Abstract
Introduction:
The effects of renin–angiotensin system blockade with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II type 1 receptor blockers (ARBs) on cancer remain inconsistent.
Methods:
We searched existing databases from 1960 to August 2015, for randomised controlled trials and observational studies (case–control studies and cohort studies) of ARB/ACEI therapy with a minimal one year of follow-up. Outcomes were incidence and mortality of cancer.
Results:
We included 14 randomised controlled trials and 17 observational studies of 3,957,725 participants (350,329 ARB/ACEI users). The users had a lower incidence of cancer in the observational studies (RR 0.82, 95% CI 0.73–0.93) but not in the randomised controlled trials (RR 1.00, 95% CI 0.92–1.08). The protection persisted for lung cancer (RR 0.85, 95% CI 0.75–0.97) but not for other sites of cancer. The relative risk of cancer associated with renin–angiotensin system blockade was reduced along with time of follow-up. Mortality reduction with ARB/ACEI was marginally significant in the observational studies (RR 0.71, 95% CI 0.55–0.93) but not in the randomised controlled trials (RR 0.99, 95% CI 0.89–1.09).
Conclusions:
The significant benefits of renin–angiotensin system blockade observed in case–control studies and cohort studies might diminish in randomised controlled trials. Clinical design, site of cancer and duration of follow-up may affect the clinical outcomes.
Keywords: Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, renin–angiotensin system, cancer, angiotensin-receptor blocker
Introduction
The renin–angiotensin system (RAS) is a key therapeutic target for diabetes mellitus, chronic kidney disorders, hypertension, heart problems, chronic obstructive pulmonary disease and stroke. RAS blockers include angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II type 1 receptor blockers (ARBs). The impacts of RAS blockade on the incidence and mortality of cancer remain debated. Some studies suggest that the use of ACEIs and ARBs may increase the risk of cancer.1 A meta-analysis showed an increased risk of cancer by ARBs compared with controlled therapy.2 Intriguingly, the US Food and Drug Administration claims no increase in the risk of cancer with ARBs.3
Angiotensin II and angiotensin II type 1 receptors (AT1) play major roles in the development and progression of cancer.4,5 Angiotensin II acts on the AT1 receptor to promote cell proliferation and angiogenesis.6–8 The expression of AT1 receptors has been reported to be upregulated in hyperplasic and cancer tissues.9,10 ACEIs prevent the generation of angiotensin II by inhibiting angiotensin-converting enzymes (ACEs) while ARBs selectively block angiotensin II binding to the AT1 receptor. These actions may have important implications for cancer development. However, the existing clinical evidence is inconsistent.2,11,12 Therefore we conducted a systematic review and meta-analysis to evaluate the impact of an RAS blockade with ACEI/ARB therapy on the risk of cancer and death.
Materials and methods
Search strategy
Candidate studies were identified through electronic literature searches of PubMed, Cochrane Library databases, Chinese National Knowledge Infrastructure (CNKI) and Wanfang databases from 1960 to August 2015. We used the following MeSH terms and keywords: ‘cancer’, ‘carcinoma’, ‘sarcoma’, ‘neoplasia’ or ‘malignancy’ in combination with ‘renin–angiotensin system’, ‘RAS’ and ‘angiotensin-receptor blocker’, ‘ARB’ or ‘angiotensin-converting enzyme inhibitor’, ‘ACEI’. A manual search of reference lists from reports of review articles, meta-analyses and original studies was performed to identify additional relevant studies.
Selection criteria
Our inclusion criteria were as follows: (a) clinical trials, including randomised controlled trials (RCTs), cohort studies and case–control studies; (b) use of ACEIs and/or ARBs in the participants; (c) incidence and/or mortality due to cancer as an outcome with detailed description of relative risk ratios (RRs), corresponding 95% confidence intervals (CIs), size of the baseline samples and years of follow-up; and (d) each study should have enrolled at least 200 participants. Literature meeting any of the following criteria was excluded: non-clinical nature, non-human studies, duplication, unclear outcome evaluation and non-original studies including reviews, letters, editorials and commentaries.
Data extraction
The extracted data included first author name, study title, year of publication date, country of origin, disease, demographic characteristics of participants, details of intervention, outcome measurements, intervention durations, incidence and mortality of cancer and RR for cancer with the corresponding 95% CI. All articles were read by two independent reviewers (JS and XZ) who extracted data from the articles according to a standardised data extraction form. Disagreements were resolved in all cases by discussion among our team members.
Quality assessment
The methodological quality of studies was assessed by the Newcastle–Ottawa scale (NOS). Using the NOS, a study is judged on three broad perspectives: the selection of the study groups, the comparability of the groups and the ascertainment of the outcome of interest.13 Studies with a score of less than 3 were considered as low quality, while scores of 4–6 were considered as moderate quality and 7–9 were considered as high quality. All studies were reviewed by two investigators (JS and X-NS). A third reviewer (H-LZ) served to resolve disputes.
Statistical analyses
This study is reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.14 Dichotomous outcome data from individual trials were analysed by using RR and corresponding 95% CI. Data were pooled using the random effects model or fixed effect model according to the heterogeneity between studies. Heterogeneity was assessed using the chi-square test, with values greater than 50% regarded as being indicative of moderate to high heterogeneity. For studies of moderate to high heterogeneity, a random effects meta-analysis model was used;15 otherwise, we used the fixed effects meta-analysis model.16 The possibility of publication bias was quantified using the Begg’s and Egger’s test.17,18 A two-tailed P>0.050 was considered to be no publication bias, followed by confirmation using the visual inspection of Begg funnel plots in which relative risks were plotted against their standard errors (SE). All the analyses were carried out using Stata 11.0SE statistical software package (StataCorp, College Station, TX, USA).
Results
Study description
Figure 1 shows the study selection process. A total of 31 studies met our inclusion criteria and involved 3,957,725 participants with hypertension, cardiomyopathy, vascular disease, breast cancer, colon cancer, lung cancer, melanoma, type 2 diabetes mellitus and glomerulonephritis. The age of the participants ranged from 18 to 80 years. All the studies were published between 1998 and 2014 (Table 1). There were 17 observational studies and 14 RCTs. Seven studies tested dual blockade with ARBs and ACEIs, 24 studies assessed ARB monotherapy and eight studies focused on ACEI monotherapy. The duration of follow-up ranged from 1.9 to 7.8 years. Sites (number) of the studies were as follows: America (13),19–31 England (5),11,32–35 Taiwan (4),12,36–38 Denmark (2),39,40 Sweden (2),41,42 Netherlands (1),43 Japan (1),44 Canada (1),45 Norway (1)46 and South Korea (1).47 Table 1 shows the characteristics of the 31 studies included in the meta-analysis.
Figure 1.
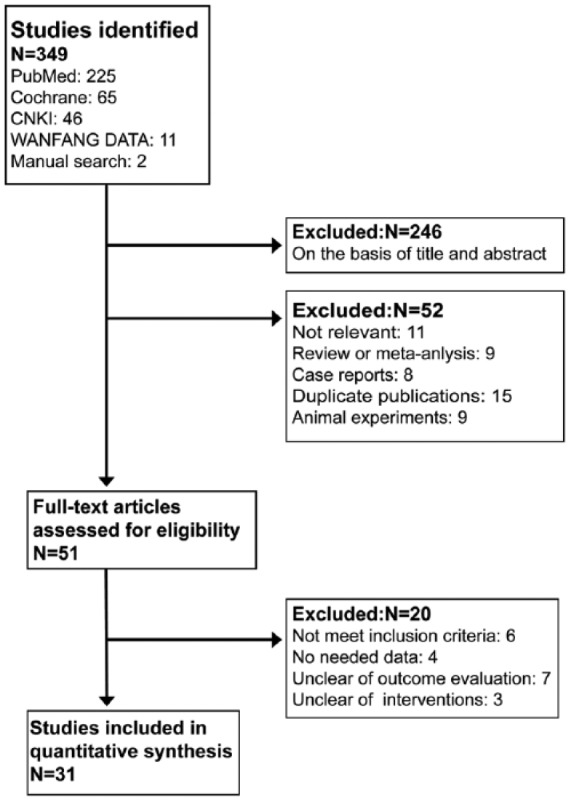
Flow chart of selection process in this study.
Table 1.
Summary of the characteristics of the included trials.
| First author, year | Study type | Country | Type of cancer | Age (year) | Participants | Intervention | Cancer outcome | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|
| Makar et al.,19 2014 | Observational studies | USA | Colorectal cancer | 70 | 31,086 | ARB, ACEI | Incidence | ⩾5.0 |
| Engineer et al.,22 2013 | Observational studies | USA | Colorectal cancer | 65 | 262 | ARB, ACEI | Mortality | 2.9 |
| Cardwell et al.,32 2014 | Observational studies | UK | Breast cancer | 65 | 16,920 | ARB, ACEI | Mortality | 3.9 |
| Colorectal cancer | 65 | 12,053 | ARB, ACEI | Mortality | 2.8 | |||
| Prostate cancer | 65 | 12,188 | ARB, ACEI | Mortality | 3.8 | |||
| Rao et al.,21 2013 | Observational studies | USA | Lung cancer | 62 | 1,228,960 | ARB | Incidence | 4.5 |
| Rao et al.,20 2013 | Observational studies | USA | Prostate cancer | 65 | 543,824 | ARB | Incidence | 6.0 |
| Koomen et al.,43 2009 | Observational studies | Netherlands | Melanoma | 67 | 6520 | ARB | Incidence | 2.7 |
| Chae et al.,23 2011 | Observational studies | USA | Breast cancer | 59 | 703 | ACEI | Incidence | 4.6 |
| Chiang et al.,36 2014 | Observational studies | Taiwan | Hypertension | 59 | 69,660 | ACEI | Incidence | 2.4 |
| Sugiura et al.,44 2012 | Randomised trials | Japan | Hypertension | 65 | 2049 | ARB | Incidence | 4.2 |
| Mortality | ||||||||
| Bhaskaran et al.,11 2012 | Observational studies | UK | Hypertension | 64 | 377,649 | ARB | Incidence | 4.6 |
| Julius et al.,26 2006 | Randomised trials | USA | Hypertension | 48 | 772 | ARB | Incidence | 3.6 |
| Julius et al.,27 2004 | Randomised trials | USA | Hypertension | 67 | 15,245 | ARB | Incidence | 4.2 |
| Huang et al.,12 2011 | Observational studies | Taiwan | Hypertension | 59 | 109,002 | ARB | Incidence | 5.7 |
| Dahlof et al.,41 2002 | Randomised trials | Sweden | Hypertension | 67 | 9193 | ARB | Incidence | 4.8 |
| Lindholm et al.,42 2001 | Randomised trials | Sweden | Hypertension | 76 | 6614 | ACEI | Incidence | 5.0 |
| Lewis et al.,29 2001 | Randomised trials | USA | Hypertension | 59 | 1715 | ARB | Incidence | 2.6 |
| Lever et al.,35 1998 | Observational studies | UK | Hypertension | 52 | 5207 | ACEI | Incidence | 6.6 |
| NAVIGATOR24 2010 | Randomised trials | USA | Cardiovascular disease | 64 | 9306 | ARB | Incidence | 5.0 |
| ONTARGET25 2008 | Randomised trials | USA | Cardiovascular disease | 66 | 23,994 | ARB | Incidence | 4.7 |
| ARB+ACEI | ||||||||
| TRANSCEND34, 2008 | Randomised trials | UK | Cardiovascular disease | 67 | 5926 | ARB | Incidence | 4.7 |
| Yusuf et al.,45 2008 | Randomised trials | Canada | Cardiovascular disease | 66 | 20,332 | ARB | Incidence | 2.5 |
| ARB+ACEI | ||||||||
| Massie et al.,31 2008 | Randomised trials | USA | Cardiovascular disease | 72 | 4128 | ARB | Incidence | 4.1 |
| ARB+ACEI | ||||||||
| Pfeffer et al.,28 2003 | Randomised trials | USA | Cardiovascular disease | 65 | 14,703 | ARB | Incidence | 2.1 |
| ARB+ACEI | ||||||||
| Dickstein et al.,46 2002 | Randomissed trials | Norway | Cardiovascular disease | 67 | 5477 | ARB | Mortality | 2.7 |
| Cohn et al.,30 2001 | Randomised trials | USA | Cardiovascular disease | 63 | 5010 | Incidence | 1.9 | |
| ARB+ACEI | ||||||||
| Chin et al.,47 2011 | Observational studies | South Korea | Glomerulonephritis | 41 | 3288 | ARB | Incidence | 2.5 |
| Mortality | ||||||||
| Chang et al.,38 2011 | Observational studies | Taiwan | Type 2 diabetes mellitus | 66 | 5104 | ARB | Incidence | 7.4 |
| Wang et al.,37 2013 | Observational studies | Taiwan | Breast, colon, lung, rectum cancer | 62 | 85,842 | ARB | Incidence | 4.8 |
| Azoulay et al.,33 2012 | Observational studies | UK | Breast, colon, lung, rectum cancer | 72 | 410,167 | ARB | Incidence | 6.4 |
| Hallas et al.,39 2012 | Observational studies | Denmark | Breast, colon, lung, rectum cancer | 69 | 597,668 | ARB, ACEI | Incidence | 7.8 |
| Pasternak et al.,40 2011 | Observational studies | Denmark | Breast, colon, lung, rectum cancer | 63 | 317,158 | ARB, ACEI | Incidence | 2.9 |
ARB: angiotensin II type 1 receptor blocker; ACEI: angiotensin-converting enzyme inhibitor.
Risk of bias within studies
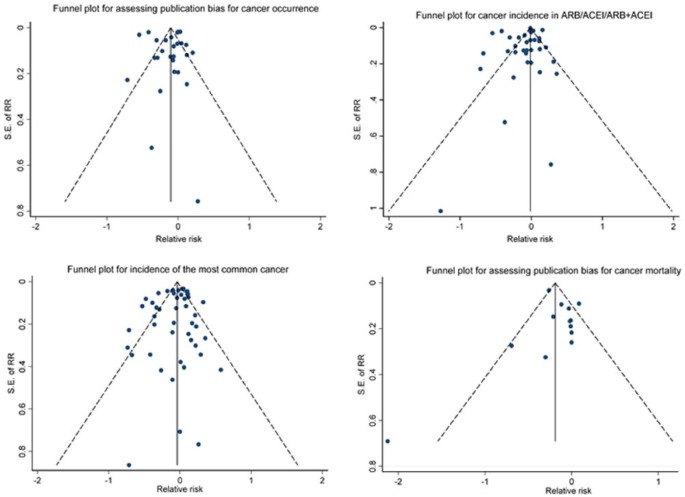
As shown in Table 2, the range in the total NOS score for the 31 studies was 6 to 9 (theoretical range 0 to 9) and the mean (SD) was 7.7 (0.77). No publication bias was evident by Begg’s test (P=0.160) and Egger’s test (P=0.790). Consistently, all the funnel plots were symmetric, indicating no publication bias (Figure 2).
Table 2.
Quality assessment of the 31 studies.
| Authors | Year | Selection score | Comparability score | Outcome score | Total score |
|---|---|---|---|---|---|
| Makar et al.19 | 2014 | 4 | 2 | 2 | 8 |
| Chiang et al.36 | 2014 | 4 | 2 | 2 | 8 |
| Cardwell et al.32 | 2014 | 3 | 2 | 2 | 7 |
| Rao et al.21 | 2013 | 3 | 2 | 3 | 8 |
| Rao et al.20 | 2013 | 3 | 2 | 3 | 8 |
| Wang et al.37 | 2013 | 4 | 1 | 2 | 7 |
| Engineer et al.22 | 2013 | 3 | 1 | 2 | 6 |
| Sugiura et al.44 | 2012 | 3 | 2 | 2 | 7 |
| Bhaskaran et al.11 | 2012 | 4 | 2 | 3 | 9 |
| Azoulay et al.33 | 2012 | 4 | 2 | 2 | 8 |
| Hallas et al.39 | 2012 | 4 | 0 | 2 | 6 |
| Pasternak et al.40 | 2011 | 4 | 2 | 2 | 8 |
| Chae et al.23 | 2011 | 3 | 2 | 2 | 7 |
| Huang et al.12 | 2011 | 4 | 2 | 3 | 9 |
| Chin et al.47 | 2011 | 3 | 2 | 2 | 7 |
| Chang et al.38 | 2011 | 4 | 2 | 3 | 9 |
| NAVIGATOR24 | 2010 | 4 | 2 | 2 | 8 |
| Koomen et al.43 | 2009 | 3 | 1 | 2 | 6 |
| ONTARGET25 | 2008 | 4 | 2 | 3 | 9 |
| TRANSCEND34 | 2008 | 4 | 2 | 3 | 9 |
| Yusuf et al.45 | 2008 | 4 | 2 | 2 | 8 |
| Massie et al.31 | 2008 | 4 | 2 | 3 | 9 |
| Julius et al.26 | 2006 | 4 | 2 | 2 | 8 |
| Julius et al.27 | 2004 | 4 | 2 | 2 | 8 |
| Pfeffer et al.28 | 2003 | 3 | 2 | 3 | 8 |
| Dahlof et al.41 | 2002 | 3 | 2 | 2 | 7 |
| Dickstein et al.46 | 2002 | 4 | 2 | 2 | 8 |
| Lindholm et al.42 | 2001 | 3 | 2 | 2 | 7 |
| Lewis et al.29 | 2001 | 4 | 1 | 2 | 7 |
| Cohn et al.30 | 2001 | 4 | 2 | 2 | 8 |
| Lever et al.35 | 1998 | 4 | 1 | 2 | 7 |
Figure 2.
Funnel plots for assessing publication bias.
Outcomes
RAS blockade on incidence of cancer
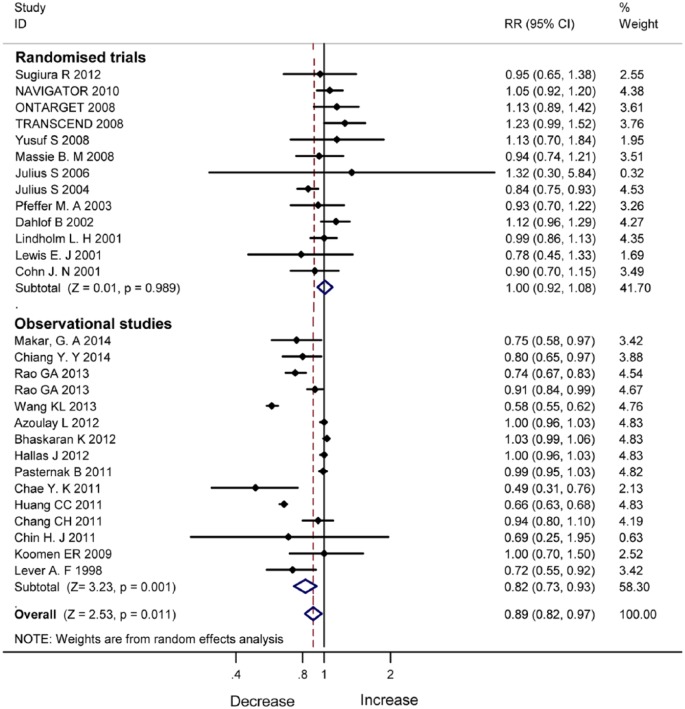
There were 28 studies reporting the incidence of cancer (Table 1). Figure 3 shows incidence reduction with ARB/ACEI in the observational studies (RR 0.82, 95% CI 0.73–0.93, P=0.001). In the RCTs, incidence reduction with ARB/ACEI was not significant (RR 1.00, 95% CI 0.92–1.08, P=0.989).
Figure 3.
Incidence reduction with angiotensin-converting enzyme inhibitor/angiotensin II type 1 receptor blocker therapy in randomised controlled trials and observational studies.
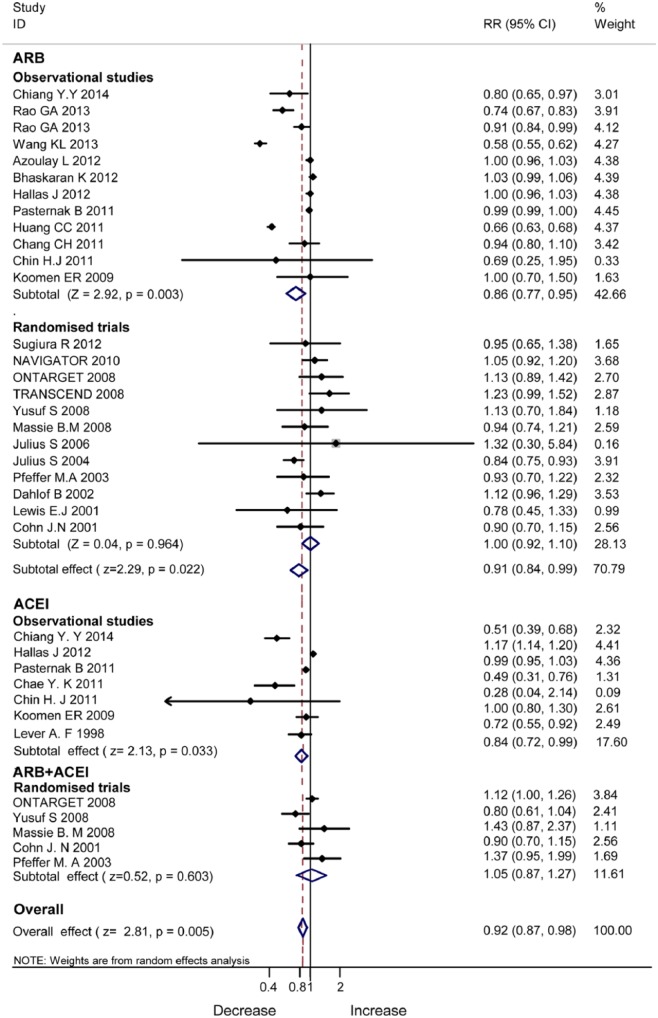
In subgroup analysis by RAS blockers, ACEIs (RR 0.84, 95% CI 0.72–0.99, P=0.033) and ARBs (RR 0.91, 95% CI 0.84–0.99, P=0.022) consistently lowered the incidence of cancer in the observational studies (Figure 4). In the RCTs, no benefits were evident by ARBs (RR 1.00, 95% CI 0.92–1.10, P=0.964) or by combined therapy of ARBs and ACEIs (RR 1.05, 95% CI 0.87–1.27, P=0.603).
Figure 4.
Subgroup analyses of monotherapy with angiotensin-converting enzyme inhibitor/angiotensin II type 1 receptor blocker therapy and dual renin–angiotensin system blockade.
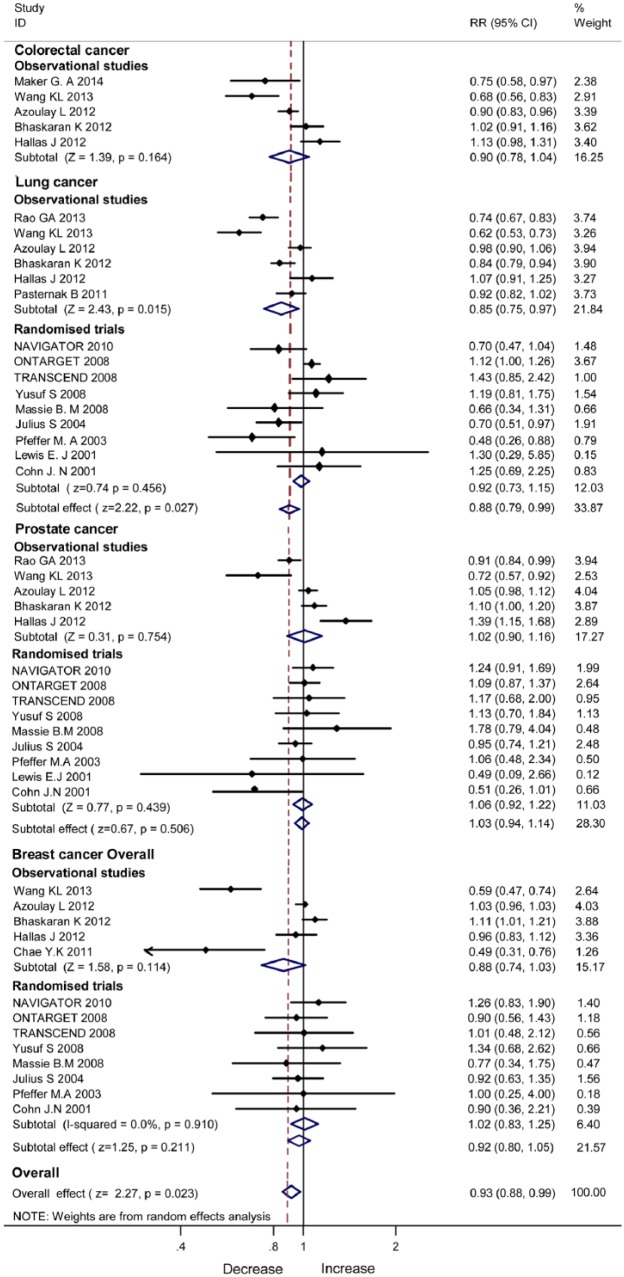
Figure 5 illustrates the pooled data of the observational studies and RCTs stratified by sites of cancer. The observational studies revealed significant incidence reduction with ARB/ACEI in lung cancer (RR 0.85, 95% CI 0.75–0.97, P=0.015) but not colorectal cancer (P=0.164), breast cancer (P=0.211) or prostate cancer (P=0.506). The RCTs showed no incidence reduction with ACEI/ARB in lung cancer, breast cancer or prostate cancer.
Figure 5.
Incidence reduction with angiotensin-converting enzyme inhibitor/angiotensin II type 1 receptor blocker therapy in site-specific cancer.
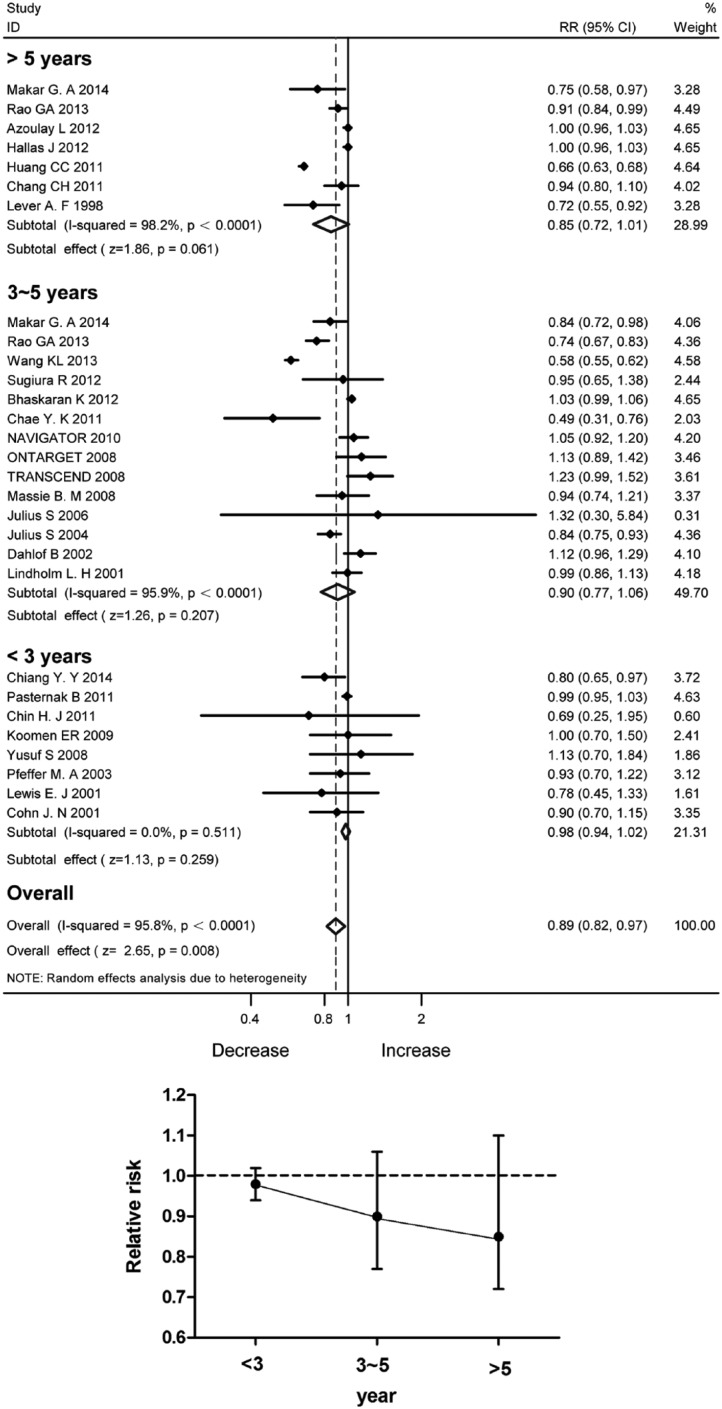
Although the pooled data of 28 studies in total disclosed significant incidence reduction with the RAS blockade (Figure 6(a)), the protective effects were not statistically significant with the duration of follow-up stratified into less than 3, 3–5 and over 5 years. Figure 6(b) demonstrates a trend of reduced RR along with the time of follow-up.
Figure 6.
Incidence reduction with angiotensin-converting enzyme inhibitor/angiotensin II type 1 receptor blocker therapy stratified by the duration of follow-up. (a) Forest plot; (b) Relative risk ratios.
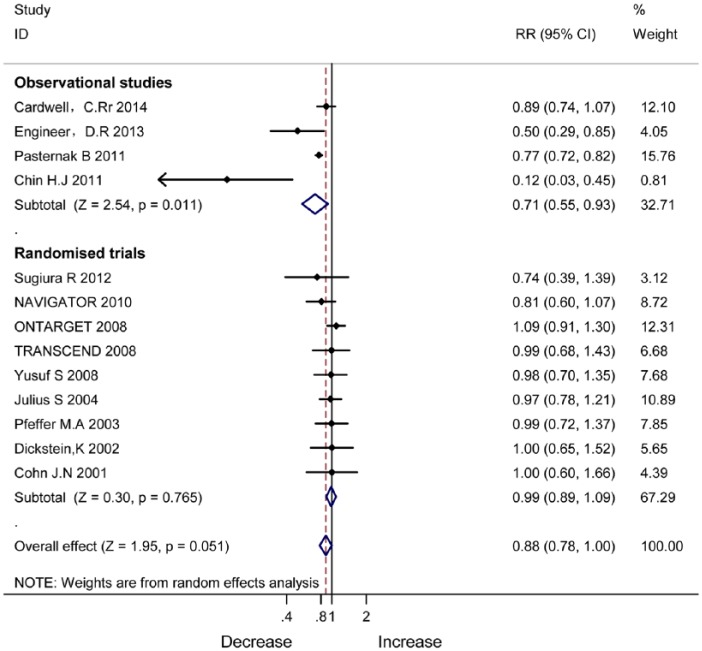
RAS blockade on mortality of cancer
Figure 7 shows mortality reduction with ARB/ACEI in the observational studies (RR 0.71, 95% CI 0.55–0.93, P=0.051). In the RCTs, mortality reduction was not significant (RR 0.99, 95% CI 0.89–1.09, P=0.765).
Figure 7.
Mortality reduction with angiotensin-converting enzyme inhibitor/angiotensin II type 1 receptor blocker therapy in observational studies and randomised controlled trials.
Differences in outcomes for cancer in observational studies and RCTs
Differences in the observational studies and RCTs included duration of follow-up and sample sizes (Table 3). The range of follow-up duration in observational studies was 2.4–7.8 years, compared to 1.9–5.0 years in RCTs. The mean duration of follow-up was 23% higher in observational studies than in RCTs. Moreover, the 17 observational studies had a sample size much larger than the 14 RCTs. The observational studies included 289,858 RAS blockade users and 3,833,261 participants, compared with 59,802 and 124,464, respectively, for the RCTs.
Table 3.
Differences in observational studies and randomised controlled trials (RCTs) included in this meta-analysis.
| Observational studies | RCTs | |
|---|---|---|
| Study included | 17 | 14 |
| Follow up (mean, years) | 4.59 (2.4–7.8) | 3.72 (1.9–5) |
| User | 289,858 | 59,802 |
| Participant | 3,833,261 | 124,464 |
| ACEI | 3 | 1 |
| ARB | 9 | 12 |
| ARB+ACEI | 0 | 5 |
| ARB or ACEI | 5 | 0 |
ARB: angiotensin II type 1 receptor blocker; ACEI: angiotensin-converting enzyme inhibitor.
Discussion
This meta-analysis reveals that the significant benefits of the RAS blockade observed in case–control studies and cohort studies might diminish in RCTs. Monotherapy with ACEI/ARB might have protective effects on the incidence and mortality of cancer in the pooled analysis of observational studies. The claimed therapeutic benefits in observational studies could not be validated in RCTs. The observed benefits of RAS blockade against the risk of cancer and death could largely result from non-randomised clinical design with a prolonged period of follow-up.
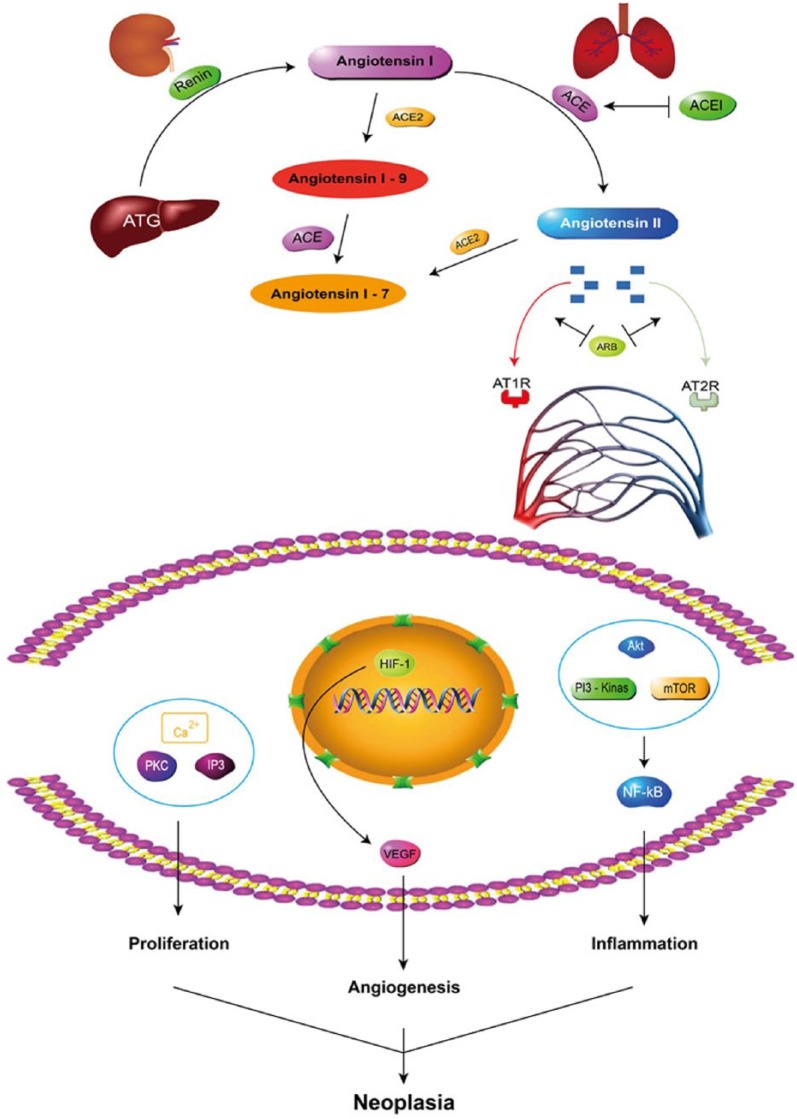
Observational studies have shown that ACEI/ARB may reduce the incidence and mortality of cancer. Angiotensin II receptors AT1 and AT2 are widely distributed in the cardiovascular system, brain, liver, kidney, adrenal cortex, muscle and connective tissue.48,49 Angiotensin II, the known key active peptide of RAS, binds the AT1 receptor to promote the initiation and progression of cancer by stimulating cell proliferation, angiogenesis and inflammation50–53 (Figure 8). The network regulation may explain the decreased risk of cancer incidence and mortality with RAS blockade.54–56 Cancer cells are angiogenesis dependent, and thus blockade of angiogenesis could limit tumour growth.57–60 Indeed, several studies have shown angiotensin II in the promotion of angiogenesis due to increased vascular endothelial growth factor expression by activation of the AT1.61–63 In contrast to angiotensin II, the angiotensin-(1-7) inhibits both angiogenesis and cell proliferation.64–66 It is well known that the ACE2–angiotensin-(1-7)–Mas axis serves as the principal counter-regulatory mechanism for the ACE–angiotensin II–AT1 axis.67 An increased ACE/ACE2 activity ratio might lead to increased angiotensin II generation and increased catabolism of angiotensin (1-7). Monotherapy with ACEIs could upregulate ACE2 expression to lower the risk of cancer.68 These findings might explain the consistent findings in the observational studies included in this meta-analysis.
Figure 8.
Current view of the renin–angiotensin system cascade.
The protection with RAS blockade against cancer in observational studies diminished in the pooled analysis of the RCTs. The difference in outcomes for cancer between the two types of studies is attributed to sample size and duration of follow-up (Table 3). The 17 observational studies included in this meta-analysis provided a larger sample size than the 14 RCTs, and had a longer average duration of follow-up. Although observational studies have inherent limitations, which might compromise real associations between tested drugs and outcomes, it is difficult to implement RCTs in real clinical settings and lifetime treatment. In the present study, RCTs included in this analysis were not conducted to evaluate the effects of ARBs and ACEIs on the risk of cancer and cancer-related death as the primary endpoints. Concerning the differences in outcomes for cancer, here we have reported cancer incidence and mortality changes separately in RCTs and observational studies.
There are limitations in this study. First, the incidence and mortality of cancer were not the primary endpoints in some of the included case–control studies. Second, lack of the original data has prevented our direct evaluation of the effects of ARB/ACEI on different ethnic groups. Finally, the exact doses and dosages were inconsistent in the 31 studies included. All these limitations might affect the implication and interpretation of the findings from the present study.
In summary, the therapeutic benefits with ACEIs and ARBs reported in case–control studies and cohort studies might diminish in RCTs. The clinical design, site of cancer and duration of follow-up may affect the clinical outcomes.
Acknowledgments
The authors would like to thank Mr Vincent Von Bokern and Golden Wilson for his English proofing. All authors agreed on the final version of the manuscript
Footnotes
Authors’ contributions: The study was designed by JS, YMH, XNS, XZH, XZ and HLZ; YMH, JS, XNS were responsible for critical revision of the article for important intellectual content and draft the manuscript; YMH, MW, YHP, XZH conducted the collection and assembly of the data; MW, YMH and WL performed the statistical analyses; MHZ, XXZ, YS and HLZ interpreted the data; JS and YMH drafted the manuscript; HLZ had primary responsibility for the final content.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work was supported by grants from the Guilin Medical University (KY2011002), Program for Innovative Research Team of Guilin Medical University (PIRTGMU) and National Natural Science Foundation of China (81270934).
References
- 1. Rosenthal T, Gavras I. Angiotensin inhibition and malignancies: a review. J Human Hypertens 2009; 23: 623–635. [DOI] [PubMed] [Google Scholar]
- 2. Sipahi I, Debanne SM, Rowland DY, et al. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncology 2010; 11: 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. FDA drug safety communication: no increase in risk of cancer with certain blood pressure drugs – angiotensin receptor blockers (ARBs). http://www.fda.gov/Drugs/DrugSafety/ucm257516.htm, 2010. (accessed 22 October 2011).
- 4. Mulrow PJ. Angiotensin II and aldosterone regulation. Regulatory Peptides 1999; 80: 27–32. [DOI] [PubMed] [Google Scholar]
- 5. de Gasparo M, Catt KJ, Inagami T, et al. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 2000; 52: 415–472. [PubMed] [Google Scholar]
- 6. Moreno M, Bataller R. Cytokines and renin–angiotensin system signaling in hepatic fibrosis. Clin Liver Dis 2008; 12: 825–852, ix. [DOI] [PubMed] [Google Scholar]
- 7. Billet S, Aguilar F, Baudry C, et al. Role of angiotensin II AT1 receptor activation in cardiovascular diseases. Kidney Int 2008; 74: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 8. Carl-McGrath S, Ebert MP, Lendeckel U, et al. Expression of the local angiotensin II system in gastric cancer may facilitate lymphatic invasion and nodal spread. Cancer Biol Ther 2007; 6: 1218–1226. [DOI] [PubMed] [Google Scholar]
- 9. De Paepe B, Verstraeten VL, De Potter CR, et al. Growth stimulatory angiotensin II type-1 receptor is upregulated in breast hyperplasia and in situ carcinoma but not in invasive carcinoma. Histochem Cell Biol 2001; 116: 247–254. [DOI] [PubMed] [Google Scholar]
- 10. Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab: TEM 2005; 16: 293–239. [DOI] [PubMed] [Google Scholar]
- 11. Bhaskaran K, Douglas I, Evans S, et al. Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ 2012; 344: e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang CC, Chan WL, Chen YC, et al. Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am J Cardiol 2011; 107: 1028–1033. [DOI] [PubMed] [Google Scholar]
- 13. Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: Wiley, 2008. [Google Scholar]
- 16. Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med 1991; 10: 1665–1677. [DOI] [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 19. Makar GA, Holmes JH, Yang YX. Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst 2014; 106: djt374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rao GA, Bottai M, Uemura H, et al. Angiotensin receptor blockers and risk of prostate cancer among United States veterans. J Clin Pharmacol 2013; 53: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rao GA, Shoaibi A, Pai SG, et al. Angiotensin receptor blockers: are they related to lung cancer? J Hypertens 2013; 31: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Engineer DR, Burney BO, Hayes TG, et al. Exposure to ACEI/ARB and beta-blockers is associated with improved survival and decreased tumor progression and hospitalizations in patients with advanced colon cancer. Transl Oncol 2013; 6: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chae YK, Valsecchi ME, Kim J, et al. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest 2011; 29: 585–593. [DOI] [PubMed] [Google Scholar]
- 24. The NAVIGATOR Study Group, McMurray JJ, Holman RR, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010; 362: 1477–1490. [DOI] [PubMed] [Google Scholar]
- 25. The ONTARGET Study Group, Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559. [DOI] [PubMed] [Google Scholar]
- 26. Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med 2006; 354: 1685–1697. [DOI] [PubMed] [Google Scholar]
- 27. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004; 363: 2022–2031. [DOI] [PubMed] [Google Scholar]
- 28. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349: 1893–1906. [DOI] [PubMed] [Google Scholar]
- 29. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860. [DOI] [PubMed] [Google Scholar]
- 30. Cohn JN, Tognoni G; Valsartan Heart Failure Trial I. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675. [DOI] [PubMed] [Google Scholar]
- 31. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 32. Cardwell CR, McMenamin UC, Hicks BM, et al. Drugs affecting the renin–angiotensin system and survival from cancer: a population based study of breast, colorectal and prostate cancer patient cohorts. BMC Med 2014; 12: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Azoulay L, Assimes TL, Yin H, et al. Long-term use of angiotensin receptor blockers and the risk of cancer. PloS one 2012; 7: e50893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. The Telmisartan Randomised AssessmeNt Study in, A. C. E. iNtolerant subjects with cardiovascular Disease Study Group, Yusuf S, Teo K, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008; 372: 1174–1183. [DOI] [PubMed] [Google Scholar]
- 35. Lever AF, Hole DJ, Gillis CR, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 1998; 352: 179–184. [DOI] [PubMed] [Google Scholar]
- 36. Chiang YY, Chen KB, Tsai TH, et al. Lowered cancer risk with ACE inhibitors/ARBs: a population-based cohort study. J Clin Hypertens (Greenwich) 2014; 16: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang KL, Liu CJ, Chao TF, et al. Long-term use of angiotensin II receptor blockers and risk of cancer: a population-based cohort analysis. Int J Cardiol 2013; 167: 2162–2166. [DOI] [PubMed] [Google Scholar]
- 38. Chang CH, Lin JW, Wu LC, et al. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: a nationwide case–control study. J Clin Oncol: official journal of the American Society of Clinical Oncology 2011; 29: 3001–3007. [DOI] [PubMed] [Google Scholar]
- 39. Hallas J, Christensen R, Andersen M, et al. Long term use of drugs affecting the renin–angiotensin system and the risk of cancer: a population-based case–control study. Br J Clin Pharmacol 2012; 74: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pasternak B, Svanstrom H, Callreus T, et al. Use of angiotensin receptor blockers and the risk of cancer. Circulation 2011; 123: 1729–1736. [DOI] [PubMed] [Google Scholar]
- 41. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 995–1003. [DOI] [PubMed] [Google Scholar]
- 42. Lindholm LH, Anderson H, Ekbom T, et al. Relation between drug treatment and cancer in hypertensives in the Swedish Trial in Old Patients with Hypertension 2: a 5-year, prospective, randomised, controlled trial. Lancet 2001; 358: 539–544. [DOI] [PubMed] [Google Scholar]
- 43. Koomen ER, Herings RM, Guchelaar HJ, et al. Melanoma incidence and exposure to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cancer Epidemiol 2009; 33: 391–395. [DOI] [PubMed] [Google Scholar]
- 44. The Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease [HIJ-CREATE] Study Group, Sugiura R, Ogawa H, et al. Candesartan-based therapy and risk of cancer in patients with systemic hypertension (Heart Institute of Japan Candesartan Randomized Trial for Evaluation in Coronary Artery Disease [HIJ-CREATE] substudy). Am J Cardiol 2012; 109: 576–580. [DOI] [PubMed] [Google Scholar]
- 45. Yusuf S, Diener HC, Sacco RL, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med 2008; 359: 1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. The Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan Study Group, Dickstein K, Kjekshus J. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Lancet 2002; 360: 752–760. [DOI] [PubMed] [Google Scholar]
- 47. Chin HJ, Oh SW, Goo HS, et al. Effect of RAAS inhibition on the incidence of cancer and cancer mortality in patients with glomerulonephritis. J Korean Med Sci 2011; 26: 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suissa S, Azoulay L. Response to Bodmer et al. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012; 35: 2665–2673. Diabetes Care 2013; 36: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang X, Chan JC. Comment on Suissa and Azoulay. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012; 35: 2665–2673. Diabetes Care 2013; 36: e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bodmer M, Jick SS, Meier CR. Comment on: Suissa and Azoulay. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012; 35: 2665–2673. Diabetes Care 2013; 36: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012; 35: 2665–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McNulty EJ, Ng W, Spertus JA, et al. Surgical candidacy and selection biases in nonemergent left main stenting: implications for observational studies. JACC Cardiovasc Intervent 2011; 4: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 53. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011; 26: 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang X, Zhao H, Sui Y, et al. Additive interaction between the renin–angiotensin system and lipid metabolism for cancer in type 2 diabetes. Diabetes 2009; 58: 1518–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Paepe B. Anti-angiogenic agents and cancer: current insights and future perspectives. Recent Patents on Anti-cancer Drug Discovery 2009; 4: 180–185. [DOI] [PubMed] [Google Scholar]
- 56. Walther T, Menrad A, Orzechowski HD, et al. Differential regulation of in vivo angiogenesis by angiotensin II receptors. FASEB J: official publication of the Federation of American Societies for Experimental Biology 2003; 17: 2061–2067. [DOI] [PubMed] [Google Scholar]
- 57. Pupilli C, Lasagni L, Romagnani P, et al. Angiotensin II stimulates the synthesis and secretion of vascular permeability factor/vascular endothelial growth factor in human mesangial cells. J Am Soc Nephrol: JASN 1999; 10: 245–255. [DOI] [PubMed] [Google Scholar]
- 58. Weidner N. The importance of tumor angiogenesis: the evidence continues to grow. Am J Clin Pathol 2004; 122: 675–677. [DOI] [PubMed] [Google Scholar]
- 59. Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology 2005; 46: 481–489. [DOI] [PubMed] [Google Scholar]
- 60. Clapp C, Thebault S, Jeziorski MC, et al. Peptide hormone regulation of angiogenesis. Physiol Rev 2009; 89: 1177–1215. [DOI] [PubMed] [Google Scholar]
- 61. Miyajima A, Kikuchi E, Kosaka T, et al. Angiotensin II type 1 receptor antagonist as an angiogenic inhibitor in urogenital cancer. Rev Recent Clin Trials 2009; 4: 75–78. [DOI] [PubMed] [Google Scholar]
- 62. Yasumatsu R, Nakashima T, Masuda M, et al. Effects of the angiotensin-I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cells. J Cancer Res Clin Oncol 2004; 130: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Imai N, Hashimoto T, Kihara M, et al. Roles for host and tumor angiotensin II type 1 receptor in tumor growth and tumor-associated angiogenesis. Lab Invest: a journal of technical methods and pathology 2007; 87: 189–198. [DOI] [PubMed] [Google Scholar]
- 64. Benndorf R, Boger RH, Ergun S, et al. Angiotensin II type 2 receptor inhibits vascular endothelial growth factor-induced migration and in vitro tube formation of human endothelial cells. Circ Res 2003; 93: 438–447. [DOI] [PubMed] [Google Scholar]
- 65. Feng Y, Wan H, Liu J, et al. The angiotensin-converting enzyme 2 in tumor growth and tumor-associated angiogenesis in non-small cell lung cancer. Oncol Rep 2010; 23: 941–948. [DOI] [PubMed] [Google Scholar]
- 66. Machado RD, Santos RA, Andrade SP. Opposing actions of angiotensins on angiogenesis. Life Sci 2000; 66: 67–76. [DOI] [PubMed] [Google Scholar]
- 67. Swanson E. Level 2 observational studies: a practical alternative to randomized trials in plastic surgery. Aesth Surg J/the American Society for Aesthetic Plastic Surgery 2016; 36: NP87–NP88. [DOI] [PubMed] [Google Scholar]
- 68. Gallagher PE, Cook K, Soto-Pantoja D, et al. Angiotensin peptides and lung cancer. CurrCancer Drug Targets 2011; 11: 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]