PubMed] [Google Scholar]
- 11. Zurbano MJ, Anguera I, Heras M, et al. Captopril administration reduces thrombus formation and surface expression of platelet glycoprotein IIb/IIa in early postmyocardial infarction stage. Arterioscler Thromb Vasc Biol 1999; 19: 1791–1795. [DOI] [PubMed] [Google Scholar]
- 12. Mitsui T, Chishima S, Odawara A, et al. Imidapril, an angiotensin converting enzyme inhibitor, inhibits thrombosis via reduction in aortic plasminogen activator inhibitor type-1 levels in spontaneously hypertensive rats. Biol Pharm Bull 1999; 22: 863–865. [DOI] [PubMed] [Google Scholar]
- 13. Kubo-Inoue M, Egashira K, Usui M, et al. Long-term inhibition of nitric oxide synthesis increases arterial thrombogenicity in rat carotid artery. Am J Physiol Heart Circ Physiol 2002; 282: H1478–1484. [DOI] [PubMed] [Google Scholar]
- 14. Pitt B, O’Neill B, Feldman R, et al. The QUinapril Ischemic Event Trial (QUIET): evaluation of chronic ACE inhibitor therapy in patients with ischemic heart disease and preserved left ventricular function. Am J Cardiol 2001; 87: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 15. The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the Europa study). Lancet 2003; 362: 782–788. [DOI] [PubMed] [Google Scholar]
- 16. Tsikouris JP, Suarez JA, Meyerrose GE, et al. Questioning a class effect: does ACE inhibitor tissue penetration influence the degree of fibrinolytic balance alteration following an acute myocardial infarction? J Clin Pharmacol 2004; 44: 150–157. [DOI] [PubMed] [Google Scholar]
- 17. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The HOPE (Heart Outcomes Prevention Evaluation) Study Investigators. N Engl J Med 2000; 342: 145–153. [DOI] [PubMed] [Google Scholar]
- 18. Petros AJ, Bogle RG, Pearson JD. Propofol stimulates nitric oxide release from cultured porcine aortic endothelial cells. Br J Pharmacol 1993; 109: 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gragasin FS, Bourque SL, Davidge ST. Propofol increases vascular relaxation in aging rats chronically treated with the angiotensin-converting enzyme inhibitor captopril. Anesth Analg 2013; 116: 775–783. [DOI] [PubMed] [Google Scholar]
- 20. Malinowska-Zaprzalka M, Wojewodzka M, Dryl D, et al. Hemodynamic effect of propofol in enalapril-treated hypertensive patients during induction of general anesthesia. Pharmacol Rep 2005; 57: 675–678. [PubMed] [Google Scholar]
- 21. Nakane M, Iwama H. A potential mechanism of propofol-induced pain on injection based on studies using nafamostat mesilate. Br J Anaesth 1999; 83: 397–404. [DOI] [PubMed] [Google Scholar]
- 22. Lawton BK, Brown NJ, Reilly CS, et al. Role of L-type calcium channels in altered microvascular responses to propofol in hypertension. Br J Anaesth 2012; 108: 929–935. [DOI] [PubMed] [Google Scholar]
- 23. Chung HG, Myung SA, Son HS, et al. In vitro effect of clinical propofol concentrations on platelet aggregation. Artif Organs 2013; 37: 51–55. [DOI] [PubMed] [Google Scholar]
- 24. Aoki H, Mizobe T, Nozuchi S, et al. In vivo and in vitro studies of the inhibitory effect of propofol on human platelet aggregation. Anesthesiology 1998; 88: 362–370. [DOI] [PubMed] [Google Scholar]
- 25. Zhu M, Ding J, Jiang H, et al. Propofol ameliorates endothelial inflammation induced by hypoxia/reoxygenation in human umbilical vein endothelial cells: role of phosphatase A2. Vascul Pharmacol 2015; 73: 149–157. [DOI] [PubMed] [Google Scholar]
- 26. Gokcinar D, Ergin V, Cumaoglu A, et al. Effects of ketamine, propofol, and ketofol on proinflammatory cytokines and markers of oxidative stress in a rat model of endotoxemia-induced acute lung injury. Acta Biochim Pol 2013; 60: 451–456. [PubMed] [Google Scholar]
- 27. De La Cruz JP, Páez MV, Carmona JA, et al. Antiplatelet effect of the anaesthetic drug propofol: influence of red blood cells and leucocytes. Br J Pharmacol 1999; 128: 1538–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wojewodzka M, Kisiel W, Stankiewicz A, et al. Potentation of experimental arteria thrombosis after quinapril and propofol treatment. In: XVIII symposium section of experimental cardiology Polish Cardiac Society, Szczyrk, Poland, 7–9 November 2013, abstract book, p. 35. [Google Scholar]
- 29. Cavalca V, Colli S, Veglia F, et al. Anesthetic propofol enhances plasma gamma-tocopherol levels in patients undergoing cardiac surgery. Anesthesiology 2008; 108: 988–997. [DOI] [PubMed] [Google Scholar]
- 30. Trapani A, Laquintana V, Lopedota A, et al. Evaluation of new propofol aqueous solutions for intravenous anesthesia. Int J Pharm 2004; 278: 91–98. [DOI] [PubMed] [Google Scholar]
- 31. Reddy KR, Chandramouli BA, Rao GS. Acute venous thrombosis caused by lipid-free propofol. Anaesthesia 2006; 61: 300–301. [DOI] [PubMed] [Google Scholar]
- 32. Dubey PK, Kumar A. Vascular complication following lipid free propofol injection. J Postgrad Med 2005; 51: 73–74. [PubMed] [Google Scholar]
- 33. Giles AR. Guidelines for the use of animals in biomedical research. Thromb Haemost 1987; 58: 1078–1084. [PubMed] [Google Scholar]
- 34. Kaminska M, Mogielnicki A, Stankiewicz A, et al. Angiotensin II via AT1 receptor accelerates arterial thrombosis in renovascular hypertensive rats. J Physiol Pharmacol 2005; 56: 571–585. [PubMed] [Google Scholar]
- 35. Ikeda K, Nara Y, Yamori Y. Indirect systolic and mean blood pressure determination by a new tail cuff method in spontaneously hypertensive rats. Lab Animals 1991; 25: 26–29. [DOI] [PubMed] [Google Scholar]
- 36. Schumacher WA, Steinbacher TE, Heran CL, et al. Effects of antithrombotic drugs in a rat model of aspirin-insensitive arterial thrombosis. Thromb Haemost 1993; 69: 509–514. [PubMed] [Google Scholar]
- 37. Guarini S. A highly reproducible model of arterial thrombosis in rats. J Pharmacol Toxicol Methods 1996; 35: 101–105. [DOI] [PubMed] [Google Scholar]
- 38. Dejana E, Villa S, de Gaetano G. Bleeding time in rats: A comparison of different experimental conditions. Thromb Haemost 1982; 48: 108–111. [PubMed] [Google Scholar]
- 39. Mant MJ. Platelet adherence to collagen: a simple, reproducible, quantitative method for its measurement. Thromb Res 1977; 11: 729–737. [DOI] [PubMed] [Google Scholar]
- 40. Gromotowicz A, Szemraj J, Stankiewicz A, et al. Study of the mechanisms of aldosterone prothrombotic effect in rats. J Renin Angiotensin Aldosterone Syst 2011; 12: 430–439. [DOI] [PubMed] [Google Scholar]
- 41. Winer J, Jung CK, Shackel I, et al. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 1999; 15: 41–49. [DOI] [PubMed] [Google Scholar]
- 42. Morishita R, Higaki J, Okunishi H, et al. Role of tissue renin angiotensin system in two-kidney, one-clip hypertensive rats. Am J Physiol 1993; 264: 510–514. [DOI] [PubMed] [Google Scholar]
- 43. Pinto YM, Paul M, Ganten D. Lessons from rat models of hypertension: from Goldblatt to genetic engineering. Cardiovasc Res 1998; 39: 77–88. [DOI] [PubMed] [Google Scholar]
- 44. Huang WC, Ploth DW, Bell PD, et al. Bilateral renal function responses to converting enzyme inhibitor (SQ 20,881) in two-kidney, one clip Goldblatt hypertensive rats. Hypertension 1981; 3: 285–293. [DOI] [PubMed] [Google Scholar]
- 45. Moncada S, Higgs EA, Vane JR. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet 1977; 1: 18–20. [DOI] [PubMed] [Google Scholar]
- 46. Bassenge E. Antiplatelet effects of endothelium-derived relaxing factor and nitric oxide donors. Eur Heart J 1991; 12: 12–15. [DOI] [PubMed] [Google Scholar]
- 47. Krotz F, Riexinger T, Buerkle MA, et al. Membrane potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol 2004; 24: 595–600. [DOI] [PubMed] [Google Scholar]
- 48. Mahaut-Smith MP. Calcium-activated potassium channels in human platelets. J Physiol 1995; 484: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kerr S, Brosnan MJ, McIntyre M, et al. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension 1999; 33: 1353–1358. [DOI] [PubMed] [Google Scholar]
- 50. Kimoto-Kinoshita S, Nishida S, Tomura TT. Decrease of endothelial nitric oxide synthase in stroke-prone spontaneously hypertensive rat cerebral cortex. Neurosci Lett 2000; 288: 103–106. [DOI] [PubMed] [Google Scholar]
- 51. Tanabe A, Naruse M, Seki T, et al. Gene expression of endothelin-1 and endothelial-type nitric oxide synthase in cardiovascular tissues of stroke-prone spontaneously hypertensive rats/Izm: effects of the angiotensin-converting enzyme inhibitor aracepril. J Cardiovasc Pharmacol 1998; 31(Suppl. 1): S395–S398. [DOI] [PubMed] [Google Scholar]
- 52. Umemoto S, Tanaka M, Kawahara S, et al. Calcium antagonist reduces oxidative stress by upregulating Cu/Zn superoxide dismutase in stroke-prone spontaneously hypertensive rats. Hypertens Res 2004; 27: 877–885. [DOI] [PubMed] [Google Scholar]
- 53. Takai S, Kirimura K, Jin D, et al. Significance of angiotensin II receptor blocker lipophilicities and their protective effect against vascular remodeling. Hypertens Res 2005; 28: 593–600. [DOI] [PubMed] [Google Scholar]
- 54. Chou TC, Yen MH, Li CY, et al. Alterations of nitric oxide synthase expression with aging and hypertension in rats. Hypertension 1998; 31: 643–648. [DOI] [PubMed] [Google Scholar]
- 55. Bachetti T, Comini L, Pasini E, et al. Ace-inhibition with quinapril modulates the nitric oxide pathway in normotensive rats. J Mol Cell Cardiol 2001; 33: 395–403. [DOI] [PubMed] [Google Scholar]
- 56. Kobayashi N, Honda T, Yoshida K, et al. Critical role of bradykinin-eNOS and oxidative stress-LOX-1 pathway in cardiovascular remodeling under chronic angiotensin-converting enzyme inhibition. Atherosclerosis 2006; 187: 92–100. [DOI] [PubMed] [Google Scholar]
- 57. Moinuddin G, Inamdar MN, Kulkarni KS, et al. Modulation of hemodynamics, endogenous antioxidant enzymes, and pathophysiological changes by angiotensin-converting enzyme inhibitors in pressure-overload rats. Hellenic J Cardiol 2011; 52: 216–226. [PubMed] [Google Scholar]
- 58. Sampaio WO, Henrique de CC, Santos RA, et al. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 2007; 50: 1093–1098. [DOI] [PubMed] [Google Scholar]
- 59. Fogari R, Zoppi A, Mugellini A, et al. Role of angiotensin II in plasma PAI-1 changes induced by imidapril or candesartan in hypertensive patients with metabolic syndrome. Hypertens Res 2011; 34: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 60. Katoh M, Egashira K, Mitsui T, et al. Angiotensin-converting enzyme inhibitor prevents plasminogen activator inhibitor-1 expression in a rat model with cardiovascular remodeling induced by chronic inhibition of nitric oxide synthesis. J Mol Cell Cardiol 2000; 32: 73–83. [DOI] [PubMed] [Google Scholar]
- 61. Mitsui T, Chishima S, Odawara A, et al. Imidapril, an angiotensin converting enzyme inhibitor, inhibits thrombosis via reduction in aortic plasminogen activator inhibitor type -1 levels in spontaneously hypertensive rats. Biol Pharm Bull 1999; 22: 863–865. [DOI] [PubMed] [Google Scholar]
- 62. Rajzer M, Wojciechowska W, Kawecka-Jaszcz K, et al. Plasma fibrin clot properties in arterial hypertension and their modification by antihypertensive medication. Thromb Res 2012; 130: 99–103. [DOI] [PubMed] [Google Scholar]
- 63. Brown NJ, Vaughan DE. Effect of activation and inhibition of the renin angiotensin system on plasma PAI-1 in humans. Hypertension 1998; 32: 965–971. [DOI] [PubMed] [Google Scholar]
- 64. Moriyama Y, Ogawa H, Oshima S, et al. Captopril reduced plasminogen activator inhibitor activity in patients with acute myocardial infarction. Jpn Circ J 1997; 61: 308–314. [DOI] [PubMed] [Google Scholar]
- 65. Labinjoh C, Newby DE, Pellegrini MP, et al. Potentiation of bradykinin-induced tissue plasminogen activator release by angiotensin-converting enzyme inhibition. J Am Coll Cardiol 2001; 38: 1402–1408. [DOI] [PubMed] [Google Scholar]
- 66. Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol 1986; 250: 822–827. [DOI] [PubMed] [Google Scholar]
- 67. Rajagopalan S, Kurz S, Munzel T. Angiotensin II-mediated hypertension in the rat increased vascular superoxide production via membrane NADH/NAGPH oxidase activation, contribution to alternation of vasomotor tone. J Clin Invest 1996; 97: 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Matoba T, Shimokawa H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Pharmacol Sci, 2003; 92: 1–6. [DOI] [PubMed] [Google Scholar]
- 69. Kirkpatrick T, Cockshott ID, Douglas EJ, et al. Pharmacokinetics of propofol (Diprivan) in elderly patients. Br J Anaesth 1988; 60: 146–150. [DOI] [PubMed] [Google Scholar]
- 70. Weisenberg M, Sessler DI, Tavdi M, et al. Dose-dependent hemodynamic effects of propofol induction following brotizolam premedication in hypertensive patients taking angiotensin-converting enzyme inhibitors. J Clin Anesth 2010; 22: 190–195. [DOI] [PubMed] [Google Scholar]
- 71. Samain E, Clichet A, Bouillier H, et al. Propofol differently alters vascular reactivity in normotensive and hypertensive rats. Clin Exp Pharmacol Physiol 2002; 29: 1015–1017. [DOI] [PubMed] [Google Scholar]
- 72. Wang Y, Zhou H, Wu B, et al. PKC isoforms distinctly regulate propofol-induced endothelium-dependent and endothelium-independent vasodilation. J Cardiovasc Pharmacol 2015; 66: 276–284. [DOI] [PubMed] [Google Scholar]
- 73. Goodman NW, Dow AC. Effects of active and passive sighs in normoxia and hyperoxia on the breathing of patients anaesthetized with infusions of propofol. Br J Anaesth 1993; 70: 536–541. [DOI] [PubMed] [Google Scholar]
- 74. Kassam SI, Lu C, Buckley N, et al. The mechanisms of propofol-induced vascular relaxation and modulation by perivascular adipose tissue and endothelium. Anesth Analg 2011; 112: 1339–1345. [DOI] [PubMed] [Google Scholar]
- 75. Bharti N, Chari P, Kumar P. Effect of sevoflurane versus propofol-based anesthesia on the hemodynamic response and recovery characteristics in patients undergoing microlaryngeal surgery. Saudi J Anaesth 2012; 6: 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]