Abstract
Objective
To evaluate the effect of prenatal exposure to selective serotonin reuptake inhibitors (SSRIs) on children’s behavioral, emotional, and social development by age 5 years, and over time since age 1.5 years.
Method
The prospective Norwegian Mother and Child Cohort Study was linked to the Medical Birth Registry of Norway. We included women who reported depressive/anxiety disorders before and/or during pregnancy. Children born to women who used SSRIs in early (weeks 0−16), mid- (weeks 17−28), or late (> week 29) pregnancy were compared to those who were unexposed. Children’s internalizing and externalizing behaviors (Child Behavior Checklist) and temperament traits (Emotionality, Activity and Shyness Temperament Questionnaire) were measured at 1.5, 3, and 5 years. Mean scores were calculated and standardized. General linear marginal structural models were fitted to account for time-varying exposure and confounders, and censoring; 3-level growth-curve models were used.
Results
A total of 8,359 mother–child dyads were included, and 4,128 children had complete outcome data at age 5 years. Children exposed to SSRIs in late pregnancy had an increased risk of anxious/depressed behaviors by age 5 years compared with unexposed children (adjusted β = 0.50, 95% CI = 0.04, 0.96). Such risk was not evident for earlier timings of exposure. There was no evidence for a substantial prenatal SSRI effect on externalizing, social, and emotional problems.
Conclusion
These findings suggest no substantial increased risk for externalizing, emotional, or social problems in preschool-aged children following prenatal SSRI exposure. Although the role of chance and potential unmeasured confounding cannot be ruled out, late-pregnancy SSRI exposure was associated with greater anxious/depressed behaviors in the offspring.
Key words: SSRI antidepressants, pregnancy, child behavior, social development, Norwegian Mother and Child Cohort Study (MoBa)
Antidepressants, mainly selective serotonin reuptake inhibitors (SSRIs), are often required to treat psychiatric disorders in pregnancy,1 with estimates of use ranging from 1% to 4% in Europe to up to 8% in the United States.2, 3 However, SSRIs cross the placenta and the blood−brain barrier and may interfere with fetal brain maturation by altering the serotonin signaling system.4, 5
It has been suggested that children prenatally exposed to SSRIs have greater internalizing and depressive-anxious behaviors at pre-school and early school age than those who are unexposed,6, 7, 8, 9 but not a more clinically problematic temperament.10 Two recent studies11, 12 have shown that at early adolescence, children prenatally exposed to SSRIs had a 25% and 84% increased hazard of receiving a diagnosis for any psychiatric disorder or depression, respectively, compared to children born to women who discontinued SSRIs before pregnancy. Findings on internalizing and externalizing behaviors are, however, inconsistent,6, 7, 8, 9, 13, 14, 15, 16 and the extent to which prenatal SSRI exposure may affect childhood-limited or persistent behavioral disorders, not least of all attention-deficit/hyperactivity disorder (ADHD) or autism spectrum disorder, in the offspring remains unresolved to date.11, 17, 18, 19, 20, 21
Because emotional and behavioral problems in early and late childhood are linked to later-life psychiatric diagnoses and poor social adjustment,22 a better understanding of the role of modifiable risk factors, such as antidepressant exposure in pregnancy on these outcomes, is crucial. Although SSRI treatment may vary during gestation, no previous study has so far explored time-dependent effects on children’s behavioral and social development, and it is not yet understood whether early or late SSRI exposures confer differential long-term risks.
Here we aimed to do the following: to explore the time-varying effect of prenatal SSRI exposure on child behavioral, emotional, and social development by age 5 years, accounting for time-dependent severity of depressive and anxiety symptoms and co-medication, and loss to follow-up; and to examine children’s developmental trajectories from age 1.5 to 5 years according to prenatal SSRI exposure status.
Method
Study Population and Data Collection
This study is based on the Norwegian Mother and Child Cohort Study (MoBa) and on records in the Medical Birth Registry of Norway (MBRN). MoBa is a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health.23 Pregnant women were recruited from all over Norway in 1999 to 2008 through a postal invitation in connection with a publicly offered routine ultrasound at 17 to 18 weeks of gestation. Data were gathered prospectively via 2 prenatal (Q1, Q3) and 4 postnatal (Q4−Q7) self-administered questionnaires (Figure 1).24 Fathers-to-be also completed one prenatal questionnaire. The current study is based on version 9 of the quality-assured data files released for research. The cohort now includes 114,500 children (41,600 by age 5 years), 95,200 mothers, and 77,300 fathers.23 The participation rate for all invited pregnancies is 41%. Of those agreeing to participate, the response rate was 92% to 95% for Q3 and Q1, and 77% for Q5.25 Women with unfavorable baseline characteristics (e.g., not in a relationship, low education level) and poorer mental health (e.g., more severe depressive symptoms) were more likely to be lost to follow-up in MoBa. This study obtained a license from the Norwegian Data Inspectorate and approval from the Regional Committee for Medical Research Ethics. All participants gave written informed consent before participation.
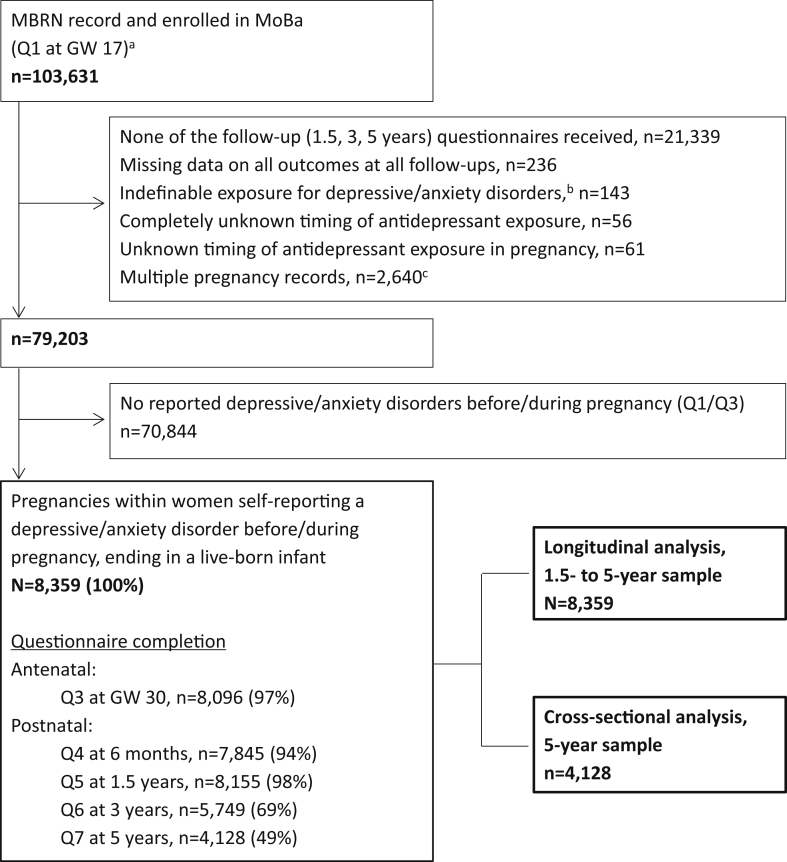
Figure 1.
Flow chart of steps to achieve the 1.5- to 5-year and the 5-year samples
Note:Conditions of exclusion may overlap. GW = gestational week; MBRN = Medical Birth Registry of Norway; Q = questionnaire.aQ1 is the first Norwegian Mother and Child Cohort Study (MoBa) questionnaire completed at gestational week 17; its completion implies enrollment in the study. About 10,000 pregnancies with an MBRN record did not complete Q1.bWomen who did not report any medication for treatment of depression/anxiety but checked the boxes related to timing of use of medication either before or during pregnancy.cIndicates 1,299 twin and 14 triplet pregnancies.
The MBRN is based on compulsory notification of all live births, stillbirths, and induced abortions.26 Data from MoBa were linked to the MBRN via the women’s personal identification numbers. Figure 1 outlines the exclusion criteria to achieve the 1.5- to 5-year and the 5-year samples.
Depressive and Anxiety Disorders
In MoBa Q1 and Q3, women were given a list of previous/concurrent illnesses, including specifically depression, anxiety, or other mental disorders (hereafter, depressive/anxiety disorders).24 To emulate the design and to conceptualize a hypothetical randomized clinical trial using observational data,27, 28, 29 this study included pregnancies within women reporting depressive/anxiety disorders before and/or during pregnancy.
Severity of Depressive and Anxiety Symptoms
The severity of maternal depressive and anxiety symptoms was measured at 2 time points in pregnancy via the short versions of The Hopkins Symptom Checklist−25 (SCL-25), namely, the 5-item (SCL-5) and 8-item (SCL-8) scales at weeks 17 and 30, respectively. These symptoms were also measured postnatally at all follow-up points.30 More information on the SCL is outlined in Supplement 1, available online.
SSRI Exposure
Information on SSRI exposure and indication for use was collected from Q1 (weeks 0−13+) and Q3 (week 13−29+).24 Women reported the name of the medication taken and timing of use in 4-week intervals throughout pregnancy. Drug classification was based on the Anatomical Therapeutic Chemical (ATC) Classification System.31 Exposure to SSRIs during pregnancy was defined as exposure to a drug belonging to the ATC group N06AB. Exposure to non-SSRIs was defined as exposure to any other antidepressant within the ATC group N06A. Co-use of SSRIs and non-SSRIs was also captured.
To reflect the temporal sequence between measurement of depressive symptoms and medication use, we defined the following time points of exposure (Figure S1, available online): early (weeks 0−16), mid- (weeks 17−28), and late (> week 29) pregnancy. Exposure at any time in pregnancy was also explored. Pregnancies exposed exclusively to non-SSRIs were grouped separately. Power analysis for the specific exposure windows is outlined in Table S1, available online.
Developmental Outcomes
Repeated assessment of children’s development was conducted at ages 1.5, 3, and 5 years by maternal report. The Child Behavior Checklist (CBCL) for preschool children (CBCL/1.5-5), a widely-used and validated measure of children’s behavior,32, 33 and the short form of the Emotionality, Activity, and Shyness Temperament Questionnaire (EAS), were used.34, 35 MoBa included selected CBCL items representing the main internalizing and externalizing domains and related subdomains (i.e., emotionally reactive, anxious/depressed, somatic complaints; attention and aggression) (Figure S2, available online).32 Mothers were asked to rate whether each item reflected their child’s behavior during the last 2 months, from never/rarely = 1 to very true/often true = 3. The Norwegian CBCL version has been shown to have satisfactory predictive validity in distinguishing between adolescents with and without psychiatric disorders (90th percentile cut-off, sensitivity: 71%, specificity: 92%).33
The EAS scale measured the temperament traits activity, emotionality, shyness, and sociability (Figure S2, available online).34 Mothers were asked to rate whether each item applied to their child’s behavior during the past 2 months, from not at all typical = 1 to very typical = 5. The internal consistency of the Norwegian version of the EAS was moderate, and the short EAS was highly correlated with the original instrument (correlation: 0.92−0.95).34 For both the CBCL and EAS, mean scores were calculated and standardized. Higher z scores indicated greater endorsement of each (sub)domain (e.g., more internalizing problems, more sociable).
Covariates
A sufficient set of confounding factors was identified with the aid of directed acyclic graphs.36 These were maternal body mass index (BMI), parity, maternal education and gross yearly income, marital status, folic acid use, smoking and alcohol use in pregnancy, illicit substance use, and paternal education (all ascertained in MoBa); co-medication in pregnancy with analgesics, anxiolytics and sedatives, antipsychotics, and non-SSRI antidepressants (Supplement 1, available online); severity of maternal depressive and anxiety symptoms in pregnancy as measured by the SCL-5/8; and lifetime history of major depression (LTH of MD), as measured in Q1 via 5 key depressive symptoms closely corresponding to the DSM-III criteria for lifetime major depression.37 Additional factors (e.g., child sex, breastfeeding, maternal postnatal mental health) were also taken into account under alternative model specifications (Table S2, available online). Information on missing values on covariates and the imputation procedure is provided in Supplement 1, available online.
Data Analysis
In the analysis at age 5 years, we fit marginal structural models (MSM) with 2 time points to account for (1) time-varying SSRI exposure; (2) time-varying confounders (i.e, depressive and anxiety symptoms in pregnancy, comedication with analgesics, anxiolytics, and sedatives), which are affected by prior SSRI treatment; and (3) loss to follow-up (Figure S1, available online).38, 39 We estimated the probability of SSRI treatment using a pooled logistic regression in which the outcome was current treatment with an SSRI in mid- or late pregnancy, and covariates were maternal baseline factors, time-varying and time-fixed confounders, and history of SSRI treatment in early pregnancy (model 1 in Table S2, available online). We also calculated the probability of remaining in the study (Table S3, available online), and then derived stabilized inverse probability of treatment weight (IPTW) and inverse probability of censoring weight (IPCW) for each pregnancy at each time point. The final stabilized weight was the product of the IPTW and IPCW. A generalized linear model with robust standard errors was fitted applying this final weight.
In the longitudinal analysis, we fitted 3-level (occasions of child’s assessment, pregnancy–child dyad, mother) growth curve models using full information maximum likelihood and an unstructured covariance, with a random intercept (levels 2 and 3), and a random slope (level 2).40 Time (i.e., child’s age in years) was scaled for gestational age and postnatal questionnaire completion date, and was modeled as continuous. Adjusted models included an interaction term between time and SSRI exposure, child’s age at baseline, and the sufficient set of confounders as fixed effects. For each exposure–outcome pair, we predicted and plotted the average adjusted standardized scores over time using the mimrgns package for Stata.
The crude and adjusted β coefficients with 95% confidence intervals represent the standardized mean difference in the developmental outcomes between children prenatally exposed to an SSRI and those unexposed to any antidepressant in the various time windows. Statistical significance was set to p < .05. All statistical analyses were performed using Stata version 14.
We used as a negative control pregnancies exposed to SSRIs in the 6-month period before pregnancy, but not during pregnancy (SSRI discontinuers). We examined the robustness of our findings in a set of sensitivity and exploratory subanalyses, as described in detail in Supplement 1, available online. To address the impact of unmeasured confounding, we applied probabilistic analysis using the bounding factor (Supplement 1, available online).41
Results
The study sample included 8,359 pregnancy–child dyads within 7,944 women (Figure 1). The women in our sample were more often disadvantaged (e.g., lower educational level, more LTH of MD) compared to the excluded group with no depressive/anxiety disorders (70,844/79,203). Baseline sociodemographic, lifestyle, and health characteristics of the 1.5- to 5-year sample (n = 8,359) and the 5-year sample (n = 4,128) are shown in Table 1 (SSRI) and Table S4 (non-SSRI; available online).
Table 1.
Characteristics of the 1.5- to 5-Year and the 5-Year Samples by Antidepressant Exposure During Pregnancy
| 1.5- to 5-Year Sample (n = 8,359)a Depressive/Anxiety Disorders |
5-Year Sample (n = 4,128)a Depressive/Anxiety Disorders |
|||
|---|---|---|---|---|
| Unexposed (n = 7,640) | SSRI Exposed (n = 605) | Unexposed (n = 3,775) | SSRI Exposed (n = 290) | |
| Maternal Characteristics | ||||
| Age (y), mean ± SD | 30.1 ± 4.9 | 30.3 ± 4.9 | 30.6 ± 4.8 | 30.8 ± 4.7 |
| BMI at conception, mean ± SD | 24.1 ± 4.5 | 24.4 ± 4.9 | 24.0 ± 4.3 | 24.3 ± 4.7 |
| Primiparous, n (%) | 3750 (49.1) | 331 (54.7) | 1944 (51.5) | 168 (57.9) |
| Married/cohabiting, n (%) | 7097 (92.9) | 532 (87.9) | 3523 (93.3) | 263 (90.7) |
| Educational level;b n (%) | ||||
| University/college | 4393 (57.5) | 332 (54.9) | 2451 (64.9) | 183 (63.1) |
| Lower than university/college | 3205 (41.9) | 273 (45.1) | 1303 (34.5) | 107 (36.9) |
| Gross yearly income;c n (%) | ||||
| Average | 5632 (73.7) | 460 (76.0) | 2624 (69.5) | 215 (74.1) |
| Low | 1134 (14.8) | 86 (14.2) | 652 (17.3) | 43 (14.8) |
| High | 636 (8.3) | 46 (7.6) | 399 (10.6) | 29 (10.0) |
| Smoking status at week 30, n (%) | ||||
| No | 5624 (73.6) | 396 (65.5) | 2967 (78.6) | 199 (68.6) |
| Yes | 809 (10.6) | 102 (16.9) | 298 (7.9) | 38 (13.1) |
| Stopped in pregnancy | 900 (11.8) | 83 (13.7) | 391 (10.4) | 43 (14.8) |
| Alcohol use in pregnancy, n (%) | ||||
| No/very limited use | 6387 (83.6) | 508 (84.0) | 3184 (84.3) | 246 (84.9) |
| Medium use | 922 (12.1) | 70 (11.6) | 455 (12.1) | 38 (13.1) |
| Weekly use | 109 (1.4) | 11 (1.8) | 60 (1.6) | 4 (1.4) |
| Folate intaked (yes), n (%) | 6318 (82.7) | 494 (81.7) | 3328 (88.2) | 248 (85.5) |
| LTH of MDe (yes), n (%) | 1701 (22.3) | 276 (45.6) | 900 (23.8) | 147 (50.7) |
| SCL-5 at GW 17, z-score ± SD | -0.03 ± 0.97 | 0.32 ± 1.18 | -0.04 ± 0.97 | 0.42 ± 1.24 |
| SCL-8 at GW 30, z-score ± SD | -0.03 ± 0.97 | 0.26 ± 1.19 | -0.03 ± 0.98 | 0.30 ± 1.18 |
| Comedication in pregnancy, n (%) | ||||
| Anxiolytics and sedatives | 204 (2.7) | 74 (12.2) | 84 (2.2) | 34 (11.7) |
| Antipsychotics | 118 (1.5) | 27 (4.5) | 60 (1.6) | 9 (3.1) |
| NSAIDs/analgesics | 4000 (52.4) | 344 (56.9) | 1990 (52.7) | 170 (58.6) |
| Antiepileptic drugs | 50 (0.7) | 16 (2.6) | 22 (0.6) | 7 (2.4) |
| Illicit substancesf | 140 (1.8) | 22 (3.6) | 62 (1.6) | 9 (3.1) |
| Child and Postpartum Characteristics | ||||
| Breastfeeding months up to child age of 6 months, mean ± SD | 5.3 ± 2.6 | 5.0 ± 2.8 | 5.5 ± 2.6 | 5.1 ± 2.8 |
| Infant sex (male), n (%) | 3923 (51.4) | 296 (48.9) | 1903 (50.4) | 143 (49.3) |
| Any malformation (yes), n (%) | 366 (4.8) | 30 (5.0) | 196 (5.2) | 13 (4.5) |
| Premature birth (yes), n (%) | 362 (4.7) | 33 (5.5) | 194 (5.1) | 18 (6.2) |
| Nursery/kindergarten attendance, n (%) | ||||
| Never between 1 and 5 years of age | 1987 (26.0) | 157 (26.0) | 587 (15.6) | 37 (12.8) |
| Any time between 1 and 5 years of age | 5200 (68.1) | 409 (67.6) | 2735 (72.5) | 214 (73.8) |
| Always between 1 and 5 years of age | 453 (5.9) | 39 (6.5) | 453 (12.0) | 39 (13.5) |
| Paternal Characteristics | ||||
| Educational level, n (%) | ||||
| University/college | 3396 (44.5) | 223 (36.9) | 1891 (50.1) | 116 (40.0) |
| Lower than university/college | 4151 (54.3) | 371 (61.3) | 1851 (49.0) | 172 (59.3) |
Note: Numbers may not add up to total due to missing values. Missing values ranged from 0.5% to 1% for maternal and paternal education, to 2% to 4% for lifetime history of major depression, alcohol use, smoking status in pregnancy, income, and body mass index (BMI) at conception. For the short version (5- and 8-item) of the Hopkins Symptom Checklist (SCL-5 and SCL-8), missing values were 5% and 12% (1- to 5-year sample) and 4% and 6% (5-year sample). NSAIDs = nonsteroidal anti-inflammatory drugs; SSRI = selective serotonin reuptake inhibitor.
Unexposed and SSRI exposed do not add up to total, as characteristics of non−SSRI-exposed participants are described in Table S4.
Includes ongoing or completed education.
Average = $14,800 to $49,900 USD; Low ≤$14,800 USD; High ≥$50,000 USD.
Use before and/or during first trimester.
Defined as Kendler's Lifetime Major Depression Scale score of ≥3 simultaneous depressive symptoms of duration of >2 weeks.
Indicates before and/or during pregnancy.
SSRIs were the most common antidepressant exposure in pregnancy (n = 290, 7.0%) in the 5-year sample, mainly as monotherapy. Depression and anxiety were the main indications for SSRI use (n = 281, 6.8%). Similar figures for the 1.5- and 3-year samples and non-SSRI monotherapy are presented in Table S5, available online. Table S6, available online, outlines the distribution of key variables in relation to missingness. Figure S3, available online, shows the severity of depressive and anxiety symptoms at week 17 and 30 by prenatal SSRI exposure.
In the analysis at age 5 years, children of mothers who used SSRI in late pregnancy had a significantly increased risk of anxious/depressed behaviors (adjusted β: = 0.50, 95% CI = 0.04, 0.95) compared to children who were unexposed (Table 2). There was no such association with other developmental outcomes, or following mid-pregnancy SSRI exposure. Tables S2, S3, and S7, available online, describe the main and alternative model specifications with corresponding weights, and the balance of covariates before and after weighting, respectively.
Table 2.
Effect of Time-Dependent Selective Serotonin Reuptake Inhibitor (SSRI) Exposure on Developmental Outcomes by Age 5 Years (N = 4,065)a
| Crude Modelsb β (95% CI) |
Weighted Modelsb,c β (95% CI) |
|
|---|---|---|
| CBCL | ||
| Internalizing Behaviors | ||
| SSRI, mid-pregnancy | 0.15 (–0.05, 0.34) | –0.14 (–0.76, 0.49) |
| SSRI, late pregnancy | 0.22 (0.01, 0.43)§ | 0.39 (–0.25, 1.02) |
| Emotionally reactive | ||
| SSRI, mid-pregnancy | –0.06 (–0.23, 0.11) | –0.02 (–0.54, 0.50) |
| SSRI, late pregnancy | –0.02 (–0.21, 0.18) | 0.05 (–0.50, 0.61) |
| Anxious/depressed | ||
| SSRI, mid-pregnancy | 0.18 (–0.05, 0.41) | –0.27 (–0.67, 0.13) |
| SSRI, late pregnancy | 0.27 (0.00, 0.53)§ | 0.50 (0.04, 0.95)‡ |
| Somatic complaints | ||
| SSRI, mid-pregnancy | 0.23 (0.02, 0.44)§ | 0.01 (–0.56, 0.58) |
| SSRI, late pregnancy | 0.24 (0.02, 0.47)§ | 0.27 (–0.30, 0.84) |
| Externalizing Behaviors | ||
| SSRI, mid-pregnancy | 0.07 (–0.11, 0.25) | –0.20 (–0.78, 0.38) |
| SSRI, late pregnancy | 0.10 (–0.09, 0.30) | 0.21 (–0.40, 0.82) |
| Attention problems | ||
| SSRI, mid-pregnancy | 0.08 (–0.11, 0.26) | –0.23 (–1.05, 0.59) |
| SSRI, late pregnancy | 0.10 (–0.10, 0.31) | 0.26 (–0.64, 1.17) |
| Aggressive behavior | ||
| SSRI, mid-pregnancy | 0.05 (–0.13, 0.23) | –0.08 (–0.44, 0.28) |
| SSRI, late pregnancy | 0.08 (–0.12, 0.27) | 0.07 (–0.30, 0.45) |
| EAS Traits | ||
| Activity | ||
| SSRI, mid-pregnancy | 0.06 (–0.13, 0.25) | –0.19 (–0.56, 0.18) |
| SSRI, late pregnancy | 0.07 (–0.14, 0.27) | 0.13 (–0.31, 0.57) |
| Emotionality | ||
| SSRI, mid-pregnancy | –0.08 (–0.27, 0.12) | –0.28 (–0.93, 0.37) |
| SSRI, late pregnancy | –0.05 (–0.26, 0.16) | 0.12 (–0.60, 0.85) |
| Shyness | ||
| SSRI, mid-pregnancy | 0.14 (–0.04, 0.33) | 0.30 (–0.29, 0.90) |
| SSRI, late pregnancy | 0.16 (–0.04, 0.36) | –0.08 (–0.79, 0.63) |
| Sociability | ||
| SSRI, mid-pregnancy | –0.17 (–0.37, 0.04) | –0.47 (–1.04, 0.10) |
| SSRI, late pregnancy | 0.16 (–0.38, 0.06) | 0.25 (–0.42, 0.92) |
Note: CBCL = Child Behavior Checklist; EAS = Emotionality, Activity and Shyness Temperament Questionnaire.
Pregnancies on non-SSRI monotherapy (n = 63) were excluded. The effect of SSRI exposure in early pregnancy cannot be estimated in the marginal structural models analysis due to the lack of measurement of depressive symptoms at baseline.
Reference: unexposed pregnancies in the corresponding time window.
Marginal structural models weighted with stabilized inverse probability of treatment and censoring weight.
p = .034; § .01 < p < .05.
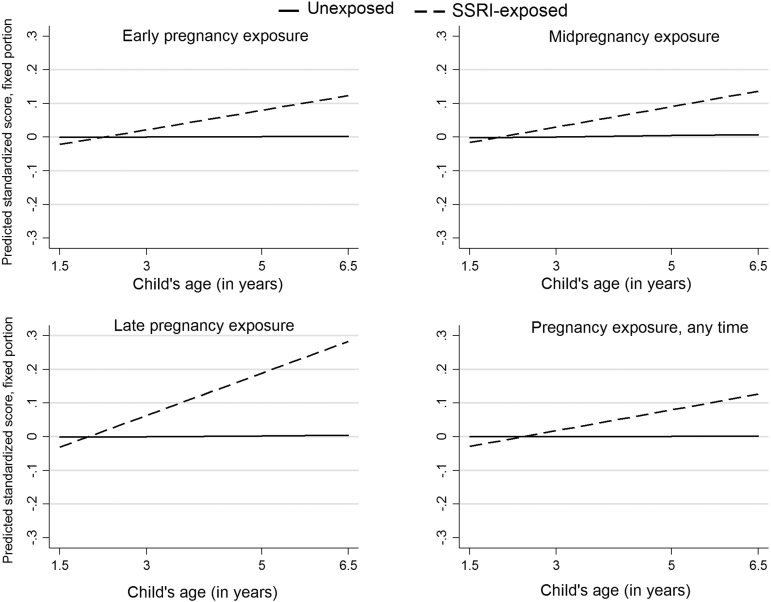
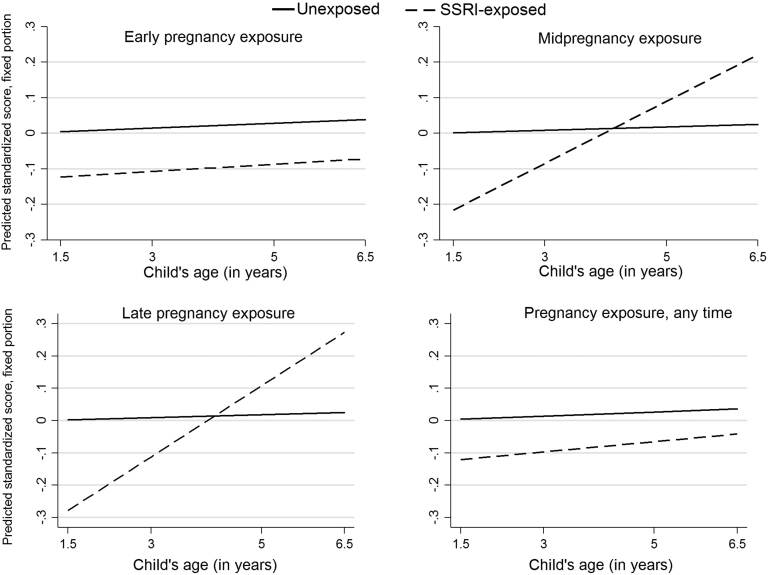
Behavioral, emotional, and social outcomes were on average assessed by age 1.6 (SD = 0.1), 3.1 (SD = 0.2) and 5.1 (SD = 0.3) years, with the highest age for Q7 completion at 6.5 years. Results of the longitudinal analysis (fixed and random effects) are presented in Tables S8 to S10, available online. For each 1-year increase in age, children exposed to SSRIs late in gestation had a 0.06 standardized effect increase on the CBCL anxious/depressed compared to unexposed children (interaction, adjusted β = 0.06, 95% CI = −0.00, 0.16; p = .058) (Figure 2), and similarly on the internalizing domain. At baseline, mid- or late in utero exposure to SSRI conferred a protective effect for attention (Figure 3), and thereby externalizing problems in the offspring; this effect attenuated as the children grew older. There was no interaction between prenatal SSRI at the various exposure windows and children’s emotional and social traits.
Figure 2.
Longitudinal trajectories on the Child Behavior Checklist (CBCL) anxious/depressed by timing of selective serotonin reuptake inhibitor (SSRI) exposurea,b
Note:aReference: unexposed pregnancies in the corresponding time window. bPredicted standardized score are estimated marginal mean standardized scores.
Figure 3.
Longitudinal trajectories on the Child Behavior Checklist (CBCL) attention by timing of selective serotonin reuptake inhibitor (SSRI) exposurea,b
Note:aReference: unexposed pregnancies in the corresponding time window. bPredicted standardized score are estimated marginal mean standardized scores.
We found no association between the SSRI discontinuer group and child CBCL outcomes (Supplement 1 and Table S11, available online). Results of the various sensitivity and subanalyses by mutually exclusive exposure groups, and by days of SSRI use, are presented in Supplement 1 and Figures S4 and S5, available online. Results of the longitudinal analyses including women with or without depressive/anxiety disorders are outlined in Figures S6 to S14, available online. The bounding factor analysis showed that an unmeasured confounder that was strongly associated (relative risk = ∼3.4−4.4) with SSRI in late pregnancy and anxious/depressed behaviors could completely explain away the observed association, but a weaker confounder could not.
Discussion
This study is the first to report the time-dependent effect of SSRI exposure on behavioral, emotional, and social outcomes in preschool children, and provides novel insights into the effect of this exposure on child-specific developmental trajectories since age 1.5 years. Although the role of chance and unmeasured confounding cannot be ruled out, we found children of mothers using SSRIs in late pregnancy to be at greater risk for anxious/depressed behavioral problems by age 5 years compared with unexposed children. Likewise, these problems seemed to increase as the children grew older following late-pregnancy SSRI exposure, and reached a substantial marginal effect only at age 5 years or more. There was no evidence for such an effect following early or mid-pregnancy exposure to SSRI. It is reassuring that prenatal SSRIs did not confer a substantial increased risk for greater externalizing behaviors in preschool-aged children or for more problematic temperament in terms of emotionality, sociability, activity, or shyness, and this was consistently evident across the various exposure windows.
The observed risk for anxious/depressed behaviors by age 5 years was of medium magnitude, corresponding to an odds ratio of 2.5,42 or translated into absolute terms, 8 children would be expected to have this behavioral problem for every 100 women treated with an SSRI in late gestation (assuming a 5% prevalence of the outcome among the unexposed).11, 43 Although some prior studies6, 8, 11, 12 accounting for maternal perinatal depression and/or familial confounders support this association, other studies13, 14, 15, 16 do not. Confounding by indication and disease severity are indeed of importance. Although the similarity in effect size between nonuse and use of SSRIs in pregnancy suggests that residual confounding by disease severity is small, this comparison could not be made in relation to late pregnancy. It is therefore possible that the severity of maternal underlying disorder partly explains our positive association.
Prior studies exploring the effect of duration and trimester of SSRI prenatal exposure on child outcomes have produced conflicting findings.12, 15, 20, 21 Timing and duration are clearly related, and we attempted to tease those effects apart by additionally exploring the SSRI effect by length and mutually exclusive windows of exposure. Our findings, coupled with those in animal models,44 may point to a plausible late-pregnancy fetal vulnerability to SSRIs and/or susceptibility to serotonin disruption. The third trimester of pregnancy is indeed a crucial time for fetal brain maturation. Alternative explanations are, however, possible: residual confounding by depression severity or stress at the very end of gestation,45, 46 the substantial genetic component of internalizing psychopathology,47 unmeasured confounding by postnatal environmental conditions such as poor parenting behavior,48, 49 or chance. Strong predictors of child psychopathology (e.g., poor parenting: odds ratio = 1.5−3.0)48, 49 and of SSRI late exposure could attenuate but not explain away our observed association. Nonetheless, there is the need to replicate this finding on late SSRI exposure and to explore its potential interplay with stressful early-life events such as poor neonatal adaptation.15, 50, 51
Prenatal SSRI exposure has not been shown to negatively affect externalizing or attention behaviors6, 7, 8, 11, 13, 18, 20, 52, 53 or temperament10 in children at various age stages independently of maternal mood disorders, which aligns with our findings at age 5 years. The literature on this topic is, however, conflicting, as other studies have identified a positive association, albeit of modest magnitude, with ADHD in the offspring.12, 17, 19 Some of these studies have cleverly addressed the bias posed by confounding by indication and/or environmental/genetic factors on this association,17, 20 yet the uptake of advanced methods38 to deal with time-varying exposures and confounders has been so far scarce.29 We found no evidence for a substantial time-dependent effect of prenatal SSRI exposure on externalizing behaviors and problematic temperament traits in preschool-aged children. In the longitudinal analysis, a protective effect of SSRI exposure in mid- or late pregnancy on externalizing and attention problems was evident at age 1.5 years, yet this effect switched in direction with the child’s increasing age. Given this trajectory, the association may be attributable to better maternal mental health in the first postpartum years following adequate pharmacotherapy during gestation, which in turn may affect reporting of externalizing psychopathology in the offspring.1, 54
A major strength of this study is that we accounted for maternal depressive/anxiety disorders, and measured their symptom severity at 2 time points in pregnancy via a validated instrument. Although the latter measurement cannot replace a clinical interview and is not designed to measure perinatal mood/anxiety specifically, it provides a reliable measure of the severity of these conditions.30, 55 We applied methods to deal with time-varying exposure and confounders, as well as study drop-out, and examined child-specific trajectories. We addressed attrition by including all cases with data at one or more of the outcome time-points and conducted multiple imputation. We carried out several sensitivity and subanalyses to explore the robustness of our findings, and explored the impact of unmeasured confounding and use of a negative control. However, residual confounding by depression severity, genetic, environmental, or familial factors cannot be ruled out.
Several limitations also need mentioning. Maternal depressive/anxiety disorders were self-reported; however, our study population was 8% of the initial data source, which equals estimates of these disorders based on clinical diagnosis.56 The early-pregnancy SSRI effect could not be estimated in the weighted analysis. Exposure misclassification could be an additional concern. Use of antidepressants was self-reported; however, the potential for exposure misclassification is lower for chronic than for short-term medications.57 Also, most women self-reporting SSRI use in MoBa did fill prescriptions for these medications.58 Information on dosage is not available in MoBa. The outcome measures on child development were parent-reported; although the risk of outcome misclassification cannot be ruled out, this was probably nondifferential. The number of CBCL items in the analysis by age 5 years was greater than in the longitudinal analysis. The MoBa study has a low response rate (41%), with a possible self-selection of the healthiest women.25 Its potential for bias has been thoroughly explored by comparing MoBa with the total Norwegian birthing population,59 and although the prevalence estimates could not necessarily be generalized, the measures of associations tested were valid in MoBa. Although we accounted for study drop-out, selection bias may have affected our results. Our small sample size precluded analyses of mutually exclusive SSRI exposure windows, non-SSRI antidepressants, or individual SSRIs, as well as sibling-control designs.
To conclude, children born to women having depressive/anxiety disorders and treated with SSRIs late in pregnancy had an increased risk of anxious/depressed behavioral problems at preschool age, compared with children of women with depressive/anxiety disorders who did not use SSRIs. This association has to be confirmed or refuted by future research, but at present may provide some insights into potentially important periods of fetal vulnerability to SSRI exposure. This potential risk needs to be balanced against a potentially detrimental effect of untreated maternal depression. There was no evidence for such an effect in relation to earlier SSRI exposure, or in relation to externalizing, social, and emotional problems; this information may assist clinicians when evaluating the risk of treatment with SSRIs at specific timing during gestation.
Acknowledgments
The authors are grateful to all the participating families in Norway who take part in this ongoing cohort study.
Footnotes
The Norwegian Mother and Child Cohort Study are supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no. N01-ES-75558), and NIH/NINDS (grant no. 1 UO1 NS 047537-01 and grant no. 2 UO1 NS 047537-06A1). This project and A.L.’s postdoctoral research fellowship are funded through the Hedvig Nordeng’s European Research Council (ERC) Starting Grant “DrugsInPregnancy” (grant no. 678033).
Dr. Wood served as the statistical expert for this research.
Disclosure: Drs. Lupattelli, Wood, Ystrom, Skurtveit, Handal, and Nordeng report no biomedical financial interests or potential conflicts of interest.
Supplemental Material
References
- 1.Hendrick V. Humana Press; Totowa, NJ: 2006. Psychiatric Disorders in Pregnancy and the Postpartum: Principles and Treatment. [Google Scholar]
- 2.Charlton R.A., Jordan S., Pierini A. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: a population-based study in six European regions. Br J Obstet Gynaecol. 2015;122:1010–1020. doi: 10.1111/1471-0528.13143. [DOI] [PubMed] [Google Scholar]
- 3.Huybrechts K.F., Palmsten K., Mogun H. National trends in antidepressant medication treatment among publicly insured pregnant women. Gen Hosp Psychiatry. 2013;35:265–271. doi: 10.1016/j.genhosppsych.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaspar P., Cases O., Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 5.Heikkine T., Ekblad U., Laine K. Transplacental transfer of citalopram, fluoxetine and their primary demethylated metabolites in isolated perfused human placenta. BJOG. 2002;109:1003–1008. doi: 10.1111/j.1471-0528.2002.01467.x. [DOI] [PubMed] [Google Scholar]
- 6.Brandlistuen R.E., Ystrom E., Eberhard-Gran M., Nulman I., Koren G., Nordeng H. Behavioural effects of fetal antidepressant exposure in a Norwegian cohort of discordant siblings. Int J Epidemiol. 2015;44:1397–1407. doi: 10.1093/ije/dyv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberlander T., Papsdorf M., Brain U., Misri S., Ross C., Grunau R. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164:444–451. doi: 10.1001/archpediatrics.2010.51. [DOI] [PubMed] [Google Scholar]
- 8.Hanley G., Brain U., Oberlander T. Prenatal exposure to serotonin reuptake inhibitor antidepressants and childhood behavior. Pediatr Res. 2015;78:174–180. doi: 10.1038/pr.2015.77. [DOI] [PubMed] [Google Scholar]
- 9.Hanley G.E., Brain U., Oberlander T.F. Infant developmental outcomes following prenatal exposure to antidepressants, and maternal depressed mood and positive affect. Early Hum Dev. 2013;89:519–524. doi: 10.1016/j.earlhumdev.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Nulman I., Rovet J., Stewart D.E. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336:258–262. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- 11.Malm H., Brown A.S., Gissler M. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry. 2016;55:359–366. doi: 10.1016/j.jaac.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Agerbo E, Ingstrup KG, et al. Antidepressant use during pregnancy and psychiatric disorders in offspring: Danish nationwide register based cohort study. [published online ahead of print September 2017] BMJ. https://doi.org/10.1136/bmj.j3668. [DOI] [PMC free article] [PubMed]
- 13.Grzeskowiak L.E., Morrison J.L., Henriksen T.B. Prenatal antidepressant exposure and child behavioural outcomes at 7 years of age: a study within the Danish National Birth Cohort. Br J Obste Gynaecol. 2016;123:1919–1928. doi: 10.1111/1471-0528.13611. [DOI] [PubMed] [Google Scholar]
- 14.Misri S., Reebye P., Kendrick K. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry. 2006;163:1026–1032. doi: 10.1176/ajp.2006.163.6.1026. [DOI] [PubMed] [Google Scholar]
- 15.Nulman I., Koren G., Rovet J. Neurodevelopment of children following prenatal exposure to venlafaxine, selective serotonin reuptake inhibitors, or untreated maternal depression. Am J Psychiatry. 2012;169:1165–1174. doi: 10.1176/appi.ajp.2012.11111721. [DOI] [PubMed] [Google Scholar]
- 16.El Marroun H., White T., Verhulst F.C., Tiemeier H. Maternal use of antidepressant or anxiolytic medication during pregnancy and childhood neurodevelopmental outcomes: a systematic review. Eur Child Adolesc Psychiatry. 2014;23:973–992. doi: 10.1007/s00787-014-0558-3. [DOI] [PubMed] [Google Scholar]
- 17.Man K.K.C., Chan E.W., Ip P. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:2350. doi: 10.1136/bmj.j2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro V.M., Kong S.W., Clements C.C. Absence of evidence for increase in risk for autism or attention-deficit hyperactivity disorder following antidepressant exposure during pregnancy: a replication study. Translat Psychiatry. 2016;6 doi: 10.1038/tp.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clements C.C., Castro V.M., Blumenthal S.R. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol Psychiatry. 2015;20:727–734. doi: 10.1038/mp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sujan A.C., Rickert M.E., Oberg A.S. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA. 2017;317:1553–1562. doi: 10.1001/jama.2017.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mezzacappa A., Lasica P.A., Gianfagna F. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: a systematic review and meta-analysis. JAMA Pediatrics. 2017;171:555–563. doi: 10.1001/jamapediatrics.2017.0124. [DOI] [PubMed] [Google Scholar]
- 22.Rutter M., Kim-Cohen J., Maughan B. Continuities and discontinuities in psychopathology between childhood and adult life. J Child Psychol Psychiatry. 2006;47:276–295. doi: 10.1111/j.1469-7610.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 23.Magnus P., Birke C., Vejrup K. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2016;45:382–388. doi: 10.1093/ije/dyw029. [DOI] [PubMed] [Google Scholar]
- 24.Norwegian Institute of Public Health. The Nowegian Mother and Child Cohort Study. Questionnaires. Norwegian Institute of Public Health. Available at: https://fhi.no/en/studies/norwegian-mother-and-child-cohort-study/for-participants-articles/questionnaires-from-moba/. Accessed August 5, 2016.
- 25.Magnus P., Irgens L.M., Haug K. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 26.Norwegian Institute of Public Health. Medical Birth Registry of Norway (MBRN). http://www.fhi.no/eway/default.aspx?pid=240&trg=Main_6664&Main_6664=6898:0:25, 7840:1:0:0:::0:0. Accessed March 11, 2015.
- 27.Hernán A.M., Alonso B.A., Logan C.R. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toh S., Manson J. An analytic framework for aligning observational and randomized trial data: application to postmenopausal hormone therapy and coronary heart disease. J Int Chinese Stat Assoc. 2013;5:344–360. doi: 10.1007/s12561-012-9073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood ME, Lapane KL, van Gelder M, Rai D, Nordeng HME. Making fair comparisons in pregnancy medication safety studies: an overview of advanced methods for confounding control [published online ahead of print October 2017]. Pharmacoepidemiol Drug Saf. https://doi.org/10.1136/bmj.j3668. [DOI] [PMC free article] [PubMed]
- 30.Strand B.H., Dalgard O.S., Tambs K., Rognerud M. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36) Nord J Psychiatry. 2003;57:113–118. doi: 10.1080/08039480310000932. [DOI] [PubMed] [Google Scholar]
- 31.WHO Collaborating Centre for Drugs Statistics Methodology. ATC/DDD index 2012. Available at: http://www.whocc.no/atc_ddd_index/. Accessed March 17, 2012.
- 32.Achenbach T. University of Vermont Department of Psychiatry; Burlington, VT: 1992. Manual for the Child Behavior Checklist/2-3 and 1992 Profile. [Google Scholar]
- 33.Novik T.S. Validity of the Child Behaviour Checklist in a Norwegian sample. Eur Child Adolesc Psychiatry. 1999;8:247–254. doi: 10.1007/s007870050098. [DOI] [PubMed] [Google Scholar]
- 34.Mathiesen K.S., Tambs K. The EAS temperament questionnaire—factor structure, age trends, reliability, and stability in a Norwegian sample. J Child Psychol Psychiatry. 1999;40:431–439. [PubMed] [Google Scholar]
- 35.Buss A.H., Plomin R. Erlbaum; Hillsdale, NJ: 1984. Temperament: early developing personality traits. [Google Scholar]
- 36.Textor J., Hardt J., Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 37.Kendler K.S., Neale M.C., Kessler R.C., Heath A.C., Eaves L.J. The lifetime history of major depression in women. Reliability of diagnosis and heritability. Arch Gen Psychiatry. 1993;50:863–870. doi: 10.1001/archpsyc.1993.01820230054003. [DOI] [PubMed] [Google Scholar]
- 38.Robins J.M., Hernan M.A., Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Hernan M.A., Brumback B., Robins J.M. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Rabe-Hesketh S., Skrondal A. 3rd ed. Stata Press; College Station, TX: 2012. Multilevel and Longitudinal Modeling Using Stata: Vol. 1: Continuous Responses. [Google Scholar]
- 41.Ding P., VanderWeele T.J. Sensitivity analysis without assumptions. Epidemiology. 2016;27:368–377. doi: 10.1097/EDE.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenblad A. In: Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R., editors. Vol 77. Wiley; Oxford, UK: 2009. pp. 478–479. (Introduction to Meta-Analysis). [Google Scholar]
- 43.Magnusson K. Interpreting Cohen's d effect size—an interactive visualization. Available at: http://rpsychologist.com/d3/cohend/. Accessed May 20, 2017.
- 44.Ansorge M.S., Zhou M., Lira A., Hen R., Gingrich J.A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 45.Barker E.D., Maughan B. Differentiating early-onset persistent versus childhood-limited conduct problem youth. Am J Psychiatry. 2009;166:900–908. doi: 10.1176/appi.ajp.2009.08121770. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor T.G., Heron J., Glover V. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adolesc Psychiatry. 2002;41:1470–1477. doi: 10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Fanous A., Gardner C.O., Prescott C.A., Cancro R., Kendler K.S. Neuroticism, major depression and gender: a population-based twin study. Psychol Med. 2002;32:719–728. doi: 10.1017/s003329170200541x. [DOI] [PubMed] [Google Scholar]
- 48.Bayer J.K., Sanson A.V., Hemphill S.A. Parent influences on early childhood internalizing difficulties. J Appl Dev Psychol. 2006;27:542–559. [Google Scholar]
- 49.Nomura Y., Wickramaratne P.J., Warner V., Mufson L., Weissman M.M. Family discord, parental depression, and psychopathology in offspring: ten-year follow-up. J Am Acad Child Adolesc Psychiatry. 2002;41:402–409. doi: 10.1097/00004583-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Klinger G., Frankenthal D., Merlob P. Long-term outcome following selective serotonin reuptake inhibitor induced neonatal abstinence syndrome. J Perinatol. 2011;31:615–620. doi: 10.1038/jp.2010.211. [DOI] [PubMed] [Google Scholar]
- 51.McEwen B.S. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oberlander T.F., Reebye P., Misri S., Papsdorf M., Kim J., Grunau R.E. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med. 2007;161:22–29. doi: 10.1001/archpedi.161.1.22. [DOI] [PubMed] [Google Scholar]
- 53.Hermansen T.K., Roysamb E., Augusti E.M., Melinder A. Behavior and inhibitory control in children with prenatal exposure to antidepressants and medically untreated depression. Psychopharmacology (Berl) 2016;233:1523–1535. doi: 10.1007/s00213-016-4248-3. [DOI] [PubMed] [Google Scholar]
- 54.Maoz H., Goldstein T., Goldstein B.I. The effects of parental mood on reports of their children's psychopathology. J Am Acad Child Adolesc Psychiatry. 2014;53:1111–1122. doi: 10.1016/j.jaac.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandanger I., Moum T., Ingebrigtsen G., Dalgard O.S., Sorensen T., Bruusgaard D. Concordance between symptom screening and diagnostic procedure: the Hopkins Symptom Checklist−25 and the Composite International Diagnostic Interview I. Soc Psychiatry Psychiatr Epidemiol. 1998;33:345–354. doi: 10.1007/s001270050064. [DOI] [PubMed] [Google Scholar]
- 56.Falah-Hassani K., Shiri R., Dennis C.L. The prevalence of antenatal and postnatal co-morbid anxiety and depression: a meta-analysis. Psychol Med. 2017;47:2041–2053. doi: 10.1017/S0033291717000617. [DOI] [PubMed] [Google Scholar]
- 57.van Gelder M.M., van Rooij I.A., de Walle H.E., Roeleveld N., Bakker M.K. Maternal recall of prescription medication use during pregnancy using a paper-based questionnaire: a validation study in the Netherlands. Drug Saf. 2013;36:43–54. doi: 10.1007/s40264-012-0004-8. [DOI] [PubMed] [Google Scholar]
- 58.Skurtveit S., Selmer R., Tverdal A., Furu K., Nystad W., Handal M. Drug exposure: inclusion of dispensed drugs before pregnancy may lead to underestimation of risk associations. J Clin Epidemiol. 2013;66:964–972. doi: 10.1016/j.jclinepi.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Nilsen R.M., Vollset S.E., Gjessing H.K. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.